Abstract
Background
The impact of serum uric acid (UA) on morbidity and mortality in hemodialysis (HD) patients is quite controversial in relation to the general population. The aim of this study was to evaluate the association of serum UA with both mortality and left ventricular hypertrophy (LVH) in HD patients.
Methods
This longitudinal study enrolled 225 prevalent HD patients who were classified into three groups according to their follow-up-averaged UA (FA-UA) levels: low FA-UA (FA-UA <400 µmol/L), intermediate/reference FA-UA (FA-UA between 400 and 450 µmol/L) and high FA-UA (FA-UA >450 µmol/L). Echocardiography was performed on a nondialysis day and the presence of LVH was defined based on a left ventricular mass index (LVMI) >131 and >100 g/m2 for men and women, respectively. The patients were followed during a 60-month period.
Results
The mean FA-UA level was 425 ± 59 µmol/L (range 294–620). There was a consistent association of higher FA-UA with better nutritional status (higher body mass index, normalized protein catabolic rate, creatinine, albumin and phosphorus), higher hemoglobin, but lower C-reactive protein and LVMI. During the 5-year follow-up, 81 patients died (36%) and the main causes of death were cardiovascular (CV) related (70%). When compared with the reference group, the hazard ratio for all-cause mortality was 1.75 [95% confidence interval (CI) 1.02–2.98; P = 0.041] in the low FA-UA group, but there was no significant association with the high FA-UA group. In contrast, FA-UA did not show an association with CV mortality neither with the lower nor with the high FA-UA group. The unadjusted odds ratio (OR) of LVH risk in the low FA-UA compared with the reference FA-UA group was 3.11 (95% CI 1.38–7.05; P = 0.006), and after adjustment for age, gender, diabetes and CV disease, ORs for LVH persisted significantly only in the low FA-UA group [OR 2.82 (95% CI 1.16–6.88,); P = 0.002].
Conclusions
Low serum UA is a mortality risk factor and is associated with LVH in HD patients. These results are in contrast with the association of UA in the general population and should be the subject of further research.
Keywords: chronic hemodialysis, left ventricular hypertrophy mortality, uric acid
INTRODUCTION
Uric acid (UA) is the end product of purine metabolism in humans, and despite being a major antioxidant in human plasma, it both correlates and predicts development of conditions associated with oxidative stress. Thus, in the general population, an elevation of serum UA has been shown to be associated with an increased risk of hypertension [1], diabetes mellitus [2], coronary heart disease [3], cardiovascular disease (CVD) [4], stroke [5] and all-cause and CV mortality [6]. An elevated UA level is commonly observed in chronic kidney disease (CKD) patients, and although two meta-analyses showed that higher serum UA levels were associated with higher all-cause and CV mortality in patients with CKD [7, 8], the role of UA in CKD progression is still under debate [9, 10].
However, regarding hemodialysis (HD) patients, the impact of UA level on their morbidity and mortality remains more obscure and quite controversial. Serum UA levels have a wide range in HD patients. Studies have reported serum UA levels ≥7 mg/dL to be present in 40–80% of all HD patients [11, 12]. The kinetics of UA clearance in HD patients is different, very complex and less investigated [13]. Although there is an inverse association with mortality in relation to the general population, there is an inverse association in relation to oxidative stress, which may be due to different physiology of UA in HD patients [14]. Many studies involving HD patients have shown an inverse association between serum UA and CV and/or all-cause mortality [11, 12, 15], however, some studies have reported either increased or no difference in mortality [16, 17]. In the HD population, the higher UA concentrations represent better nutritional status [11, 12, 18], and concentrations were inversely correlated with markers of malnutrition and inflammation. In this association, UA shows a paradoxical correlation with some traditional CV risk factors and uremia-related factors.
Left ventricular hypertrophy (LVH) with multifactorial etiology is an important predictor of CV mortality and morbidity in HD patients. In the last decade, numerous cross-sectional clinical studies have investigated the association between UA and LVH in the general population, hypertensive cohorts and patients with diabetes and renal failure [19–21]. The Pressioni Arteriose Monitorate E Loro Associazioni 10-year follow-up study [22] is the first study that showed UA is a predictor of long-term echocardiographic changes from normal left ventricular mass index (LVMI) to LVH in a community sample. However, to our knowledge, there is no information in the literature on the clinical effect of longitudinal changes in serum UA levels on LVH in HD patients, and hence this is the first clinical study to assess the relationship between serum UA level and LVH in HD patients.
Therefore the aim of the current 5-year follow-up study was not only to evaluate the association between serum UA level and all-cause or CV mortality, but also with LVH in patients treated at a single HD center.
MATERIALS AND METHODS
Study design
This observational 5-year prospective longitudinal study was conducted at a single outpatient dialysis center. The study was approved by our institutional ethics committee. All patients were informed about the aim of the study and participated voluntarily after providing written consent.
Study population, comorbidity index and follow-up
A total of 225 prevalent HD patients on regular HD treatment for at least 3 months were recruited for the study. Patient demographics, etiology of ESRD, duration of maintenance HD and comorbid conditions on enrolment were obtained from the medical records at our unit. We determined the comorbidity index for atherosclerotic CVD using the scoring system for each of these diseases, namely coronary heart disease, cerebrovascular disease and peripheral vascular disease. The comorbidity index of individual patients is scored with a value ranging from 0 to 3, with 0 indicating the absence of disease and increasing values indicating an increasing severity of disease [23, 24]. The follow-up period of patients was from January 2010 to December 2014. Patients transferred to alternative renal replacement therapies (kidney transplantation or peritoneal dialysis) were censored from the survival analysis. CV mortality was defined as death resulting from coronary heart disease, sudden death, stroke or complicated peripheral vascular disease.
