Abstract
Long-acting injectable (LAI) pre-exposure prophylaxis (PrEP) has the potential to facilitate adherence and transform HIV prevention. However, little LAI PrEP research has occurred among women, who face unique barriers. We conducted 30 in-depth interviews with HIV-negative women from 2017–2018 across six sites (New York; Chicago; San Francisco; Atlanta; Washington, DC; Chapel Hill) of the Women’s Interagency HIV Study. Interviews were recorded, transcribed, and analyzed using thematic content analysis. Few women expressed interest in PrEP and when prompted to choose a regimen, 55% would prefer LAI, 10% daily pills, and 33% said they would not take PrEP regardless of formulation. Perceived barriers included: 1) the fear of new—and perceived untested—injectable products and 2) potential side effects (e.g., injection-site pain, nausea). Facilitators included: 1) Believing shots were more effective than pills; 2) ease and convenience; and 3) confidentiality. Future studies should incorporate women’s LAI PrEP-related experiences to facilitate uptake.
Keywords: Long-acting injectable (LAI), pre-exposure prophylaxis (PrEP), women, HIV/AIDS, prevention, qualitative research
INTRODUCTION
In 2017, adolescent and adult women constituted 19% of new HIV diagnoses and 23% of new AIDS cases in the United States (U.S.)[1]. Women living with HIV comprise nearly one-quarter of all persons living with HIV in the U.S.[2]. Although HIV incidence has declined among women overall since 2010, HIV incidence has not decreased among women 55 and older[2]. In addition, racial and ethnic disparities remain stark: in 2017, Black women constituted 59% of new HIV diagnoses among women despite being just 13% of the female population; white women constituted 20% of new HIV diagnoses but 77% of the female population[2].
Women have historically been underrepresented in HIV research compared to men and face myriad barriers, including gender-specific barriers, to HIV prevention[3,4]. These multi-level barriers include those resulting from structural inequalities, such as high demand/low control labor (e.g., sex work) and limited access to health insurance and drug treatment programs. At a clinic level, providers are less likely to ask women about their HIV-related risk behaviors and discuss HIV-related prevention options[7]. In addition, gendered dynamics and social norms may increase women’s HIV vulnerability and, if infected, ability to access adequate treatment. These can include women’s desire to conceive, the stress of managing caretaking demands[5,6], and poor family communication and support[6].
Women need HIV prevention strategies that can be feasibly and consistently integrated into their lives, and that do not rely on a partner’s permission or participation (e.g., such as male condoms). One such strategy is oral pre-exposure prophylaxis (PrEP), which for women engaging in vaginal intercourse involves a daily pill comprised of tenofovir disoproxyl fumarate (TDF) and emtricitabine (FTC)[8]. Oral PrEP trials have demonstrated reductions in HIV incidence from 44%−75% among heterosexual men and women and serodiscordant heterosexual couples[9,10]. After controlling for adherence, one study found a 92% reduction in HIV incidence among male participants[11], but the two trials conducted exclusively among women, FEM-PrEP and VOICE, failed to demonstrate efficacy[12,13]. This was due to low levels of adherence: fewer than one-quarter of women in the FEM-PrEP trial reached the target tenofovir blood levels[14].
The Food and Drug Administration (FDA) approved oral PrEP for men who have sex with men (MSM) in July 2012[15] and in May 2014, the Centers for Disease Control and Prevention (CDC) issued guidelines for providers to offer PrEP to women, “at substantial risk of HIV acquisition” due to drug injection or sexual risk [16]. While oral PrEP has generally been well-received, overall uptake remains lower than anticipated. PrEP uptake is particularly low among youth, African American and Hispanic individuals and women [20]. CDC estimates suggest that 1.1 million U.S. adults are at substantial risk of HIV acquisition, and thus indicated for PrEP use, including 176,670 heterosexual women[17]. PrEP use among women is disproportionately low compared to their HIV prevention need. Women constitute fewer than 5% of people who use PrEP in the U.S. [18,19], and the prevalence of PrEP use was at least 3 times lower for women than men relative to the number of new HIV diagnoses[19]. Among PrEP users, women’s average length of use was 5.8 months compared to 8.4 months for men[21,22]. Racial disparities also exist: white women were four times more likely to have received PrEP than Black women[20].
These low levels of oral PrEP uptake were due to several factors. At a structural level, fourteen states in the U.S. have yet to adopt Medicaid expansion under the Affordable Care Act (ACA)[23]. This has significant implications for PrEP access in these non-expansion states since insured patients are four-times more likely to use PrEP than uninsured patients[24]. However, even when medication assistance or insurance programs do cover PrEP, many do not cover the associated doctors’ visits, counseling sessions, and blood tests—which are recommended under CDC guidelines and often included in authorization requirements[25,26]. Further, even medication assistance programs generally include income requirements and assistance caps[27].
Additional barriers to oral PrEP use can include individual-level factors such as fear of side effects, low perceived risk of HIV acquisition[28] and barriers related to daily pill-taking, such as forgetfulness and pill fatigue[3,4], food insecurity[29], drug use[30], medical mistrust[31] and stigmatization[3,32]; structural-level factors include transportation and employment[21,22]. Women also face additional gender-specific adherence barriers to pill taking such as caregiving demands[33], pregnancy interactions[34] and low perceived self-efficacy. Individuals who take oral PrEP often face adherence-related challenges as a result of intolerable dosing regimens, medication side effects, and daily life impediments[35]. In addition, a 2017 study reported that 26% of primary providers have never heard of PrEP and only 28% felt comfortable with the prescription process.[36] This places the burden on patients to know about and request PrEP, which may be even more challenging for women who are often not the target of PrEP advertising, and for whom PrEP awareness is therefore limited[36]. Given the multi-level factors that impact women’s interest in, and access to PrEP, we applied Bronfenbrenner’s[37] (1979) ecological model, adapted from a model tailored to oral PrEP uptake[38], to frame our analytic approach (Figure 1).
