Abstract
Introduction
Thyroid eye disease (TED) is an autoimmune disease that causes retro-orbital inflammation and subsequent proptosis, corneal exposure, strabismus, and variable vision changes. European studies have shown that TED can severely impact quality of life (QOL), but little is known about the QOL of patients with TED in the USA. Given that patient QOL influences TED severity classifications and subsequent treatment, understanding physician-perceived patient QOL is extremely important.
Methods
This retrospective chart review (conducted in 2018) examined QOL in US patients with moderate-to-severe TED, as reported by treating physicians who regularly manage patients with TED (≥ 5 patients in prior 12 months). The physicians graded patients’ overall QOL (7-point Likert scale; 1 = “not at all impaired”, 7 = “extremely impaired”), assessing mental health, vision changes, and ocular structural signs/symptoms. Patient demographics and clinical findings were examined to understand the impact of disease presentation on physician-perceived QOL.
Results
Medical record data of 714 US patients with moderate-to-severe TED were provided by 181 physicians (73 endocrinologists, 108 ophthalmologists). Patients had a mean age of 49.4 (standard deviation [SD] 13.6) years, and 102 cases (14%) were severe. Anxiety and/or depression was reported in 36% of patients (an increase from the 18.9% prevalence reported for the USA in 2017 by the US National Institute of Mental Health; P < 0.001). The mean physician-reported QOL impact score was 4.1 (SD 1.5). Furthermore, 62 and 89% of patients with moderate and severe TED, respectively, had a high physician-perceived QOL impact (≥ 4). The higher QOL impact group had significantly higher rates of pain symptoms, visual disturbances (including diplopia), and orbito-facial structural changes. Higher disease activity and severity were associated with lower physician-perceived QOL.
Conclusion
Patients’ QOL, as evaluated by US physicians, is highly impacted by the activity and severity of TED. Additionally, mental health issues were more frequently reported by patients with TED than in the general US population. Ocular pain, strabismus, and diplopia appear to be main drivers of physician-perceived QOL impairment in this sample of US patients with TED.
Keywords: Graves’ ophthalmopathy, Quality of life, Thyroid eye disease
Plain Language Summary
Little is known on how thyroid eye disease (TED) affects patient quality of life (QOL) in the USA. Patient QOL can affect how TED is treated; consequently, it is important to understand how physicians perceive QOL in patients with TED. We evaluated 714 patients, as reported by physicians, with this rare condition to better understand QOL in US patients with TED. The medical records of 612 patients with moderate TED and 102 patients with severe TED were examined. QOL impact was rated from 1 to 7, with 1 being “not at all impaired” and 7 being “extremely impaired.” Overall QOL, as assessed by treating physicians, is heavily impacted by both moderate and severe TED in US patients, with these patients also reported to have a higher frequency of mental health diagnoses than reported in the general US adult population. Higher levels of inflammation on and around the eye and more severe disease led to a higher QOL impairment. More specifically, pain, visual disturbances (including double vision), and changes to the face and tissues around the eye all negatively affected QOL.
Key Summary Points
| Why carry out this study? |
| Limited data exist on the impact of thyroid eye disease (TED) on quality of life (QOL) in US patients, particularly physician-perceived QOL which directly influences TED severity classification and treatment decisions. |
| This large-scale study of a rare condition sought to examine QOL in US patients with moderate-to-severe TED and to determine influencing factors. |
| What was learned from the study? |
| QOL is heavily impacted by both moderate and severe TED in US patients. |
| Higher disease activity and more severe disease were associated with a greater QOL impact, more specifically, orbital pain, visual disturbances (including diplopia), and orbito-facial structural changes. |
Digital Features
This article is published with digital features, including a summary slide and plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13110254.
Introduction
Thyroid eye disease (TED) is an autoimmune condition that begins with progressive inflammation, frequently referred to as ‘active’ or ‘acute’ TED. Inflammation and/or expansion of orbital and retro-orbital tissues can lead to subsequent ocular pain, proptosis, diplopia, exposure keratopathy, and optic neuropathy [1, 2]. After approximately 1–3 years, TED becomes chronic and less active with varying levels of long-term sequelae, such as proptosis and diplopia [3, 4]. Only about 2% of patients with moderate-to-severe TED are ever considered to be free of TED [5], which will hopefully change with newer, more targeted therapies. Therefore, many patients experience significant changes in their quality of life (QOL) due to changes in their appearance, visual alterations, inability to cope, and mental/psychiatric manifestations [6–8]. Studies have shown that patients with TED have an equivalent or poorer QOL than patients with diabetes, emphysema, or heart failure [9].
