Abstract
Approximately one-half of patients with substance use disorders (SUD) experience chronic pain. Yet how patients perceive the relationship between their substance use and chronic pain remains poorly understood. We sought to identify how patients with comorbid SUD and chronic pain describe the relationship between, and mechanisms linking, these conditions. We conducted qualitative interviews with 34 patients engaged in SUD treatment who were also diagnosed with chronic pain. Interviews were transcribed verbatim and coded by both primary and secondary coders. Qualitative content analysis guided coding and analysis. Patient interviews revealed three primary pathways. One group of participants described SUD as developing independently from their experiences of chronic pain. A second group of participants described turning to substances to self-manage or cope with the physical and emotional aspects of chronic pain. A third group of participants described encounters with opioid medications as the causal agent initiating a SUD. Our findings build upon research that has identified chronic pain and SUD as developmentally similar and mutually reinforcing, by revealing the ways in which patients themselves understand and experience the interconnections between their substance use and chronic pain.
Introduction
Chronic pain is common among patients diagnosed with substance use disorders (SUD), with prevalence estimates ranging from 50–60% [11,13]. The high rate of co-occurrence has prompted investigations of the temporal ordering of chronic pain and SUD to better understand the relationship between the two diagnoses and improve treatment outcomes. A number of explanatory pathways have been suggested. First, patients might “self-medicate” chronic pain with licit or illicit substances, which leads to SUD [13,1]. Second, as described in the diathesis stress model, chronic pain may cause a stress response, which then activates an underlying propensity to SUD [13,6]. Third, risky lifestyle choices associated with SUD may lead to injury and subsequent development of chronic pain [10,13]. This research views chronic pain and SUD as relatively distinct disorders that develop in reaction to one another. More recently, neurobiological research has found that chronic pain and SUD share elements of a common neural system, both emerge as a maladaptive, learned response, and share chronic stress as a common precondition [4, 8, 9]. There may be substantial overlap in the neurodevelopmental pathways and symptoms of each condition, and their co-occurrence may be attributable to dysregulation of the pain-reward neural circuitry.
Patients have also provided insight into the inter-relationship between pain and SUD. One study, examining patients’ explanations for the initiation of OUD specifically, provides a starting point to understand the link between chronic pain and SUD more generally. In a secondary analysis of data collected as part of a larger study of 283 patients with OUD [15], 43% of participants, unprompted, described the processes through which their OUD was initiated. Of the five pathways authors identified, three were related to pain control. Specifically, patients described 1) Misuse/abuse of opioid medications to control untreated pain, 2) Exposure to opioids during acute pain treatment, and 3) Exposure to opioid medications for chronic pain management. In addition, patients described two pathways to OUD that did not involve physical pain: 4) Opioids for the treatment of emotional pain and 5) Recreational use of opioids [19]. It is unclear if these pathways linking pain and the development of OUD mirror those of patients diagnosed with SUD more generally.
In this study, we conducted qualitative interviews with 34 patients diagnosed with chronic pain who were actively receiving treatment for SUD (e.g., alcohol, methamphetamine, opioids, polysubstance) to draw out rich description from the patients’ perspectives on the relationship between experiences of chronic pain and SUD. Qualitative data allow us to evaluate whether mechanisms described in prior research are reflective of the experiences of our sample [11,13] and suggest additional explanatory pathways and processes to inform future neurobiological and epidemiologic research. Identifying the pathways through which chronic pain is linked with SUD can help also inform the design of targeted health care interventions that address these frequently comorbid conditions.
Methods
Participants and Procedures
Participants were recruited from an outpatient SUD treatment program located at a single VA medical center. Program clinicians referred patients with histories of chronic pain to research staff who subsequently confirmed patient eligibility through electronic health record review and a brief telephone interview. Eligible patients were (1) at least 18 years old, (2) currently receiving care in the SUD treatment program, (3) had a chronic musculoskeletal pain diagnosis documented in the electronic health record in the past 5 years, and (4) reported average pain intensity in the past week and the past 3 months of at least 4 on a 0–10 numeric rating scale where 0 = no pain and 10 = worst pain imaginable. Exclusion criteria included having a pain diagnosis secondary to cancer or pending pain-related disability claim. This study was approved by the Institutional Review Board of the participating VA hospital and all participants signed written informed consent.