Laboratory measurements, dialysis parameters and echocardiography
All patients were on 4–4.5 h standard thrice-weekly HD at a blood flow rate of 250 mL/min and a dialysis solution flow rate of 500 mL/min. Dialysis treatments were performed with high-flux biocompatible dialyzer membranes with surface areas of 1.4–1.8 m2. None of the patients was receiving UA-lowering agents such as allopurinol during the follow-up period.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured routinely before each dialysis session. Pulse pressure (PP) was evaluated by the formula (SBP − DBP). Echocardiography was performed in all of these patients prior to starting the study, on a nondialysis day. Left ventricular end-diastolic diameter, left ventricular end-systolic diameter, interventricular septal thickness, left atrial diameter and left ventricular posterior wall thickness were measured. LVMI was calculated by dividing LVM by the body surface area. LVH was defined as an LVMI >131 g/m2 in men and >100 g/m2 in women. Left ventricular mass was calculated using the Devereux modified method {i.e. LVM = 1.04[(IVSTd + LVIDd + LVPWTd)3−LVIDd3]−13.6 g}, where IVSTd is interventricular septal thickness in doastole, LVIDd is the left ventricular internal dimension in diastole and LVPWT is the left ventricular posterior wall thickness in diastole [25].
Venous blood samples collected pre- and postdialysis at the start of the study and then on the first Monday or Tuesday of each month during the follow-up period were used for analysis and included hemoglobin, albumin, creatinine, serum calcium, phosphorus, potassium, UA, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and C-reactive protein (CRP). For all these monthly laboratory analyses, an average of 35.7 values were taken per patient. Serum total intact parathyroid hormone was measured at 6 months and ferritin quarterly. Brain natriuretic peptide (BNP) was measured only at the beginning of the study, and blood samples were drawn from vascular access just before HD. Repetitive blood samples of UA during the follow-up period, collected with other laboratory analyses (regular intervals of every month in a period of 60 months for patients who survived and to the last laboratory for patients who died during the follow-up period) were used to calculate the follow-up-averaged UA (FA-UA) to represent the UA level more precisely for HD patients. Dietary protein intake was also estimated by using the normalized protein catabolic rate (nPCR) calculation from patient’s urea generation rate by urea kinetic modeling [26]. Single-pool model urea kinetics were used to estimate the nPCR. The Kt/V was calculated by the Daugirdas method [27]. Postdialysis blood urea nitrogen from the same dialysis session was measured to calculate the delivered dose of dialysis (Kt/V) during a treatment session.
Statistical analysis
Data were summarized as mean ± SD. Student’s t-test was used to analyze the differences between two mean values. A the linear regression analysis was performed to elucidate the associations between FA-UA level and each variable. The strength of the linear correlation between FA-UA and LVH was tested by Pearson’s correlation coefficient. Categorical data were compared between groups by the chi-square test. To compare the characteristics according to FA-UA level, participants were stratified into three groups of FA-UA levels as follows: FA-UA <400 µmol/L (low FA-UA group), FA-UA 400–450 µmol/L (intermediate FA-UA group) and FA-UA >450 µmol/L (high FA-UA group). The intermediate FA-UA group patients served as the reference group. The groups were compared using analysis of variance. Survival curves were assessed using Kaplan–Meier analysis (logrank test) to explore the association between all-cause and CV mortality across three FA-UA categories. Analysis of the receiver operating characteristic (ROC) curves and the area under the ROC curves (AUC) was performed to evaluate the FA-UA levels as a predictor of all-cause mortality in the study populations. To calculate the relative risk of death, hazard ratios (HRs) and 95% confidence intervals (CIs) were obtained by Cox proportional hazards models. The odds ratios (ORs) and their 95% CIs of the risk of LVH according to the three groups of FA-UA levels were calculated by logistic regression, having the intermediate FA-UA group as a reference. Data were adjusted for age and gender, then for age, gender, diabetes and CV diseases. P-values <0.05 were considered statistically significant. SPSS statistical software (version 14.0; SPSS, Chicago, IL, USA) was used for the analysis.
RESULTS
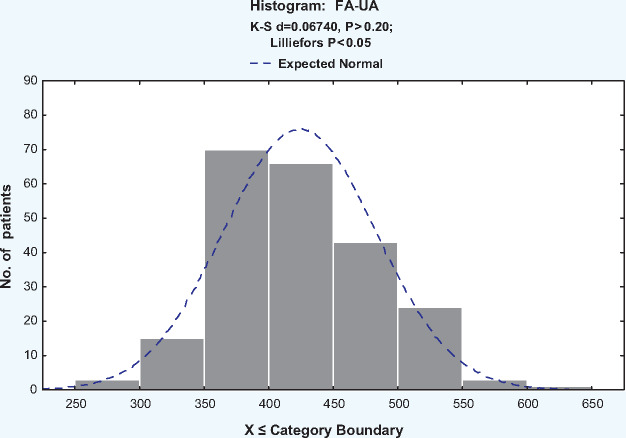
The baseline characteristics of the study population are shown in Table 1. The mean baseline UA level was 414 ± 89 µmol/L and the mean 5-year FA-UA level was 425 ± 59 µmol/L (range 294–620; in our laboratory, the normal value of UA is 150–450 μmol/L). In our study we used the FA-UA to represent the UA level in HD patients. Figure 1 reveals FA-UA as approximately normally distributed, with a mean of 424.0 µmol/L. The mean FA-UA level in male patients (431 ± 58 µmol/L) was higher than that in female patients (415 ± 60 µmol/L, P = 0.048). Patients with diabetes and CVD had significantly lower FA-UA than patients who did not have diabetes and CVD (393 ± 54 versus 431 ± 58 µmol/L, P = 0.000; 411 ± 59 versus 431 ± 59 µmol/L, P = 0.000).