Figure 1:
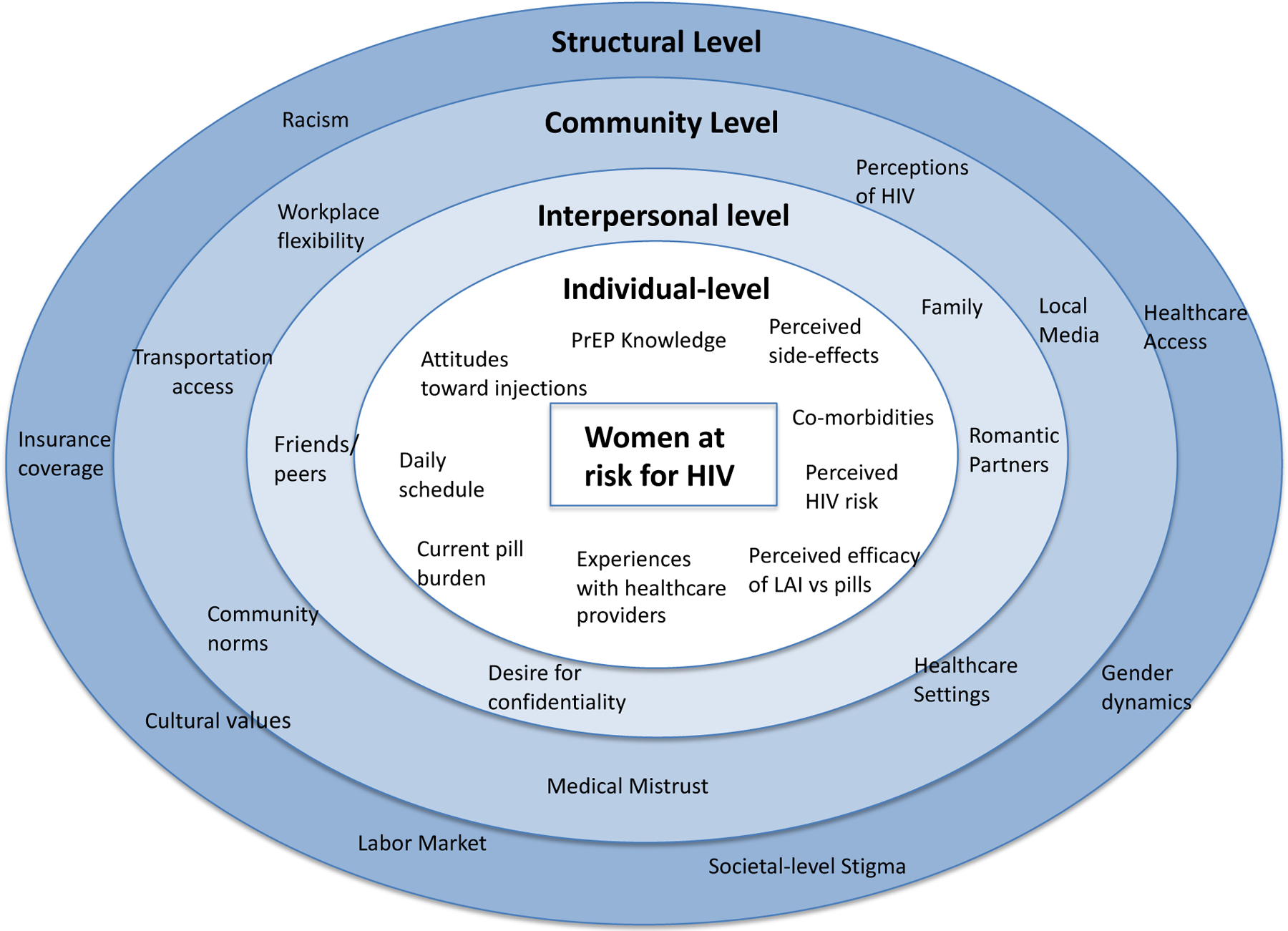
Ecological model of factors that impact women’s interest in LAI PrEP
Long-acting injectable (LAI) PrEP is an alternative to oral PrEP and may facilitate adherence by requiring less frequent dosing. Multiple LAI PrEP formulations are currently in Phase III trials, and would be administered in a clinical setting through bi-monthly injections[40]. Research in Phase II clinical trials found that LAI PrEP was generally well-tolerated and has high acceptability (~80%) despite individuals’ frequent experiences of injection-site pain [41–43]. Results from HIV Prevention Trials Network (HPTN) 083, which compared long-acting injectable cabotegravir (CAB) to daily oral tenofovir/emtricitabine (TDF/FTC) (Truvada) among 4,570 cisgender men and transgender women who have sex with men in seven countries across the world, showed that LAI PrEP was superior to daily oral PrEP[44]. FDA approval will be sought in early 2021. An additional LAI PrEP trial (HPTN 084) among cisgender women in ongoing. Alternative long-acting PrEP options, including implants, patches, and monthly pills[45], are in earlier trial stages.
Although women face unique barriers to oral PrEP use[28,35,46], they are underrepresented in HIV prevention clinical trials (particularly pregnant women); this means that the trial data from primarily men does not represent their needs. As such, women do not equally benefit from technological advances that aim to improve HIV prevention and treatment[47]. Because the majority of LAI PrEP trials have occurred among MSM,[41,48] and pregnant women were excluded, pregnancy-related interactions[49] remain unexplored. In addition, because women’s interest in PrEP may be complicated by socio-structural issues such as medical mistrust, stigmatization, cost, and transportation[28], we do not know how women will respond to LAI PrEP or the characteristics of women most likely to adhere to LAI PrEP. Oral PrEP studies have identified barriers that LAI PrEP may help alleviate (e.g., around privacy and confidentiality) and, within this context, we conducted in-depth interviews with women across six cities in the U.S. to explore their interest in using LAI PrEP, with a focus on perceived barriers and facilitators to uptake.
METHODS
Data were collected from the Women’s Interagency HIV Study (WIHS), the largest national prospective cohort study of women living with HIV and at risk for HIV infection in the US.[50] HIV-negative women were eligible for WIHS enrollment if they had a history of sexually transmitted infections (STIs) or behavioral or demographic characteristics that increased their risk of acquiring HIV (e.g., sex without a condom with three or more men, trading sex, injection drug use or use of crack cocaine, cocaine, heroin or methamphetamine)[51]. The biennial WIHS visits included a physical examination and interviewer-administered questionnaire that addresses medical history and psychosocial factors. This sub-study conducted qualitative in-depth interviews with HIV-negative women (5 per site; n=30 total) at six WIHS sites: Atlanta, Georgia; San Francisco, California; Washington, D.C.; Chapel Hill, North Carolina; Bronx, New York; and Chicago, Illinois. Women were purposively sampled from each site to reflect those with a range of experience by age, relationship status and employment status. Eligibility criteria included being a participant of the WIHS study and being willing to consent to the interview.
