Abstract
Purpose of Review
Lipoprotein apheresis is the most effective means of lipid-lowering therapy. However, it’s a semi-invasive, time consuming, and chronic therapy with variable adherence. There are still no specific guideline recommendations for the management of patients on lipid apheresis. The purpose of this review is to discuss the clinical indications and major drawbacks of lipid apheresis in the light of recent evidence.
Recent Findings
Lipoprotein apheresis should be initiated at early ages and performed frequently to receive the expected cardiovascular benefits. However, in clinical practice, most patients experience ineffective apheresis and fail to reach lipid targets. This real-world failure is due to several factors including late diagnosis, delayed referral, and improper frequency of procedures. All these denote that awareness is still low among physicians. Another important factor is the semi-invasive, time consuming nature of the apheresis, leading to high refusal and low adherence rates. Moreover, apheresis decreases quality of life and increases the risk of depression. Mental status is also deteriorated in patients with familial hypercholesterolemia on lipid apheresis. New effective lipid lowering agents are underway with promising cardiovascular results.
Summary
To overcome the drawbacks, a structured approach, including standardized protocols for lipoprotein apheresis with regular cardiovascular follow-up is warranted. New effective lipid lowering agents with documented cardiovascular benefit, should be integrated into the treatment algorithms of patients on lipoprotein apheresis.
Keywords: Apheresis, Atherosclerosis, Familial hypercholesterolemia, Guidelines, Low-density lipoprotein cholesterol, Lipoprotein(a)
Introduction
With the introduction of lipid lowering agents (LLA), there has been a significant reduction in the risk of cardiovascular (CV) disease (CVD) and premature deaths. However, there are many patients with refractory familial hypercholesterolemia (FH) who are far from the recommended treatment goals. FH is a genetic disease characterized by cumulative lifetime exposure to high cholesterol levels leading to premature atherosclerosis [1–3]. Homozygous patients (HoFH) usually have low density lipoprotein (LDL)-cholesterol levels >500 mg/dL (13 mmol/L) and generally present with potentially fatal CVD within the early decades of life [2•]. The basis of the management of HoFH is early and efficient in LDL-cholesterol lowering. On the other hand, conventional LLA remain ineffective in most of the patients, as the underlying genetic mutations mostly result in deficient or defective LDL-receptors [3••]. Lipoprotein apheresis (LA) which covers the extracorporeal selective elimination of apolipoprotein (apo)-B containing lipoproteins, is the most effective therapy if combined with conventional LLA for HoFH in adults and children [4,5].
Physically elimination of lipoproteins was first introduced in 1960’s when the available anti-lipid agents were not effective in lowering lipid levels [5, 6]. Since then, extracorporeal elimination initially with non-selective plasma exchange and subsequently with selective removal of apo-B containing lipoproteins is in use for the treatment of patients with refractory FH. The newer techniques are highly selective with elimination of only apo-B100 containing lipoproteins without removing high density lipoprotein (HDL)-cholesterol and other essential proteins [5, 7].
The long-term benefits of LA are documented with regard to prevention of the progression of atherosclerosis. LA treatment decreases inflammation and blood viscosity while improves endothelial functions [8, 9]. However, it’s a semi-invasive, time consuming, chronic expensive therapy with variable adherence [2, 10, 11]. Furthermore, there are still no specific guideline recommendations for the management of patients on LA therapy. This review is aimed to discuss the clinical indications, side effects, and major drawbacks in the light of recent evidence, current guidelines, and our 20-year of lipid clinic experience with FH patients on LA therapy [10•].
Indications and Contraindications of Lipoprotein Apheresis
LA therapy is primarily indicated for the elimination of severely elevated apo-B containing lipoproteins in clinical settings including FH, familial apo-100 defect, polygenic hypercholesterolemia, familial combined hypercholesterolemia, and isolated lipoprotein (a) [Lp(a)] elevation [12]. LA is not a preferred method of extracorporeal elimination of high triglycerides. Triglycerides with large particle size, increase the transmembrane pressure during the procedure causing hemolysis by forming clots. Therefore, plasmapheresis should be performed in severe life-threatening hypertriglyceridemia.
Indications for LA are determined according to both plasma LDL-cholesterol levels and response to medical treatment. The presence and severity of atherosclerotic CVD is the major driving factor in terms of initiation of LA. Due to the lack of outcome trials, standardized recommendations of the current guidelines for initiating LA therapy are still lacking. However, all related guidelines define LA as a lifesaving last step therapy for refractory severe hypercholesterolemia as in HoFH [13••]. For HoFH, unresponsiveness to LLA is accepted as an indication for initiating apheresis. The indications for heterozygous FH (HeFH) are less clear and vary from country to country (Table 1). The definition of maximally tolerated possible LLA also vary with the different reimbursement strategies of both the drugs and the LA.
Table 1.
Indications of Lipid apheresis (Countries in alphabetical order)
| Australia |
Homozygous FH: - LDL-C > 270 mg/dL (7.0 mmol/L) on maximally tolerated possible drug therapy Heterozygous FH: - CVD and LDL-C ≥ 193 mg/dL (5.0 mmol/L) on maximally tolerated possible drug therapy Alternative criteria (homozygous FH and heterozygous FH): < 50% reduction on maximal possible drug therapy |
| Germany |
Homozygous FH: Severe hypercholesterolaemia (including heterozygous FH and others): LDL-C elevated on maximally tolerated possible drug therapy (considering the overall risk of the patient) - Primary Prevention: LDL-C > 160 mg/dL (4.1 mmol/L) and CVD in family history - Secondary Prevention: LDL-C > 120–130 mg/dL (3.1–3.4 mmol/L) High Lipoprotein(a): - Progressive CVD detected by clinically or imaging despite optimal control of all the other risk factors and lipoprotein(a) ≥ 60 mg/dL (independent of LDL-C levels) |
| Japan |
Homozygous FH: Heterozygous FH: - Total cholesterol ≥250 mg/dL (6.5 mmol/L) on maximally tolerated possible drug therapy |
| National Lipid Association |
Homozygous FH*: - LDL-C ≥ 300 mg/dL (7.8 mmol/L) (or non-HDL-C ≥ 330 mg/dL) Heterozygous FH*: - LDL-C ≥ 300 mg/dL (7.8 mmol/L) (or non-HDL-C ≥ 330 mg/dL) and 0 to 1 risk factors - LDL-C ≥ 200 mg/dL (5.2 mmol/L) (or non-HDL-C ≥ 230 mg/dL) and high- risk characteristics, such as 2 risk factors or high Lp(a) ≥50 mg/dL using an isoform insensitive assay - LDL-C ≥ 160 mg/dL (4.1 mmol/L) (or non-HDL-C ≥ 190 mg/dL) and very high-risk characteristics (established CHD, other cardiovascular disease, or diabetes) |
| Spain |
Homozygous FH: Heterozygous FH: - LDL-C ≥ 200 mg/dL (5.2 mmol/L) with CVD or - LDL-C ≥ 300 mg/dL (7.8 mmol/L) without CVD |
| Turkey |
Homozygous FH: - LDL-C > 500 mg/dL (12.9 mmol/L) Heterozygous FH: - LDL-C > 300 mg/dL (7.8 mmol/L) with no CVD - LDL-C > 200 mg/dL (5.2 mmol/L) with CVD High Lipoprotein(a): - Progressive CVD detected by clinically or imaging despite optimal control of all the other risk factors and lipoprotein(a) ≥ 60 mg/dL (independent of LDL-C levels) Any other criteria available for lipoprotein apheresis in any worldwide guidelines is also valid for Turkey in patients with at least 6 months of proper diet and maximum tolerated conventional lipid lowering agents |
| United Kingdom |
Homozygous FH: - LDL-C reduction <50% on maximally tolerated possible drug therapy or - LDL-C > 350 mg/dL (9.1 mmol/L) Other hypercholesterolaemia (including heterozygous FH): -CVD progression and LDL-C ≥ 190 mg/dL (4.9 mmol/L) or lower if lipoprotein(a) elevated or LDL-C reduction <40% |
| United States |
Homozygous FH**: - LDL-C > 500 mg/dL (12.9 mmol/L) Heterozygous FH**: - LDL-C > 300 mg/dL (7.8 mmol/L) (< 1 additional risk factor) or - LDL-C > 200 mg/dL (5.2 mmol/L) and (≥ 2 additional risk factors or additional high lipoprotein(a)), presence of CAD - LDL ≥ 160 mg/dL (4.1 mmol/L) (if at very high risk) *All patients: Unresponsive or intolerant to medical and life style changes with lipid lowering diet for 6 months |
FH: Familial hypercholesterolemia, LDL-C: Low density lipoprotein cholesterol, CVD: Cardiovascular disease
(Modified from: Curr Atheroscler Rep. 2019;21:26. 10.1007/s11883-019-0787-5; 10.1007/s11883-019-0787-5; Creative Commons user license https://creativecommons.org/licenses/by/4.0/) [7•]
There are only two major contraindications of LA: bleeding diathesis and heparin-hypersensitivity. In low birth body weight, in other words, at very young ages LA could be risky. However, in the literature, there are reports of successful and safe LA treatments in very young, even at the age of 3.5 years [14–16]. Pregnancy is not a contraindication for LA.
