Abstract
The incidence of myocardial infarction (MI) increases every year worldwide. Better diagnostic and prognostic biomarkers for clinical applications are the consistent pursuit of MI research. In addition to electrocardiogram, echocardiography, coronary angiography, etc., circulating biomarkers are essential for the diagnosis, prognosis, and treatment effect monitoring of MI patients. In this review, we assessed both strength and weakness of MI circulating biomarkers including: (1) originated from damaged myocardial tissues including current golden standard cardiac troponin, (2) released from non-myocardial tissues due to MI-induced systems reactions, and (3) preexisted in blood circulation before the occurrence of MI event. We also summarized newly reported MI biomarkers. We proposed that the biomarkers preexisting in blood circulation before MI incidents should be emphasized in research and development for MI prevention in near future.
Keywords: serum biomarkers, myocardial infarction, MI diagnosis, MI prognosis, circulating biomarkers
Introduction
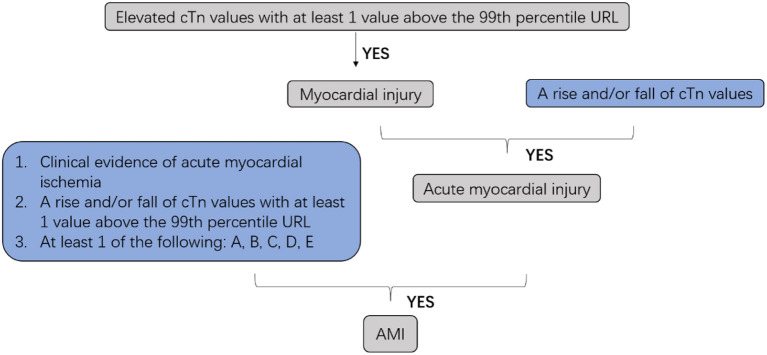
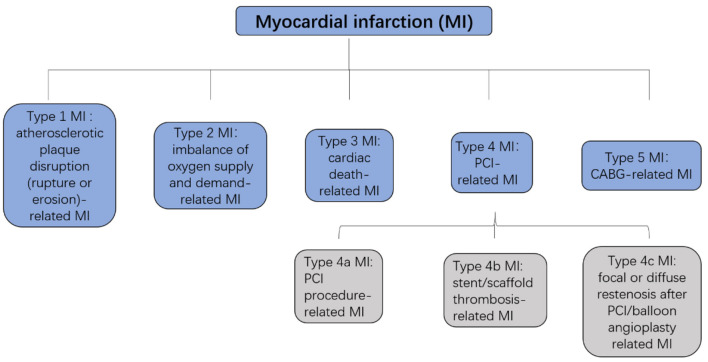
Cardiovascular diseases are a leading cause of mortality in humans, and nearly 20 million individuals worldwide die from acute cardiovascular events every year. Myocardial infarction (MI), also known as a heart attack, is a myocardial injury caused by myocardial ischemia (1). In 2018, the fourth Universal Definition of Myocardial Infarction emphasized the difference between acute myocardial infarction (AMI) and myocardial injury and divided MI into five types (2, 3) (Figures 1, 2).
Figure 1.
Clinic defined acute myocardial infarction (AMI). cTn, cardiac troponin; URL, upper reference limit; AMI, acute myocardial infarction; A: Symptoms of myocardial ischemia; B: New ischemic ECG changes; C: Development of pathological Q waves; D: Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; E: Identification of a coronary thrombus by angiography or autopsy (not for types 2 or 3 MIs).
Figure 2.
Five types of MI. MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
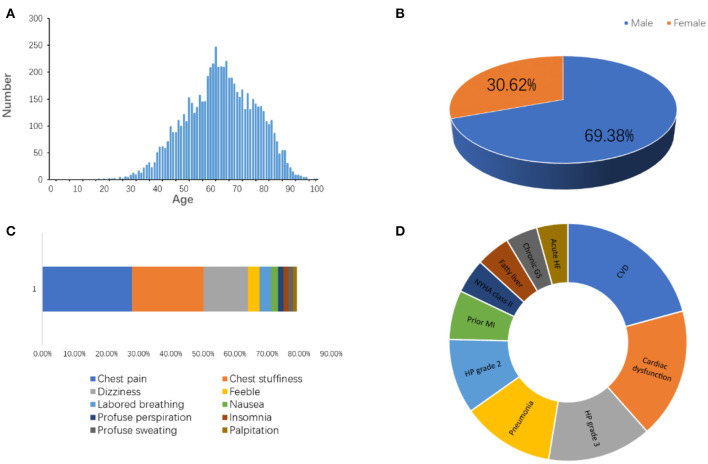
Approximately 1.5 million individuals in the United States suffer from MI every year (4). We have followed the information of MI patients admitted to the Affiliated Hospital of Qingdao University in China from January 2015 to August 2020. The total incidents, patient age, gender, symptoms, and companion diagnosis are summarized in Figure 3, which are consistent with published knowledge that the MI patients are largely male ranging from 60 to 75 years old with typical symptoms, such as chest pain, chest stuffiness, and dizziness.
Figure 3.
The information of patients diagnosed as MI admitted to the Affiliated Hospital of Qingdao University from 2015 to 2020. (A) Age distribution. (B) Gender distribution. (C) Symptoms. (D) Companion diagnosis. CVD, cardiovascular disease; HP, high blood pressure; MI, myocardial infarction; GS, gastritis; HF, heart failure.
The high incidence of MI results in financial burdens to both families and society and affects the life quality of MI patients.
In addition to ECG, echocardiography, coronary angiography, etc., circulating biomarkers are essential for MI diagnosis. The MI circulating biomarkers have gone through a long process from discoveries to clinical applications. Table 1 summarizes the milestone of the MI biomarker research and development during the past 70 years.
Table 1.
The milestones of biomarker discoveries for myocardial infarction.
| Years | Milestone | References |
|---|---|---|
| 1954 | AST released from the necrotic cardiac myocytes to the circulation could be helpful in diagnosing AMI | (5) |
| 1954–1955 | LDH was considered as a useful marker in the diagnosis of AMI | (6, 7) |
| 1978 | CK activity was considered a better predictor of myocardial injury and an independent indicator in the diagnosis of AMI for 20 years | (8) |
| 1982 | Myoglobin occurs in the first 30 min while it has less specificity | (9) |
| 1982 | S100 gradually comes into spotlight and is related to MI | (10–14) |
| 1992 | Subtypes of IL play crucial roles in diagnosis and prognosis of MI | (15–21) |
| 1993 | “Decreased immunoreactivity for H-FABP may be a good histological marker of damaged cardiomyocytes” | (22) |
| 1997 | IGF may protect cardiac function after AMI | (23) |
| 1998 | cMyC is expressed in the heart specially | (24–27) |
| 2001 | uACR is associated with increased long-term risk of cardiovascular and total mortality in survivors of MI | (28) |
| 2003 | Atherosclerosis is proved to be associated with alterations in MMP activity | (29) |
| 2003 | MiRNAs are associated to MI | (30–41) |
| 2004 | VEGF is associated with diagnosis, infarction size, and clinical outcomes of MI | (42–45) |
| 2007 | Hs-cTn is current golden standard for AMI diagnosis | (46) |
| 2013 | HPA is associated with acute MI accompanied with elevated white blood cell count | (47) |
| 2013 | Copeptin: a new marker in cardiology | (48) |
| 2014 | Ischemia-reperfusion injury has been proved to decrease MG53 in heart | (49) |
| 2015 | PIK3C2A could affect angiogenesis contributing to the pathophysiology of coronary artery disease | (50) |
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK, creatine kinase; IL, interleukin; H-FABP, heart type fatty acid-binding protein; IGF, insulin-like growth factor 1; cMyC, cardiac myosin-binding protein C; VEGF, vascular endothelial growth factor; hs-cTn, high sensitivity cardiac troponin; HPA, heparanase.
The AMI circulating biomarkers can be divided into three categories: (1) the biomarkers originated from damaged myocardial tissues and released into blood circulation (Table 2); (2) biomarkers with increased levels in blood circulation due to systems reactions after the MI events (Table 3); and (3) biomarkers with abnormal serum levels before the occurrence of MI event (Table 4). Both the strength and weakness of these MI circulating biomarkers will be discussed in the following sections. We also discussed the strengths and weaknesses of newly reported MI biomarkers. We proposed that the biomarkers preexisted in blood circulation before MI incidents should be emphasized in research and development for MI prevention in near future.
Table 2.
Biomarkers originated from myocardial tissue.
| Abbreviation | Full name | Characteristics | Remarks | References |
|---|---|---|---|---|
| LDH | Lactate dehydrogenase |
*Low sensitivity and specificity *Distinguish acute from subacute MI in patients with positive troponins and negative CK or CK-MB |
LDH1:LDH2 ratio >1 is specific for AMI | (51) |
| CK | Creatine kinase |
*Higher sensitivity and specificity than LDH *Cannot detect minor myocardial injury |
*MB2:MB1 ≥1.5 is in favor of AMI *CK-MB relative index (CK-MB/total CK*100) could be used to diagnose MI *Total CK and CK-MB are related to infarction size and prognosis of MI | (5, 52–54) |
| – | Myoglobin |
*Has no specificity so negative values are more meaningful than positive *Rises early after MI |
Used to evaluate infarction size and reperfusion | (5, 9, 55) |
| cTn | Cardiac troponin | Highest sensitivity and specificity among biomarkers applied to clinic | Golden standard | (5, 46) |
| H-FABP | Heart type fatty acid-binding protein |
*Sense post-ischemic myocardial reperfusion injury *Prognose relatively long-term post-ischemia |
High negative predictive value of H-FABP test can help to rule out AMI earlier | (56–62) |
| cMyC | Myosin-binding protein C | Rise and fall more rapidly after myocardial injury | To rule in/out AMI more effectively among those presenting early after symptom onset | (27, 63) |
Table 3.