The effect of TED on patient QOL has been widely studied in Europe. However, only a few studies have evaluated the impact of TED on patients’ QOL in the USA. Similar to results of European studies [10, 11], Bradley et al. [12] found impaired QOL in patients with TED, with diplopia being an influencing factor. Schotthoefer and Wallace [13] demonstrated QOL improvement following surgical correction of TED-related strabismus. Lastly, Paulsen et al. [14] found that dry eye disease, which is highly present in patients with TED, significantly lowers QOL.
To the best of our knowledge, no prior studies have examined how physicians in the USA perceive QOL in their patients with TED. QOL assessment can influence treatment decisions and patient–physician relationships, both of which are of particular importance for patients with TED because physician-perceived QOL helps define disease severity and provides justification for utilizing certain immunosuppressive, surgical, and/or biological therapies [15]. In the study reported here, we examined the impact of physician-reported QOL on patients with moderate-to-severe TED. Patient clinical data were also examined to better understand the drivers of QOL impact.
Methods
This study was given exempt status by the Western Institutional Review Board (Puyallup, WA, USA; registration no.: IRB00000533), waiving the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964, and its later amendments. Ophthalmologists and endocrinologists who completed the survey were reimbursed for their time.
Ophthalmologists and endocrinologists who regularly manage patients with TED (at least 5 patients within the prior 12 months) completed an online survey between 1 September and 31 December 2018 regarding their TED patient population and treatment practices. Physicians were then asked to complete data collection forms (only de-identified data collected) using clinical records of up to four patients with moderate-to-severe TED. The survey forms also included questions on physicians’ perceptions on how TED impacted patient QOL, such as “To what degree does TED impair this patient?”, in terms of overall QOL and subscale measures (ability to attend work/school, ability to function in social situations, ability to participate and enjoy day-to-day activities, ability to drive and psychological well-being). Responses were scored using a Likert scale, ranging from 1 (“not at all impaired”) to 7 (“extremely impaired”). The survey was programmed in and administered via an established platform (Confirmit; Oslo, Sweden), and physicians were recruited from existing market research panels (via an independent third party [Biovid; Bristol, PA, USA]). A link to the screening questionnaire was sent via email to physicians. Those wishing to participate simply opted in by clinking on the link.
The median overall QOL impact was calculated and used as the cut-off point to divide patients into higher and lower QOL impact groups. Patient characteristics, pain, visual symptoms, and orbito-facial structural changes (e.g., proptosis, soft tissue changes, extraocular muscle involvement) were then compared between groups in an effort to understand how each of these factors potentially influenced QOL.
TED severity was reported by physicians as moderate or severe. TED activity for each patient was determined by calculating the clinical activity score (CAS), with 1 point given for each of the following signs/symptoms: pain in the primary gaze, pain with eye movement, eyelid swelling, eyelid redness, conjunctival swelling, conjunctival redness, and caruncle swelling [16]. In this survey, six of seven measures were used to calculate CAS because information on caruncle status was not available.
Statistical Analysis
Differences in patient and TED characteristics were compared using two-tailed chi-square tests for categorical variables (GraphPad QuickCalcs [online version]; Graphpad Software Inc., San Diego, CA, USA; accessed October 2020) and two-tailed Student’s t tests for continuous variables (Microsoft Excel, version 1808 [Office 2019 Professional]; Microsoft Corp., Redmond, WA, USA). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using Microsoft Excel. Statistical significance was defined as P < 0.05.
Results
Physician Characteristics
A total of 899 physicians expressed interest in participating in the study. Of these, 183 (20%) met the inclusion criteria and completed the survey; 240 (27%) did not complete screening questions, 365 (41%) did not meet inclusion criteria, and 111 qualified physicians (12%) did not complete the survey. Two completed surveys did not meet quality control standards and were excluded. Therefore, surveys from 181 physicians were included in analyses.
Patient Characteristics
Physicians provided de-identified data on 714 patients who were diagnosed with moderate-to-severe TED. A total of 108 ophthalmologists reported on 432 patients (61%) and 73 endocrinologists reported on 282 patients (39%). Patient characteristics are summarized in Table 1. Most patients were women (65%) and mean age at the time of data collection was 49.4 (standard deviation [SD] 13.6) years. Severe disease was reported in 102 (14%) patients. Overall QOL impact was 4.1 (SD 1.5) (median 4, range 1–7). There was no difference in mean overall QOL score between patients of endocrinologists (4.1 [SD 1.4]) and those of ophthalmologists (4.1 [SD 1.6]). At the time of data collection, 555 patients (78%) were euthyroid. A formal diagnosis of Graves’ disease was given in 487 patients (68%) and of Hashimoto’s thyroiditis in 25 patients (4%); 12 patients (2%) were diagnosed with both conditions.
Table 1.