Forty-six patients were referred to the study by clinicians within the SUD treatment program in which patients received care. We were unable to contact seven referred patients, one patient declined participation following informed consent, and four patients agreed to participate but did not attend the scheduled interview appointment and were not able to be rescheduled. A total of 34 patients completed interviews.
Data collection
All participants completed a single one-on-one structured interview with the study’s principal investigator. Interviews occurred face-to-face in a private office located in the SUD treatment program where participants received care. Interviews were audio recorded, and participants received a $30 store gift card for completing the interview.
The primary purpose of patient interviews was to ascertain patients’ experiences of pain and its relationship to their substance use. Sample questions included the following: “How are you currently managing your pain?” “What approaches have worked the best at managing pain? If you are not currently using these approaches, what are the reasons?” “For some people, pain is closely related to their use of alcohol or other substances. How is pain related to your own alcohol or drug use?” “How has pain impacted your recovery from alcohol or drugs?”
Data Analysis
Interviews were transcribed verbatim by a member of the study team. The study PI reviewed all transcripts for completeness and accuracy as they were transcribed. In order to develop a codebook, four members of the research team carefully read and independently coded a selection of five interview transcripts. Codes were developed inductively from the data (i.e., open coded) following conventional content analysis [8]. Utilizing Atlas.ti version 7, each coder “tagged” sections of text that described concepts emergent from the data with a descriptive label, or code. Examples of codes utilized include the following: pain story, barriers to pain management, and pain and recovery. The group jointly reviewed the five transcripts to discuss each coders’ code list and coding decisions. Coders jointly agreed upon a final code list and created a definition of all codes, which were summarized in a code book.
The team then divided all transcripts, including those that had been previously coded, and assigned a primary and secondary coder to each. All transcripts were coded by the primary coder, and coding was reviewed by the secondary coder to identify discrepancies or additions. Any differences that were present between the coders were resolved through consensus methods.
Once all documents were coded, queries were run on codes that described participants’ descriptions of the relationship between their pain and substance use. These queries produced reports that included all data tagged with the identified codes. We carefully reviewed and sorted the identified text into categories, and analyzed and compared textual excerpts within categories to develop key themes. Key themes, and the data from which they were developed, were discussed at a group meeting. All team members agreed on the centrality of the key themes identified. Finally, illustrative quotes relating to key themes were selected for inclusion in this manuscript.
Results
Participants were, on average, 51 years old (range: 28 years to 70 years). The majority (88%) were cis-male gender, with the remaining 12% cis-female gender. One participant identified as multiracial, one as Hawaiian non-Hispanic, and one as White Hispanic; the remaining participants identified as White non-Hispanic. Alcohol was the primary “substance of choice” for 68% of participants, with 17%, 11%, and 3% endorsing opioids, methamphetamine, and cannabis as primary substances of choice, respectively. Sixteen participants (47%) reported abuse of more than one substance, not including tobacco. On average, participants reported pain severity in the past 3 months as 6 on the 0–10 numeric rating scale, indicating moderate to severe chronic pain.
Table 1 summarizes themes and subthemes linking patients’ experiences of chronic pain with substance use identified through this research, while Figure 1 provides a visual depiction of the identified pathways characterizing the relationship between chronic pain and substance use. Primary themes identified include Independent Onset of Chronic Pain and SUD, Self-Medication of Physical and Emotional Pain, and Opioid Medication for Pain Catalyzes Substance Use. Qualitative data supporting themes and subthemes are presented below.
Table 1.