Biomarkers induced by MI incidence.
| Abbreviation | Full name | Characteristics | Remarks | References |
|---|---|---|---|---|
| ILs | Interleukins | *Targeting IL-1 could be a novel therapy pericarditis associated with inflammasome activation after MI *Related to cardiac remodeling |
IL-1Ra may have a predictive effect on MI | (15, 17, 64–66) |
| IGF-1 | Insulin-like growth factor 1 | *Reduce adverse cardiac remodeling *Improve ventricular arrhythmia |
Cannot be used to diagnose MI | (67–72) |
| VEGF | Vascular endothelial growth factor | *An independent risk factor for adverse clinical outcomes after AMI *Associate with infarct size in patients with AMI |
Different subtypes have various effect | (42, 44, 45) |
| MMPs | Matrix metalloproteinases | Circulating MMP-28, a predictor for short-term prognosis in patients with MI | – | (73) |
Table 4.
Biomarkers preexisted before MI occurred.
| Abbreviation | Full name | Characteristics | Remarks | References |
|---|---|---|---|---|
| Glc | Glucose | Significantly increased in AMI | Lack specificity for MI | (4) |
| AST | Aspartate aminotransferase | Previously used MI biomarker | Lack specificity for MI | (5) |
| RNAs | RNAs (including microRNAs and LncRNAs) | Involved in every aspects of MI | Next generation biomarker for MI | (30–41) |
| S100 | – | S100 family are essential in diagnosis and prognosis of MI. | – | (74–76) |
| HPA | Heparanase | Participate in mediating pathological process of MI | A predictive marker for high thrombus burden in patients with STEMI | (47, 77) |
| PIK3C2A | – | Low expression of PIK3C2A gene is an independent risk factor and could serve as a potential biomarker to predict risk of AMI | – | (78) |
| Copeptin | – | Combinated with hs-cTnI to detect suspected ACS patients with low hs-cTnI | Gender-specific | (79, 80) |
| Mitsugumin 53 | MG53 | Elevated serum MG53 levels shows a significant adverse outcome after a 3-year follow-up among patients with STEMI | – | (81) |
| The serum albumin-to-creatinine ratio | sACR | An independent prognostic marker and a useful marker for early risk stratification of patients with AMI | – | (82) |
Biomarkers
Biomarkers Originated From Myocardial Tissues
Lactate Dehydrogenase
Lactate dehydrogenase (LDH) was considered as a useful biomarker in diagnosing AMI (51, 83). LDH has five isoenzymes. LDH-1 is expressed in the heart but it is not heart-specific (83). Circulating LDH-1 increases within 6–12 h from onset of chest pain. It peaks at 1–3 days and returns to normal within 8–14 days. Due to its low sensitivity and specificity, LDH is only used to distinguish acute from subacute MI in patients with positive troponins while CK and CK-MB are negative (51). Moreover, a LDH-1:LDH-2 ratio >1 is reported to be specific for diagnosing AMI (51).
Creatine Kinase
Creatine kinase (CK) activity was considered a better predictor of myocardial injury and an independent indicator of AMI for 20 years (8). CK is a dimeric enzyme, consisting of two subunits, M and B, and has three isoenzymes, CK-BB (CK1), CK-MB (CK2), and CK-MM (CK3) (52). Among them, only CK-MB is found in the heart, but CK-MB is also detected in other organs, such as uterus, tongue, etc. (84). When released into the blood, CK-MB can be divided into two groups, MB1 and MB2. When AMI occurs, MB2 passes into blood companying with significant change in the MB2:MB1 ratio. An MB2:MB1 ratio ≥1.5 is considered as an indicator of AMI (5). CK-MB is an excellent biomarker in diagnosis of AMI during the first 6 h, and at the same time, the negative predictive value during the first 6 h is 97% (5). Furthermore, it was reported that the CK-MB relative index (CK-MB/total CK × 100) could be used for diagnosis of MI. If this index is 2.5% or above, CK-MB has a great possibility released from heart (53). Finally, the total CK and CK-MB levels are correlated with infarct size and provide possibility to predict prognosis. CK-MB, however, cannot detect minor myocardial damage (54).
Myoglobin
Myoglobin is a biomarker for early detection and/or exclusion of cardiac injury because the serum level of myoglobin rises in the first 30 min after the onset of an acute event (9). Negative values are more meaningful in the clinic than positive values due to its low-specificity (55).
Cardiac Troponin
Increased serum cardiac troponin (cTn) level is the gold standard for AMI diagnosis. Combined changes of cTn with clinical manifestations and ECG could initially identify AMI in the early stage after the onset of chest pain, which can decrease mortality significantly.
Cardiac troponin I (cTnI) is presented in cardiac muscle tissue. Cardiac troponin T (cTnT) is expressed in both skeletal and cardiac myocytes. There is no report that cTnI increases after non-cardiac tissues are damaged while cTnT is more complicated because elevated cTnT may be derived from skeletal muscles. Thus, cTnI is more specific in diagnosing MI (2, 85, 86). High sensitive (hs)-cTn assays measure cTn concentrations 5- to 100-fold lower than conventional assays. Anda et al. suggested that use of risk stratification thresholds for hs-cTnl could identify patients with suspected acute coronary syndrome and at least 2 h of symptoms as low risk at presentation irrespective of age and sex (87). Due to higher sensitivity and specificity compared with others biomarkers, cTnI plays an important role in diagnosis of MI and high-sensitivity (hs)-cTn assays are routinely used in clinic (46).
Heart Type Fatty Acid-Binding Protein
Fatty acid-binding proteins (FABPs) belong to a family of proteins that are responsible for the transportation of fatty acids and lipophilic materials into or out of cells (88). There are several types of tissue-specific FABPs, including heart-type, liver-type, intestinal-type, epidermal-type, brain-type, ileal-type, myelin-type, adipocyte-type, and testis-type FABPs (88). Heart type fatty acid-binding protein (H-FABP) is a small (15 kDa) soluble protein. Despite not being cardiac-specific, H-FABP plays an essential part in metabolism of fatty acid (FA) inside cardiomyocytes and present at high concentrations in cardiomyocytes cytoplasm (89). Other tissues such as skeletal muscle, brain, and kidney also produce it, although at a lower concentration than in myocardium (90). Watanabe indicated that a “decreased immunoreactivity for H-FABP may be a good histological biomarker of damaged cardiomyocytes” (22). Serum concentration of H-FABP increases in the first 1–2 h after symptom onset, which provides possibility for early diagnosis of AMI, and peak concentration is achieved in approximately 5–10 h, after which it returns to its reference range within 24–36 h (91). Notably, H-FABP demonstrates different sensitivity and specificity at different cutoff, and the highest sensitivity of H-FABP is measured at 4 h (88%) (56). However, its sensitivity and specificity are lower than hs-cTn and thereby it cannot be used as a standalone biomarker for AMI diagnosis. Further studies may help to determine the most precise cutoff point of H-FABP for AMI diagnosis and its additional usage in cardiovascular emergencies (57).
Recently, more attention has been paid on H-FABP as a biomarker for immediate myocardial injury and even for relatively long-term post-ischemic prognosis. Although H-FABP has relatively poor effects on AMI diagnosis, there is evidence that H-FABP plays an important role in evaluating in-stent restenosis and achievement of heart reperfusion after ischemic attack (57, 58, 92). Huang et al. (59) also found H-FABP to be a more sensitive biomarker than cTnI and CK-MB for sensing post-ischemic myocardial reperfusion injury. Additional analysis showed that H-FABP could be a biomarker that can independently predict adverse cardiac events on different levels and provide information for a risk evaluation for clinicians (60, 93, 94). Furthermore, a high negative predictive value of H-FABP test can help to rule out AMI earlier (61), which can reduce hospitalizations and expenses. It is worth mentioning that in 2018, Jo et al. proved that H-FABP would be a more useful biomarker to detect myocardial ischemic injury than CK-MB and cTnT (95). Based on the properties of H-FABP, the combination of H-FABP with other biomarkers, for instance, H-FABP at the early stage and cTnI at the late stage of AMI, may achieve better diagnostic and prognostic significance.
Myosin-Binding Protein C
The myosin-binding protein C family consists of three isoforms (96). Notably, the cardiac myosin-binding protein C (cMyC) is expressed in the heart specifically (24, 25). cMyC is more abundant in myocardial tissue and in blood circulation than cTn. cMyC is essential in assembly and function of cardiac sarcomere (26, 27, 63). Due to the delayed appearance of cTn, patients who demonstrate acute chest pain need to test repeatedly to determine AMI while cMyC appears earlier and rises faster in AMI patients (97). Thus, cMyC has an advantage over cTn for early diagnosis of AMI.