Characteristics of patients labeled as having moderate-to-severe thyroid eye disease
| Patient characteristics | All patients (N = 714) | Moderate TED (n = 612) | Severe TED (n = 102) | P valuea |
|---|---|---|---|---|
| Number (%) of patients of an ophthalmologist | 432 (61) | 370 (61) | 62 (61) | > 0.999 |
| Number (%) of women | 466 (65) | 398 (65) | 68 (67) | 0.835 |
| Age, mean (SD), years | 49.4 (13.6) | 49.5 (13.7) | 49.4 (12.7) | 0.949 |
| Number (%) of euthyroid patients | 555 (78) | 477 (78) | 78 (76) | 0.840 |
| Number (%) of patients with Graves’ disease | 487 (68) | 405 (66) | 82 (80) | 0.006 |
| TED duration, mean (SD), years | 4.2 (5.1) | 4.2 (5.1) | 4.2 (5.2) | 0.979 |
| Number (%) of patients with higher overall QOL impact (score ≥ 4) | 473 (66) | 382 (62) | 91 (89) | < 0.001 |
| Overall QOL impact score, mean (SD)b | 4.1 (1.5) | 4.0 (1.5) | 5.2 (1.3) | < 0.001 |
| Ability to attend work/school, mean (SD) | 3.5 (1.7) | 3.3 (1.6) | 4.8 (1.6) | < 0.001 |
| Ability to function in social situations, mean (SD) | 4.0 (1.6) | 3.8 (1.6) | 5.1 (1.4) | < 0.001 |
| Ability to participate in daily activities, mean (SD) | 3.8 (1.6) | 3.6 (1.5) | 5.0 (1.4) | < 0.001 |
| Ability to drive, mean (SD) | 3.6 (1.7) | 3.3 (1.7) | 5.0 (1.5) | < 0.001 |
| Psychological well-being, mean (SD) | 4.1 (1.5) | 3.9 (1.5) | 5.2 (1.3) | < 0.001 |
| Number (%) of patients with mental health issuesc | 260 (36) | 230 (38) | 30 (29) | 0.140 |
| Number (%) of patients with anxiety | 188 (26) | 174 (28) | 14 (14) | 0.003 |
| Number (%) of patients with depression | 131 (18) | 109 (18) | 22 (22) | 0.442 |
QOL Quality of life, SD standard deviaiton, TED thyroid eye disease
aComparisons of patients with moderate and severe TED were performed using a 2-tailed Student’s t test or chi-square test, as appropriate
bOverall and subscale QOL were scored by physicians using a Likert scale, ranging from 1 (not at all impaired) to 7 (extremely impaired)
cAnxiety and/or depression
Comparison of Patients with Moderate and Severe TED
Patients with severe TED had a significantly higher mean negative QOL impact score than those with moderate TED (5.2 [SD 1.3] vs. 4.0 [SD 1.5], P < 0.001). Additionally, 89 and 44% of patients with severe TED had an overall QOL impact score ≥ 4 and ≥ 6, respectively. Among those with moderate TED, 62 and 16% had an overall QOL impact score ≥ 4 and ≥ 6, respectively. All examined QOL subscales had a mean impact score of ≥ 3.5, with “psychological well-being” having the highest impact score (mean 4.1 [SD 1.5]). All subscale QOL measures were rated significantly higher in patients with severe TED than in those with moderate TED, indicating a perceived poorer QOL (Table 1). Diagnosis of anxiety and/or depression was noted in 260 patients (36%), with 188 patients (26%) having anxiety, 131 patients (18%) having depression, and 59 patients [8%] having both. Anxiety was significantly more prevalent in patients with moderate TED than in those with severe TED (28 vs. 14%, P = 0.003; OR 2.50, 95% CI 1.38−4.51, P = 0.001), but depression was similarly reported in both groups (18 and 22%, P = 0.442; OR 0.79, 95% CI 0.47−1.32, P = 0.182).
Effect of TED Signs and Symptoms on QOL Impact
Approximately two-thirds (66%) of patients were categorized into the higher QOL impact group (overall QOL score ≥ 4; Table 2). Patient age and the proportion of women did not differ between the two groups, but duration of TED was shorter in the higher QOL impact group.
Table 2.