Methods of Extracorporeal Lipoprotein Elimination
There are several techniques of extracorporeal elimination of lipoproteins (Table 2). In four of these methods; double or cascade filtration plasmapheresis, immune-adsorption, dextran sulphate adsorption, and heparin-induced extracorporeal LDL precipitation (HELP); first the cellular elements of blood are separated and returned to the patient and then LDL eliminated from the plasma. The other two methods, direct perfusion with dextran sulfate (DALI) and whole blood adsorption with polyacrylate (lipocollect), remove LDL from plasma by using whole blood, without separating the blood cellular elements [12].
Table 2.
Comparison between different Methods of Lipoprotein apheresis (% reduction rates using 1 plasma volume)
| Selective removal from whole blood | |||||||
|---|---|---|---|---|---|---|---|
| Plasmapheresis | Double or Cascade filtration plasmapheresis | Immunoadsorption | Dextran sulphate immunoadsorption | HELP | DALI | Whole blood adsorption with polyacrylate lipocollect | |
| Main method | Plasma exchange | LDL is cleaned from the plasma passing through the filtration columns by considering the particle size | Circulating LDL, VLDL, and Lp (a) are cleared using polyclonal sheep anti-apoB antibodies | ApoB containing lipoproteins are electrostatically bound to dextran sulfate and removed from the circulation | With the help of heparin, LDL particles in the plasma are precipitated | Treatment with whole blood without separating plasma | Treatment with whole blood without separating plasma |
| Lipoproteins and fibrinogen mean reduction in percent of original concentration (%) | |||||||
| LDL | 72 | 65 | 65 | 73–80 | 69 | 67 | 61 |
| HDL | 65 | 40 | 22 | 10 | 14 | 11 | 22 |
| Apolipoprotein B | 69 | 59 | 56 | 62 | 53 | 55 | 51 |
| Apolipoprotein A1 | 68 | 45 | 20 | 16 | 12 | 25 | 25 |
| Lipoprotein (a) | 68 | 52 | 53 | 72 | 50 | 50 | 61 |
| Fibrinogen | 58 | 36 | 23 | 16 | 44 | 25 | 39 |
| Advantages |
Quick and well-tolerated elimination of pathologic substances Cheap |
Semiselectivity Cheaper |
High Selectivity and effectiveness Easy process, regeneration, and reusability of columns |
High Selectivity and effectiveness |
High Selectivity, effectiveness, fast processing capability, regeneration, and reusability |
High Selectivity, effectiveness, and simple technology, easy and fast use, reusable columns, low blood volume |
High Selectivity, effectiveness, simple technology, easy and fast use, reusable columns |
| Disadvantages | Unselectivity, danger of infection, bleeding, and risks of human albumin | Danger of infection and low effectiveness |
Expensive technology decreasing colon efficiency in repeated use, sterility control requirement, cooling process requirement |
Expensive technology (disposable kit) Special cell separator required, potential side effects in patients on ACE inhibitors |
Expensive technology (single-use expensive kit) Special device required, maximum 3 l of plasma can be cleaned in each session, its use in pediatrics is limited or not due to its high minimum inlet flow rate (> 40 ml / min) |
Expensive, special device requirement compared to other methods, potential side effects in patients on ACE inhibitors | Expensive, special device requirement than other methods, cooling of the columns, side effect potential in patients on ACE inhibitors |
|
Side Effects Total percent (%) |
- Infection, bleeding |
2% Hypotension, Fatigue, Edema, Protein loss |
< 2% Hypotension, Nausea, Vertigo, Sheep antibodies |
0.3–3.6% Hypotension, Paresthesia, Pain, Nausea, Vertigo Bradykinin ↑, Coagulation ↓ |
3.05% Hypotension, Coagulation ↓ Angina, Headache, Nausea, Fatigue, Edema, Eye pressure ↑ |
3.85% Hypotension, Nausea, Vomiting, Chest pain, Flushing Bradykinin ↑ |
- Bradykinin ↑ |
HELP: Heparin-extracorporeal LDL precipitation, DALI: Direct perfusion with dextran sulfate, LDL: Low density lipoprotein cholesterol, HDL: High density lipoprotein cholesterol
All methods lower LDL-cholesterol to a similar extent averaging over 60% during a single procedure; however HDL-cholesterol reduction is more remarkable with double filtration [5, 7, 12]. All available techniques decrease fibrinogen levels and blood viscosity. This reduction is more pronounced in either double or cascade filtration plasmapheresis and HELP methods with an almost 60% reduction of fibrinogen levels during a single procedure [12, 17]. LA methods differ in cost, re-usability, applicability, and complication rates (Table 2). Hemoperfusion systems are more user-friendly, meanwhile immunoadsorption is relatively cheap with re-usable columns.
Cardiovascular Effects of Lipoprotein Apheresis
The major goal of therapy is to reduce the burden of atherosclerotic CVD in HoFH. In addition to selective removal of the circulating atherogenic lipoproteins, LA also decreases the number of inflammatory proteins and thrombogenic factors and improves endothelial functions [4, 5]. LA also has an impact on gene translation and transcription by affecting cytokine receptor functions [9]. Moreover, proteomic analysis from the post-apheresis wastes denoted the favorable effects on coagulation factors, inflammatory factors, adhesion molecules, complements, glycoproteins, apolipoproteins, and immune globulins [18].