It is reported that cMyC rises and falls more rapidly after AMI in patients with vascular risk factors and/or underlying chronic heart disease (98). The ability to distinguish patients suffering MI or acute chest pain by cMyC is similar to that of hs-cTnT and hs-cTnI and superior to s-cTnI (99). In patients presenting <3 h of chest pain onset, cMyC is superior to hs-cTnT (99). In short, cMyC is a promising AMI biomarker with the strengths of ruling in/out AMI more effectively at early onset.
Biomarkers Induced by MI Incidence
Interleukins
Interleukins (ILs) are a group of cytokines that are expressed by leukocytes. ILs can be divided into four major groups based on distinguishing structural features. The human genome encodes more than 50 interleukins and related proteins.
IL-1 family is a group of 11 cytokines, which induces a complex network of proinflammatory cytokines via expression of integrins on leukocytes and endothelial cells, and regulates and initiates inflammatory responses. In 2004, Patti et al. evaluated that interleukin-1 receptor antagonist (IL-1Ra) increased early in patients with AMI, especially in those with premonitory infarction and symptom onset ≤3 h, and preceded other biomarkers of necrosis (15). IL-1Ra may be an important early adjuvant toward diagnosis of AMI in the emergency department.
IL-32 is a newly discovered inflammatory cytokine with eight isoforms in most mammals. IL-32 is found to be highly expressed in human atherosclerotic plaques and significantly increased in patients with heart failure after MI (16, 17). Furthermore, the soluble IL-1 receptor 2 (sIL-1R2), IL-1, IL-6 plays an important role in myocardial remodeling after MI (64, 65, 100). For the time being, the IL-related therapy of MI is effective, indicating that further research on ILs is needed for MI diagnosis and therapy.
Insulin-Like Growth Factor 1
Insulin-like growth factor 1 (IGF-1) is an anabolic hormone that controls growth and metabolism of many cell types. In 1997, Scheinowitz indicated that IGF may protect cardiac function after AMI (23). The majority of circulating IGF-1 molecules combine with IGF binding proteins (IGFBPs), which can modulate IGF-1 binding to the IGF-1 receptor (IGF1R) (101). Evidence shows that IGF-1 levels are correlated with occurrence of coronary heart disease, which functions possibly by affecting atherosclerosis progression (102).
Free IGF-1 is inferior to CK-MB as an indicator of myocardial damage (103). However, IGF-1 plays an important role in some other aspects of MI. IGF-1 can affect vascular function and atherosclerosis by anti-inflammatory and anti-apoptotic actions as well as by stimulating angiogenesis (67–69, 104). At the same time, there is also evidence indicating that IGF-1 has indirect effects on the cardiovascular system by increasing insulin sensitivity (69–71). On the one hand, IGF-1 prevents recruitment of monocytes/macrophages from atherosclerotic plaques, production of proinflammatory cytokines, conversion of macrophages into lipid-laden foam cells, and extracellular matrix degradation. IGF-1 also promotes smooth muscle cell (SMC) migration, proliferation, and SMC-dependent matrix deposition, all of which may contribute to IGF-1-induced reduction in plaque burden and increase in plaque stability (72). By the mechanism described above, IGF-1 stabilizes plaque so as to decrease MI events. Furthermore, IGF-1 treatment is effective to reduce adverse cardiac remodeling after cardiac ischemia/reperfusion injury, when IGF-1 is administered systemically (105). Finally, Yao et al. found that combination of hepatocyte growth factor (HGF) and IGF-1 promote connexin 43 expression and improve ventricular arrhythmia after MI in a rat model (106), which may exhibit therapeutic potential for ventricular arrhythmias after MI.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF) is a highly specific growth factor for vascular endothelial cells, which can promote vascular permeability, extracellular matrix denaturation, vascular endothelial cell migration, proliferation, and angiogenesis. VEGF family include VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor (PGF).
VEGF-A, which is actively produced in damaged myocardium to foster angiogenesis and tissue repair, is a major effector of endothelial junction disruption and vascular leakage (42, 107, 108). Clinical studies on VEGF-A in patients with AMI have demonstrated that low levels of circulating VEGF-A are an independent risk factor for adverse clinical outcomes after AMI (43, 44). VEGF-A 165b is the main anti-angiogenic isoform of VEGF-A and associates with infarct size in patients with AMI and dysregulated VEGF-A 165b in aging endothelial cells contribute to the risk of coronary heart disease (109–111). Study shows that the assessment of VEGF-A 165b combined with VEGF-A may predict main adverse cardiovascular events (MACEs) in clinical practice (112) and VEGF-A 165b might play a negative regulatory role in AMI as an inhibitor of angiogenesis in myocardium. According to that, therapy aimed at VEGF-A 165b might be significant to reperfusion of myocardium after STEMI (109). In addition, a low VEGF-C value may independently predict all-cause mortality in patients with suspected or known CHD (113).
In summary, VEGF has little effect on diagnosis of MI while it has significant effect on prognosis and MI treatment.
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a group of zinc ion-dependent proteases that degrade collagen and proteoglycans and play an important role in the development of atherosclerosis (114). MMPs play a pivotal role in post-myocardial infarction cardiac remodeling as well as in the development of adverse outcomes (115). Circulating MMP-28, a new member in the family of MMPs, could be considered a predictor for short-term prognosis in patients with myocardial infarction (73).
Biomarkers Preexisted Before MI Occurred
Glucose
A recent study reported that in a well-treated contemporary population of AMI patients, 42% of patients without diabetes have elevated admission plasma glucose levels and AMI event rates are increased with the elevated admission plasma glucose levels (116). In addition, fasting blood glucose levels are significantly increased in patients suffering AMI (117). These findings highlight the importance to research and develop the circulating biomarkers usable for MI prevention.
Aspartate Aminotransferase
Aspartate Aminotransferase (AST) catalyzes the reversible transfer of an α-amino group between aspartate and glutamate. AST plays an important role in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, and red blood cells. In 1954, AST is established as a biomarker in assisting AMI diagnosis (5). However, it was abandoned because increased serum AST levels are associated with various diseases and also present in both pre- and after AMI events.
RNAs
microRNAs
A whole new understanding of miRNA began in 2003 when Ambros showed that miRNA participates in regulating development process in worms (118). MicroRNAs (miRNAs/miRs) are a class of tissue-specific or cell-specific small (19–25 nucleotides) non-coding RNAs, which have effects on various biological processes including cell growth, proliferation, differentiation, and apoptosis (119, 120). MicroRNAs are circulating in plasma/serum and have been tested as biomarkers for cardiovascular diseases (30). Among them, microRNA-499 has been shown to be expressed in myocardium and skeletal muscle in mammals, and the blood samples from patients have high levels of microRNA-499 before the AMI events (31, 32). Circulating miR-499 has good sensitivity and specificity for differentiating AMI from non-AMI (0.84 and 0.97, respectively) and it is considered for early diagnosis of AMI (33).
By detecting plasma concentration of microRNA-145 in patients, Zhang et al. indicated that low microRNA-145 levels correlate inversely with the severity of AMI (34). They also speculated that circulating microRNA-145 might not only be of use in diagnosing MI but could also potentially be helpful in prognosticating cardiac function and the risk of developing heart failure. In 2015, Jia et al. indicated that miR-125b-5p and miR-30d-5p have a value for early diagnosis of AMI, and miR-30d-5p might37 have a higher diagnostic value than cTnI (35). MicroRNA-208b (34), microRNA-133a (121), miR-486 as well as miR-150 (36), and microRNA-21 (37) may be novel biomarkers used for the diagnosis of AMI.
Long Non-coding RNAs
Zhong et al. indicated that differential expression of long non-coding RNAs (LncRNAs) would be helpful to understand molecular mechanism of AMI and might be useful biomarkers for non-invasive diagnostic application (122). LncRNAs are a set of RNA transcripts containing more than 200 nucleotides, which cannot transcript protein but have same effects with miRNAs (123). It has been reported that lncRNAs are involved in regulation of cardiac development, pathogenesis of heart failure, and the role of cardiovascular aging (38, 39). Previous studies demonstrated that the serum levels of three lncRNAs, namely H19, MIAT, and MALAT1, are significantly increased in AMI patients when compared with healthy volunteers, which indicates that these lncRNAs are promising biomarkers for the diagnosis of AMI (124).
S100 Protein
S100 belongs to the family of EF-hand proteins and is a calcium-binding protein with a low molecular weight of 10–12 kD. Its amino acid sequence is highly conserved in vertebrates and it has high homology with calmodulin and other EF-hand type calcium-binding proteins. At present, there are at least 21 different types of S100 proteins. They are also named as damage-associated molecular pattern molecules (DAMPs). S100 protein consists of two isomeric subunits(α/β) with αα, αβ, and ββ combinations (125).
S100 proteins are normally present in cells derived from the neural crest, chondrocytes, adipocytes, myoepithelial cells, macrophages, Langerhans cells, dendritic cells, and keratinocytes. It has been shown that heart and skeletal muscle are rich in S100A while most S100B is found in the brain (10, 126). S100 proteins have been implicated in a variety of intracellular and extracellular functions. S100 proteins are involved in regulation of protein phosphorylation, transcription factors, Ca2+ homeostasis, the dynamics of cytoskeleton constituents, enzyme activities, cell growth and differentiation, and the inflammatory response.