Characteristics of patients with moderate-to-severe thyroid eye disease who had a higher and lower quality of life impact
| Patient characteristics | All patients (N = 714) | Overall QOL impact < 4 (n = 241) | Overall QOL impact ≥ 4 (n = 473) | P valuea |
|---|---|---|---|---|
| Number (%) of patients of an ophthalmologist | 432 (61) | 150 (62) | 282 (60) | 0.551 |
| Number (%) of women | 466 (65) | 157 (65) | 309 (65) | > 0.999 |
| Age, mean (SD), years | 49.4 (13.6) | 49.8 (14.5) | 49.2 (13.2) | 0.445 |
| Number (%) of euthyroid patients | 555 (78) | 185 (77) | 370 (78) | 0.728 |
| Number (%) of patients with Graves’ disease | 487 (68) | 162 (67) | 325 (69) | 0.749 |
| TED duration, mean (SD), years | 4.2 (5.1) | 5.0 (5.8) | 3.8 (4.7) | 0.006 |
| Clinical activity score, mean (SD)b | 2.3 (1.8) | 1.5 (1.5) | 2.7 (1.9) | < 0.001 |
| Number (%) of patients with severe disease | 102 (14) | 11 (5) | 91 (19) | < 0.001 |
| Number (%) of patients with mental health issuesc | 260 (36) | 77 (32) | 183 (39) | 0.092 |
| Number (%) of patients with anxiety | 188 (26) | 60 (25) | 128 (27) | 0.595 |
| Number (%) of patients with depression | 131 (18) | 35 (15) | 96 (20) | 0.075 |
aComparison between higher and lower QOL impact groups performed using 2-tailed Student’s t test or chi-square test as appropriate
bTED severity was reported by physicians as moderate or severe. TED activity for each patient was determined by calculating the clinical activity score (CAS), with 1 point given for each of the following signs/symptoms: pain in the primary gaze, pain with eye movement, eyelid swelling, eyelid redness, conjunctival swelling, conjunctival redness, and caruncle swelling. In this survey, data for caruncle swelling were not available and thus the CAS ranged from 1 to 6
cIndicates reporting of anxiety and/or depression
Mental Health Issues
Anxiety and depression were highly reported in both QOL impact groups (anxiety 25 and 27%, depression 15 and 20%; Table 2). Patients were further stratified by overall QOL impact (groups 1–7 representing an overall QOL impact score of 1–7, respectively) to examine trends between overall QOL impact and anxiety or depression. The proportion of patients with reported anxiety was relatively steady across all QOL impact score groups, but depression progressively increased from 12% in group 1 (not at all impaired) to 25% in group 7 (extremely impaired).
Ocular Pain
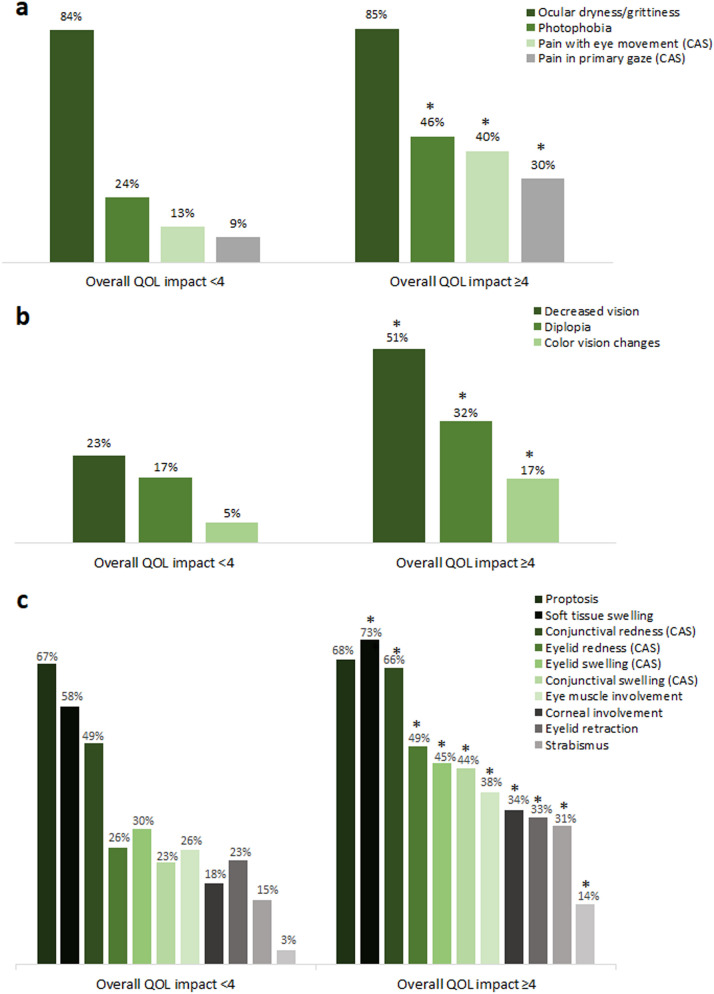
Most ocular signs and symptoms of TED were reported more often in patients with a higher QOL impact score. Ocular pain symptoms associated with TED were significantly more prevalent in the higher QOL impact group than in the lower QOL impact group (photophobia 46 vs. 24%, OR 2.71, 95% CI 1.92−3.84; pain with eye movement 40 vs. 13%, OR 4.59, 95% CI 3.02−6.98; pain in primary gaze 30 vs. 9%, OR 4.36, 95% CI 2.70−7.04; all P < 0.001; Fig. 1a). However, discomforts associated with exposure keratopathy were equally and highly present in both QOL impact groups (ocular dryness/grittiness 85 and 84%; excessive tearing 60 and 56%; results not shown).