Relationship between chronic pain and substance use
| Causal Path | Illustrative quote |
|---|---|
| Independent Onset of Chronic Pain and SUD | |
| SUD Prior to Pain | “I mean I drank all my life…one way or another, off and on…but I’d say the last 15, 20 years it’s gotten progressively worse due to stress and depression… I just started drinking more and more. And with the pain… it was just another excuse. I felt like I had to drink to kill the pain.” |
| Pain Recognized in Sobriety | “When I first started using the meth and coke, I just started doing it because I liked it…And then I got clean in 1984…And I relapsed in ‘92. When I relapsed that’s right around the time…I got diagnosed with the back problems. During my sobriety is when I was really feeling that pain. I went through a divorce and I ended up relapsing… That’s when I realized that if I started getting high on meth, I wasn’t suffering the pain nearly as much.” |
| Self-Medication of Physical and Emotional Pain | |
| Substance Used to Treat Pain | “I mean my only thing I really knew to do, and I basically developed my own thing, is I just basically drink a lot of alcohol. Daily. I became a daily drinker…That’s how I managed pain, how I managed to get to sleep, and also other stuff. Just being a post-deployment person.” |
| Opioid Medication for Pain Catalyzes Substance Use | |
| Brief Exposure to Opioids | “…[it] was just like a one or two-time thing… but, I realized how it made me feel. And [in] the military I got pain medication a couple times when I was in Iraq. But not for long periods of time… I would always tell the doctors, I don’t want to get on this stuff but I’ll use it temporarily… And then after I came back from Iraq… that’s when I started self-medicating. And honestly, I used my back as an excuse… I personally don’t think that pain medication helped my back at all. But it helped me not care. And I liked pain medication.” |
| Rapid Opioid Taper Reveals SUD | … the guy was an idiot. But he gave me what I wanted [opioids] And it was easy for me to agree with him and justify it, “Oh he’s a doctor, he knows better than I do”…and then of course they [VA staff] were all happy to tell me… that he no longer works here. There was no, “well we’re gonna cut you down slowly.” So two days later I was buying heroin. |
Figure 1.
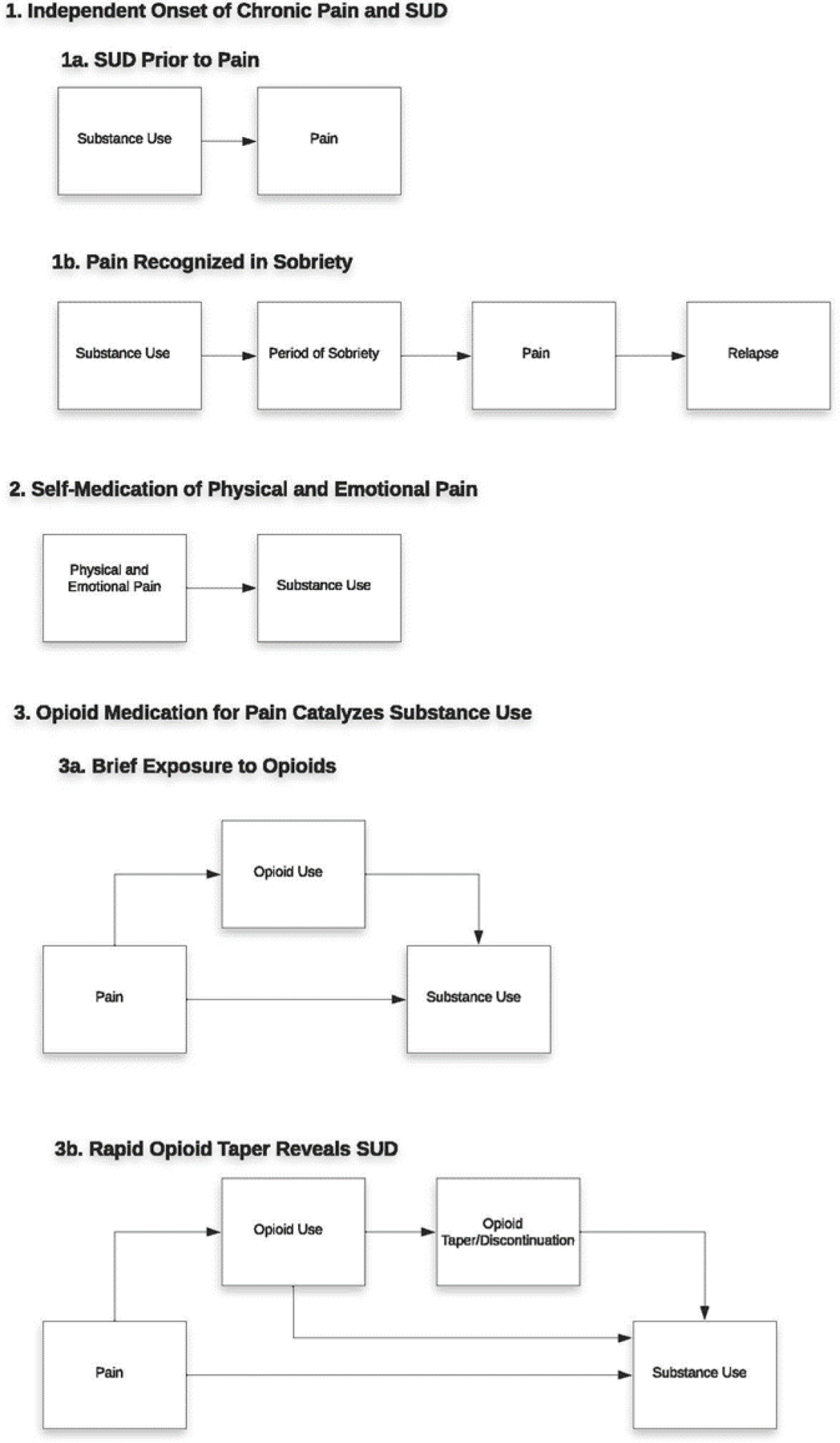
A visual depiction of the identified pathways characterizing the relationship between chronic pain and substance use.