Several members of the S100 protein family are useful as biomarkers for certain cancers (127). Further, S100 proteins are biomarkers for inflammatory diseases and can mediate inflammation and act as antimicrobials (128). Serum S100A0 is a useful biomarker for diagnosing AMI, which is also better than CK-MB for differentiating AMI from angina pectoris (11). S100A1 is abundant in the heart, especially ventricular cardiomyocytes (12), which can be used to detect the postmortem diagnosis of AMI at an early stage (13). S100A4 expression protects cardiac myocytes against myocardial ischemia and is required for stabilization of cardiac function after MI (14). In 2011, serum levels of S100B, S100A6, S100P, and soluble receptor for advanced glycation endproduct (sRAGE) were analyzed in 882 patients. It was found that serum levels of S100B, S100A6, and S100P are associated with ischemic myocardial injury in acute coronary syndrome (ACS), and expression of these S100 proteins is related to myocardial infarct size (129). As for S100B, it plays an important role in down-regulating cardiac myocyte hypertrophy and is an attractive therapeutic target for treatment of cardiovascular disease (74). In addition, S100B expression may affect cardiac metabolism in diabetic post-MI remodeling and function (75).
More attention should be directed toward S100 as early diagnostic biomarker for MI prevention.
Heparanase
Heparanase (HPA) is an endo-b-D-glucuronidase capable of degrading heparan sulfate (HS) and heparin side chains (130), whose expression is particularly in placenta, platelet, keratinocyte, and active cells of the immune system. HPA plays a role in tumor growth, angiogenesis, cell invasion, and activation of the coagulation system (131, 132). Here we mainly discuss its effects on atherosclerosis, stenosis, and thrombosis, which are all associated with arterial plaque development and rupture (133).
Blich et al. found that HPA levels increase nearly 9-fold in patients with AMI while 3-fold in patients with stable angina (SA) compared to healthy individuals (47). They found that high levels of HPA promotes plaque toward vulnerability, which is pathological basis for MI. Furthermore, HPA functions as a mediator to enhance expression of tissue factor and generation of factor Xa, which are two critical components in blood coagulation (77, 134). Thrombosis caused by rapid blood clotting might lead to disruption of coronary blood blow thereby onset of AMI. In addition, HPA is a predictive biomarker for high thrombus burden in patients with STMI (135). HPA levels are associated with plaque vulnerability and progression and may thus be considered as a pre-diagnostic biomarker and potentially therapeutic target for prevention of acute heart diseases.
PIK3C2A
PIK3C2A belongs to phosphoinositide 3-kinases (PI3Ks), which is a family of enzymes that phosphorylate the 3′-OH position of the inositol ring of phosphatidylinositol (PI), and regulate a broad range of signaling pathways (136). A retrospective study showed that the level of PIK3C2A gene expression in patients with AMI is significantly lower than that of healthy individuals. Low expression of PIK3C2A is an independent risk factor and could serve as a potential biomarker to predict the risk of AMI (78).
Copeptin
Copeptin, a neuropeptide, has attracted interest for its use as part of a dual marker strategy in combination with cTn for the early rule-out of chest pain patients with suspected NSTEMI (137). A large pooled cohort showed that copeptin below cut-off in combination with hs-cTn below the upper limit of normal range may be used in more than 2.4-times more patients presenting with suspected acute coronary syndrome than a single biomarker strategy based on very low hs-cTn (79). Furthermore, men with suspected NSTEMI have higher copeptin levels, and certain predictors of copeptin elevation are gender-specific (80).
Mitsugumin 53
Mitsugumin 53 (MG53), a muscle-specific protein belonging to the tripartite motif family, has been demonstrated to protect the heart against oxidative injury. A study demonstrated that elevated serum MG53 levels have a significant adverse outcome after a 3-year follow-up among patients with STEMI. The measurement of MG53 could be used as a novel biomarker to improve the current means of risk stratification of AMI (81).
The Urine Albumin-To-Creatinine Ratio
The urine albumin-to-creatinine ratio (uACR) has been verified to be independently associated with increased long-term risks of cardiovascular and total mortality in survivors of MI (28). The serum albumin-to-creatinine ratio (sACR) was found to be an independent prognostic biomarker in patients with AMI on admission to the emergency department and a useful biomarker for early risk stratification of patients with AMI (82).
The Other Functions of MI Biomarkers
Except for MI diagnosis, the biomarkers mentioned above are also involved in atherosclerosis progression, evaluation of cardiac function, prognosis, etc. (Table 5).
Table 5.
Other biological functions of MI circulating biomarkers.
| Biomarkers | Application value | References | |
|---|---|---|---|
| S100 | S100A8 | Prevents myocardial ischemia and stabilize cardiac function after MI | (14) |
| S100B, S100A6, S100P |
*Associate with ischemic myocardial injury in ACS * Relate to myocardial infarct size |
(129) | |
| S100B |
*Down-regulate cardiac myocyte hypertrophy and apoptosis *Affect myocardial metabolism |
(74, 75) | |
| IL | sIL-1R2, IL-1, IL-6 | Associated with myocardial remodeling after MI | (64, 65, 100) |
| H-FABP |
*Involved in in-stent restenosis *Evaluate the degree of heart reperfusion after ischemic attack *Predict adverse cardiac events and provide risk evaluation |
(57, 58, 60, 92–94) | |
| IGF-1 |
*Affect atherosclerosis progression * Reduce adverse cardiac remodeling after cardiac I/R injury * Improve ventricular arrhythmia after myocardial infarction combined with HGF |
(102, 105, 106) | |
| microRNAs | microRNA-145 | Prognosticate cardiac function and the risk of developing HF | (40) |
| microRNA-1,microRNA-133, microRNA-208 | Diagnose sudden death due to early AMI sensitively | (138) | |
| microRNA-124 | Reduce cardiomyocyte apoptosis following MI | (41) | |
| microRNA-208b | Reduces post-infarction myocardial fibrosis in | (139) | |
| microRNA−93 | Inhibit cardiac remodeling and HF | (140) | |
| miR-24-3p | Exert cardioprotective effects in myocardial I/R injury | (141) | |
| VEGF | VEGF-A | Inversely correlated with LVEF after MI at 6-month follow-up | (45) |
| VEGF-C | Predict all-cause mortality independently | (113) |
Many circulatory factors are associated with function and prognosis in patients with cardiovascular diseases (142, 143). Many other functions for potential serum MI biomarkers have been reported. For example, the levels of protooncogene tyrosine-protein kinase (SRC), C-C motif chemokine ligand 17 (CCL17), and chymotrypsin C (CTRC) are all significantly decreased in extracellular vesicles (EVs) lysates from MI patients but remain unaltered in the normal control plasma samples (144). The EVs isolated from plasmas provide additional diagnostic value and improve pathophysiological understanding compared to plasma alone in the context of MI, but further study on EVs is required. Moreover, by using proteomic analysis, elevated serum levels of Pregnancy Zone Protein (PZP) and Leucine-Rich Alpha-2-Glycoprotein (LRG) are shown to be independent risk factors for early-onset MI. Therefore, inflammation-associated LRG and PZP may be novel MI biomarkers (145). Furthermore, there is evidence indicating that galectin-3 may be a promising biomarker for evaluation of severity and prognosis of AMI (146). Recently, it was demonstrated that the levels of microparticles (MPs), especially CD31+CD42−EMPs and CD144+EMPs, have the order of normal subjects < stable angina < unstable angina < MI, indicating that MPs have the potential capacity to distinguish stable angina, unstable angina, and MI (147).
Conclusions and Outlook
MI is an aging-related systems disease caused by multiple genetic and environmental factors in addition to lifestyles (148, 149). It is understandable that high-sensitivity cardiac troponin (hs-cTn) is the current golden standard for AMI diagnosis since it is the specific heart tissue damage product. However, other circulating biomarkers are needed for prevention, prognosis, and treatment effect monitoring purposes.
Both glucose and HPA have pre-diagnostic properties. It has been proved that fasting blood glucose is an independent risk factor for Gensini score in AMI patients (150). In addition, a cohort study has shown that MI with diabetes mellitus tend to develop cardiogenic shock and have worse outcomes (151). A 10-year cohort study demonstrates that relatively high but clinically normal serum glycated hemoglobin A1c (HbA1c) and thyrotropin (TSH) may increase risk of coronary heart disease (CHD) (152). Most cancer biomarkers, such as CA199 and carcinoembryonic antigen (CEA), are either specific glycan structures or heavily glycosylated proteins (153). Among them, elevated carbohydrate antigen 125 (CA125) can be used to predict mortality risk at 6 months following AMI (154), which is consistent with our previous findings that the circulating CA125 levels are higher in fibrosis-associated diseases than in most types of cancers (155).
We classified the MI biomarkers for their diagnostic, prognostic, and preventive purposes and also discussed other biological functions of the biomarkers in current review. However, certain biomarkers belong to more than one category listed in Tables 1–5. Since the development of MI is a long process accompanied with multiple changes of the systems, biomarkers preexisting in blood circulation before MI incidents should be emphasized in research and development for MI prevention in the near future.