Participants provided informed oral consent prior to each interview and interviews lasted approximately 60 minutes. For the purpose of maintaining complete anonymity, written consent was not required, as a signed consent form would have been the only document linking the participant’s name to the study. Interviews were conducted in English, digitally recorded and professionally transcribed. Interviews were conducted by two master’s-level research associates, one of whom also led the data analysis with the first author. Data collection occurred from November 2017 to October 2018 and participants were compensated $50. IRB approval was obtained at all participating sites prior to interview initiation. In addition to the qualitative interview, participants were asked about their age, race/ethnicity, educational attainment, relationship status and insurance coverage. We also asked women about their product preference using the following question: “Given the choice between shots of PrEP every two months and daily pills to prevent HIV (i.e., PrEP), which would you prefer? With option categories including: 1) Shots of PrEP every two months; 2) Daily pills (i.e., oral PrEP); 3) No preference; or 4) won’t take PrEP regardless of formulation” (Table 1).
Table I:
Demographic Characteristics
| Characteristic | Total (N=30) | Median | Percentage |
|---|---|---|---|
| Age (32–72) years | 51 | ||
| 32–39 | 7 | 23% | |
| 40–49 | 8 | 27% | |
| 50–59 | 10 | 33% | |
| 60+ | 5 | 17% | |
| Race | |||
| Black/African-American | 23 | 77% | |
| Caucasian | 2 | 7% | |
| Hispanic | 0 | 0% | |
| Mixed | 4 | 13% | |
| Other (Native American) | 1 | 3% | |
| Education | |||
| Less than high school | 10 | 33% | |
| Completed high school/GED | 8 | 27% | |
| Some college | 8 | 27% | |
| College or graduate school | 4 | 13% | |
| Household Incomea | $10,800 | ||
| $0 - $11,999 | 16 | 57% | |
| $12,000+ | 12 | 43% | |
| Relationship Status | |||
| Single | 6 | 20% | |
| Dating < 6 months | 3 | 10% | |
| Dating > 6 months | 9 | 30% | |
| Married/long-term partnership | 12 | 40% | |
| Children | |||
| Has children | 26 | 87% | |
| Does not have children | 4 | 13% | |
| Insurance | |||
| Uninsured | 4 | 13% | |
| Public insurance | 22 | 73% | |
| Private insurance | 4 | 13% | |
| Other insurance | 0 | 0% | |
| Previous knowledge of PrEP | |||
| Knew of PrEP | 17 | 57% | |
| Did not know of PrEP | 13 | 43% |
some values missing/unanswered
Interview domains focused on women’s knowledge of, attitudes, and beliefs toward PrEP, with a specific focus on long-acting injectable PrEP. Questions in the interview guide were organized around Bronfenbrenner’s ecological model to ensure that we captured the full landscape of potential multi-level barriers and facilitators. Since not all women were familiar with LAI PrEP, we included a brief description (Supplemental Table 1). Interview questions were open-ended and explored women’s experience with injectable medication, related knowledge and attitudes, and perceived barriers and facilitators to LAI PrEP. Additionally, each woman was asked a series of 15 quantitative questions to assess their preference for LAI versus daily pills and any potential barriers.
Data were analyzed using thematic content analysis[52,53], with the ecological framework used to organize analysis. Three members of the study team conducted line-by-line open coding on the first five interviews to develop a provisional coding scheme focused primarily on identifying women’s attitudes about injectable PrEP as well as their perceived barriers and facilitators towards its use. Thematic codes based on existing literature were subsequently added to ensure that theory-based and emergent concepts were included. These team members then cross-coded a random sample of 10 additional transcripts to refine the code dictionary and to develop a codebook. This codebook was reviewed and amended by other team members[54]. While all transcripts were coded to ensure the inclusion of all women’s experiences, thematic saturation for all codes was reached after coding approximately two-thirds (i.e., 20) of the interviews. Thematic saturation was reached earlier for codes related to desirability of LAI PrEP and perceived HIV risk and confidentiality; themes around medical mistrust and side effects took longer to reach. Analyses were conducted to explore potential axes of difference such as age, region, and race/ethnicity. Two coders then independently applied this final coding scheme to all interview transcripts, and ongoing discussions were scheduled to resolve any discrepancies. Double-coding transcripts increased the validity of the findings; inter-rater reliability was high.
RESULTS
Participants’ average age was 51 (range 34–72) and the majority was women of color (93%) (Table I). The majority was in a relationship (70%) and had children (87%). Just over half (17/30) had heard of PrEP. A study among HIV-negative women in WIHS found that in the year prior to the index visit, 36% reported >1 male sexual partner, 6.7% had a partner living with HIV, 38.4% had a new partner, 19.1% reported consistent condom use and 18.2% reported crack, cocaine, or heroin use.[55] The women sampled for this qualitative study reported similar substance use and sexual health patterns, yet expressed an overall low perceived risk of acquiring HIV based on their substance use and sexual behaviors. Women shared how their low perceived risk of HIV made them less interested in PrEP regardless of its formulation. Specific barriers to LAI PrEP uptake included medical mistrust, injection-related side effects, administration location, and more frequent doctors’ visits, while facilitators included beliefs that shots were more effective than pills, convenience, and confidentiality.
Barriers to LAI PrEP Uptake
Perceived Risk of Acquiring HIV:
Participants’ primary barrier to PrEP uptake was low perceived benefit, regardless of formulation. The women we interviewed did not see themselves as at risk for HIV, which obviated the need for PrEP, “I don’t want to take another pill for another thing that…won’t happen to me. I don’t see myself ever being exposed to that” (Black, 50–591, San Francisco). While they understood the utility of PrEP, most women spoke of their lack of potential risk behaviors (e.g., unprotected sex or needle sharing) that might expose them to HIV: “Nothing to prevent because after a while I’m going to stop having sex with anybody. It [PrEP] don’t serve no significance for me” (Black, 60–69, Bronx). Most women, however, said that they would have considered PrEP when they were younger, had more sexual partners, or were injecting drugs. When prompted to choose a regimen, 55% of women would prefer LAI PrEP, 10% oral PrEP, 3% no preference and 33% would refuse any formulation of PrEP.
Medical mistrust:
Women voiced apprehension toward LAI PrEP due to fears of new—and perceived untested—injectable products. Women expressed a desire to wait until LAI PrEP had been on the market for an extended period to ensure its safety and efficacy: “We’re all an experiment. We’re like guinea pigs, you know? You don’t know for real if it’s gonna be effective. I mean they say it is, but who’s to say that it really will be?” (Black, 50–59, San Francisco). In addition to feeling that LAI PrEP was not adequately tested even after clinical trials, 60% of women were afraid that PrEP would stop working: “Even though you said that it does prevent HIV, I’m scared if I take it, I might get it. It might be un-effective, so I wouldn’t trust it” (Black, 40–49, San Francisco). Lastly, women were afraid that LAI PrEP might create additional medical problems that the doctors might not disclose, “I hope it wouldn’t create some other kinds of medical problems, issues in my body. Then down the line they’re talking about I got this, that, and the other and it’s got 45 letters in it. You can’t even pronounce it and you, come to find out it come from that [PrEP]” (Black, 50–59, Chicago). This limited women’s overall enthusiasm for LAI PrEP and interest in taking it.