Fig. 1.
Prevalence of pain symptoms (a), vision-related symptoms (b), and structural signs (c) associated with thyroid eye disease in patients with a higher and lower quality of life (QOL) impact score. Asterisks indicate a statistically significant difference between groups as calculated using a 2-tailed chi-square test (a, b: all P < 0.001; c: all P ≤ 0.009). CAS Clinical activity score measure (see section “Methods” for details)
Vision-Related Symptoms and Structural Signs
All examined vision-related symptoms were significantly more prevalent in the higher QOL impact group than in the lower QOL impact group (all P < 0.001; Fig. 1b), including decreased vision (51 vs. 23%, OR 3.49, 95% CI 2.46−4.95), diplopia (32 vs. 17%, OR 2.27, 95% CI 1.54–3.33), and color vision changes (17 vs. 5%, OR 3.62, 95% CI 1.97−6.66). The structural signs and symptoms of TED were also examined and compared (Fig. 1c). With the exception of proptosis, which was equally and highly prevalent in both groups (67 and 68%), all structural signs were reported significantly more often in the higher QOL impact group than in the lower QOL impact group.
The proportion of patients with decreased vision (reported as decreased visual acuity, blurred vision, and/or vision loss) progressively increased from 15 to 75% as the overall QOL impact score increased from 1 to 7. Similar findings were observed for eye muscle involvement (from 24 to 75%). More specifically, reporting of diplopia increased from 21 to 57% and reporting of strabismus increased from 6 to 50% as the overall QOL impact score increased from 1 to 7.
Effect of TED Activity on Perceived QOL
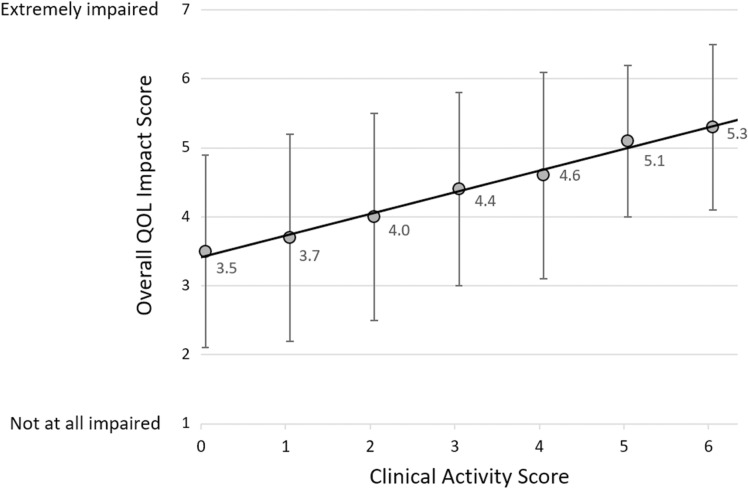
Thyroid eye disease was more active in the higher QOL impact group (mean CAS: 2.7 [SD 1.9] vs. 1.5 [SD 1.5]; CAS ≥ 3: 53 vs. 23%, OR 3.74, 95% CI 2.63−5.30; all P < 0.001). Patients were grouped by CAS (range 0–6 [caruncle status not reported]) to further examine the influence of disease activity on QOL impact. The overall QOL impact score progressively increased with increasing CAS (Fig. 2).
Fig. 2.
Relationship between the overall QOL impact score and CAS. The best-fit line is shown (y = 0.311x + 3.413, r = 0.375; 714 patients, P < 0.001). The CAS was calculated using six of seven measures because caruncle status was not reported. Data points represent mean overall QOL impact score and error bars represent 1 standard deviation
Patients with a higher QOL impact score reported all examined CAS components more frequently (Fig. 1a, c). This was particularly true of orbital pain (pain in primary gaze and/or with eye movement), which was reported nearly threefold more often in the higher QOL impact group (47 vs. 17%, OR 4.52, 95% CI 3.08−6.64; both P < 0.001).