Author Contributions
YW drafted the manuscript and drew the figures. NP, YA, MX, LT, and LZ revised the manuscript for important intellectual content. YW and LZ were responsible for manuscript concept and design and edited the final manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Alexander Lu of St. Louis University in the United States for English editing of the manuscript.
Footnotes
Funding. This research was supported by the Natural Science Foundation of China (Grants 81672585) and the Taishan Scholar Fellowship to LZ.
References
- 1.Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 3.Chapman AR, Adamson PD, Shah ASV, Anand A, Strachan FE, Ferry AV, et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. (2020) 141:161–71. 10.1161/CIRCULATIONAHA.120.047269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu SS, Jiang JJ, Zhang Y, Chen TT, Zhu M, Fang CF, et al. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J Thorac Dis. (2019) 11:3962–72. 10.21037/jtd.2019.08.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydin S, Ugur K, Aydin S, Sahin I, Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. (2019) 15:1–10. 10.2147/VHRM.S166157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladue JS, Wroblewski F, Karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science. (1954) 120:497–9. 10.1126/science.120.3117.497 [DOI] [PubMed] [Google Scholar]
- 7.Wroblewski F, Ladue JS. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. (1955) 90:210–3. 10.3181/00379727-90-21985 [DOI] [PubMed] [Google Scholar]
- 8.Knudsen J, Steenstrup B, Byrjalsen I, Hildebrandt P, Sorensen S. At what level of serum total creatine kinase activity can measurement of serum creatine kinase MB isoenzyme activity be omitted in suspected myocardial infarction? Scand J Clin Lab Invest. (1989) 49:661–5. 10.3109/00365518909091542 [DOI] [PubMed] [Google Scholar]
- 9.Klocke FJ, Copley DP, Krawczyk JA, Reichlin M. Rapid renal clearance of immunoreactive canine plasma myoglobin. Circulation. (1982) 65:1522–8. 10.1161/01.CIR.65.7.1522 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki F, Nakajima T, Kato K. Peripheral distribution of nervous system-specific S-100 protein in rat. J Biochem. (1982) 92:835–8. 10.1093/oxfordjournals.jbchem.a133996 [DOI] [PubMed] [Google Scholar]
- 11.Usui A, Kato K, Sasa H, Minaguchi K, Abe T, Murase M, et al. S-100ao protein in serum during acute myocardial infarction. Clin Chem. (1990) 36:639–41. 10.1093/clinchem/36.4.639 [DOI] [PubMed] [Google Scholar]
- 12.Remppis A, Greten T, Schafer BW, Hunziker P, Erne P, Katus HA, et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. BBA Mol Cell Res. (1996) 1313:253–7. 10.1016/0167-4889(96)00097-3 [DOI] [PubMed] [Google Scholar]
- 13.Bi HT, Yang Y, Huang JY, Li YM, Ma CL, Cong B. Immunohistochemical detection of S100A1 in the postmortem diagnosis of acute myocardial infarction. Diagn Pathol. (2013) 8:84. 10.1186/1746-1596-8-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doroudgar S, Quijada P, Konstandin M, Ilves K, Broughton K, Khalafalla FG, et al. S100A4 protects the myocardium against ischemic stress. J Mol Cell Cardiol. (2016) 100:54–63. 10.1016/j.yjmcc.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patti G, D'Ambrosio A, Mega S, Giorgi G, Zardi EM, Zardi DM, et al. Early interleukin-1 receptor antagonist elevation in patients with acute myocardial infarction. J Am Coll Cardiol. (2004) 43:35–8. 10.1016/j.jacc.2003.07.032 [DOI] [PubMed] [Google Scholar]
- 16.Heinhuis B, Popa CD, van Tits BL, Kim SH, Zeeuwen PL, van den Berg WB, et al. Towards a role of interleukin-32 in atherosclerosis. Cytokine. (2013) 64:433–40. 10.1016/j.cyto.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Damen MSMA, Popa CD, Netea MG, Dinarello CA, Joosten LAB. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis. (2017) 264:83–91. 10.1016/j.atherosclerosis.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Fischer E, Van Zee KJ, Marano MA, Rock CS, Kenney JS, Poutsiaka DD, et al. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood. (1992) 79:2196–200. 10.1182/blood.V79.9.2196.bloodjournal7992196 [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. (1996) 87:2095–147. 10.1182/blood.V87.6.2095.bloodjournal8762095 [DOI] [PubMed] [Google Scholar]
- 20.Rothwell N, Allan S, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. (1997) 100:2648–52. 10.1172/JCI119808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loppnow H, Werdan K, Reuter G, Flad HD. The interleukin-1 and interleukin-1 converting enzyme families in the cardiovascular system. Eur Cytokine Netw. (1998) 9:675–80. [PubMed] [Google Scholar]
- 22.Watanabe K, Wakabayashi H, Veerkamp JH, Ono T, Suzuki T. Immunohistochemical distribution of heart-type fatty acid-binding protein immunoreactivity in normal human tissues and in acute myocardial infarct. J Pathol. (1993) 170:59–65. 10.1002/path.1711700110 [DOI] [PubMed] [Google Scholar]
- 23.Scheinowitz M, Abramov D, Eldar M. The role of insulin-like and basic fibroblast growth factors on ischemic and infarcted myocardium: a mini review. Int J Cardiol. (1997) 59:1–5. 10.1016/S0167-5273(96)02902-6 [DOI] [PubMed] [Google Scholar]
- 24.Fougerousse F, Deleozide AL, Fiszman MY, Schwartz K, Beckmann JS, Carrier L. Cardiac myosin binding protein C gene is specifically expressed in heart during murine and human development. Circ Res. (1998) 82:130–3. 10.1161/01.RES.82.1.130 [DOI] [PubMed] [Google Scholar]
- 25.Kuster DWD, Barefield D, Govindan S, Sadayappan S. A sensitive and specific quantitation method for determination of serum cardiac myosin binding protein-C by electrochemiluminescence immunoassay. Jove J Vis Exp. (2013) 50786:1–8. 10.3791/50786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aye TT, Scholten A, Taouatas N, Varro A, Van Veen TAB, Vos MA, et al. Proteome-wide protein concentrations in the human heart. Mol Biosyst. (2010) 6:1917–27. 10.1039/c004495d [DOI] [PubMed] [Google Scholar]
- 27.Marjot J, Kaier TE, Martin ED, Reji SS, Copeland O, Iqbal M, et al. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin Chem. (2017) 63:990–6. 10.1373/clinchem.2016.264648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berton G, Cordiano R, Palmieri R, Cucchini F, De Toni R, Palatini P. Microalbuminuria during acute myocardial infarction - a strong predictor for 1-year mortality. Eur Heart J. (2001) 22:1466–75. 10.1053/euhj.2000.2582 [DOI] [PubMed] [Google Scholar]
- 29.Plutzky J. The vascular biology of atherosclerosis. Am J Med. (2003) 115:55–61. 10.1016/j.amjmed.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Jing Q. Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol Sin. (2018) 39:1110–9. 10.1038/aps.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, et al. Plasma MicroRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. (2010) 56:1183–5. 10.1373/clinchem.2010.144121 [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Zhang LZ, Su T, Li H, Huang Q, Wu D, et al. Kinetics of plasma microRNA-499 expression in acute myocardial infarction. J Thorac Dis. (2015) 7:890–6. 10.3978/j.issn.2072-1439.2014.11.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao JY, Yu HR, Yan P, Zhou XH, Wang Y, Yao YH. Circulating microRNA-499 as a diagnostic biomarker for acute myocardial infarction: a meta-analysis. Dis Markers. (2019) 2019:6121696. 10.1155/2019/6121696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang WQ, Xie BQ. A meta-analysis of the relations between blood microRNA-208b detection and acute myocardial infarction. Eur Rev Med Pharmaco. (2017) 21:848–54. [PubMed] [Google Scholar]
- 35.Jia KG, Shi P, Han XJ, Chen TN, Tang HX, Wang J. Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol Med Rep. (2016) 14:184–94. 10.3892/mmr.2016.5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R, Lan C, Pei H, Duan GY, Huang L, Li L. Expression of circulating miR-486 and miR-150 in patients with acute myocardial infarction. BMC Cardiovasc Disor. (2015) 15:51. 10.1186/s12872-015-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Liu YJ, Liu T, Zhang H, Yang SJ. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur Rev Med Pharmaco. (2016) 20:323–9. [PubMed] [Google Scholar]
- 38.Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res. (2014) 115:668–77. 10.1161/CIRCRESAHA.115.303836 [DOI] [PubMed] [Google Scholar]
- 39.Ma YJ, Huang DS, Yang FF, Tian M, Wang YM, Shen D, et al. Long noncoding RNA highly upregulated in liver cancer regulates the tumor necrosis factor-alpha-induced apoptosis in human vascular endothelial cells. DNA Cell Biol. (2016) 35:296–300. 10.1089/dna.2015.3203 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Cheng YJ, Sara JDS, Liu LJ, Liu LP, Zhao X, et al. Circulating MicroRNA-145 is associated with acute myocardial infarction and heart failure. Chin Med J Peking. (2017) 130:51–6. 10.4103/0366-6999.196573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Z, Du S, Shen S, Wang L. MicroRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1beta. J Cell Physiol. (2020) 235:2710–21. 10.1002/jcp.29175 [DOI] [PubMed] [Google Scholar]