As a result of these pleiotropic effects, development and progression of atherosclerotic CVD and aortic fibrosis are prevented with LA therapy [5, 19, 20]. Since the initial studies, LA therapy has significantly improved the survival in HoFH patients compared to their non-treated HoFH relatives [21•]. LA was notably associated with lower rates of CV events in HeFH patients with established CVD in comparison to LLA [22]. Two-year follow-up of HeFH patients with angina has revealed a substantial prolongation of time to 1-mm ST-depression during exercise with LA therapy compared to LLA [23].
There are also favorable consequences of LA on plaque regression, and improved tissue and organ perfusions documented with different imaging techniques [21–25]. These improvements on aortic and coronary angiographic findings have been documented as early as in the first year of LA [26]. Besides, a single session LA prior to percutaneous coronary intervention (PCI) combined with multiple times of LA after PCI was shown to be associated with a restenosis rate of 18% compared to 52% restenosis in the controls who did not receive peri-PCI LA [27].
While evaluating the CV effects of LA, we have to keep in mind that demonstrating the positive effects on LA on CV outcomes is difficult, since comparative treatments (placebo / sham apheresis) are not ethically appropriate and the number of HoFH patients in clinical follow-up is small. Therefore, most of the CV results are generated from observational and/or retrospective studies, HeFH patients and generally on surrogate markers [5, 7].
Despite the regular LA, progression or de novo development of CVD are reported in 25–35% [28]. Albeit all the favorable effects, progression of atherosclerosis can be explained by the short-lived decreases in lipid levels after apheresis. Indeed, Bangalore S et al. have previously addressed the association between visit-to-visit LDL-cholesterol variability and the increased risk of CV outcomes [29••]. Julius et al. showed that a younger age at the start of therapy was associated with less CV events during regular LA [30•].
Effects on Cholesterol Depositions
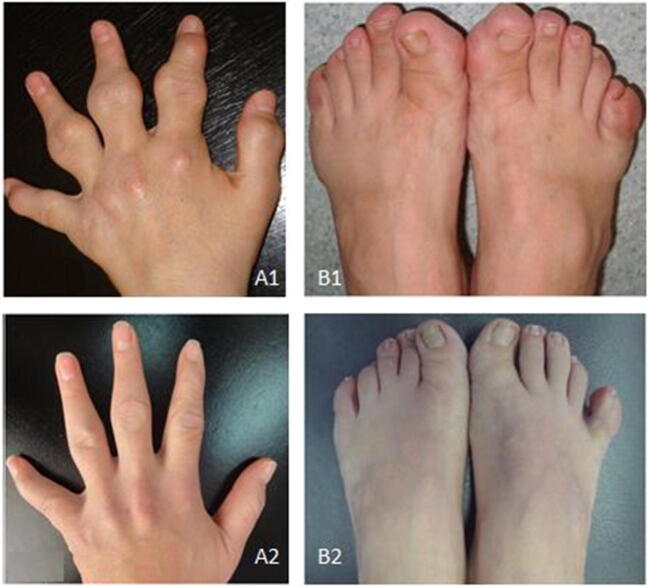
Although there is an eye catching improvement in lipid levels within the first 3–6 months of LA therapy, “delipidation” namely the mobilization of the intracellular cholesterol requires long-term apheresis with appropriate techniques and frequency. Xanthomas generally regress in two years [10, 31] (Fig. 1). The disappearance of xanthomas is generally faster in children (within 6–12 months).
Fig. 1.
Pictures present the regressed and completely vanished tendon xanthomas within the 2 years of effective LA therapy in a young patient with homozygous familial hypercholesterolemia. Tendon xanthomas on her right hand (A1) and feet (B1) before the initiation of apheresis when she was 22 years old. Completely vanished tendon xanthomas on her hand (A2) and feet (B2) after 2 years of regular weekly apheresis [10•]. Unfortunately, she was referred to apheresis more than 10 years after the initial symptoms of hypercholesterolemia. Although tendon xanthomas were regressed as a result of effective cholesterol reduction with regular apheresis, she died at the age of 27 years due to atherosclerotic complications of hypercholesterolemia. (With permission from: Kayikcioglu M et al. Turk Kardiyol Dern Ars. 2014;42:599–611. 10.5543/tkda.2014.09633) [10•]
Ideal Age for Initiation Lipoprotein Apheresis and Frequency of Procedures
Due to cumulative burden of high-cholesterol levels since birth, all guidelines strongly recommend the early initiation of intensive lipid-lowering therapy including LA for patients with HoFH [3, 13, 32]. The ideal age for starting LA therapy is <6–7 years before the development of aortic root involvement [2–4]. In patients with late initiated LA after age of 10 years, the progression of aortic atheroma to stenosis cannot be prevented even the LDL goals attained [2, 3]. The mechanism of the progression of aortic stenosis is probably the accelerated degeneration and calcification process that cannot be reversed only with LDL lowering [2, 10]. Also, diagnosis and/or initiation of LA therapy at younger age, is associated with less CV events and with better quality of life, lower anxiety, and greater emotional well-being possibly denoting better adaptation compared to patients with delayed diagnosis [33•]. However, in the A-HIT1 study, mean age at first LA was 21 ± 12 years due to a 7.37 ± 7.1 years delay between the diagnosis and first LA treatment [2•].
The Procedure of Lipoprotein Apheresis
Good vascular access is essential to allow efficient processing of an adequate plasma volume for removal of lipoproteins. For vascular access, peripheral veins could be used, but considering the long duration of the procedure, arteriovenous fistula is generally preferred [34••]. However, our experience with arterio-venous fistula is more challenging. Many experienced centers prefer vein-to-vein access.
The duration of LA procedure is about 2–4 h. Procedure is repeated continuously weekly or bi-weekly according to the clinical characteristics and LDL-cholesterol levels. The general consensus for HoFH is to perform LA weekly [34••]. In our lipid clinic, we prefer to initiate LA treatment twice-weekly to afford a more faster cholesterol removal from the tissues (delipidation), and based on the average LDL-cholesterol levels, and then continue either weekly or bi-weekly manner. While determining the frequency of the procedure and the duration of each procedure, the patient’s compliance, the severity of the disease (clinical and laboratory), and CVD status should be considered. For an effective apheresis procedure, approximately 1.5 times the plasma volume is recommended to be treated [34••].
A single LA procedure can lower plasma LDL-cholesterol by 45–76% as compared with pre-treatment levels, with not much variation between the apheresis techniques [5, 7, 34]. The acute reduction in LDL-cholesterol with a regular LA has shown to result in a time-averaged LDL-reduction of ~48% between apheresis intervals [28]. However, do to the nature of treatment immediately after the rapid LDL decline, levels begin to increase again within days and gradually plateau in the second week.
Efficacy of Apheresis and Goals of Treatment
The treatment targets in FH patients should be determined according to the presence of CVD. The EAS/ESC Dyslipidemia Guidelines recommend a treatment target of LDL-cholesterol <55 mg/dL (1.4 mmol/L) for patients FH with documented CVD [13••]. And for FH patients in primary prevention LDL target is determined according to the presence of additional major risk factors such as hypertension, obesity etc. LDL-cholesterol should be <70 mg/dL (1.8 mmol/L) for primary prevention of FH patients with no additional risk factors. If an additional risk factor is present, then the LDL goal is <55 mg/dL (1.4 mmol/L) for FH patients without CVD. Targeting non-HDL is not recommended as HDL-cholesterol levels are unreliable immediately after LA especially with double-filtration [34••].