Effect of TED Severity on Perceived QOL
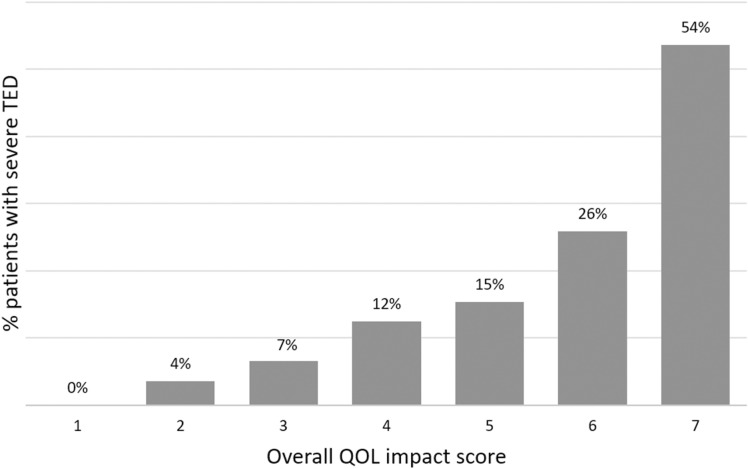
Severe disease was reported more often in patients with a higher QOL impact score (Table 2). Furthermore, the proportion of patients with severe TED progressively increased from 0 to 54% as the overall QOL score increased from 1 (not at all impaired) to 7 (extremely impaired; Fig. 3). Although physicians made the determinations on TED severity, clinical parameters described by the American Thyroid Association (ATA) [16] and European Group on Graves’ Orbitopathy (EUGOGO) [15] that contribute to TED severity classifications were examined. Factors that progressively increased with overall QOL impact score included eye muscle involvement (24–75%), corneal involvement (18–61%), and the presence of compressive optic neuropathy (0–25%).
Fig. 3.
The proportion of patients with severe thyroid eye disease in patients at each overall QOL impact level. QOL impact was rated on a 7-point Likert scale with 1 = not at all impaired and 7 = extremely impaired. TED Thyroid eye disease
Discussion
The current study uniquely examined physician-perceived QOL in a large group of patients with moderate-to-severe TED in the USA (714 patients). Our analyses indicate that patient QOL is highly negatively impacted in both moderate and severe disease. To the best of our knowledge, this is the first study of its kind to include information from patients in the USA. Other QOL studies performed in Europe [9–11, 17–19], Asia [20–22], Australia [23], and South America [24, 25] have also shown a high impact of TED on patients’ QOL.
The current study found that TED-associated visual function changes, pain, and structural changes were significantly different between the higher and lower QOL impact groups. Patients in the lower QOL impact group had a longer TED duration (5.0 [SD 5.8] vs. 3.8 [SD 4.7] years, P = 0.006) and a significantly lower CAS (1.5 [SD 1.5] vs. 2.7 [SD 1.9], P < 0.001) than those in the higher QOL impact group. Therefore, the lower QOL impact group may have been closer to disease quiescence (or chronic disease), which can be accompanied by a spontaneous improvement in some TED inflammatory signs and symptoms [3, 26–28]. This conclusion is supported by a longer duration of TED disease in this group as compared with the higher impact group. Nonetheless, the lower QOL impact group continued to present with a wide array of signs and symptoms of the disease despite the lower CAS score.
The mean overall QOL impact score significantly increased with increasing CAS. All examined CAS elements were reported significantly more often in patients with a higher QOL impact score, but differences in the presence of ocular pain (in the primary gaze and/or with eye movement) were the most pronounced. Therefore, ocular pain is likely a significant driver of QOL decrement in patients with TED. These findings are in agreement with prior studies which demonstrated that increasing disease activity [11, 21, 22, 24, 25] and ocular pain [10, 11] significantly impair patient QOL. It should be noted that smaller, earlier published studies did not find a significant correlation between patient-perceived QOL and disease activity [20, 29]. This discrepancy may be related to a lower number of included patients in previous studies and/or differences between physician and patient QOL perceptions [30]. Further, Sabini et al. [5] reported that some patients with continuing symptoms apparently develop a psychological adaptation to the changes in appearance or to the functional limitations of TED and, therefore, may not report QOL changes.
As expected, significantly more patients had severe disease in the higher QOL impact group than in the lower QOL impact group, and this proportion progressively increased with increasing overall QOL impact score. With the exception of proptosis, which was reported similarly in both groups, all examined ATA-defined severity measures (proptosis, diplopia, soft tissue involvement, corneal exposure, optic nerve status) [16] were reported significantly more often in the higher QOL impact group. These findings suggest that overall disease severity, rather than any one severity measure, drive physician-perceived QOL impact in patients with TED. This conclusion is supported by patient-perceived QOL studies that show an increased QOL impact in patients with more severe disease [21–25].
On the other hand, eye muscle involvement, and the resulting sequelae (e.g., strabismus and diplopia), have been previously shown to specifically negatively influence QOL in TED patients [10–12, 22, 24]. Decreases in QOL associated with strabismus have been shown to be correlated with the level of diplopia [31]. In the current study, eye muscle involvement, strabismus, and diplopia were all reported significantly more often in patients with a higher QOL impact score. The reported frequency of each progressively increased with overall QOL impact score, with 75% of extremely impaired patients having some form of eye muscle involvement.