Independent Onset of SUD and Chronic Pain
“I mean I drank all my life…”
For some participants, problematic substance use preceded their experiences of chronic pain. They described social drinking, a daily marijuana habit or methamphetamine use that was relaxing, fun, or just part of their social milieu. In this description, physical pain was not a motivation for use, at least not initially. One participant described the slow process by which his alcohol use increased over time:
… I mean I drank all my life. One way or another, off and on. And most of the time it was just casual and you know, a drink there, and I worked construction and when you work construction you drink beer. And in the military I drank beer. You know I didn’t do it off and on all the time, but I’d say the last 15, 20 years it’s gotten progressively worse due to stress and depression. What have you. I just started drinking more and more. And with the pain… it was just another excuse. I felt like I had to drink to kill the pain… (61 year-old, white non-Hispanic, cis-male, with alcohol use disorder and low back and shoulder pain)
For this participant, what began as social drinking slowly increased over time as he increasingly turned to alcohol to address uncomfortable feelings and untreated mental health issues. After he developed chronic pain, his experience with pain became an additional rationale among others that he relied on to justify his alcohol use.
Another participant described a similar process, in which substance use was initially unrelated to his experience of physical pain, “I started by using cocaine and alcohol. And I didn’t plan to medicate for pain by doing that. I just started using cocaine and alcohol at the same time. And I found really quickly that I felt good, I felt like I had energy, and I felt really positive.” (40 year-old, white non-Hispanic, cis-male, with alcohol, cocaine and methamphetamine use disorders and knee and low back pain).
He went on to describe that it was only later that he recognized that substances dulled his pain and allowed him to feel “normal.”
“During my sobriety is when I was really feeling that pain…”
A closely related pathway in which participants’ problematic substance use preceded the onset of chronic pain was one in which chronic pain was initially identified during a period of sobriety. Participants described past substance use as so all-encompassing that, prior to their sobriety, they were simply unaware of their pain or, as several participants described, “numb” to it. One participant described this process of awakening to his pain:
When I first started using the meth and coke, I just started doing it because I liked it. I didn’t know at the time, but I also was diagnosed with ADHD later on, in the early ‘90’s. So now I can correlate the two. Amphetamines help me focus more, slow down my head, wasn’t spinning out as much. And then I got clean in 1984, I got sober for 14 years. And [then] I relapsed… When I relapsed that’s right around the time the VA diagnosed me with… I got diagnosed with the back problems. During my sobriety is when I was really feeling that pain. I went through a divorce and I ended up relapsing after almost 14 years sober. That’s when I realized that if I started getting high on meth, I wasn’t suffering the pain nearly as much. (62 year-old, white non-Hispanic, cis-male, with methamphetamine and cocaine use disorders and low back pain)
Diagnosed with back problems during a relapse, he describes first becoming aware of this pain in his sobriety. And while the emotional toll of divorce seemed to spur his relapse initially—he turned to substances to deal with divorce—in his period of relapse he learned through experience that methamphetamine was effective in treating his physical pain as well.
Self-Medication of Pain
“I mean my only thing I really knew to do…is…just basically drink a lot of alcohol.”
Other participants described first turning to substances, particularly alcohol, explicitly to manage or alleviate their experiences of chronic pain. For many, alcohol helped to address not just their physical experiences of pain, but emotional pain and suffering as well. One participant described this pathway to problematic use:
Patient: I mean my only thing I really knew to do, and I basically developed my own thing, is I just basically drink a lot of alcohol. Daily. I became a daily drinker.
Interviewer: Okay. And that was how you managed pain was through alcohol?