Due to cyclic nature of LDL and lipoprotein levels, there is no ideal practical measure of lipoproteins for monitoring LA. An acute LDL-cholesterol reduction of at least 60% from baseline is accepted as an efficacy criterion of a LA procedure [28, 34]. LDL-cholesterol concentration displays a pattern similar to saw-tooth during regular LA treatment [35]. Therefore, interpretation of LDL-cholesterol levels at regular intervals is important in assessing the efficacy of LA as a long-term intermittent therapy.
The Kroon formula calculating the time-averaged LDL-cholesterol between two LA procedures adjusting for the non-linear rebound of LDL-cholesterol, suggested as a surrogate parameter to evaluate the attainment of LDL goals; LDLmean = LDLmin x K (LDLmax-LDLmin), where LDLmin = LDL-cholesterol immediately after LA, LDLmax = LDL-cholesterol immediately prior to LA; and K is coefficient which is 0.73 for HeFH and 0.66 for HoFH [28, 34, 36].
Current consensus for interval mean decrease of LDL-cholesterol is <254 mg/dL (6.7 mmol/L) (>65% reduction) for HoFH, <101 mg/dL (2.6 mmol/L) (>60% reduction) for HeFH, and < 50 mg/dL for high Lp (a). However, current ESC/EAS dyslipidemia targets for FH are far below these targets [1, 13, 34]. Increasing the frequency of the procedures and/or use of concomitant LLA could alleviate the rebounds of LDL-cholesterol following LA procedures and help to get the goals recommended in guidelines [5, 34].
In clinical practice, even in experienced centers, patients may fail to reach LDL-cholesterol targets. A-HIT1 study showed that most patients experience ineffective LA and fail to attain LDL goals, even in a country where LA is widely available and full reimbursed [2•]. Of note, A-HIT1 is a nationwide registry conducted in 19 LA centers to provide insight into the real-world management of patients with HoFH undergoing LA in Turkey. LDL-cholesterol levels were on target only in 5.7% of the A-HIT1 population, meanwhile, mean frequency of LA sessions was every 19 (range 7–90) days. Though the high rate of patient awareness about treatment targets, 85% of them were not willing to increase LA frequency [2, 11, 33]. None of the apheresis centers had a standardized approach for LA and 70% of the attending physicians were unaware of the individual patient’s target LDL-cholesterol levels. The lack of awareness among physicians specialized on apheresis and semi-invasive time-consuming nature of LA were probably the major reasons of the failure of LA in attaining LDL goals.
Concomitant Anti-Lipid Therapy
Combined therapy of high intensity statins with ezetimibe may lower cholesterol by up to 40% in HoFH patients receiving LA [37, 38]. Even though the LDL goals cannot be attained, survival analysis in patients with HoFH before and after the introduction of statins showed significant benefit [39•]. Therefore, all patients should be offered maximum tolerated doses of statins combined with ezetimibe [34••]. Interestingly, we experienced patients with phenotypically severe HoFH, who could easily get LDL-cholesterol goals with only intense doses of statins.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors could be effective in HoFH patients depending on the LDL-receptor activity [40]. The LDL-cholesterol reduction with PCSK9 inhibitors might be variable ranging from 7% to 56%, in receptor defective patients even with the same mutation [41•]. Therefore, unless patients are known to be receptor negative, a therapeutic trial is recommended if treatment goals cannot be attained [34••]. Patients with a response of 10–15% LDL-cholesterol reduction (or interval mean LDL) should continue PCSK9 inhibitors. Evolocumab has been approved for HoFH treatment in adults and children >12 years of age and should be injected subcutaneously after the LA procedure. Recently, the efficacy of alirocumab has been shown as an additional 17.9% LDL-cholesterol reduction in 6 HoFH patients on LA therapy in the ODYSSEY HoFH Trial [42].
Lomitapide, a microsomal triglyceride transfer protein inhibitor, should be considered for adults with HoFH, who have failed to reach treatment targets while on a combined therapy of apheresis and standard LLA and have had a trial of evolocumab [34••]. It is currently used as adjunctive therapy for HoFH with or without LA. According to real world clinical experience, LDL goal attainment rate is 68% and 42% for targets of LDL-cholesterol < 100 mg/dL (2.5 mmol/L) and 70 mg/dL (1.8 mmol/L), respectively [43•]. In our experience, even low doses of lomitapide could reduce the frequency of LA. There are also cases in literature with cessation of LA procedure with this agent [44•].
Mipomersen, an antisense oligonucleotide inhibitor targeting ApoB has also been shown to be effective in HoFH patients. Although it is not approved in Europe, it is approved and available in other countries such us the USA. In a prospective randomized controlled phase II single center trial of 15 patients on mipomersen has decreased the pre-apheresis LDL-cholesterol and Lpa levels significantly, meanwhile seven patients have discontinued the drug due to side effects [45].
There are also studies ongoing with new anti-lipid agents bypassing LDL-receptors for patients with HoFH on LA [46]. In ECLIPSE study, Evinacumab provided a similar reduction (almost 50%) in LDL-cholesterol levels regardless of whether patients were being treated with apheresis [47•]. Indeed, results of Orion 5 with Inclisiran on HoFH patients with or without LA will be available in 2021 [48].
Bempedoic acid, a new, first-in-class oral ATP-citrate lyase (ACLY) inhibitor, is introduced as an effective and safe LDL-cholesterol lowering agent especially in patients with statin intolerance [49]. Its combination with LA (with or without statins) seems to be reasonable; however no data of this issue is available yet.
Adverse Effects
With the newer techniques, the side effects of LA therapy are low, generally <5%. The most frequent side effect is mild to severe hypotension due to bradykinin release and especially seen in dextran sulphate-based adsorption and haemoperfusion methods. Concomitant use of an angiotensin-converting enzyme (ACE) inhibitor is contraindicated to prevent the severe hypotension, since these agents inhibit the catabolism of bradykinin. Therefore, patients should not receive ACE-inhibitors 24 h prior to LA procedure or an angiotensin receptor blocker should be preferred [34••].
Fast or large volume removal during the LA procedure could lead to severe hypotension or hypoperfusion especially in patients with moderate to severe aortic stenosis. Other side effects are gastrointestinal symptoms due to hypotension or hypocalcaemia (as a consequence of use of citrate), anemia due to iron-deficiency on chronic treatment and apoferritin loss, tendency to bleeding due to loss of fibrinogen, and infections due to loss of immunoglobulins [2, 5, 34]. Allergic reactions due to the removal of substrates and heparin might be observed. Complications related to vascular access are also highly prevalent and significant reasons for the low adherence to LA [10, 11].
Effect of Apheresis on Quality of Life, Psychosocial Life, and Patients’ Perspective
An important challenge of LA is its consequences on psychosocial life. In line with the time-consuming, invasive nature of LA that requires frequent and long-distance travel, Bruckert et al. have shown the disturbance of family and social life in patients receiving regular LA [50•]. Similarly, A-HIT1 study showed that adult HoFH patients on regular LA, experience significantly impaired quality of life associated with an increased risk of depression [11]. Patient survey revealed that most of the patients were suffering not only from HoFH but also from drawbacks of apheresis. For most of these patients, LA was a difficult-to-bare treatment; the major complaint was related to pain and needles in 34.8%, time spent for apheresis in 27.5%, and both in 17.4% [2, 11, 33]. In line with patients’ perspective, the refusal and low compliance rates are high even in experienced centers [10•]. Because of chronicity of the disease and peculiar characteristics of the apheresis therapy, depressive mood develops nearly in all patients which worsen noncompliance to treatment [11, 33]. All of these factors may adversely affect the expected benefits of LA. For the success of LA therapy, it is essential to relax the patient psychologically and to provide continuous support. Thus, it is suggested that LA centers might consider offering routine psychological consultations to support patients during their treatment journey [11, 33].