Our study had several limitations. First, the retrospective nature of this study may have introduced a selection bias. Second, physician-perceived QOL, and not patient-perceived QOL, was examined. A prior study demonstrated that physicians tend to underestimate patient QOL impact [30] and place different emphasis than patients or their caregivers on which clinical signs/symptoms most impact QOL [30, 32]. However, understanding how physicians perceive patient QOL is important, as QOL impact can influence TED severity grading and subsequent treatment decisions. This sentiment has been previously reflected by others [7], who have stated the importance of clinicians understanding which TED-related signs and symptoms most heavily impact their patients. Third, this study used a Likert scale to evaluate QOL impact, which has a long history of use since its introduction in 1932 [33] and is the basis for well-established QOL instruments, including those developed by the World Health Organization [34]. Further, a 7-point scale has been shown to balance ease of use with score reliability, internal consistency, and test–retest reliability [35]. Additionally, a TED-specific QOL questionnaire is available to gain patient’s perspective on QOL impact (i.e., the GO-QOL [29]), but a validated instrument to obtain a physician’s perspective has not yet been developed. Therefore, future prospective studies that examine and compare physician- and patient-perceived QOL with TED-specific questionnaires are needed to better understand TED-related QOL impact and to identify physician/patient perception differences.
Conclusions
The findings presented here suggest that increasing disease activity, disease severity, and vision changes significantly influence physician-perceived patient QOL. The current study adds to the growing body of evidence that shows a high impact of TED on patients’ QOL and supports the importance of QOL evaluations when caring for patients with TED.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fees were funded by Horizon Therapeutics plc (Lake Forest, IL, USA).
Medical Writing, Editorial and Other Assistance
Editorial assistance in the preparation of this article was provided by Naina Barretto, PhD of Horizon Therapeutics. Support for this assistance was funded by Horizon.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Preliminary findings from this study were presented at the 2020 annual meeting of the American Association of Clinical Endocrinologists (virtual presentation only, May 7–10, 2020).
Disclosures
Yao Wang, Anu Sharma, and Guy Massry declare that they have no conflict of interest. Lissa Padnick-Silver, Megan Francis-Sedlak, Robert J. Holt, and Colleen Foley are employees of and hold stock in Horizon. Raymond S. Douglas is a consultant for Horizon Therapeutics plc.
Compliance with Ethics Guidelines
This study was given exempt status by the Western Institutional Review Board (Puyallup, WA, USA; registration no.: IRB00000533), waiving the requirement of informed consent. All study conduct adhered to the tenets of the Declaration of Helsinki of 1964, and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:284–290. doi: 10.1016/S0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson AJ, Hintschich C. Clinical manifestations. In: Wiersinga WM, Kahaly GJ, editors. Graves' orbitopathy: a multidisciplinary approach—questions and answers. 3. Basel: Karger; 2017. pp. 1–25. [Google Scholar]
- 3.Hales IB, Rundle FF. Ocular changes in Graves' disease. A long-term follow-up study. Q J Med. 1960;29:113–126. [PubMed] [Google Scholar]
- 4.Bartalena L, Tanda ML. Clinical practice. Graves' ophthalmopathy. N Engl J Med. 2009;360:994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 5.Sabini E, Leo M, Mazzi B, et al. Does Graves' orbitopathy ever disappear? Answers to an old question. Eur Thyroid J. 2017;6:263–270. doi: 10.1159/000477803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estcourt S, Vaidya B, Quinn A, Shepherd M. The impact of thyroid eye disease upon patients' wellbeing: a qualitative analysis. Clin Endocrinol (Oxf) 2008;68:635–639. doi: 10.1111/j.1365-2265.2007.03087.x. [DOI] [PubMed] [Google Scholar]
- 7.Coulter I, Frewin S, Krassas GE, Perros P. Psychological implications of Graves' orbitopathy. Eur J Endocrinol. 2007;157:127–131. doi: 10.1530/EJE-07-0205. [DOI] [PubMed] [Google Scholar]
- 8.Estcourt S, Quinn AG, Vaidya B. Quality of life in thyroid eye disease: impact of quality of care. Eur J Endocrinol. 2011;164:649–655. doi: 10.1530/EJE-11-0055. [DOI] [PubMed] [Google Scholar]
- 9.Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves' ophthalmopathy is markedly decreased: measurement by the medical outcomes study instrument. Thyroid. 1997;7:885–889. doi: 10.1089/thy.1997.7.885. [DOI] [PubMed] [Google Scholar]