Table 1.
Clinical and laboratory parameters of the study population
| Parameters | Mean ± SD | Range |
|---|---|---|
| Age (years) | 48.9 ± 15.1 | 17–81 |
| Gender (male/female), n/n | 133/90 | – |
| Diabetes, n (%) | 38 (17.0) | – |
| CVD, n (%) | 57 (30.8) | – |
| HD vintage (months) | 106 ± 72 | 3–311 |
| BMI (kg/m2) | 24.0 ± 4.3 | 16.2–45.8 |
| SBP (mmHg) | 136 ± 23 | 84–198 |
| DBP (mmHg) | 81 ± 15 | 40–120 |
| PP (mmHg) | 55 ± 15 | 24–106 |
| Hemoglobin (g/L) | 106.9 ± 12.7 | 62–138 |
| Single-pool Kt/V | 1.24 ± 0.21 | 0.71–1.76 |
| Follow-up UA (µmol/L) | 425 ± 59 | 294–620 |
| Urea (mmol/L) | 29.2 ± 3.6 | 17.8–39 |
| Creatinine (µmol/L) | 919.9 ± 203.9 | 450–1670 |
| Albumin (g/L) | 38.5 ± 3.2 | 26.7–46.9 |
| PCR (g/kg/day) | 1.03 ± 0.14 | 0.58–1.43 |
| iPTH (pg/mL) | 216.5 ± 231.1 | 15–1670 |
| HDL cholesterol (mmol/L) | 0.99 ± .27 | 0.4–1.80 |
| LDL cholesterol (mmol/L) | 2.66 ± 0.87 | 1.2–7.8 |
| Calcium (mmol/L) | 2.29 ± 0.14 | 1.89–2.71 |
| Phosphorus (mmol/L) | 1.50 ± 0.34 | 0.63–2.7 |
| Potassium (mmol/L) | 5.99 ± 0.60 | 4.4–8.0 |
| BNP (pg/mL) | 1321 ± 1366 | 109.7–8237.6 |
| LVMI (g/m2) | 138.4 ± 49.7 | 54–438 |
| CRP (mg/L) | 15.9 ± 23.8 | 0.35–117 |
iPTH, intact parathyroid hormone.
FIGURE 1.
Distribution of FA-UA concentration.
The FA-UA level of patients with atherosclerotic events (coronary heart disease, cerebrovascular disease and peripheral vascular disease) was compared with that of patients who did not have the condition. A statistically significant difference (present versus absent) was found for coronary heart disease (416 ± 57 versus 441 ± 59 µmol/L, P = 0.002) and peripheral vascular disease (411 ± 61 versus 435 ± 56 µmol/L, P = 0.002), but not for cerebrovascular disease (421 ± 58 versus 426 ± 59 µmol/L, P = 0.616).
Linear regression analysis showed that FA-UA showed a significant positive association with BMI, hemoglobin, creatinine, nPCR, length of HD in hours, ultrafiltration and phosphorus but was negative with age, HDL, BNP and LVH (the table is not shown). Of the patients stratified into three groups, 87 patients (38.7%) were in the low, 67 (29.8%) in the intermediate and 71 (31.6%) in the high FA-UA group. The characteristics of the whole cohort and comparisons across the three FA-UA groups are summarized in Table 2. As the FA-UA level decreased, the participants were older. The low FA-UA level was significantly associated with the lower BMI, nPCR and hemoglobin and higher HDL cholesterol versus the high FA-UA level, but also with lower serum phosphorus and albumin levels and higher CRP versus the intermediate FA-UA level. Ultrafiltration increases with increasing FA-UA levels. Patients in the low FA-UA group had significantly lower creatinine and length of HD but higher LVMI compared with patients in the intermediate and high groups. With regards to BNP in the three groups of FA-UA there was borderline significance.
Table 2.