LA is a hospital or healthcare center dependent treatment that can be easily disrupted by a disaster as we experienced during the COVID-19 pandemic [51•]. Either due to the occupation of the centers heavily by COVID patients or the fear of getting infected during the procedures, most of the patients could not get access to LA therapy in the course of the pandemic facing them to increased risk of CV events.
Apheresis Therapy for High Lipoprotein a
Lp(a) is an apo-B containing lipoprotein with an additional plasminogen like domain leading to increased thrombosis. High levels of Lp(a) is documented as an independent risk factor for CV events and aortic sclerosis [13, 52]. More than 90% of the levels of Lp(a) are determined genetically. Lifestyle measures and conventional LLA do not have much effect on Lp(a) levels. Therefore, for many years, LA is used as the most effective Lp(a) lowering therapy with 50–75% reduction [13, 52, 53]. The special immune-adsorption polyclonal antibody columns are available since 1993 for the selective elimination of Lp(a) [53••]. However, the awareness of Lp(a) as a CV risk factor is extremely low among both physicians and patients, consequently Lp(a) apheresis is not a widely available therapy. In recent years with the development of new anti-Lp(a) agents, the popularity of Lp(a) and its treatments including LA increased [54•]. Though the evidence is generated from small sized studies, Lp(a) apheresis reduces the inflammatory and prothrombotic proteins, and ameliorates the risk of CV events after 2–5 years of therapy [53, 55, 56]. But, clinical benefits of LA therapy for isolated high Lp(a) is still unclear and we need more evidence to define the treatment targets of Lp(a).
The Follow-Up of Patients on LA Therapy
Since the major goal of LA therapy is to reduce the burden of atherosclerosis, CV evaluation should be the major component of regular follow-up. It’s obvious that the follow-up of patients on regular LA therapy, should be performed by a multi-disciplinary team including lipid specialist (or endocrinologist, or pediatric metabolism specialist), cardiologist [57], specialists of apheresis (nephrologists or hematologists), and psychiatrist (or psychologist) etc. Although there are no clearly defined criteria and guideline recommendations, follow-up measures and their timings are extremely important for the detection and monitoring of early atherosclerosis and aortic involvement in these patients. Baseline evaluation should cover CV risk assessment, electrocardiography, echocardiography, and investigation of aortic stenosis and aneurysm [58].
Table 3 displays our standardized approach to patients on LA therapy, as an example. The type and frequency of procedures for CV evaluation should be determined based on the individual patient’s risk level, requirements, and the attitude of the following clinic. In our clinic, we prefer more frequent evaluation with CV imaging modalities. Our baseline evaluation includes lipid profile [total cholesterol, LDL-cholesterol, HDL-cholesterol, non-HDL-cholesterol, and Lp (a)], laboratory analysis (transaminases, creatinine, urea, albumin, fasting blood glucose, electrolytes, thyroid stimulating hormone, hemoglobin, and platelet counts), and screening tests for the detection of atherosclerosis. Measurement of the thickness of Achilles tendon (ultrasonography or x-ray) is also a component of our baseline evaluation. In the follow-up, except thyroid stimulating hormone, all biochemical measurements and ECG are repeated every 3 months. Echocardiography is performed semi-annually or annually depending on the presence of valve involvement. Ultrasonography of the carotid and renal arteries, retinal examination, and measurement of Achilles tendon thickness are performed annually. Exercise stress tests are performed in asymptomatic patients annually unless the presence of ischemic symptoms. Coronary CT including calcium score and angiography is not used on routine basis, we prefer to use in patients with moderate CV risk. In children, we do not prefer performing coronary CT due to the risk of radiation and in case of ischemia in young children we perform direct conventional coronary angiography. Monitoring of hemoglobin and ferritin levels with transferrin saturation is recommended along with iron supplementation for the prevention of anemia with long-term LA. We calculate time averaged LDL monthly, and evaluate patients in CV prevention clinic every 3 months.
Table 3.
Baseline and follow-up assessment and timing for the management of patients requiring/on lipid apheresis therapy used in the Lipid Clinic of Ege University Cardiology Department
| Diagnosis | Follow-up | Description | |
|---|---|---|---|
| History and physical examination | + | + | Physical examination in every 3 months |
| Family history (premature CV events, xanthoma, xanthelasma, consanguinity, etc.) | + | – | A detailed family history and lipid profile of all family members should be obtained if possible family screening should be conducted |
| Lipid profile (total cholesterol, LDL-C, HDL-C, non-HDL-C, triglycerides, lipoprotein a) | + | + |
A full lipid profile is being monitored in every 3 months; only LDL-C levels are measured before and after the apheresis sessions unless there are other lipid abnormalities If Lp(a) normal at baseline, no further measurement needed |
| Time averaged LDL-C calculation | – | + | In every 4 LA procedure |
| Biochemical analysis | + | + |
Baseline analyses include transaminases, creatinine, albumin, FBG, electrolytes, TSH, hemoglobin, and platelet counts In the follow-up except TSH all measurements in every 3 months. Ca + 2, hemoglobin, and platelet counts and albumin are measured before and immediately post apheresis procedures |
| Screening of CV risk factors | + | + | In every 3 months |
| ECG | + | + | In every 3 months |
| Echocardiography | + | + | Semiannually or annually based on the presence of valve involvement |
| Carotid Doppler USG | + | + | Annually |
| Renal artery Doppler USG | + | ± | Annually (biannually if the patient has no problem regarding renal artery involvement) |
| Retinal examination | + | + | Annually |
| Measurement of the thickness of Achilles tendon (USG or x-ray) | + | ± | Annually |
| Exercise test (stress test) | + | ± | In asymptomatic patient, annually |
|
Coronary calcium score CT angiography |
± | ± |
Not on routine basis depending on the clinical findings of the individual patients In patients with advanced disease especially not on regular apheresis, or late initiation of apheresis treatment, we perform CT angiography at baseline |
| Coronary angiography | – | – | Not on routine basis depending on the clinical findings of the individual patients |
| Cardiac MR imaging | ± | ± |
Not on routine basis If valvular involvement or low ejection fraction is detected in echocardiography, we perform MR imaging at baseline, too Reexamination of the patient with MR imaging is based on the clinical findings |
| Education of patient and family for FH and LA | + | + | At baseline and then every 6 months |
| Group medical visits and motivational interviews | + | + |
Every 3 months, group medical visits are conducted with family members An experienced nurse conducts the motivational interviews at baseline and in non-adherent patients regularly |
| Educational courses on apheresis and FH | – | + |
Semi-annually All patients followed in the center with their family members are invited to a big meeting and receive education for FH, apheresis, healthy nutrition, healthy life style, physical activity, and how to live with FH and apheresis |
| Psychiatric (psychologic) evaluation | + | + | Baseline and annually |
CRP C-reactive protein, Ca + 2 calcium, RFT renal function tests, FBG fasting blood glucose, TSH thyroid stimulating hormone, CT computed tomography, MR magnetic resonance, USG ultrasonography, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, FH familial hypercholesterolemia, LA lipoprotein apheresis, CV cardiovascular
Patient education and team work are important in alleviating the drawbacks and increase the adherence to LA therapy. We perform group medical visits with patients and their families, and big educational courses, for all LA patients followed in the center. During these courses patients receive detailed information on FH, LA, CVD, healthy life-style measures, drugs, treatment goals, and how to live with FH and apheresis from a team of coaches consisting of experienced nurses, dietician, physiotherapist, and a psychologist.