- 42.Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. (2019) 234:17690–703. 10.1002/jcp.28395 [DOI] [PubMed] [Google Scholar]
- 43.Matsudaira K, Maeda K, Okumura N, Yoshikawa D, Morita Y, Mitsuhashi H, et al. Impact of low levels of vascular endothelial growth factor after myocardial infarction on 6-month clinical outcome - results from the nagoya acute myocardial infarction study. Circ J. (2012) 76:1509–16. 10.1253/circj.CJ-11-1127 [DOI] [PubMed] [Google Scholar]
- 44.Niu JM, Han X, Qi HX, Yin J, Zhang ZQ, Zhang ZT. Correlation between vascular endothelial growth factor and long-term prognosis in patients with acute myocardial infarction. Exp Ther Med. (2016) 12:475–9. 10.3892/etm.2016.3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia R, Bouleti C, Sirol M, Logeart D, Monnot C, Ardidie-Robouant C, et al. VEGF-A plasma levels are associated with microvascular obstruction in patients with ST-segment elevation myocardial infarction. Int J Cardiol. (2019) 291:19–24. 10.1016/j.ijcard.2019.02.067 [DOI] [PubMed] [Google Scholar]
- 46.Ladenson JH. A personal history of markers of myocyte injury [myocardial infarction]. Clin Chim Acta. (2007) 381:3–8. 10.1016/j.cca.2007.02.039 [DOI] [PubMed] [Google Scholar]
- 47.Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, et al. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscl Throm Vas. (2013) 33:E56. 10.1161/ATVBAHA.112.254961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morawiec B, Kawecki D. Copeptin: a new marker in cardiology. J Cardiovasc Med. (2013) 14:19–25. 10.2459/JCM.0b013e3283590d59 [DOI] [PubMed] [Google Scholar]
- 49.Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol. (2014) 69:67–74. 10.1016/j.yjmcc.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Zhang Y, Chen Y, Xiang Y, Xie Y. Role of the microRNA, miR-206, and its target PIK3C2alpha in endothelial progenitor cell function - potential link with coronary artery disease. FEBS J. (2015) 282:3758–72. 10.1111/febs.13372 [DOI] [PubMed] [Google Scholar]
- 51.Schmiechen NJ, Han C, Milzman DP. ED use of rapid lactate to evaluate patients with acute chest pain. Ann Emerg Med. (1997) 30:571–7. 10.1016/S0196-0644(97)70071-4 [DOI] [PubMed] [Google Scholar]
- 52.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. BBA Mol Basis Dis. (2006) 1762:164–80. 10.1016/j.bbadis.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 53.Keffer JH. Myocardial markers of injury - evolution and insights. Am J Clin Pathol. (1996) 105:305–20. 10.1093/ajcp/105.3.305 [DOI] [PubMed] [Google Scholar]
- 54.Hawkins RC, Tan HL. Comparison of the diagnostic utility of CK, CK-MB (activity and mass), troponin T and troponin I in patients with suspected acute myocardial infarction. Singapore Med J. (1999) 40:680–4. [PubMed] [Google Scholar]
- 55.Mair J, Artner-Dworzak E, Lechleitner P, Morass B, Smidt J, Wagner I, et al. Early diagnosis of acute myocardial infarction by a newly developed rapid immunoturbidimetric assay for myoglobin. Br Heart J. (1992) 68:462–8. 10.1136/hrt.68.11.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalay N, Yarlioglues M, Ardic I, Kaya MG, Vardar A, Inanc T, et al. The role of heart-type fatty acid-binding protein in predicting properties of coronary atherosclerosis in patients with acute coronary syndrome. Coronary Artery Dis. (2010) 21:435–40. 10.1097/MCA.0b013e32833db539 [DOI] [PubMed] [Google Scholar]
- 57.Chen K, Chen QJ, Wang LJ, Liu ZH, Zhang Q, Yang K, et al. Increment of HFABP level in coronary artery in-stent restenosis segments in diabetic and nondiabetic minipigs: HFABP overexpression promotes multiple pathway-related inflammation, growth and migration in human vascular smooth muscle cells. J Vasc Res. (2016) 53:27–38. 10.1159/000446652 [DOI] [PubMed] [Google Scholar]
- 58.de Lemos JA, Antman EM, Morrow DA, Llevadot J, Giugliano RP, Coulter SA, et al. Heart-type fatty acid binding protein as a marker of reperfusion after thrombolytic therapy. Clin Chim Acta. (2000) 298:85–97. 10.1016/S0009-8981(00)00259-X [DOI] [PubMed] [Google Scholar]
- 59.Huang ZY, Zhong XW, Irwin MG, Ji SY, Wong GT, Liu YA, et al. Synergy of isoflurane preconditioning and propofol postconditioning reduces myocardial reperfusion injury in patients. Clin Sci. (2011) 121:57–69. 10.1042/CS20100435 [DOI] [PubMed] [Google Scholar]
- 60.Suzuki M, Hori S, Noma S, Kobayashi KJ. Prognostic value of a qualitative test for heart-type fatty acid-binding protein in patients with acute coronary syndrome. Int Heart J. (2005) 46:601–6. 10.1536/ihj.46.601 [DOI] [PubMed] [Google Scholar]
- 61.Bivona G, Agnello L, Bellia C, Lo Sasso B, Ciaccio M. Diagnostic and prognostic value of H-FABP in acute coronary syndrome: still evidence to bring. Clin Biochem. (2018) 58:1–4. 10.1016/j.clinbiochem.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 62.Body R, Carlton E, Sperrin M, Lewis PS, Burrows G, Carley S, et al. Troponin-only manchester acute coronary syndromes (T-MACS) decision aid: single biomarker re-derivation and external validation in three cohorts. Emerg Med J. (2017) 34:349. 10.1136/emermed-2016-205983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. (2010) 48:866–75. 10.1016/j.yjmcc.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jing R, Long TY, Pan W, Li F, Xie QY. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur Rev Med Pharmaco. (2019) 23:6283–91. 10.26355/eurrev_201907_18450 [DOI] [PubMed] [Google Scholar]
- 65.Orrem HL, Shetelig C, Ueland T, Limalanathan S, Nilsson PH, Husebye T, et al. Soluble IL-1 receptor 2 is associated with left ventricular remodelling in patients with ST-elevation myocardial infarction. Int J Cardiol. (2018) 268:187–92. 10.1016/j.ijcard.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 66.Yang ZC, Shi L, Xue Y, Zeng T, Shi Y, Lin YZ, et al. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin Chim Acta. (2019) 497:104–9. 10.1016/j.cca.2019.07.019 [DOI] [PubMed] [Google Scholar]
- 67.Li YX, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscl Throm Vas. (2003) 23:2178–84. 10.1161/01.ATV.0000099788.31333.DB [DOI] [PubMed] [Google Scholar]
- 68.Hutter R, Sauter BV, Reis ED, Roque M, Vorchheimer D, Carrick FE, et al. Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation. (2003) 107:1658–63. 10.1161/01.CIR.0000058169.21850.CE [DOI] [PubMed] [Google Scholar]
- 69.Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Murota S. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis. (1992) 92:141–9. 10.1016/0021-9150(92)90273-J [DOI] [PubMed] [Google Scholar]
- 70.Sesti G, Sciacqua A, Cardellini M, Marini MA, Maio R, Vatrano M, et al. Plasma concentration of IGE-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. (2005) 28:120–5. 10.2337/diacare.28.1.120 [DOI] [PubMed] [Google Scholar]
- 71.Yakar S, Liu JL, Fernandez AM, Wu YP, Schally AV, Frystyk J, et al. Liver-specific IGF-1 gene deletion leads to muscle insulin insensitivity. Diabetes. (2001) 50:1110–8. 10.2337/diabetes.50.5.1110 [DOI] [PubMed] [Google Scholar]
- 72.Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res. (2019) 45:6–16. 10.1016/j.ghir.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou K, Li YM, Xu YW, Guo R. Circulating matrix metalloproteinase-28 levels are related to GRACE scores and short-term outcomes in patients with acute myocardial infarction. Biomed Res Int. (2020) 1–8. 10.1155/2020/9206703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsoporis JN, Mohammadzadeh F, Parker TG. S100B: a multifunctional role in cardiovascular pathophysiology. Amino Acids. (2011) 41:843–7. 10.1007/s00726-010-0527-1 [DOI] [PubMed] [Google Scholar]
- 75.Mohammadzadeh F, Desjardins JF, Tsoporis JN, Proteau G, Leong-Poi H, Parker TG. S100B: role in cardiac remodeling and function following myocardial infarction in diabetes. Life Sci. (2013) 92:639–47. 10.1016/j.lfs.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 76.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin evaluation and infection therapy: thrombolysis in myocardial infarction (PROVE IT-TIMI 22) trial. Am Heart J. (2008) 155:49–55. 10.1016/j.ahj.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nadir Y, Brenner B, Gingis-Velitski S, Levy-Adam F, Ilan N, Zcharia E, et al. Heparanase induces tissue factor pathway inhibitor expression and extracellular accumulation in endothelial and tumor cells. Thromb Haemostasis. (2008) 99:133–41. 10.1055/s-0037-1608919 [DOI] [PubMed] [Google Scholar]