Patient: Yeah. That’s how I managed pain, how I managed to get to sleep, and also other stuff. Just being a post-deployment person. (35 year-old, white Hispanic, cis-male, with alcohol use disorder and neck and shoulder pain)
Alcohol was a coping strategy he utilized to address his chronic pain and the myriad emotional challenges associated with being, as he described, “a post-deployment person.” Similarly, another interview participant described using opioids to address the physical and emotional aspects of his pain:
Interviewer: How did pain impact your use of opioids?
Patient: I think at first it was more a subconscious thing. I didn’t even realize that I had as much pain as I did… It’s always been… I played sports my whole life, I was a good, cut kid, I joined the Army. You don’t whine about stuff like that. So I would just… I would take opiates to cope with my pain, I would take opiates to cope with my depression and my anxiety, my lack of self-confidence and stuff. Just opiates made all that shit go away (31 year-old, white Non-Hispanic, cis-male, with opioid and cannabis use disorders and low back pain)
For this participant, opiates provided a means for him to treat the pain that he did not feel comfortable voicing or addressing in any other way.
Opioid Medication for Pain Catalyzes Substance Use
“I realized how it made me feel…”
Pain also initiated participants’ substance misuse indirectly, as exposure to prescribed opioid medications for pain opened up an awareness of the effects of the medication, and resulted in craving for additional opioids. One participant described how a brief exposure to opioids in college, for treatment of a sports injury, catalyzed his addiction years later. He described his reaction to receiving opioids initially:
…[it] was just like a one or two-time thing… but, I realized how it made me feel. And [in] the military I got pain medication a couple times when I was in Iraq. But not for long periods of time. But I knew how it made me feel and I would always tell the doctors, I don’t want to get on this stuff but I’ll use it temporarily… And then after I came back from Iraq… that’s when I started self-medicating. And honestly, I used my back as an excuse. I mean, I don’t, I personally don’t think that pain medication helped my back at all. But it helped me not care. And I liked pain medication. (33 year-old, white non-Hispanic, cis-male, with opioid use disorder and shoulder and low back pain)
An awareness of both the pain-numbing effects of medication, as well as the positive feelings the medication instilled in him, led this participant to problematic use following his deployment, and the challenges he faced at that time. The benefits of the medication, however, eroded over time, and the participant turned to heroin before seeking treatment.
“There was no, ‘well we’re gonna cut you down slowly…’”
For others, the pathway from opioid medication to addiction was first uncovered when changes to opioid prescriptions were initiated, either during an abrupt opioid taper or full discontinuation. One participant described his experience working with a clinician who prescribed him more opioids than he likely needed:
… the guy was an idiot. But he gave me what I wanted. And it was easy for me to agree with him and justify it, “Oh he’s a doctor, he knows better than I do.” And so, I just continued to use as an excuse and then of course they were all happy to tell me, you know, that he no longer works here. There was no, “Well we’re gonna cut you down slowly.” So two days later I was buying heroin. (59 year-old, white non-Hispanic, cis-male, with heroin use disorder and lower leg pain)
This participant’s misuse of the medication began initially with a clinician who may have been prescribing a higher opioid dose than was necessary. The clinician’s compliance with his requests also validated the patient’s use of the medication in his own mind. Once the provider left the clinic, other clinicians would not continue to prescribe opioids for the participant. However, rather than initiating a slow taper, they abruptly discontinued his medication, and he turned to street drugs.
Another participant described a similar process wherein a new clinician abruptly discontinued his opioid prescription. This participant turned to alcohol as a substitute. He explains, “… left me to my own devices which caused me to start drinking more and more and more. And actually, they turned me into an alcoholic.” (53 year-old, white non-Hispanic, cis-male, with alcohol use disorder and shoulder pain). It is not clear from the description what motivated the participant’s turn to alcohol, whether it was dependency on the medication, or a need to treat his own pain. Regardless, the participant links his problematic substance use with the discontinuation of his opioid medication.
Discussion
Interviews with patients diagnosed with chronic pain and actively engaged in SUD treatment revealed three primary perceived pathways linking pain and substance use. One group of participants described SUD as developing independently from their experiences of chronic pain. For these participants, physical pain had been a rationale through which they had justified their substance use, both to themselves, as well as others. Some of these participants also described first recognizing that they experienced chronic pain in a period of sobriety. A second group of participants described turning to substances to self-manage chronic pain. Alcohol, opioids and methamphetamine were described as dulling, numbing or allowing participants to cope with the physical and emotional aspects of pain that they experienced. A third group of participants described encounters with opioid medications as the causal agent initiating a SUD. This could occur through exposure to the medication, taking more medication than prescribed, or in response to an opioid taper or discontinuation that proceeded abruptly and did not allow the body to acclimate to a reduced level of medication.