- 10.Kahaly GJ, Hardt J, Petrak F, Egle UT. Psychosocial factors in subjects with thyroid-associated ophthalmopathy. Thyroid. 2002;12:237–239. doi: 10.1089/105072502753600205. [DOI] [PubMed] [Google Scholar]
- 11.Kahaly GJ, Petrak F, Hardt J, Pitz S, Egle UT. Psychosocial morbidity of Graves' orbitopathy. Clin Endocrinol (Oxf) 2005;63:395–402. doi: 10.1111/j.1365-2265.2005.02352.x. [DOI] [PubMed] [Google Scholar]
- 12.Bradley EA, Sloan JA, Novotny PJ, Garrity JA, Woog JJ, West SK. Evaluation of the National Eye Institute visual function questionnaire in Graves' ophthalmopathy. Ophthalmology. 2006;113:1450–1454. doi: 10.1016/j.ophtha.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Schotthoefer EO, Wallace DK. Strabismus associated with thyroid eye disease. Curr Opin Ophthalmol. 2007;18:361–365. doi: 10.1097/ICU.0b013e32827038f2. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the management of Graves' orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 17.Elberling TV, Rasmussen AK, Feldt-Rasmussen U, Hording M, Perrild H, Waldemar G. Impaired health-related quality of life in Graves' disease. A prospective study. Eur J Endocrinol. 2004;151:549–555. doi: 10.1530/eje.0.1510549. [DOI] [PubMed] [Google Scholar]
- 18.Terwee C, Wakelkamp I, Tan S, Dekker F, Prummel MF, Wiersinga W. Long-term effects of Graves' ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146(6):751–757. doi: 10.1530/eje.0.1460751. [DOI] [PubMed] [Google Scholar]
- 19.Wiersinga WM. Quality of life in Graves' ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:359–370. doi: 10.1016/j.beem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Bahmani-Kashkouli M, Pakdel F, Astaraki A, et al. Quality of life in patients with thyroid eye disease. J Ophthalmic Vis Res. 2009;4:164–168. [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YJ, Lim HT, Lee SJ, Lee SY, Yoon JS. Assessing Graves' ophthalmopathy-specific quality of life in Korean patients. Eye (London) 2012;26:544–551. doi: 10.1038/eye.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin IC, Lee CC, Liao SL. Assessing quality of life in Taiwanese patients with Graves' ophthalmopathy. J Formos Med Assoc. 2015;114:1047–1054. doi: 10.1016/j.jfma.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Park JJ, Sullivan TJ, Mortimer RH, Wagenaar M, Perry-Keene DA. Assessing quality of life in Australian patients with Graves' ophthalmopathy. Br J Ophthalmol. 2004;88:75–78. doi: 10.1136/bjo.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villagelin D, Romaldini J, Andrade J, et al. Evaluation of quality of life in the Brazilian Graves' disease population: focus on mild and moderate Graves' orbitopathy patients. Front Endocrinol (Lausanne) 2019;10:192. doi: 10.3389/fendo.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delfino LC, Zunino A, Sapia V, Croome M, Ilera V, Gauna AT. Related quality of life questionnaire specific to dysthyroid ophthalmopathy evaluated in a population of patients with Graves' disease. Arch Endocrinol Metab. 2017;61:374–381. doi: 10.1590/2359-3997000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menconi F, Profilo MA, Leo M, et al. Spontaneous improvement of untreated mild Graves' ophthalmopathy: Rundle's curve revisited. Thyroid. 2014;24:60–66. doi: 10.1089/thy.2013.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rundle FF. Management of exophthalmos and related ocular changes in Graves' disease. Metabolism. 1957;6:36–48. [PubMed] [Google Scholar]
- 28.Agapitos PJ, Hart IR. Long-term follow-up of ophthalmic Graves' disease. CMAJ. 1987;136:369–372. [PMC free article] [PubMed] [Google Scholar]
- 29.Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves' ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998;82:773–779. doi: 10.1136/bjo.82.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson KA, Dowling AJ, Abdolell M, Tannock IF. Perception of quality of life by patients, partners and treating physicians. Qual Life Res. 2000;9:1041–1052. doi: 10.1023/A:1016647407161. [DOI] [PubMed] [Google Scholar]
- 31.Beauchamp GR, Felius J, Stager DR, Beauchamp CL. The utility of strabismus in adults. Trans Am Ophthalmol Soc. 2005;103:164–171. [PMC free article] [PubMed] [Google Scholar]
- 32.Ysrraelit MC, Fiol MP, Gaitan MI, Correale J. Quality of life assessment in multiple sclerosis: different perception between patients and neurologists. Front Neurol. 2017;8:729. doi: 10.3389/fneur.2017.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22:5–55. [Google Scholar]
- 34.Skevington SM. Measuring quality of life in Britain: introducing the WHOQOL-100. J Psychosom Res. 1999;47:449–459. doi: 10.1016/S0022-3999(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 35.Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol (Amst) 2000;104:1–15. doi: 10.1016/S0001-6918(99)00050-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.