Conclusions
Apheresis treatment, which has been in use for more than 45 years, is still the most effective means of lowering LDL-cholesterol levels in patients with refractory FH and high Lp(a) levels [4, 5]. LA not only selectively removes the circulating apo-B containing atherogenic lipoproteins, but also reduces inflammatory markers, oxidative stress, and thrombogenic factors, and improves endothelial functions [5, 7–9, 34]. Regular LA treatment can effectively and safely induce the regression of xanthomas, retard the progression of atherosclerotic lesions, and improves survival [2, 3, 10]. Regular LA with intermittent nature leads to a sawtooth pattern of LDL-cholesterol levels [35]. The extent of the LDL-cholesterol rebound can be improved with increasing the frequency of the procedures and also with concomitant use of LLA [5, 34].
To afford all the expected benefits, LA should be performed frequently; i.e. the ideal is on a weekly manner. The initiation age is also associated with the incidence of CV events during regular LA therapy. Indeed, LA should be initiated before the age of 6–7 to prevent the progression of aortic root atheroma [2, 3, 10]. However, in real clinical practice, most patients experience ineffective LA and fail to reach LDL targets even in countries where LA widely available [2, 11]. This real-world care failure is due to several factors including late diagnosis, delayed referral to apheresis, and improper frequency of LA procedures. All these denote that awareness is still low among physicians. Another important factor is the semi-invasive, time consuming, chronic nature of the LA, leading to high refusal and low adherence rates [2, 5, 10]. To overcome all these drawbacks, a structured approach, including standardized protocols for LA treatment with regular CV follow-up by an experienced multidisciplinary team is warranted [2, 33, 59]. CV risk assessment and time average LDL-cholesterol levels should be monitored closely. New effective LLA with documented CV benefit, should be inoculated to the treatment algorithms of patients on LA therapy either due to refractory FH or high Lp(a) levels.
Compliance with Ethical Standards
Conflict of Interest
Meral Kayikcioglu has received research grants for investigator-initiated trials from a variety of companies including Amgen, Amyryt Pharma, and AstraZeneca; investigator fees for RCTs from Sanofi, Esperion, and Amgen; and honorarium for advisory boards for Amgen, Novartis via her institution (Ege University) within the last 3 years.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Statin Drugs
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Feingold KR, Grunfeld C. Lipoprotein apheresis. [updated 2020 Jan 18]. In: Feingold KR, Anawalt B, Boyce a, et al., editors. Endotext [internet] South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 2.• Kayikcioglu M, Tokgozoglu L, Yilmaz M, Kaynar L, Aktan M, Durmuş RB, et al. A nation-wide survey of patients with homozygous familial hypercholesterolemia phenotype undergoing LDL-apheresis in Turkey (A-HIT 1 registry). Atherosclerosis. 2018;270:42-48. 10.1016/j.atherosclerosis.2018.01.034. Turkish HoFH registry data of patients on lipoprotein apheresis providing insight to real life management. [DOI] [PubMed]
- 3.• Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 201421;35:2146–57. 10.1093/eurheartj/ehu274. The latest consensus report on the management of homozygous familial hypercholesterolaemia. [DOI] [PMC free article] [PubMed]
- 4.Moriarty PM, Hemphill L. Lipoprotein apheresis. Cardiol Clin. 2015;33:197–208. doi: 10.1016/j.ccl.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Stefanutti C, Thompson GR. Lipoprotein apheresis in the management of familial hypercholesterolaemia: historical perspective and recent advances. Curr Atheroscler Rep. 2015;17:465. doi: 10.1007/s11883-014-0465-6. [DOI] [PubMed] [Google Scholar]
- 6.• de Gennes JL, Touraine R, Maunand B, Truffert J, Laudat P. Homozygous cutaneo-tendinous forms of hypercholesteremic xanthomatosis in an exemplary familial case. Trial of plasmapheresis an heroic treatment. Bull Mem Soc med Hôp Paris. 1967;118: 1377–402. First report of plasmapheresis as a therapy of high cholesterol levels. [PubMed]
- 7.• Thompson G, Parhofer KG. Current role of lipoprotein apheresis. Curr Atheroscler rep. 2019;21:26. 10.1007/s11883-019-0787-5. An up-to-date review of current developments and studies of lipoprotein apheresis especially describing the kinetics of LDL-cholesterol rebound. [DOI] [PMC free article] [PubMed]
- 8.Koziolek MJ, Mueller GA. Impact of LDL-apheresis on inflammation and microcirculation. Atheroscler Suppl. 2009;10:56–58. doi: 10.1016/S1567-5688(09)71812-4. [DOI] [PubMed] [Google Scholar]
- 9.• Stefanutti C, Morozzi C, Petta A. Lipid and low-density-lipoprotein apheresis. Effects on plasma inflammatory profile and on cytokine pattern in patients with severe dyslipidemia. Cytokine. 2011;56:842-9. 10.1016/j.cyto.2011.08.027. An important report of the effect of lipoprotein apheresis on cytokine levels and inflammation. [DOI] [PubMed]
- 10.• Kayikcioglu M, Kısmalı E, Can L, Payzin S. Long-term follow-up in patients with homozygous familial hypercholesterolemia; 13-year experience of a university hospital lipid clinic. Turk Kardiyol Dern Ars. 2014;42:599-611. 10.5543/tkda.2014.09633. A long term single center experience of lipoprotein apheresis of patients with homozygous familial hypercholesterolaemia. [DOI] [PubMed]
- 11.• Kayikcioglu M, Kuman-Tunçel O, Pirildar S, Yílmaz M, Kaynar L, et al. Clinical management, psychosocial characteristics, and quality of life in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis in Turkey: Results of a nationwide survey (A-HIT1 registry). J Clin Lipidol. 2019;13:455–467. 10.1016/j.jacl.2019.02.001. A recent report of the effect of lipoprotein apheresis on quality of life and depression in a large series of patients with homozygous familial hypercholesterolaemia. [DOI] [PubMed]
- 12.Bambauer R, Bambauer C, Lehmann B, Latza R, Schiel R. LDL-apheresis: technical and clinical aspects. Sci World J. 2012;2012:314283–314219. doi: 10.1100/2012/314283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•• Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 ;41:111–188. 10.1093/eurheartj/ehz455. Current guidelines of dyslipidemia. [DOI] [PubMed]
- 14.• Palcoux JB, Atassi-Dumont M, Lefevre P, Hequet O, Schlienger JL, Brignon P, et al. Low-density lipoprotein apheresis in children with familial hypercholesterolemia: follow-up to 21 years. Ther Apher dial. 2008;12:195–201. 10.1111/j.1744-9987.2008.00574.x. An important report of long term safety and efficacy of lipoprotein apheresis in children with homozygous familial hypercholesterolaemia. [DOI] [PubMed]