Characteristics of HD patients with different serum UA concentrations
| Characteristics | FA-UA (µmol/L) |
P-value | ||
|---|---|---|---|---|
| Low (FA-UA <400 µmol/L) (n = 87) | Intermediate (FA-UA 400–450 μmol/L) (n = 67) | High (FA-UA >450 μmol/L) (n = 71) | ||
| Age (years) | 53.8 ± 14.5* | 49.7 ± 14.6* | 42.4 ± 14.0* | 0.0000 |
| HD vintage (months) | 98.5 ± 73.5 | 105.6 ± 69.1 | 115.3 ± 74.0 | NS |
| Diabetes, n (%) | 23 (26.4) | 9 (13.4) | 6 (8.5) | 0.007 |
| CVD, n (%) | 34 (39.1) | 24 (35.8) | 15 (21.1) | 0.039 |
| BMI (kg/m2) | 23.2 ± 3.4* | 23.9 ± 4.6 | 25.2 ± 4.8* | 0.016 |
| SBP (mmHg) | 137 ± 23 | 140 ± 21 | 132 ± 24 | NS |
| DBP (mmHg) | 80 ± 15 | 84 ± 14 | 80 ± 16 | NS |
| Length of HD (minutes) | 236 ± 10* | 241 ± 12* | 243 ± 11* | 0.001 |
| Hemoglobin (g/L) | 104.0 ± 12.9* | 107.1 ± 12.9 | 110.0 ± 11.6* | 0.012 |
| Ultrafiltration (L) | 2.9 ± 0.7* | 3.1 ± 0.8* | 3.6 ± 0.8* | 0.000 |
| Single-pool Kt/V | 1.25 ± 0.23 | 1.23 ± 0.19 | 1.24 ± 0.19 | NS |
| nPCR (g/kg/day) | 1.08 ± 0.144* | 1.11 ± 0.12 | 1.14 ± 0.12* | 0.008 |
| Creatinine (µmol/L) | 810.6 ± 163.2* | 959.8 ± 156.2* | 1013.2 ± 228.0* | 0.000 |
| Calcium (mmol/L) | 2.27 ± 0.12 | 2.32 ± 0.16 | 2.28 ± 0.15 | NS |
| Phosphorus (mmol/L) | 1.41 ± 0.33* | 1.56 ± 0.35* | 1.53 ± 0.34 | 0.018 |
| Albumin (g/L) | 37.7 ± 3.6* | 39.3 ± 2.5* | 38.7 ± 3.1 | 0.008 |
| HDL cholesterol (mmol/L) | 1.04 ± 0.30* | 1.02 ± 0.21 | 0.93 ± 0.26* | 0.025 |
| LDL cholesterol (mmol/L) | 2.68 ± 0.82 | 2.67 ± 0.83 | 2.60 ± 0.97 | NS |
| iPTH (pg/mL), median (IQR) | 88.3 (51–233) | 138.7 (69–297) | 177 (102–309) | NS |
| BNP (pg/mL), median (IQR) | 935 (548–1894) | 860 (358–1349) | 698 (369–1527) | 0.054 |
| CRP (mg/L), median (IQR) | 10.3 (3.7–23)* | 5.7 (3.8–11.9)* | 8.2 (3.8–17.9) | 0.044 |
| LVMI (g/m2) | 153.1 ± 59.9* | 131.6 ± 41.0* | 131.2 ± 44.5* | 0.029 |
Data are presented as mean ± SD unless stated otherwise.
Low group = 1, intermediate group = 2, high group = 3/age (1 versus 3, P = 0.000; 2 versus 3, P = 0.008), BMI (1 versus 3, P = 0.011), nPCR (1 versus 3, P = 0.005), HDL cholesterol (1 versus 3, P = 0.024), hemoglobin (1 versus 3, P = 0.007), phosphorus (1 versus 2, P = 0.019), albumin (1 versus 2, P = 0.006), CRP (1 versus 2, P = 0.032), ultrafiltration (1 versus 3, P = 0.00; 2 versus 3, P = 0.001), creatinine (1 versus 2, P = 0.000; 1 versus 3, P = 0.000), length of HD (1 versus 2, P = 0.000; 1 versus 3, P = 0.034), LVH (1 versus 2, P = 0.042; 1 versus 3, P = 0.038).
iPTH, intact parathyroid hormone; IQR, interquartile range; NS, not significant.
Serum FA-UA and mortality
Of the 225 HD patients during the 5-year follow-up, 81 patients died (36%) and the main causes of death were CV related [57 patients (70%)]. The FA-UA level of survivors (432 ± 58 µmol/L) was higher than that of those who died of all-cause (410 ± 59 µmol/L) and CV causes (407 ± 59 µmol/L) and the difference was statistically significant (P = 0.009 and P = 0.005). But this difference was not statistically significant in terms of baseline UA level (survivors versus died of all-cause and CV causes, 418 ± 95 versus 407 ± 78 and 401 ± 81; P = 0.371 and P = 0.230).
Patients with FA-UA <400 µmol/L had a significantly higher all-cause (logrank, P = 0.003) and CV mortality (logrank, P = 0.004) rate compared with those with FA-UA 400–450 µmol/L and FA-UA >450 µmol/L (Figure 2). The AUC in the ROC was 0.61 (95% CI 0.53–0.68). The cut-off point for FA-UA as a predictor of the 5-year all-cause mortality according to the ROC curve was 379.5 μmol/L (the figure is not shown). When compared with the reference group, the HR for all-cause mortality was 1.75 (95% CI 1.02–2.98, P = 0.041) in the low FA-UA group, but there was no significant association with the high FA-UA group. In contrast, FA-UA did not show an association with CV mortality with the low or the high FA-UA group (Table 3).
FIGURE 2.