Response to injection-related side effects and administration location:
Most women questioned whether PrEP’s ability to prevent HIV was enough to outweigh any potential side effects: two-thirds of women reported being somewhat or very concerned about related side effects. Some participants explicitly invoked side effects as a rationale for eschewing PrEP, with just over half (53%) describing themselves as somewhat or very concerned about injection site pain, “I would do the pill; I’ve done enough blood draws to have bruising and it’s not a cute look” (Black, 50–59, San Francisco). In addition to injection-related side effects, a few women expressed a strong dislike of needles, enough to refuse LAI medication, “A lot of people don’t like needles, you know? There are people very frightened” (Black, 50–59, San Francisco). Nearly one-quarter of women raised issues about whether the injection might interfere with a pregnancy, “If I was to become pregnant, I wouldn’t want something to just be in my system like that” (Black, 30–39, Chapel Hill).
Many women had experience receiving an injection in their buttocks (e.g., antibiotics) and while they generally disliked it, 66% said that the injection location would not deter them from using LAI PrEP. For some, however, it was incredibly important, “I can deal with the soreness and tenderness in my arm, but the tenderness and soreness in my butt. It’s gonna be a little hard to sit back, I’d sit down real soft…” (Black, 30–39, Atlanta). In all, 25% said that they would be much more likely to take LAI PrEP if they could get the injection somewhere else besides the buttocks, while 20% said they would not take LAI PrEP regardless of where it was administered.
Where and how to access LAI:
While oral PrEP requires a doctor’s visit every three months, LAI PrEP—in its current form—would require visits every 8 weeks. Women were divided about the feasibility of more frequent clinic visits. Those in cities with extensive transportation—like New York—voiced little concern, while women in Atlanta, North Carolina, and Washington D.C. said the frequency would be challenging: “There are very few ways for people to get there. So, they’ll probably have to take a bus or maybe a couple of buses if they don’t drive, and that would make access hard” (Black, 40–49, D.C.). This anticipated barrier might be addressed if LAI PrEP could be offered in other locales (e.g., in local pharmacies), though women indicated a strong preference for receiving LAI PrEP from their doctor. Preferring a doctor’s office was important for confidentiality, as well as to address any potential side effects: “I don’t think everybody would want to show their butts to anybody. It would be more effective in a private setting with their own doctors. I don’t think Walgreens needs to see everybody. I don’t think people would feel comfortable” (Black, 50–59, San Francisco). Even though women acknowledged that pharmacies are more ubiquitous and easier to access than doctors’ offices, they would still prefer to receive LAI PrEP from their doctor.
Facilitators to LAI PrEP Uptake
Shots are more effective than pills:
Women frequently noted that, “the shot would be more effective” (Black, 50–59, Chapel Hill), particularly because, “it goes straight to your bloodstream. The pill form takes a couple of hours to get in your system; the shot form is better” (Black, 30–39, Atlanta). In addition to perceived effectiveness, women expressed a preference for the shot because it would not require navigating challenges that might come with daily pill taking, “If it’s a pill do I take as soon as I get out of bed in the morning? Do I take it with food? With the shot it don’t matter if empty stomach. With the pill, a lot might matter” (Black, 60–69, Chapel Hill). This also suggests that shots may be easier to take than pills, a theme many women expanded upon.
Ease and convenience:
Many women described living somewhat hectic lives, which might challenge their ability to consistently take oral PrEP. They described LAI PrEP as particularly beneficial because, even with multiple reminders, adherence to oral PrEP could be challenging, “even with your best effort you still forget. You can set the clock. You can put the little seven-day pill thing there. But you don’t forget appointments where they give you your injection and see you in two months” (Black, 50–59, Bronx). Taking LAI PrEP would also eliminate the need for women to carry pills if they spent the night elsewhere, “What if I forget to take my pills two or three days, or just say I go out of town and I left my medication at home? I forgot, so now I’m opening myself up? I would rather take the shot” (Biracial, 50–59, Atlanta). When asked to choose a regimen, most women said they would prefer LAI PrEP, precisely because of the ease and convenience of not having to remember daily pills.
Confidentiality:
A primary benefit of LAI PrEP would be confidentiality. Women expressed worry that others might find the PrEP pills and think they were HIV-positive, “People see you with a pill bottle and, ‘Girl, what you taking these pills for?’ They don’t want to hear, ‘I’m taking these to prevent myself from getting HIV.’ They be like, ‘Girl, yeah, okay. You got it’” (Black, 40–49, Chicago). An additional perceived benefit of LAI PrEP was that few people would question a doctor’s visit: “It’s easy to make up something, ‘I’m getting a shot for my diabetes, I’m getting a shot for asthma’, Getting the shot would be preferable to carrying around a pill” (Biracial, 60–69, Chicago). While women would need to go to the clinic for their injections, they felt able to provide multiple other reasons for the visit to friends and family. This demonstrates the increased confidentiality that LAI PrEP would confer compared to oral PrEP, which could help increase uptake.
DISCUSSION
The FDA approved oral PrEP in 2012, but fewer people have used PrEP than public health officials had hoped. In addition, use is particularly low among women and racial/ethnic minorities[35,56]. As a result, researchers are developing LAI PrEP with a goal to facilitate uptake and increase adherence. Results from the HPTN 083 study with nearly 5,000 cis-gender men and transgender women show that LAI PrEP was superior to oral PrEP, highlighting the need for additional research across diverse populations to ensure equitable access during scale-up. There has been a dearth of both clinical trials and qualitative work assessing women’s perceptions regarding LAI PrEP [41], though a current study (HPTN 084) is comparing LAI and oral PrEP among cis-gender women in Sub-Sahara Africa. This presents a critical moments for complementary studies that explore women’s perceptions toward LAI PrEP and the unique advantages or burdens they may encounter, particularly outside of clinical trials and in diverse geographical contexts. This multi-site qualitative study explored how predominantly racially-and ethnically-diverse women in the US think about, understand, and would engage with LAI PrEP.