- 15.Stefanutti C, Julius U. Lipoprotein apheresis: state of the art and novelties. Atheroscler Suppl. 2013;14:19–27. doi: 10.1016/j.atherosclerosissup.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 16.• Stefanutti C, Lanti A, Di Giacomo S, Mareri M, De Lorenzo F, Landolfo A. At al. Therapeutic apheresis in low weight patients: technical feasibility, tolerance, compliance, and risks. Transfus Apher Sci. 2004;31:3-10. 10.1016/j.transci.2004.01.010. The initial report of lipoprotein apheresis in very low weight patients. [DOI] [PubMed]
- 17.• Schuff-Werner P, Schütz E, Seyde WC, Eisenhauer T, Janning G, Armstrong VW, et al. Improved haemorheology associated with a reduction in plasma fibrinogen and LDL in patients being treated by heparin-induced extracorporeal LDL precipitation (HELP). Eur J Clin invest. 1989;19:30-7. 10.1111/j.1365-2362.1989.tb00192.x. A historical first report documenting the effect of lipoprotein apheresis on fibrinogen and blood viscosity. [DOI] [PubMed]
- 18.Yuasa Y, Osaki T, Makino H, Iwamoto N, Kishimoto I, Usami M, Minamino N, Harada-Shiba M. Proteomic analysis of proteins eliminated by low-density lipoprotein apheresis. Ther Apher Dial. 2014;18:93–102. doi: 10.1111/1744-9987.12056. [DOI] [PubMed] [Google Scholar]
- 19.Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, et al; Pro(a)LiFe Study Group*. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128:2567–76. 10.1161/CIRCULATIONAHA.113.002432 [DOI] [PubMed]
- 20.Lefort B, Saheb S, Bruckert E, Giraud C, Hequet O, Hankard R. Impact of LDL apheresis on aortic root atheroma in children with homozygous familial hypercholesterolemia. Atherosclerosis. 2015;239:158–162. doi: 10.1016/j.atherosclerosis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 21.•.Thompson GR, Miller JP, Breslow JL. Improved survival of patients with homozygous familial hypercholesterolemia treated with plasma exchange. BMJ 1985;291:1671-3. A historical first report documenting improvement in survival of patients with homozygous familial hypercholesterolemia with lipoprotein apheresis. [DOI] [PMC free article] [PubMed]
- 22.Mabuchi H, Koizumi J, Shimizu M, Kajinami K, Miyamoto S, Ueda K, et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Am J Cardiol. 1998;82:1489–95. 10.1016/s0002-9149(98)00692-4. [DOI] [PubMed]
- 23.Kroon AA, Aengevaeren WR, van der Werf T, Uijen GJ, Reiber JH, Bruschke AV, Stalenhoef AF. LDL-apheresis atherosclerosis regression study (LAARS). Effect of aggressive versus conventional lipid lowering treatment on coronary atherosclerosis. Circulation. 1996;93:1826–1835. doi: 10.1161/01.cir.93.10.1826. [DOI] [PubMed] [Google Scholar]
- 24.Aengevaeren WR, Kroon AA, Stalenhoef AF, Uijen GJ, van der Werf T. Low density lipoprotein apheresis improves regional myocardial perfusion in patients with hypercholesterolemia and extensive coronary artery disease. LDL-apheresis atherosclerosis regression study (LAARS) J Am Coll Cardiol. 1996;28:1696–1704. doi: 10.1016/s0735-1097(96)00388-9. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki M, Hiramori K, Imaizumi T, Kitabatake A, Hishida H, Nomura M, Fujii T, Sakuma I, Fukami K, Honda T, Ogawa H, Yamagishi M. Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein-apheresis in familial hypercholesterolemia: the low density lipoprotein-apheresis coronary morphology and reserve trial (LACMART) J Am Coll Cardiol. 2002;40:220–227. doi: 10.1016/s0735-1097(02)01955-1. [DOI] [PubMed] [Google Scholar]
- 26.Stefanutti C, Vivenzio A, Di Giacomo S, Mazzarella B, Bosco G, Berni A. Aorta and coronary angiographic follow-up of children with severe hypercholesterolemia treated with low-density lipoprotein apheresis Transfusion. 2009;49:1461–70. [DOI] [PubMed]
- 27.Yamaguchi H, Lee YJ, Daida H, et al. Effectiveness of LDL-apheresis in preventing restenosis after percutaneous transluminal coronary angioplasty (PTCA): LDL-apheresis angioplasty restenosis trial (L-ART). Chem Phys Lipids. 1994;67–68:399–403. [DOI] [PubMed]
- 28.Thompson GR, Barbir M, Davies D, Dobral P, Gesinde M, Livingston M, Mandry P, Marais AD, Matthews S, Neuwirth C, Pottle A, le Roux C, Scullard D, Tyler C, Watkins S. Efficacy criteria and cholesterol targets for LDL apheresis. Atherosclerosis. 2010;208:317–321. doi: 10.1016/j.atherosclerosis.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 29.•• Bangalore S, Breazna A, DeMicco DA, Wun CC. Messerli FH; TNT steering committee and investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J am Coll Cardiol. 2015;65:1539-48. 10.1016/j.jacc.2015.02.017. The first report of the increased cardiovascular risk associated with the variability in LDL-cholesterol levels. [DOI] [PubMed]
- 30.•.Julius U, Kuss S, Tselmin S, Schatz U, Bornstein SR. Why Some Patients Undergoing Lipoprotein Apheresis Therapy Develop New Cardiovascular Events? J Cardiovasc Dev Dis. 2020;7:E25. Published 2020 Jul 16. doi:10.3390/jcdd7030025A recent study evaluating the factors associated with the development of cardiovascular events during regular lipoprotein apheresis. [DOI] [PMC free article] [PubMed]
- 31.•• Harada-Shiba M, Arai H, Oikawa S, Ohta T, Okada T, Okamura T, et al. Guidelines for the management of familial hypercholesterolemia. J Atheroscler Thromb. 2012;19:1043-60. 10.5551/jat.14621. Japanese guidelines for the management of familial hypercholesterolemia. [DOI] [PubMed]
- 32.Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–268. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 33.• Kuman Tunçel Ö, Kayıkçıoğlu M, Pırıldar Ş, Yılmaz M, Kaynar L, Aktan M, et al. Mental status and physical activity in patients with homozygous familial hypercholesterolemia: a subgroup analysis of a nationwide survey (A-HIT1 registry). J Clin Lipidol. 2020;14:361-370.e2. 10.1016/j.jacl.2020.04.006. The first report of demonstrating the impaired mental status in patients with homozygous familial hypercholesterolemia on lipoprotein apheresis. [DOI] [PubMed]
- 34.•• France M, Rees A, Datta D, Thompson G, Capps N, Ferns G. Et al; for HEART UK medical scientific and research committee. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016;255:128-139. 10.1016/j.atherosclerosis.2016.10.017. The most recent statement on the management of homozygous familial hypercholesterolaemia. [DOI] [PubMed]
- 35.•• Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing Committee of the American Society for apheresis: the eighth special issue. J Clin Apher. 2019;34:171-354. 10.1002/jca.21705. Current American guidelines for therapeutic apheresis in clinical practice. [DOI] [PubMed]
- 36.•• Kroon AA, van't Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis. 2000;152:519–26. 10.1016/s0021-9150(00)00371-3. The first report of time averaged (interval mean) LDL calculation as an efficacy parameter of lipoprotein apheresis. [DOI] [PubMed]