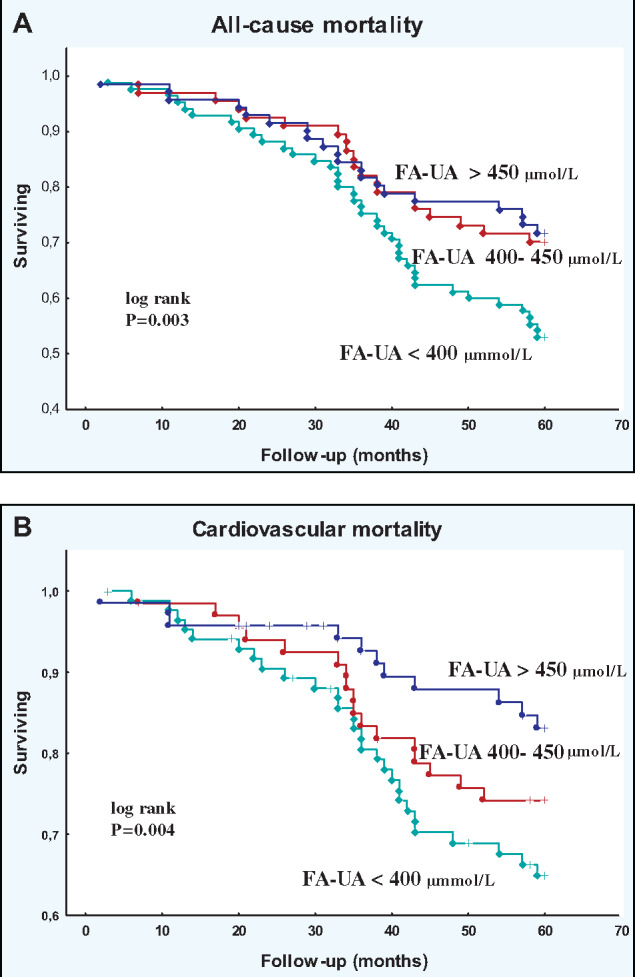
Kaplan–Meier survival curves for all follow-up study patients according to low, intermediate and high FA-UA groups: (A) all-cause and (B) CV mortality.
Table 3.
HR (95% CI) of all-cause and CV mortality
| HR | 95% CI | P-value | |
|---|---|---|---|
| All-cause mortality | |||
| FA-UA 400–450 µmol/L (reference) | |||
| FA-UA <400 µmol/L versus 400–450 µmol/L | 1.75 | 1.02–2.98 | 0.041 |
| FA-UA >450 μmol/L versus 400–450 µmol/L | 0.85 | 0.51–1.75 | 0.276 |
| CV mortality | |||
| FA-UA 400–450 µmol/L (reference) | |||
| FA-UA <400 µmol/L versus 400–450 µmol/L | 1.45 | 0.79–2.64 | 0.222 |
| FA-UA >450 μmol/L versus 400–450 µmol/L | 0.61 | 0.28–1.31 | 0.200 |
Serum FA-UA and LVH
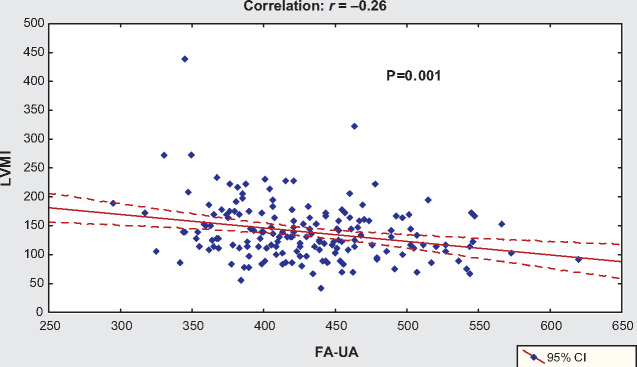
Mean LVMI was 138.4 ± 49.7 g/m2 (123.2 ± 42.9 for women and 147.5 ± 51.5 for men). FA-UA showed a significant negative association with LVH (R2 = 0.069, β = −0.233, β = −0.262, P = 0.001) (Figure 3). The mean FA-UA level in patients with LVH (417 ± 54 µmol/L) was lower than in those without LVH (448 ± 56 µmol/L, P = 0.000). Compared with patients without LVH, patients with LVH were older (42.0 ± 13.2 versus 49.5 ± 14.5, P = 0.000) and with lower BMI (24.9 ± 4.4 versus 23.3 ± 3.8, P = 0.013), length of HD (4.1 ± 0.2 versus 4.0 ± 0.2 h, P = 0.008), ultrafiltration (3.5 ± 0.8 versus 3.1 ± 0.8, P = 0.000), hemoglobin (111.7 ± 8.8 versus 107.1 ± 11.9 g/L, P = 0.011), creatinine (1041.2 ± 200.8 versus 904.5 ± 175.4 μmol/L, P = 0.000) and albumin levels (39.7 ± 2.4 versus 38.4 ± 3.3 g/L, P = 0.005), but higher SBP (129 ± 21 versus 139 ± 21 mmHg, P = 0.001), DBP (79 ± 14 versus 84 ± 15 mmHg, P = 0.024), PP (50 ± 12 versus 55 ± 14 mmHg, P = 0.011), BNP (1027 ± 1616 versus 2136 ± 3744 pg/mL, P = 0.000) and CRP (9.2 ± 15.4 versus 14.0 ± 16.1 mg/L, P = 0.034) (the table is not shown).
FIGURE 3.
Correlation between serum FA-UA and LVH.
Table 4 shows the OR for the risk of LVH in the lowest and highest tertiles of FA-UA compared with the intermediate FA-UA group. In unadjusted analysis, the OR for the risk of LVH in the low FA-UA group compared with the intermediate group was 3.11 (95% CI 1.38–7.05, P = 0.006). After adjusting for age and gender, then by entering diabetes and CV diseases, the OR for the risk of LVH remained significant only in the low FA-UA group compared with the intermediate group [OR 3.02 (95% CI 1.29–7.05), P = 0.011 and OR 2.82 (95% CI 1.16–6.88), P = 0.23] (Table 4).
Table 4.