Despite PrEP’s increasing availability and inclusion in many state Medicaid formularies, just over half of the women we interviewed had heard of PrEP. Women shared a near uniform view that LAI PrEP was a useful option for others, but that it was not relevant for their lives. This was due to low levels of perceived HIV risk, primarily due to being in monogamous partnerships. However, previous research suggests a potential disconnect between perceived HIV risk and actual HIV risk[57]. While 33% of women would not consider PrEP regardless of its formulation, when asked to choose, the majority would prefer LAI PrEP over oral PrEP. Women’s preferences were driven by their unique life experiences, perceived risk for HIV, and health and employment status. For example, women who were employed and had children stressed that not having to remember a daily pill led to a preference for LAI, while others noted that they would like LAI PrEP because it would facilitate confidentiality and not require them to tell their partner. Others believed that shots were more effective that pills and so would choose LAI PrEP. However, other women focused on potential barriers to LAI PrEP. For example, many participants stated concerns about potential side effects due to the long-acting nature of the medication. Qualitative work with men in LAI PrEP trials also identified side effects as a primary concern, although most men felt that the benefits outweighed the side effects[41]. Fear of side effects also existed with oral PrEP[38,48], but with that formulation individuals could stop taking it if they developed side effects. Similar to oral PrEP, women also described medical mistrust as a consistent barrier[28]. Medical mistrust is a demonstrated barrier to oral PrEP uptake, which may significantly impact individuals’ ability to trust that a new, longer acting medication (i.e., LAI PrEP) is safe and effective[58,59]. Medical mistrust might also be particularly salient for women of color given the U.S.’ history of forced sterilization campaigns that often used injections[60].
LAI PrEP would require more frequent clinic visits than oral PrEP (every two months instead of every three months), which was particularly concerning for participants in regions with minimal public transportation (particularly in the south and rural areas[61]); limited access to public transportation, inflexible work schedules and inconvenient PrEP dispensing locations are known barriers to oral PrEP implementation[62,63]. While participants overwhelmingly preferred to receive LAI PrEP from their physician, additional research should explore whether LAI PrEP delivery at local pharmacies, in addition to doctors’ offices, may improve accessibility.
Finally, just under half of the women we interviewed had never heard of PrEP: lack of knowledge about PrEP is a significant barrier to use, particularly among women[46,62,64]. The majority did not consider themselves as at risk for acquiring HIV, even though over a third was either single or in a new relationship. In addition, one-third of our sample constitute the only female demographic for whom HIV incidence is not decreasing (i.e., women over 55)[1]. Previous work has shown that older adult women do not see themselves at risk for HIV[65] and often lack knowledge about HIV risk[66,67] However, HIV prevention programs rarely target women over 50[68,69] and healthcare providers rarely communicate with this demographic about sexual risk[70]. The fact that they did not see PrEP as useful is an important finding that demonstrates what will be needed to facilitate PrEP uptake among all women, not just those who seek it out. While women did not see PrEP as useful to them, they spoke to whether they would have used PrEP at different points in their lives.
These findings demonstrate that continued efforts must be made to improve PrEP awareness among women, particularly older adult women, and the need for patient-provider communications that offer information about PrEP in an appropriate and non-stigmatizing way. Studies have identified potential approaches, including PrEP risk-reduction counseling specifically tailored to women’s life course events and partnership dynamics[71,72], as well as enhanced integration of PrEP programs within family planning clinics[73] and non-sexual health-specific community services[72]. In addition, LAI PrEP uptake will be most successful if barriers at all levels of the ecological framework are addressed. Specifically, public health campaigns and patient-provider interactions must include individual-level factors such as perceived side-effects and current pill burden, interpersonal factors such as desires for confidentiality and caregiving-related barriers, community-level factors such as workplace flexibility and ability to access transportation, and structural-level factors such as gender dynamics.
Despite barriers, most women would prefer LAI PrEP over oral PrEP; many of the potential barriers reported by other studies on women’s acceptance of oral PrEP—such as the need to take pills every day, carrying them around, need to hide them from others including partners--can be overcome by LAI PrEP. Similar to studies among men[41], participants in this study felt that LAI PrEP would be more effective because it eliminated the need to take pills at a specific time or to remember them while traveling. Women also felt that shots provided greater confidentiality, which was a concern given the stigma around HIV. Participants described fear that friends, family, or sexual partners might see their pills and presume their HIV status or certain sexual behaviors, whereas a bi-monthly shot could be done in the privacy of a doctor’s office[41,43].
There were some notable differences between women in this study and previous work among men. Among men, primarily MSM, a benefit of injectable PrEP was that it would provide a safeguard in the case of hook-ups or casual sexual encounters[76,77]. Although this may be the case for some women, it was not a primary finding, perhaps because of different sexual patterns between women and MSM or age differences in individuals interviewed[76,77]. Additionally, women may have less power to negotiate sexual relationships and condom use than men[78,79]. LAI PrEP may provide a safeguard for women whose partners refuse condoms or for those who believe their partners may be unfaithful, while providing confidentiality in ways that minimize the fear of retaliation. This study suggests that LAI PrEP may be a useful option for women by providing them with more autonomy over their bodies and control of their health.
Strengths and Limitations:
This study involved women across six diverse sites, including women living in different contexts (i.e. urban versus rural, North versus South) who may have different concerns and levels of access to PrEP. Individuals that are eligible for clinical trials—and therefore included in most current LAI PrEP research—often have unique characteristics that differ from the general population. The experiences of women in this study may therefore be more representative of women at risk for HIV than those included in other studies and clinical trials; one study found that approximately 50% of women in WIHS would be excluded from clinical trials[80]. Our findings also highlight a group of women not often included but in need of HIV prevention and potentially PrEP (i.e., women over 50). Though LAI PrEP was described to participants, and any resulting questions were answered, many participants were not aware of PrEP prior to the study and therefore did not have an extended period to think through the potential benefits and limitations of LAI PrEP. Finally, women enrolled in the WIHS cohort study – and particularly those who have been participating in WIHS for over two decades – may trust the care they get and their providers more than the general population, suggesting that findings about medical mistrust may actually underestimate barriers within the general population.
CONCLUSIONS
This study provides critical evidence that women perceive unique benefits and drawbacks to LAI PrEP across individual-, community-, and structural-levels, and it is therefore crucial to include their perspectives in research. Women of color in the U.S. are at particularly high risk for HIV, and we must continue to gain an understanding of how prevention measures can be scaled up in ways that can be easily incorporated in their daily lives. Future studies should incorporate more women, particularly those who are younger and are in high risks groups, to comprehensively explore their unique concerns and to facilitate uptake.