- 37.Marais AD, Raal FJ, Stein EA, Rader DJ, Blasetto J, Palmer M, Wilpshaar W. A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis. 2008;197:400–406. doi: 10.1016/j.atherosclerosis.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Gagné C, Gaudet D, Bruckert E, Ezetimibe Study Group Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 39.•.Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202-7. 10.1161/CIRCULATIONAHA.111.042523. The first report of improved survival with statin therapy in patients with homozygous familial hypercholesterolemia even though LDL targets were not attained. [DOI] [PubMed]
- 40.Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–2120. doi: 10.1161/CIRCULATIONAHA.113.004678. [DOI] [PubMed] [Google Scholar]
- 41.• Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al; TESLA Investigators. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. 10.1016/S0140-6736(14)61374-X. The first randomized clinical trial of PCSK9 inhibitors in patients with homozygous familial hypercholesterolemia. [DOI] [PubMed]
- 42.Blom DJ, Harada-Shiba M, Rubba P, Gaudet D, Kastelein JJP, Charng MJ, Pordy R, Donahue S, Ali S, Dong Y, Khilla N, Banerjee P, Baccara-Dinet M, Rosenson RS. Efficacy and safety of Alirocumab in adults with homozygous familial hypercholesterolemia: the ODYSSEY HoFH trial. J Am Coll Cardiol. 2020;76:131–142. doi: 10.1016/j.jacc.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 43.• Underberg JA, Cannon CP, Larrey D, Makris L, Blom D, Phillips H. Long-term safety and efficacy of lomitapide in patients with homozygous familial hypercholesterolemia: Five-year data from the Lomitapide Observational Worldwide Evaluation Registry (LOWER). J Clin Lipidol. 2020;S1933–2874(20)30251–8. doi:10.1016/j.jacl.2020.08.006The report of long term real life data of lomitapide registry. [DOI] [PubMed]
- 44.• Stefanutti C. Lomitapide-a Microsomal Triglyceride Transfer Protein Inhibitor for Homozygous Familial Hypercholesterolemia [published correction appears in Curr Atheroscler Rep. 2020 Jul 15;22(8):41]. Curr Atheroscler Rep. 2020;22:38. doi:10.1007/s11883-020-00858-4The most recent review on the efficacy and safety of lomitapide in homozygous familial hypercholesterolemia. [DOI] [PMC free article] [PubMed]
- 45.Waldmann E, Vogt A, Crispin A, Altenhofer J, Riks I, Parhofer KG. Effect of mipomersen on LDL-cholesterol in patients with severe LDL-hypercholesterolaemia and atherosclerosis treated by lipoprotein apheresis (The MICA-Study). Atherosclerosis. 2017 Apr;259:20–25. Erratum in: Atherosclerosis. 2018 Aug;275:461–462. [DOI] [PubMed]
- 46.Kersten S. Bypassing the LDL receptor in familial hypercholesterolemia. N Engl J Med. 2020;383:775–776. doi: 10.1056/NEJMe2023520. [DOI] [PubMed] [Google Scholar]
- 47.• Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P. Et al; ELIPSE HoFH investigators. Evinacumab for homozygous familial hypercholesterolemia. N Engl J med. 2020;383:711-720. 10.1056/NEJMoa2004215. First randomized clinical trial of evinocumab in homozygous familial hypercholesterolemia. [DOI] [PubMed]
- 48.https://clinicaltrials.gov/ct2/show/NCT03851705?term=ORION+5&rank=1
- 49.Paton DM. Bempedoic acid: effect of ATP-citrate lyase inhibition on low-density lipoprotein cholesterol and other lipids. Drugs Today (Barc). 2020;56:573–82. [DOI] [PubMed]
- 50.• Bruckert E, Saheb S, Bonté JR, Coudray-Omnès C. Daily life, experience and needs of persons suffering from homozygous familial hypercholesterolaemia: insights from a patient survey. Atheroscler Suppl. 2014;15:46-51. 10.1016/j.atherosclerosissup.2014.07.006. An important study defining the perception of patients with homozygous familial hypercholesterolaemia on lipoprotein apheresis. [DOI] [PubMed]
- 51.• Kayikcioglu M, Tokgozoglu L, Tuncel OK, Pirildar S, Can L. Negative impact of COVID-19 pandemic on the lifestyle and management of patients with homozygous familial hypercholesterolemia. J Clin Lipidol. 2020;14:751–5. 10.1016/j.jacl.2020.09.002. First report of the impact of COVID-19 on patients with familial hypercholesterolemia. [DOI] [PMC free article] [PubMed]
- 52.••.Cegla J, Neely RDG, France M, Ferns G, Byrne CD, Halcox J. Et al; HEART UK medical, scientific and research committee. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis. 2019;291:62-70. 10.1016/j.atherosclerosis.2019.10.011. Consensus statement of Heart UK on the management of high lipoprotein a. [DOI] [PubMed]
- 53.••. Pokrovsky SN, Afanasieva OI, Ezhov MV. Therapeutic Apheresis for Management of Lp(a) Hyperlipoproteinemia. Curr Atheroscler Rep. 2020;22:68. 10.1007/s11883-020-00886-0. The most recent up to date review of the role of apheresis in the management of high Lp(a) written by the investigators who developed the selective apheresis for Lp(a). [DOI] [PubMed]
- 54.•.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–83 The first study documented that anti-sense therapy can decrease Lp(a) levels effectively to a greater extent than apheresis. [DOI] [PubMed]
- 55.•.Safarova MS, Ezhov MV, Afanasieva OI, Matchin YG, Atanesyan RV, Adamova IY, et al. Effect of specific lipoprotein(a) apheresis on coronary atherosclerosis regression assessed by quantitative coronary angiography. Atheroscler Suppl. 2013;14:93–9. 10.1016/j.atherosclerosissup.2012.10.015. The first controlled clinical trial of the specific and sustained removal of Lp(a) diminishing the signs of coronary atherosclerosis. [DOI] [PubMed]
- 56.•.Poller WC, Berger A, Dreger H, Morgera S, Enke-Melzer K. Lipoprotein apheresis in patients with peripheral artery disease and lipoprotein(a)-hyperlipoproteinemia: 2-year follow-up of a prospective single center study. Atheroscler Suppl. 2017;30:174–9. 10.1016/j.atherosclerosissup.2017.05.007The first results of the clinical efficacy of lipoprotein apheresis for the treatment of patients with stenotic atherosclerosis of the lower limb arteries. [DOI] [PubMed]
- 57.Watts GF, Sullivan DR. Van Bockxmeer FM, Poplawski N, Hamilton-Craig I, Clifton PM, et al; familial Hypercholesterolaemia Australasia network. A new model of care for familial hypercholesterolaemia: what is the role of cardiology? Heart Lung Circ. 2012;21:543–550. doi: 10.1016/j.hlc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R. Brown WV, et al. International Familial Hypercholesterolemia Foundation Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation Eur J Prev Cardiol. 2015;22:849–854. doi: 10.1177/2047487314533218. [DOI] [PubMed] [Google Scholar]
- 59.Stefanutti C, Julius U, Watts GF, Harada-Shiba M, Cossu M, Schettler VJ, et al; MIGHTY MEDIC Multinational Society. Toward an international consensus-Integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol. 2017;11:858–871.e3. 10.1016/j.jacl.2017.04.114. [DOI] [PubMed]