Our findings partially align with prior research. Of the three dominant pathways outlined by Ilgen et al (2010), (self-medication of chronic pain, the stress of chronic pain activating underlying SUD, and injuries relating to risky behaviors resulting in pain), only self-medication was endorsed by our participants. Our findings are more consistent with recent qualitative research by Stumbo et al. (2017), which identified brief exposure to opioid medication as a catalyst to future addiction, as well as rapid taper or abrupt discontinuation as leading some patients to seek out alternative substances to address symptoms of withdrawal or opioid dependence. Findings from the current study suggest that these identified pathways may not be limited to patients diagnosed with opioid use disorder, but rather characterize pathways to other SUDs as well, including alcohol use disorder.
Our findings build upon research that has identified chronic pain and SUD as neurodevelopmentally similar and mutually reinforcing [4,9], by revealing ways in which patients themselves understand the interconnections between their experiences of stress, substance use and chronic pain. Participants’ descriptions of their experiences of, and pathways to, pain and SUD align with a growing body of research identifying chronic pain, in part, as a disorder of emotion, as well as one of learning and cognition [2, 3], and highlights patients’ perceptions of substances as a tool for relieving both physical and emotional aspects of pain. Uncovering patient perceptions is important both for generating hypotheses for future observational research, as well as building clinical understanding of patient experiences, which may inform the communication strategies, treatments and interventions utilized in their care. Findings also suggest that treatment resources and educational curricula within SUD programs may need to incorporate contemporary understandings of pain and SUD, moving away from describing pain and emotion as distinct constructs, and instead describe them as manifestations of shared neurobiological mechanisms.
Study findings have several implications for direct patient care. First, our results highlight the importance of providing integrated treatment for chronic pain and SUD, rather than treating each of these disorders independently. Integrated treatments have been shown to be both feasible and acceptable, and linked with improvements in both pain outcomes (pain intensity, pain-related functioning, pain interference) as well as SUD outcomes (alcohol and other substance use, cravings) [5,14,16], but remain poorly integrated within current treatment paradigms. Second, alcohol was the primary substance of choice for the majority of our participants. Not only does alcohol have analgesic properties [20], it is a readily available, relatively socially acceptable strategy that patients may use to distract from painful thoughts, experiences and sensation [20]. Clinicians working with patients experiencing chronic pain should be aware of the possibility that patients may rely on alcohol, as well as other substances, to address or temper chronic pain, and that untreated or undertreated pain may serve as a trigger for relapse to substance use.
Finally, the results from our third group of participants align with a growing body of research [7,19,21] emphasizing the importance of limiting initial exposure to opioids for the treatment of chronic pain, proceeding slowly with a taper or discontinuation using shared decision-making processes, and considering opioid agonist therapies such as buprenorphine for those with opioid use disorder. While a slow and steady patient-centered opioid taper, supplemented by a robust infrastructure of mental health and non-pharmacological pain treatments, may not prevent patients from turning to street drugs or other substances, it could make substance use less likely.
Limitations
This study was based on interviews conducted with a sample of largely white, male veteran patients receiving active treatment for SUD. Research conducted with a different patient population, such as those with SUD who are not enrolled in specialty addictions treatment, or a more socio-demographically diverse sample, may find a different set of patient-identified pathways linking chronic pain and substance use. Second, most patients in this sample identified alcohol as their primary substance of abuse, with smaller proportions of patients identifying other substances such as opioids, methamphetamine, and cannabis. A strength of this study is the diversity of SUDs represented in the sample, but this also precludes examination of SUD-specific pathways related to pain. That our findings in a predominantly alcohol-using sample align with prior research in patients with opioid use disorders [18], lends confidence that results may not be specific to type of SUD. Third, as a qualitative study, the goal of this research is to provide rich description from the patient’s perspective regarding the linkage between pain and substance use; however, our data do not allow us to make claims regarding the frequency of the pathways we have described. Future research should pursue which of the pathways presented are most common, and whether these findings can be generalized to a broader patient population. Finally, while we present the pathways linking pain and SUD as our interview participants described them to us, the views presented may have been shaped by individual biases or cognitive distortions linked with individual characteristics, social contexts or otherwise. Future studies may consider obtaining reports from family members and clinicians familiar with patients’ disease course to corroborate patient reports.