Supplementary Material
Table II:
Participants’ statements regarding the acceptability and feasibility of LAI PrEP
| Theme | Impact | Quote |
|---|---|---|
| Perceived risk of acquiring HIV | Barrier | “I don’t need to take PrEP. I have abstinence tolerance and, I’m not involved with none of the other shenanigans. I ain’t trying to catch that. So, I don’t need to take PrEP to prevent it [HIV]. It’s prevented already by Jesus.” (Other race, 45, Chicago). |
| “Ever since I was growing up. I don’t pop pills and I don’t do injections. So I only take the medicines that my doctor prescribe, not no other doctor. And if my doctor even ask me about that one I probably wouldn’t deal with needles still.” (Black, 41, San Francisco) | ||
| Medical mistrust | Barrier | “Let’s say we’ve been doing this. We’ve been taking the injections and 10 years from now I have HIV, and it wasn’t because I missed a shot or I missed a pill. It just didn’t work. And then how would you handle somebody going through that mentally after they trusted this process?” (Black, 54, San Francisco) |
| Fears of injection-related side effects | Barrier | “I don’t like it [getting injections]. It wasn’t good. So, I’m not going to inject anything into my body that necessarily doesn’t have to be there. That’s why I don’t take the flu shot” (Black, 56, D.C.) |
| “Well, I really didn’t like the pain, you know, so if I really concentrate on the pain, I wouldn’t take the shots if I really concentrate on how bad they hurt, especially in the stomach.” (Black, 57, Chapel Hill) | ||
| Administration location | Barrier | “Not in the butt. I don’t want them messing with my butt over there. It’s not-- it don’t feel the same. If I can get it in my arm, yeah.” (Black, 44, San Francisco) |
| “I think I wouldn’t want the shot. Because I mean one in each butt cheek, it’s going to be kind of hard to sit down. I would rather take the pills if I had to.” (Black, 53, Atlanta) | ||
| Where and how to access LAI | Barrier | “Transportation, mostly transportation. And certain areas in Atlanta that you go in might not want to go in there.” (Black, 46, Atlanta). |
| Facilitator | “I think it would be good if it’s in a doctor’s office, less conspicuous [than a pharmacy]. Like, if you say, I’m going to this Walgreens and getting this shot. Okay, what are you getting that shot for? It’s not a clinic. I think a doctor’s office is most conventional and less conspicuous” (Mixed, 62, Chicago) | |
| Shots are more effective than pills | Facilitator | “I mean, you know it’s in your system, so you wouldn’t have to worry for at least a month or two, before you get your next shot, because it’s already in your system to block whatever supposed to stop you from getting HIV.” (Black, 56, D.C.) |
| Ease and convenience | Facilitator | “It lasts longer than the pill. The pill you got to take every day. The injection you only have to take every two months.” (Mixed, 65, Bronx) |
| “I might forget to take the pill, and then I might have myself at risk if I want to have sex that day, and I don’t have the pill in me” (Black, 56, D.C.) | ||
| Confidentiality of shots | Facilitator | “Maybe they’re dating someone, and someone sees a bottle of pills in their purse and they’re like, “What’s that for?” You’re at risk? Now you’re taking these pills.” And so, I can see that being an issue of carrying around a bottle of medicine.” (Black, 49, D.C.). |
| “That’s about it: that it won’t be really known. You don’t have to tell your partner that you’re trying to protect yourself, if you have a relationship and it’s not open-open, where you all can sit at the table and talk about any and everything. You know.” (Black, 45, Chicago) |
Funding
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR). Dr. Philbin is supported by K01DA039804A
Footnotes
Conflicts of Interest
Adaora A. Adimora-has received funding from Viiv, Merck, and Gilead, including a grant from Gilead; Anandi N. Sheth declares that Gilead has given research grants to her institution, but none are related to her current work. For the remaining authors none were declared.
Ethics Approval
IRB approval was obtained at all participating sites prior to interview initiation. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study prior to the interview.
Consent to Publish
Not applicable.
Ages are reported in ranges by decade, rather than specific ages, in order to maintain participant confidentiality.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2017 [Internet]. Washington, DC; 2018. November p. 129 Report No.: 29. Available from: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf [Google Scholar]
- 2.Centers for Disease Control and Prevention. Women | Gender | HIV by Group | HIV/AIDS | CDC [Internet]. CDC.gov. 2018. [cited 2018 May 21]. Available from: https://www.cdc.gov/hiv/group/gender/women/index.html
- 3.Rice WS, Turan B, Fletcher FE, Nápoles TM, Walcott M, Batchelder A, et al. A Mixed Methods Study of Anticipated and Experienced Stigma in Health Care Settings Among Women Living with HIV in the United States. AIDS Patient Care STDs. 2019;33:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turan B, Rice WS, Crockett KB, Johnson M, Neilands TB, Ross SN, et al. Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV: the mediating role of depression. AIDS. 2019;33:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerrigan D, Mantsios A, Gorgolas M, Montes ML, Pulido F, Brinson C, et al. Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS ONE. 2018;13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellins CA, Kang E, Leu CS, Havens JF, Chesney MA. Longitudinal Study of Mental Health and Psychosocial Predictors of Medical Treatment Adherence in Mothers Living with HIV Disease. AIDS PATIENT CARE STDs. 2003;17:407–16. [DOI] [PubMed] [Google Scholar]
- 7.Bradley E, Hoover K. Improving HIV Preexposure Prophylaxis Implementation for Women: Summary of Key Findings From a Discussion Series with Women’s HIV Prevention Experts. Womens Health Issues. 2019;1:3–7. [DOI] [PubMed] [Google Scholar]
- 8.Laurence J. Pre-Exposure Prophylaxis (PrEP) for HIV: Opportunities, Challenges, and Future Directions. AIDS Patient Care STDs. 2018;32:487–9. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367:423–34. [DOI] [PubMed] [Google Scholar]
- 11.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010;363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2015;372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012;367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson KA, Baeten JM, Mugo NR, Bekker L-G, Celum CL, Heffron R. Tenofovir-based Oral PrEP Prevents HIV Infection among Women. Curr Opin HIV AIDS. 2016;11:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U. S. Food and Drug Administration. FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2012.