OR for the risk of LVH in the lowest and highest tertiles of FA-UA compared with the intermediate tertile (reference)
| FA-UA | OR | 95% CI | P-value |
|---|---|---|---|
| Unadjusted | |||
| FA-UA 400–450 µmol/L | 1 | ||
| FA-UA <400 µmol/L versus 400–450 µmol/L | 3.11 | 1.38–7.05 | 0.006 |
| FA-UA >450 μmol/L versus 400–450 µmol/L | 0.93 | 0.45–1.94 | 0.851 |
| Adjusted for age and gender | |||
| FA-UA 400–450 µmol/L | 1 | ||
| FA-UA <400 µmol/L versus 400–450 µmol/L | 3.02 | 1.29–7.05 | 0.011 |
| FA-UA >450 μmol/L versus 400–450 µmol/L | 1.21 | 0.56–2.63 | 0.624 |
| Adjusted for age, gender, diabetes and CVDs | |||
| FA-UA 400–450 µmol/L | 1 | ||
| FA-UA <400 µmol/L versus 400–450 µmol/L | 2.82 | 1.16–6.88 | 0.023 |
| FA-UA >450 µmol/L versus 400–450 µmol/L | 1.34 | 0.60–2.99 | 0.473 |
DISCUSSION
In this 5-year follow-up study we found an inverse association between FA-UA and all-cause mortality in 225 prevalent HD patients and for the first time it was demonstrated that hypouricemia is associated with LVH in this cohort.
Initially, in our study, we explored the relationship between the FA-UA level and mortality in 225 prevalent HD patients. Until now, in most studies, elevated serum UA levels were associated with lower all-cause and/or CV mortality [11, 12, 15, 18, 28–30], however, some studies reported either increased or no difference [16, 17] in mortality. The principal finding of our results was that when compared with the reference group (FA-UA 400–450 μmol/L), the HR for all-cause mortality was 1.75 in the low FA-UA group, but there was no significant association with the high FA-UA group. In contrast, FA-UA did not show an association with CV mortality in either the low or high FA-UA group. In the ROC curve analysis, FA-UA with a cut-off point of 379.5 μmol/L was identified as a predictor of all-cause mortality, but we think that further analysis with higher sensitivity and specificity may yield better results.
In HD patients, the physiology of UA and its clinical impact is very different from the other types of renal replacement therapy. In HD patients, there is no universally accepted definition of hyperuricemia, so UA levels have a wide range and are significantly different among studies. In our study, the mean FA-UA level was 425 ± 59 µmol/L (range 294–620), and it is quite similar to the value of the Dialysis Outcomes and Practice Patterns Study [11]. Most studies in which elevated serum UA levels were associated with lower mortality relied on the pre-HD serum concentration of UA obtained as a single and baseline measurement of UA. One of the important differences in our study is that we used repeated measurement of UA at regular monthly intervals during the follow-up period. However, the time-averaged UA concentrations, i.e. pre- and post-HD measurements, might be the best predictors of clinical outcomes [31], but the validity of such analyses is still unclear, because the rate of UA rebound after HD has not been evaluated.
It has already been confirmed that UA is a good marker of nutrition in HD patients. In line with previous studies [11, 12, 18, 30], we also found that a high UA level is related to higher nPCR, serum levels of creatinine, phosphorus and albumin and greater BMI. The additional significant finding in our study is that, in the survivor patients, there was an increase in serum UA levels from 418 when analyzing only at the baseline level to 432 µmol/L as a result of FA-UA levels. This finding suggests that higher FA-UA levels relative to lower baseline serum UA levels in the survivor patients may be a result of improvement in nutritional status in HD patients. Beberashvili et al. [30] also showed that the longitudinal increase in UA levels over time in HD patients is accompanied by an improvement in nutritional status and lower mortality rates. However, nutritional features of serum UA warrant additional studies, especially after the results of the study of Park et al. [28], where hypouricemia showed a higher mortality risk among incident dialysis patients with low nPCR but not among those with high nPCR. It is known that uremic malnutrition, i.e. ‘protein-energy wasting’, is a very common condition in HD patients, and the question is whether UA belongs to the group of CV risk factors that are paradoxically associated with the outcome in HD patients, such as low BMI and hypocholesterolemia [32].
The link between UA and inflammation in the HD population has not been sufficiently investigated and is still controversial. In a study with an incident HD population, UA was positively associated with serum CRP levels [33], while a retrospective longitudinal study did not observe any association between UA and CRP levels over time [30]. Our study did not find a direct correlation between FA-UA and CRP, but in the grouping analysis we observed that patients in the low FA-UA group had a significantly higher CRP compared with patients in the intermediate and high FA-UA group.
Although this study showed that patients with coronary heart disease and peripheral vascular disease, as well as those who died of CV causes, had significantly lower FA-UA levels, there was no association between serum UA level and CV mortality in the survival analysis. An interesting finding of this study is that contrary to the serum FA-UA, when only the baseline value of UA was analyzed, there was no statistically significant difference between survivors and those who died of all-cause and CV causes. This result confirms the speculation that UA-mediated biological effects are dependent upon the uremic milieu in HD patients. However, our prevalent cohort was exposed to a very different time period from the initiation of HD to start of the follow-up period. All of this also limits the possibility of analyzing the impact of the HD milieu on changes in UA over time, due to the presence of the temporal bias of this prevalent cohort. Future studies with incident patients may clarify this phenomenon, that is, changes in UA levels over time from the initiation of HD versus baseline levels.