Conclusion
This study contributes to a growing body of research describing the relationship between chronic pain and experiences of SUD by describing patient perceptions of this relationship. This research reveals an alignment between contemporary neurodevelopmental models of pain and addiction and patients’ understanding of these co-occurring disorders, emphasizing the need for integrated chronic pain and SUD treatment modalities that address the deep interplay between these conditions.
Acknowledgments
We would like to thank Anders Herreid-O’Neil for research assistance. This work was supported by Career Development Award IK2HX001516 from the U.S. Department of Veterans Affairs Health Services Research and Development (PI Lovejoy). Dr. Wyse’s time was supported by grant number K12HS026370 from the Agency for Healthcare Research and Quality and Career Development Award 1IK2HX003007 from the U.S. Department of Veterans Affairs Health Services Research and Development (PI Wyse). The work was also supported by resources from the VA Health Services Research and Development-funded Center to Improve Veteran Involvement in Care at the VA Portland Health Care System (CIN 13–404). The views expressed in this article are those of the authors and do not represent the official views of the U.S. Department of Veterans Affairs, the U.S. Government or the Agency for Healthcare Research and Quality.
Footnotes
Authors report no conflicts of interest.
Literature
- 1.Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. Gen Intern Med 2016;31(5):486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 2015;87(3):474–491. doi: 10.1016/j.neuron.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152(3 Suppl):S49–S64. doi: 10.1016/j.pain.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantyne JC, Sullivan MD. Discovery of endogenous opioid systems: what it has meant for the clinician’s understanding of pain and its treatment. Pain 2017. December 1;158(12):2290–300. [DOI] [PubMed] [Google Scholar]
- 5.Barry DT, Beitel M, Cutter CJ, Fiellin DA, Kerns RD, Moore BA, Oberleitner L, Madden LM, Liong C, Ginn J, Schottenfeld RS. An evaluation of the feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy for opioid use disorder and chronic pain. Drug Alcohol Depend 2019;194:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom med 2002;64(5):773–786. [DOI] [PubMed] [Google Scholar]
- 7.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience & Biobehavioral Reviews 2012. November 1;36(10):2179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016. January 6;89(1):11–36. [DOI] [PubMed] [Google Scholar]
- 10.Gatchel RJ, Dersh J. Psychological disorders and chronic pain: Are there cause-and-effect relationships. Psychological approaches to pain management: A practitioner’s handbook 2002:51–30.
- 11.Hser Y-I, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D. Chronic pain among patients with opioid use disorder: Results from electronic health records data. J Subst Abuse Treat 2017;77:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 13.Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton J. The timing of onset of pain and substance use disorders. Am J Addict 2010;19(5):409–415. [DOI] [PubMed] [Google Scholar]
- 14.Ilgen MA, Bohnert AS, Chermack S, Conran C, Jannausch M, Trafton J, Blow FC. A randomized trial of a pain management intervention for adults receiving substance use disorder treatment. Addiction 2016;111(8):1385–1393. [DOI] [PubMed] [Google Scholar]
- 15.McCarty D, Perrin NA, Green CA, Polen MR, Leo MC, Lynch F. Methadone maintenance and the cost and utilization of health care among individuals dependent on opioids in a commercial health plan. Drug Alcohol Depend 2010;111(3):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morasco BJ, Greaves DW, Lovejoy TI, Turk DC, Dobscha SK, Hauser P. Development and preliminary evaluation of an integrated cognitive-behavior treatment for chronic pain and substance use disorder in patients with the hepatitis C virus. Pain Med 2016;17(12):2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain 2017;158 Suppl 1(Suppl 1):S43–S49. doi: 10.1097/j.pain.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stumbo SP, Yarborough BJH, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017;73:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Opioid Therapy for Chronic Pain Work G. VA/DoD clinical practice guideline for opioid therapy for chronic pain Washington D.C.: U.S. Department of Veterans Affairs/U.S. Department of Defense;2017https://www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf [Google Scholar]
- 20.Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B. Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. J Pain 2017;18(5):499–510. doi: 10.1016/j.jpain.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016;374(13):1253–1263. [DOI] [PubMed] [Google Scholar]