- 16.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2017 update – clinical providers’ supplement [Internet]. Washington, DC: Centers for Disease Control and Prevention; 2018. March p. 59 Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2017.pdf [Google Scholar]
- 17.Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850–857.e9. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg ES, Marcus JL. Progress and pitfalls in measuring HIV preexposure prophylaxis coverage in the United States. Ann Epidemiol. 2018;28:830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegler AJ, Mouhanna F, Giler RM, Weiss K, Pembleton E, Guest J, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis–to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush S, Magnuson D, Rawlings MK, Hawkins T, McCallister S, Mera Giler R. Racial Characteristics of FTC/TDF for Pre-exposure Prophylaxis (PrEP) Users in the US. Boston, MA; 2016. [Google Scholar]
- 21.Hicks S. Staying on PrEP is Significantly Different for PrEP Users With Commercial Insurance Versus Medicaid [Internet]. TheBodyPRO.com. 2019. [cited 2019 Apr 8]. Available from: http://www.thebodypro.com/content/81655/staying-on-prep-commercial-insurance-vs-medicaid.html
- 22.Huang Y-LA. Persistence with HIV preexposure prophylaxis in the United States, 2012–2016 [Internet]. Seattle, Washington; 2019. [cited 2019 Apr 8]. Available from: http://www.croiwebcasts.org/console/player/41212?crd_fl=0&ssmsrq=1554747561744 [Google Scholar]
- 23.The Kaiser Family Foundation. Status of State Action on the Medicaid Expansion Decision [Internet]. Henry J Kais. Fam. Found 2019. [cited 2019 Apr 8]. Available from: https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/ [Google Scholar]
- 24.Patel RR, Mena L, Nunn A, McBride T, Harrison LC, Oldenburg CE, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS ONE [Internet]. 2017. [cited 2019 Sep 13];12 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5448799/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almanza K. PrEP Services and Approaches: It’s More Than Just Meds [Internet]. 2016. [cited 2019 Apr 8]. Available from: https://bphc.hrsa.gov/sites/default/files/bphc/qualityimprovement/clinicalquality/presentations/prep-svcs-approaches-slides.pdf
- 26.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2017 update – a clinical practice guideline [Internet]. Washington, DC: Centers for Disease Control and Prevention; 2018. March p. 77 Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf [Google Scholar]
- 27.Centers for Disease Control and Prevention. Paying for PrEP [Internet]. CDC.gov. 2018. [cited 2019 Apr 8]. Available from: https://www.cdc.gov/actagainstaids/campaigns/prescribe-hiv-prevention/patient-materials/index.html
- 28.Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, Attitudes, and Likelihood of Pre-Exposure Prophylaxis (PrEP) Use Among US Women at Risk of Acquiring HIV. AIDS Patient Care STDs. 2014;29:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinelli MA, Frongillo EA, Sheira LA, Palar K, Tien PC, Wilson T, et al. Food Insecurity is Associated with Poor HIV Outcomes Among Women in the United States. AIDS Behav. 2017;21:3473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter A, Roth EA, Ding E, Milloy M-J, Kestler M, Jabbari S, et al. Substance Use, Violence, and Antiretroviral Adherence: A Latent Class Analysis of Women Living with HIV in Canada. AIDS Behav. 2018;22:971–85. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman SC, Eaton L, Kalichman MO, Grebler T, Merely C, Welles B. Race-based medical mistrust, medication beliefs and HIV treatment adherence: test of a mediation model in people living with HIV/AIDS. J Behav Med. 2016;39:1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalichman SC, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. J Behav Med. 2003;26:315–32. [DOI] [PubMed] [Google Scholar]
- 33.Murphy DA, Roberts KJ, Martin DJ, Marelich W, Hoffman D. Barriers to antiretroviral adherence among HIV-infected adults. Aids Patient Care STDs. 2000;14:47–58. [DOI] [PubMed] [Google Scholar]
- 34.Lachat MF, Scott CA, Relf MV. HIV and pregnancy: Considerations for nursing practice. MCN Am J Matern Nurs. 2006;31:233–240. [DOI] [PubMed] [Google Scholar]
- 35.Sidebottom D, Ekström AM, Strömdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV – how can we improve uptake and adherence? BMC Infect Dis [Internet]. 2018. [cited 2019 Mar 6];18 Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-018-3463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petroll AE, Walsh JL, Owczarzak JL, McAuliffe TL, Bogart LM, Kelly JA. PrEP Awareness, Familiarity, Comfort, and Prescribing Experience among US Primary Care Providers and HIV Specialists. AIDS Behav. 2017;21:1256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronfenbrenner U. The Ecology of Human Development. Boston, MA: Harvard University Press; 1979. [Google Scholar]
- 38.Philbin MM, Parker CM, Parker RG, Wilson PA, Garcia J, Hirsch JS. The Promise of Pre-Exposure Prophylaxis for Black Men Who Have Sex with Men: An Ecological Approach to Attitudes, Beliefs, and Barriers. AIDS Patient Care STDs. 2016;30:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellowski JA, Price DM, Harrison AD, Tuthill EL, Myer L, Operario D, et al. A Systematic Review and Meta-analysis of Antiretroviral Therapy (ART) Adherence Interventions for Women Living with HIV. AIDS Behav [Internet]. 2018. [cited 2019 Apr 16]; Available from: 10.1007/s10461-018-2341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.HIV.gov. Long-acting PrEP [Internet]. HIV.gov. 2019. [cited 2019 Mar 29]. Available from: https://www.hiv.gov/hiv-basics/hiv-prevention/potential-future-options/long-acting-prep
- 41.Kerrigan D, Mantsios A, Grant R, Markowitz M, Defechereux P, La Mar M, et al. Expanding the Menu of HIV Prevention Options: A Qualitative Study of Experiences with Long-Acting Injectable Cabotegravir as PrEP in the Context of a Phase II Trial in the United States. AIDS Behav. 2018;22:3540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4:e331–e340. [DOI] [PubMed] [Google Scholar]
- 43.Murray MI, Markowitz M, Frank I, Grant RM, Mayer KH, Hudson KJ, et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: Patient perspectives from the ECLAIR trial. HIV Clin Trials. 2018;19:129–38. [DOI] [PubMed] [Google Scholar]
- 44.HIV Prevention Trials Network. Long-acting injectable cabotegravir is highly effective for the prevention of HIV infection in cisgender men and transgender women who have sex with men | The HIV Prevention Trials Network [Internet]. 2020. [cited 2020 Jul 2]. Available from: https://www.hptn.org/news-and-events/press-releases/long-acting-injectable-cabotegravir-highly-effective-prevention-hiv
- 45.Flexner CW. New prevention products in the pipeline: Implants and transdermal drug deliver systems for HIV prevention. Mexico City; 2019. [Google Scholar]
- 46.Aaron E, Blum C, Seidman D, Hoyt MJ, Simone J, Sullivan M, et al. Optimizing Delivery of HIV Preexposure Prophylaxis for Women in the United States. AIDS Patient Care STDs. 2018;32:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiv TL. New PrEP formulation approved…but only for some. Lancet HIV. 2019;6:e723. [DOI] [PubMed] [Google Scholar]
- 48.John SA, Whitfield THF, Rendina HJ, Parsons JT, Grov C. Will Gay and Bisexual Men Taking Oral Pre-exposure Prophylaxis (PrEP) Switch to Long-Acting Injectable PrEP Should It Become Available? AIDS Behav. 2018;22:1184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIVAIDS Auckl NZ. 2019;11:179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute of Allergy and Infectious Diseases (NIAID). Women’s Interagency HIV Study (WIHS) [Internet]. ClinicalTrials.gov. 1994. [cited 2019 Mar 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT00000797
- 51.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M-C, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buetow S. Thematic Analysis and Its Reconceptualization as ‘Saliency Analysis.’ J Health Serv Res Policy. 2010;15:123–5. [DOI] [PubMed] [Google Scholar]
- 53.Glaser BG, Strauss AL. The discovery of grounded theory : strategies for qualitative research. 1967.