The strength of this study is our finding showing that hypouricemia is associated with LVH in HD patients, which has not been studied until now, so it is difficult to make a comparative analysis with other studies. It is known that ∼70% of patients who start dialysis have LVH [34] and many risk factors are involved in the progression of this condition, such as age, gender, race, diabetes, primary renal disease, BMI, dialysis vintage, hypertension, anemia, inflammation, interdialytic weight gain, left ventricular volume overload and type of vascular access [35–38]. In the last decade, numerous cross-sectional clinical studies in the general population have investigated the association between UA and LVH, with controversial results [19, 20]. Recently, Cuspidi et al. [22], in a 10-year follow-up study, found that each 1 mg/dL increase in UA produced a 26% higher risk of incident LVH in subjects of the general population.
To the best of our knowledge, no information is available from previous studies about the association between UA and LVH in HD patients. Our results showed a significant negative association between LVH and FA-UA and also showed that patients in the low FA-UA group had significantly higher LVMI of 153 g/m2. But the most significant finding of the present analysis is that the OR for the risk of LVH in the low FA-UA group compared with the intermediate group remained significant after adjustment of data for confounders, including age, gender, diabetes and CVDs.
In order to explain the unusual association of UA, we will refer to several hypotheses that relate to the effects of higher serum UA levels in HD patients. Experimental and clinical research has shown that UA may function either as an antioxidant (primarily in plasma) or a pro-oxidant (primarily within the cell) [39]. Therefore the metabolic acidosis present in HD patients may confer lower intracellular concentrations of UA by enhancing intracellular to extracellular efflux of UA. Also, hypothetical analysis of time-averaged concentrations of UA (pre- and post-HD measurements) shows that patients with normal or low serum concentrations of UA before HD would have much lower weekly time-averaged UA levels, which could nadir below the optimal UA concentration needed to execute antioxidant functions. In contrast, patients with high pre-HD concentrations of UA would attain near-normal weekly time-averaged UA levels that could be sufficient to exert its antioxidant effects [14]. However, this is an assumption because, as we have already said, the rate of UA rebound after HD has not been evaluated. One experimental study [40] reported that hyperuricemia increases myocardial oxidative stress and endothelin-1 levels, as one of the possible mechanisms by which UA mediates LVH [41]. But the experimental study of Hsu et al. [42] concluded that UA ameliorated indoxyl sulfate–induced endothelial damage and that a high-normal level of UA was beneficial for HD patients. Based on this study, where UA reduced uremic toxin–induced oxidative stress in endothelial cells, we can suggest that a high-normal level of UA reduced myocardial hypertrophy and interstitial fibrosis, which is why our patients had less LVMI. Hsu et al. [42] suggest that a high-normal UA level in HD patients may be a compensatory mechanism that counteracts the oxidative damage and vascular toxicity from uremic toxins like indoxyl sulfate. Once again we emphasize that the uremic milieu in HD patients changes the influence of UA.
There are several limitations to our study. First, our study is observational with a relatively small number of HD patients from a single center, thus causation cannot be established and the results should be interpreted with caution. Second, our prevalent cohort with a dialytic vintage of 106 ± 72 months might not represent the typical HD population. Third, there is no universally accepted definition of hyperuricemia in HD patients, and even though our stratification of three UA groups was based on repeated measurement of UA at regular monthly intervals during the follow-up period, it can be debated that pre-HD serum UA values alone might not be the best predictors of clinical outcomes. Finally, the most significant limiting factor may be that we could not include the repetitive measurement of LVH so we could provide more reliable evidence of the impact of UA on LVH. Hence all these may contribute to different results.
CONCLUSION
In summary, our study shows that prolonged exposure to hypouricemia is not only associated with higher mortality, but also with LVH in HD patients. This unexpected paradoxical association can only be explained by the hypothesis that the uremic milieu in HD patients changes the influence of UA. However, these results do not prove causality, only association, and should be the subject of further research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Grayson PC, Kim SY, LaValley M et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res 2011; 63: 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bandaru P, Shankar A. Association between serum uric acid levels and diabetes mellitus. Int J Endocrinol 2011; 2011: 604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li M, Hu X, Fan Y et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 2016; 6: 19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kivity S, Kopel E, Maor E et al. Association of serum uric acid and cardiovascular disease in healthy adults. Am J Cardiol 2013; 111: 1146–1151 [DOI] [PubMed] [Google Scholar]
- 5. Li M, Hou W, Zhang X et al. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis 2014; 232: 265–270 [DOI] [PubMed] [Google Scholar]
- 6. Zhao G, Huang L, Song M et al. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis 2013; 231: 61–68 [DOI] [PubMed] [Google Scholar]
- 7. Luo Q, Xia X, Li B et al. Serum uric acid and cardiovascular mortality in chronic kidney disease: a meta-analysis. BMC Nephrol 2019; 20: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia X, Luo Q, Li B et al. Serum uric acid and mortality in chronic kidney disease: a systematic review and meta-analysis. Metab Clin Exp 2016; 65: 1326–1341 [DOI] [PubMed] [Google Scholar]
- 9. Kumagai T, Ota T, Tamura Y et al. Time to target uric acid to retard CKD progression. Clin Exp Nephrol 2017; 21: 182–192 [DOI] [PubMed] [Google Scholar]