- 78.Tan BC, Liu M, Yang YS, Liu L, Meng FB. Low expression of PIK3C2A gene A potential biomarker to predict the risk of acute myocardial infarction. Medicine. (2019) 98:e15061. 10.1097/MD.0000000000015061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giannitsis E, Slagman A, Hamm CW, Gehrig S, Vollert JO, Huber K. Copeptin combined with either non-high sensitivity or high sensitivity cardiac troponin for instant rule-out of suspected non-ST segment elevation myocardial infarction. Biomarkers. (2020) 25:649–58. 10.1080/1354750X.2020.1833084 [DOI] [PubMed] [Google Scholar]
- 80.Vargas KG, Tajsic M, Latsuzbaia A, Bastian S, Andric T, Kassem M, et al. Gender-based differences of copeptin alone or combined with troponin for early rule-out of non-ST-elevation myocardial infarction. Am J Emerg Med. (2020). 10.1016/j.ajem.2020.08.053 [DOI] [PubMed] [Google Scholar]
- 81.Xie HY, Yan ZJ, Feng S, Zhu TQ, Zhu ZB, Ni JW, et al. Prognostic value of circulating MG53 Levels in acute myocardial infarction. Front Cardiovasc Med. (2020) 7:596107. 10.3389/fcvm.2020.596107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H, Zhang JN, Yu J, Li DZ, Jia Y, Cheng YS, et al. Prognostic value of serum albumin-to-creatinine ratio in patients with acute myocardial infarction Results from the retrospective evaluation of acute chest pain study. Medicine. (2020) 99:e22049. 10.1097/MD.0000000000022049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinova D, Rosival I, Avidar Y, Bogin E. Lactate dehydrogenase isoenzyme distribution and patterns in chicken organs. Res Vet Sci. (1999) 67:309–12. 10.1053/rvsc.1999.0317 [DOI] [PubMed] [Google Scholar]
- 84.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, et al. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. (1985) 313:1050–4. 10.1056/NEJM198510243131704 [DOI] [PubMed] [Google Scholar]
- 85.Rittoo D, Jones A, Lecky B, Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases. J Am Coll Cardiol. (2014) 63:2411–20. 10.1016/j.jacc.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 86.Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. (2011) 58:1819–24. 10.1016/j.jacc.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 87.Bularga A, Lee KK, Stewart S, Ferry AV, Chapman AR, Marshall L, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation. (2019) 140:1557–68. 10.1161/CIRCULATIONAHA.119.042866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. (2006) 47:39–48. 10.1007/BF03194597 [DOI] [PubMed] [Google Scholar]
- 89.Alhadi HA, Fox KAA. Do we need additional markers of myocyte necrosis: the potential value of heart fatty-acid-binding protein. QJM Int J Med. (2004) 97:187–98. 10.1093/qjmed/hch037 [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol. (2017) 14:135–50. 10.11909/j.issn.1671-5411.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Otaki Y, Watanabe T, Kubota I. Heart-type fatty acid-binding protein in cardiovascular disease: a systemic review. Clin Chim Acta. (2017) 474:44–53. 10.1016/j.cca.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 92.Das DK, Barua PK, Jones RM. Release of fatty acid-binding protein from ischemic-reperfused rat heart and its prevention by mepacrine. Biochim Biophys Acta. (1991) 1073:394–401. 10.1016/0304-4165(91)90148-A [DOI] [PubMed] [Google Scholar]
- 93.O'Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, et al. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. (2006) 114:550–7. 10.1161/CIRCULATIONAHA.106.641936 [DOI] [PubMed] [Google Scholar]
- 94.Ishii J, Ozaki Y, Lu JC, Kitagawa F, Kuno T, Nakano T, et al. Prognostic value of serum concentration of heart-type fatty acid-binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clin Chem. (2005) 51:1397–404. 10.1373/clinchem.2004.047662 [DOI] [PubMed] [Google Scholar]
- 95.Jo MS, Lee J, Kim SY, Kwon HJ, Lee HK, Park DJ, et al. Comparison between creatine kinase MB, heart-type fatty acid-binding protein, and cardiac troponin T for detecting myocardial ischemic injury after cardiac surgery. Clin Chim Acta. (2019) 488:174–8. 10.1016/j.cca.2018.10.040 [DOI] [PubMed] [Google Scholar]
- 96.Kaier TE, Twerenbold R, Puelacher C, Marjot J, Imambaccus N, Boeddinghaus J, et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation. (2017) 136:1495–508. 10.1161/CIRCULATIONAHA.117.028084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaier TE, Alaour B, Marber M. Cardiac myosin-binding protein C-from bench to improved diagnosis of acute myocardial infarction. Cardiovasc Drugs Ther. (2019) 33:221–30. 10.1007/s10557-018-6845-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. (2015) 110:23. 10.1007/s00395-015-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang XW, Tian HW, Qiao SB. Letter by Jiang et al. regarding article, “direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction”. Circulation. (2018) 138:543. 10.1161/CIRCULATIONAHA.117.032597 [DOI] [PubMed] [Google Scholar]
- 100.Bageghni SA, Hemmings KE, Yuldasheva NY, Maqbool A, Gamboa-Esteves FO, Humphreys NE, et al. Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight. (2019) 5:e125074. 10.1172/jci.insight.125074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allard JB, Duan CM. IGF-binding proteins: why do they exist and why are there so many? Front Endocrinol. (2018) 9:117. 10.3389/fendo.2018.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease - a population-based case-control study. Circulation. (2002) 106:939–44. 10.1161/01.CIR.0000027563.44593.CC [DOI] [PubMed] [Google Scholar]
- 103.Anastasilakis AD, Koulaxis D, Upadhyay J, Pagkalidou E, Kefala N, Perakakis N, et al. Free IGF-1, Intact IGFBP-4, and PicoPAPP-A are altered in acute myocardial infarction compared to stable coronary artery disease and healthy controls. Horm Metab Res. (2019) 51:112–9. 10.1055/a-0794-6163 [DOI] [PubMed] [Google Scholar]
- 104.Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, et al. Insulin-like growth factor-1 receptor deficiency in macrophages accelerates atherosclerosis and induces an unstable plaque phenotype in apolipoprotein E-deficient mice. Circulation. (2016) 133:2263. 10.1161/CIRCULATIONAHA.116.021805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heinen A, Nederlof R, Panjwani P, Spychala A, Tschaidse T, Reffelt H, et al. IGF1 treatment improves cardiac remodeling after infarction by targeting myeloid cells. Mol Ther. (2019) 27:46–58. 10.1016/j.ymthe.2018.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao J, Ke J, Zhou Z, Tan G, Yin Y, Liu M, et al. Combination of HGF and IGF-1 promotes connexin 43 expression and improves ventricular arrhythmia after myocardial infarction through activating the MAPK/ERK and MAPK/p38 signaling pathways in a rat model. Cardiovasc Diagn Ther. (2019) 9:346–54. 10.21037/cdt.2019.07.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pannitteri G, Petrucci E, Testa U. Coordinate release of angiogenic growth factors after acute myocardial infarction: evidence of a two-wave production. J Cardiovasc Med. (2006) 7:872–9. 10.2459/01.JCM.0000253831.61974.b9 [DOI] [PubMed] [Google Scholar]
- 108.Kranz A, Rau C, Kochs M, Waltenberger J. Elevation of vascular endothelial growth factor-A serum levels following acute myocardial infarction. Evidence for its origin and functional significance. J Mol Cell Cardiol. (2000) 32:65–72. 10.1006/jmcc.1999.1062 [DOI] [PubMed] [Google Scholar]
- 109.Hueso L, Rios-Navarro C, Ruiz-Sauri A, Chorro FJ, Nunez J, Sanz MJ, et al. Dynamics and implications of circulating anti-angiogenic VEGF-A(165)b isoform in patients with ST-elevation myocardial infarction. Sci Rep. (2017) 7:9962. 10.1038/s41598-017-10505-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peiris-Pages M. The role of VEGF 165b in pathophysiology. Cell Adh Migr. (2012) 6:561–8. 10.4161/cam.22439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF(165)b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. (2004) 64:7822–35. 10.1158/0008-5472.CAN-04-0934 [DOI] [PubMed] [Google Scholar]
- 112.Harada K, Kikuchi R, Ishii H, Shibata Y, Suzuki S, Tanaka A, et al. Association between the ratio of anti-angiogenic isoform of VEGF-A to total VEGF-A and adverse clinical outcomes in patients after acute myocardial infarction. IJC Heart Vasc. (2018) 19:3–7. 10.1016/j.ijcha.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wada H, Suzuki M, Matsuda M, Ajiro Y, Shinozaki T, Sakagami S, et al. VEGF-C and mortality in patients with suspected or known coronary artery disease. J Am Heart Assoc. (2018) 7:e010355 10.1161/JAHA.118.010355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Myasoedova VA, Chistiakov DA, Grechko AV, Orekhov AN. Matrix metalloproteinases in pro-atherosclerotic arterial remodeling. J Mol Cell Cardiol. (2018) 123:159–67. 10.1016/j.yjmcc.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 115.Yabluchanskiy A, Li YJ, Chilton RJ, Lindsey ML. Matrix metalloproteinases: drug targets for myocardial infarction. Curr Drug Targets. (2013) 14:276–86. 10.2174/1389450111314030002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Frobert O, et al. Elevated admission glucose is common and associated with high short-term complication burden after acute myocardial infarction: insights from the VALIDATE-SWEDEHEART study. Diabetes Vasc Dis Res. (2019) 16:582–4. 10.1177/1479164119871540 [DOI] [PubMed] [Google Scholar]