- 54.MacQueen K, McLellan E, Kay K. Codebook development for team-based qualitative analysis. Cult Anthropol Methods. 1998;10:31–6. [Google Scholar]
- 55.Liu C, Hu H, Goparaju L, Plankey M, Bacchetti P, Weber K, et al. Sexual Serosorting among Women with or at Risk of HIV Infection. AIDS Behav. 2011;15:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koren DE, Nichols JS, Simoncini GM. HIV Pre-Exposure Prophylaxis and Women: Survey of the Knowledge, Attitudes, and Beliefs in an Urban Obstetrics/Gynecology Clinic. AIDS Patient Care STDs. 2018;32:490–4. [DOI] [PubMed] [Google Scholar]
- 57.Maughan-Brown B, Venkataramani AS. Accuracy and determinants of perceived HIV risk among young women in South Africa. BMC Public Health [Internet]. 2017. [cited 2019 Oct 16];18 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5520344/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eaton LA, Driffin DD, Smith H, Conway-Washington C, White D, Cherry C. Psycho-social factors related to willingness to use pre-exposure prophylaxis for HIV prevention among Black men who have sex with men attending a community event. Sex Health. 2014;11:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cahill S, Taylor SW, Elsesser SA, Mena L, Hickson D, Mayer KH. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2017;29:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sofair Á N, Kaldjian LC. HISTORY OF MEDICINE Eugenic Sterilization and a Qualified Nazi Analogy: [DOI] [PubMed]
- 61.Siegler AJ, Bratcher A, Weiss KM. Geographic Access to Preexposure Prophylaxis Clinics Among Men Who Have Sex With Men in the United States. Am J Public Health. 2019;109:1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP Implementation Through Multilevel Interventions: A Synthesis of the Literature. AIDS Behav. 2018;22:3681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elopre L, Kudroff K, Westfall AO, Overton ET, Mugavero MJ. Brief Report: The Right People, Right Places, and Right Practices: Disparities in PrEP Access Among African American Men, Women, and MSM in the Deep South. J Acquir Immune Defic Syndr 1999. 2017;74:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel AS, Goparaju L, Sales JM, Mehta CC, Blackstock OJ, Seidman D, et al. Brief Report: PrEP Eligibility Among At-Risk Women in the Southern United States: Associated Factors, Awareness, and Acceptability. J Acquir Immune Defic Syndr 1999. 2019;80:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and Aging: State of Knowledge and Areas of Critical Need for Research. A Report to the NIH Office of AIDS Research by the HIV and Aging Working Group. JAIDS J Acquir Immune Defic Syndr. 2012;60:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zablotsky D, Kennedy M. Risk Factors and HIV Transmission to Midlife and Older Women: Knowledge, Options, and the Initiation of Safer Sexual Practices. JAIDS J Acquir Immune Defic Syndr. 2003;33:S122. [DOI] [PubMed] [Google Scholar]
- 67.Smith TK. Sexual Protective Strategies and Condom Use in Middle-aged African American Women: A Qualitative Study. J Assoc Nurses AIDS Care. 2015;26:526–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman CL. Women 50 and Older and HIV: Prevention and Implications for Health Care Providers. J Gerontol Nurs. SLACK Incorporated; 2017;43:29–34. [DOI] [PubMed] [Google Scholar]
- 69.Taylor TN, Munoz-Plaza CE, Goparaju L, Martinez O, Holman S, Minkoff HL, et al. “The Pleasure Is Better as I’ve Gotten Older”: Sexual Health, Sexuality, and Sexual Risk Behaviors Among Older Women Living With HIV. Arch Sex Behav. 2017;46:1137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beaulaurier R, Fortuna K, Lind D, Emlet CA. Attitudes and Stereotypes Regarding Older Women and HIV Risk. J Women Aging. Routledge; 2014;26:351–68. [DOI] [PubMed] [Google Scholar]
- 71.Namey E, Agot K, Ahmed K, Odhiambo J, Skhosana J, Guest G, et al. When and why women might suspend PrEP use according to perceived seasons of risk: implications for PrEP-specific risk-reduction counselling. Cult Health Sex. 2016;18:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakasone S, Young I, Estcourt C, Calliste J, Flowers P, Ridgway J, et al. Risk perception, safer sex practices and PrEP enthusiasm: barriers and facilitators to oral HIV pre-exposure prophylaxis in Black African and Black Caribbean women in the UK. Sex Transm Infect. 2020;0:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garfinkel D, Alexander K, McDonald-Mosley R, Willie T, Decker M. Predictors of HIV-related risk perception and PrEP acceptability among young adult female family planning patients. AIDS Care. 2017;29:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goparaju L, Experton LS, Praschan NC, Warren-Jeanpiere L, Young MA, Kassaye S. Women want pre-exposure prophylaxis but are advised against it by their HIV-positive counterparts. J AIDS Clin Res. 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goparaju L, Praschan NC, Warren-Jeanpiere L, Experton LS, Young MA, Kassaye S. Stigma, Partners, Providers and Costs: Potential Barriers to PrEP Uptake among US Women. J AIDS Clin Res [Internet]. 2017. [cited 2019 Oct 16];8 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5708581/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macapagal K, Moskowitz D, Li DH, Carrion A, Bettin E, Fisher CB, et al. Hookup app use, sexual behavior, and sexual health among adolescent men who have sex with men in the United States. J Adolesc Health Off Publ Soc Adolesc Med. 2018;62:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ybarra ML, Mitchell KJ. A national study of lesbian, gay, bisexual (LGB) and non-LGB youth sexual behavior online and in-person. Arch Sex Behav. 2016;45:1357–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peasant C, Sullivan TP, Ritchwood TD, Parra GR, Weiss NH, Meyer JP, et al. Words can hurt: The effects of physical and psychological partner violence on condom negotiation and condom use among young women. Women Health. 2018;58:483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McLaurin-Jones T, Lashley M-B, Marshall V. Minority College Women’s Views on Condom Negotiation. Int J Environ Res Public Health [Internet]. 2016. [cited 2019 Jun 26];13 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4730431/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols: AIDS. 2005;19:1885–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