- 10. Nacak H, van Diepen M, Qureshi AR et al. Uric acid is not associated with decline in renal function or time to renal replacement therapy initiation in a referred cohort of patients with Stage III, IV and V chronic kidney disease. Nephrol Dial Transplant 2015; 30: 2039–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latif W, Karaboyas A, Tong L et al. Uric acid levels and all cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol 2011; 6: 2470–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bae E, Cho HJ, Shin N et al. Lower serum uric acid level predicts mortality in dialysis patients. Medicine (Baltimore) 2016; 95: e3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanholder RC, De Smet RV, Ringoir SM. Assessment of urea and other uremic markers for quantification of dialysis efficacy. Clin Chem 1992; 38: 1429–1436 [PubMed] [Google Scholar]
- 14. Murea M, Tucker BM. The physiology of uric acid and the impact of end-stage kidney disease and dialysis. Semin Dial 2019; 32: 47–57 [DOI] [PubMed] [Google Scholar]
- 15. Lee SM, Lee AL, Winters TJ et al. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am J Nephrol 2009; 29: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu SP, Pai MF, Peng YS et al. Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 457–462 [DOI] [PubMed] [Google Scholar]
- 17. Muela HC, De Lima JJ, Gowdak LH et al. Prognostic value of serum uric acid in patients on the waiting list before and after renal transplantation. Int J Nephrol 2015; 2015: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beberashvili I, Sinuani I, Azar A et al. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition 2015; 31: 138–147 [DOI] [PubMed] [Google Scholar]
- 19. Iwashima Y, Horio T, Kamide K et al. Uric acid, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertension 2006; 47: 195–202 [DOI] [PubMed] [Google Scholar]
- 20. Cuspidi C, Valerio C, Sala C et al. Lack of association between serum uric acid and organ damage in a never-treated essential hypertensive population at low prevalence of hyperuricemia. Am J Hypertens 2007; 20: 678–685 [DOI] [PubMed] [Google Scholar]
- 21. Zeng C, Cheng D, Sheng X et al. Increased serum uric acid level is a risk factor for left ventricular hypertrophy but not independent of eGFR in patients with type 2 diabetic kidney disease. J Diabetes Res 2017; 2017: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cuspidi C, Facchetti R, Bombelli M et al. Uric acid and new onset left ventricular hypertrophy: findings from the PAMELA population. Am J Hypertens 2017; 30: 279–285 [DOI] [PubMed] [Google Scholar]
- 23. Greenfield S, Apolone G, McNeil BJ et al. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement: comorbidity and outcomes after hip replacement. Med Care 1993; 31: 141–154 [DOI] [PubMed] [Google Scholar]
- 24. Cheung AK, Sarnak MJ, Yan G et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 2000; 58: 353–362 [DOI] [PubMed] [Google Scholar]
- 25. Devereux RB, Alonso DR, Lutas EM et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458 [DOI] [PubMed] [Google Scholar]
- 26. Depner TA, Daugirdas JT. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol 1996; 7: 780–785 [DOI] [PubMed] [Google Scholar]
- 27. Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol 1993; 4: 1205–1213 [DOI] [PubMed] [Google Scholar]
- 28. Park C, Obi Y, Streja E et al. Serum uric acid, protein intake and mortality in hemodialysis patients. Nephrol Dial Transplant 2017; 32: 1750–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim CS, Jin DC, Yun YC et al. Relationship between serum uric acid and mortality among hemodialysis patients: retrospective analysis of Korean end-stage renal disease registry data. Kidney Res Clin Pract 2017; 36: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beberashvili I, Erlich A, Azar A et al. Longitudinal study of serum uric acid, nutritional status, and mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2016; 11: 1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toida T, Sato Y, Komatsu H et al. Pre- and postdialysis uric acid difference and risk of long-term all-cause and cardiovascular mortalities in Japanese hemodialysis patients; Miyazaki Dialysis Cohort Study. Blood Purif 2019; 47: 50–55 [DOI] [PubMed] [Google Scholar]
- 32. Kalantar-Zadeh K, Block G, Humphreys MH et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003; 63: 793–808 [DOI] [PubMed] [Google Scholar]
- 33. Suliman ME, Johnson RJ, García-López E et al. J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 2006; 48: 761–771 [DOI] [PubMed] [Google Scholar]
- 34. Foley RN, Parfrey PS, Harnett JD et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47: 186–192 [DOI] [PubMed] [Google Scholar]
- 35. McMahon LP, Parfrey PS. Cardiovascular aspects of chronic kidney disease In: Brenner BM (ed). Brenner and Rector’s the Kidney, 8th edn Philadelphia, PA: Saunders: Elsevier, 2008, 1697–1727 [Google Scholar]
- 36. Mostovaya IM, Bots ML, van den Dorpel MA et al. Left ventricular mass in dialysis patients, determinants and relation with outcome. Results from the COnvective TRansport STudy (CONTRAST). PLoS One 2014; 9: e84587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekinci C, Karabork M, Siriopol D et al. Effects of volume overload and current techniques for the assessment of fluid status in patients with renal disease. Blood Purif 2018; 46: 34–47 [DOI] [PubMed] [Google Scholar]
- 38. Cataliotti A, Malatino LS, Jougasaki M et al. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc 2001; 76: 1111–1119 [DOI] [PubMed] [Google Scholar]
- 39. Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008; 27: 608–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen CC, Hsu YJ, Lee TM. Impact of elevated uric acid on ventricular remodeling in infarcted rats with experimental hyperuricemia. Am JPhysiol Heart Circ Physiol 2011; 301: 1107–1117 [DOI] [PubMed] [Google Scholar]
- 41. Kuwabara M, Sato Y, Kanbay M et al. Uric acid and left ventricular hypertrophy: a potentially new modifiable target? Am J Hypertens 2017; 30: 229–231 [DOI] [PubMed] [Google Scholar]
- 42. Hsu WL, Li SY, Liu JS et al. High uric acid ameliorates indoxyl sulfate-induced endothelial dysfunction and is associated with lower mortality among hemodialysis patients. Toxins (Basel) 2017; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]