- 117.Zhang QH, Zhao G, Yang NL, Zhang LJ. Fasting blood glucose levels in patients with different types of diseases. Prog Mol Biol Transl. (2019) 162:277–92. 10.1016/bs.pmbts.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 118.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. (2003) 113:673–6. 10.1016/S0092-8674(03)00428-8 [DOI] [PubMed] [Google Scholar]
- 119.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004) 116:281–97. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 120.Kloosterman WP, Plasterk RHA. The diverse functions of MicroRNAs in animal development and disease. Dev Cell. (2006) 11:441–50. 10.1016/j.devcel.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 121.Zhu L, Liu F, Xie H, Feng J. Diagnostic performance of microRNA-133a in acute myocardial infarction: a meta-analysis. Cardiol J. (2018) 25:260–7. [DOI] [PubMed] [Google Scholar]
- 122.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. (2018) 362:911–7. 10.1126/science.aau3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhai H, Li XM, Liu F, Chen BD, Zheng H, Wang XM, et al. Expression pattern of genome-scale long noncoding RNA following acute myocardial infarction in Chinese Uyghur patients. Oncotarget. (2017) 8:31449–64. 10.18632/oncotarget.16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang XM, Li XM, Song N, Zhai H, Gao XM, Yang YN. Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother. (2019) 118:109208. 10.1016/j.biopha.2019.109208 [DOI] [PubMed] [Google Scholar]
- 125.Isobe T, Okuyama T. The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem. (1978) 89:379–88. 10.1111/j.1432-1033.1978.tb12539.x [DOI] [PubMed] [Google Scholar]
- 126.Kato, Kimura S, Haimoto H, Suzuki F. S100a0 (alpha alpha) protein: distribution in muscle tissues of various animals and purification from human pectoral muscle. J Neurochem. (1986) 46:1555–60. 10.1111/j.1471-4159.1986.tb01776.x [DOI] [PubMed] [Google Scholar]
- 127.Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. (2008) 35:1014–9. 10.1111/j.1600-0560.2007.00953.x [DOI] [PubMed] [Google Scholar]
- 128.Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids. (2011) 41:789–96. 10.1007/s00726-010-0666-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai XY, Lu L, Wang YN, Jin C, Zhang RY, Zhang Q, et al. Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia-reperfusion injury. Atherosclerosis. (2011) 217:536–42. 10.1016/j.atherosclerosis.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 130.Freeman, Parish CR. Human platelet heparanase: purification, characterization and catalytic activity. Biochem J. (1998) 330:1341–50. 10.1042/bj3301341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. BBA Rev Cancer. (2001) 1471:M99–M108. 10.1016/S0304-419X(01)00017-8 [DOI] [PubMed] [Google Scholar]
- 132.Nadir Y, Brenner B. Heparanase-a link between coagulation, angiogenesis, and cancer. Rambam Maimonides Med J. (2012) 3:e0002. 10.5041/RMMJ.10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vlodavsky, Blich M, Li JP, Sanderson RD, Ilan N. Involvement of heparanase in atherosclerosis and other vessel wall pathologies. Matrix Biol. (2013) 32:241–51. 10.1016/j.matbio.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baker AB, Gibson WJ, Kolachalama VB, Golomb M, Indolfi L, Spruell C, et al. Heparanase regulates thrombosis in vascular injury and stent-induced flow disturbance. J Am Coll Cardiol. (2012) 59:1551–60. 10.1016/j.jacc.2011.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gurbuz AS, Ozturk S, Efe SC, Yilmaz MF, Yanik RE, Yaman A, et al. Heparanase is a predictive marker for high thrombus burden in patients with ST-segment elevation myocardial infarction. Biomarkers. (2019) 24:600–6. 10.1080/1354750X.2019.1628809 [DOI] [PubMed] [Google Scholar]
- 136.Mountford JK, Petitjean C, Putra HWK, McCafferty JA, Setiabakti NM, Lee H, et al. The class II PI 3-kinase, PI3KC2 alpha, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. (2015) 6:6535. 10.1038/ncomms7535 [DOI] [PubMed] [Google Scholar]
- 137.Giannitsis E, Clifford P, Slagman A, Ruedelstein R, Liebetrau C, Hamm C, et al. Multicentre cross-sectional observational registry to monitor the safety of early discharge after rule-out of acute myocardial infarction by copeptin and troponin: the Pro-Core registry. BMJ Open. (2019) 9:e028311. 10.1136/bmjopen-2018-028311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pinchi E, Frati P, Aromatario M, Cipolloni L, Fabbri M, La Russa R, et al. miR-1, miR-499 and miR-208 are sensitive markers to diagnose sudden death due to early acute myocardial infarction. J Cell Mol Med. (2019) 23:6005–16. 10.1111/jcmm.14463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou CY, Cui QT, Su GB, Guo XL, Liu XC, Zhang J. MicroRNA-208b alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med Sci Monitor. (2016) 22:1808–16. 10.12659/MSM.896428 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Li K, Lin T, Chen LD, Wang N. MicroRNA-93 elevation after myocardial infarction is cardiac protective. Med Hypotheses. (2017) 106:23–5. 10.1016/j.mehy.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 141.Tan H, Qi J, Fan BY, Zhang J, Su FF, Wang HT. MicroRNA-24-3p attenuates myocardial ischemia/reperfusion injury by suppressing RIPK1 expression in mice. Cell Physiol Biochem. (2018) 51:46–62. 10.1159/000495161 [DOI] [PubMed] [Google Scholar]
- 142.Rullman E, Melin M, Mandic M, Gonon A, Fernandez-Gonzalo R, Gustafsson T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin Res Cardiol. (2020) 109:655–72. 10.1007/s00392-019-01554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferreira JP, Pizard A, Machu JL, Bresso E, Rocca HB, Girerd N, et al. Plasma protein biomarkers and their association with mutually exclusive cardiovascular phenotypes: the FIBRO-TARGETS case-control analyses. Clin Res Cardiol. (2020) 109:22–33. 10.1007/s00392-019-01480-4 [DOI] [PubMed] [Google Scholar]
- 144.Gidlof O, Evander M, Rezeli M, Marko-Varga G, Laurell T, Erlinge D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci Rep. (2019) 9:8991. 10.1038/s41598-019-45473-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xuan, Li H, Li LL, Tian QW, Wang Q, Zhang BB, et al. Screening and identification of pregnancy zone protein and leucine-rich alpha-2-glycoprotein as potential serum biomarkers for early-onset myocardial infarction using protein profile analysis. Proteom Clin Appl. (2019) 13:e1800079. 10.1002/prca.201800079 [DOI] [PubMed] [Google Scholar]
- 146.Li MX, Yuan Y, Guo K, Lao Y, Huang XS, Feng L. Value of galectin-3 in acute myocardial infarction. Am J Cardiovasc Drug. (2020) 20:333–42. 10.1007/s40256-019-00387-9 [DOI] [PubMed] [Google Scholar]
- 147.Wang B, Li T, Han X, Li Y, Cheng W, Wang L, et al. The level of circulating microparticles in patients with coronary heart disease: a systematic review and meta-analysis. J Cardiovasc Transl Res. (2019) 13:702–12. 10.1007/s12265-019-09945-7 [DOI] [PubMed] [Google Scholar]
- 148.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. (2011) 17:320–9. 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kasper P, Martin A, Lang S, Kutting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 1–17. (2020). 10.1007/s00392-020-01709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharda M, Sharma N, Meena SR, Meena JK, Kumar P. Effect of on admission blood glucose level and HbA1c value on short term prognosis in acute STEMI. J Assoc Physicians India. (2019) 67:84–5. [PubMed] [Google Scholar]
- 151.Echouffo-Tcheugui JB, Kolte D, Khera S, Aronow HD, Abbott JD, Bhatt DL, et al. Diabetes mellitus and cardiogenic shock complicating acute myocardial infarction. Am J Med. (2018) 131:778–86 e1. 10.1016/j.amjmed.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 152.Li H, Cui Y, Zhu Y, Yan H, Xu W. Association of high normal HbA1c and TSH levels with the risk of CHD: a 10-year cohort study and SVM analysis. Sci Rep. (2017) 7:45406. 10.1038/srep45406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu M, Lan Y, Lu A, Ma X, Zhang L. Glycan-based biomarkers for diagnosis of cancers and other diseases: PAST, present, and future. Progress Mol Biol Transl Sci. (2019) 162:1–24. 10.1016/bs.pmbts.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 154.Falcao F, Oliveira F, Cantarelli F, Cantarelli R, Brito Junior P, Lemos H, et al. Carbohydrate antigen 125 for mortality risk prediction following acute myocardial infarction. Sci Rep. (2020) 10:11016. 10.1038/s41598-020-67548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang M, Zhang Y, Fu J, Zhang L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Progress Mol Biol Transl Sci. (2019) 162:241–52. 10.1016/bs.pmbts.2018.12.012 [DOI] [PubMed] [Google Scholar]