Estimates suggest that, despite recent decreases in their prescribing and usage [11,28], several million opioid prescriptions are dispensed in the United States annually to manage pain in children and adolescents [9,13,14]. Even when used correctly, prescribed opioids pose significant risks for common and potentially serious adverse drug effects (ADEs). Children and adolescents are particularly vulnerable to ADEs and opioid misuse. For instance, a recent study found that approximately one in 2600 opioid prescriptions dispensed to children aged 2–17 years was associated with a subsequent opioid-related emergency room visit, hospital admission or death [4]. Further, 71% of these ADEs were related to therapeutic use of the prescribed opioid and 7%, unintentional overdose. Teens are at additional risk for misuse of prescription opioids, most often as they attempt to self-manage pain [10,27,29].
Incorrect usage and misuse, combined with parental uncertainty and lack of knowledge, increases the risks that prescription opioids pose for children. For instance, 1 in 10 parents has admitted to giving more than the prescribed analgesic doses when treating their children’s pain [15,43]. Experimental and claims data have revealed that many parents continue to give prescribed opioids during high risk situations, failing to recognize early signs of toxicity (i.e., excessive sedation) – in some cases, even as their children were dying [7,21,26,46]. On the other hand, parents who are uncertain about analgesic risks may undertreat their children’s pain [34]. We have found that risky prescription opioid decisions not only reflect lack of knowledge [48], but parents’ strong preferences to relieve their children’s pain [49]. Thus, strategies to reduce prescription opioid-related ADEs for children must address risk understanding while supporting parents’ abilities to effectively manage their children’s pain.
Earlier strategies to educate parents on prescription opioid use emphasized the importance of achieving pain relief while minimizing the significance of opioid risks [17, 40]. Such strategies likely created an imbalanced approach to parental analgesic decision-making, leaving many children vulnerable to opioid-related ADEs. We developed our educational intervention, the Scenario-Tailored Opioid Messaging Program (STOMP), to rebalance parental analgesic decision-making with dual goals to enhance their safe use while acknowledging their potential benefit to relieve pain [47]. We previously tested the educational intervention in a non-clinical sample of community parents [47].(CITE) The purpose of this study was to determine, in a clinical sample, whether the STOMP educational intervention would improve parental risk knowledge and perceptions, ensure the safety of scenario-based opioid decisions, and improve parents’ analgesic self-efficacy and child outcomes.
Our primary aims were to determine whether, compared to a control group, parents who received the STOMP educational intervention would:
exhibit improved awareness and heightened risk perceptions of potentially serious opioid-related ADEs
-
gain improved analgesic self-efficacy toward managing their children’s pain and ADEs
Our secondary aims were to determine whether parents who received STOMP would
make safer scenario-based opioid use decisions
administer fewer opioid doses to their children after surgery, when controlled for important child and pain-related factors.
Methods
This randomized, controlled study addressed the second aims of our larger, longitudinal clinical trial (NCT03287622). We previously reported findings from our clinical trial aim that tested the effect of STOMP on parental left-over opioid disposal.(CITE) In contrast to our previous work, this paper addresses the effects of differing aspects of the STOMP educational intervention on parents’ perceived risks of opioid-related adverse effects and on the child’s pain outcomes.
Procedures:
With approval from the institutional review board (HUM00127009) and comprehensive written consent from parents (and assent from children) we recruited parent-child dyads from our tertiary care, dedicated pediatric hospital setting. We approached all families whose children, aged 5–17 years were scheduled to undergo ambulatory or short-stay surgery and expected to receive an opioid prescription to manage postoperative pain. We screened and excluded dyads when children were scheduled to undergo major surgery associated with a longer hospital stay (>48 hours), had a chronic pain condition (lasting > 3 months) or who were cognitively unable to reliably self-report pain (i.e., < 5 years of age or developmentally delayed); or when parents could not read or comprehend English. We also excluded those who did not receive an opioid prescription for home use or who had a surgical complication that kept them in the hospital for longer than expected.
Dyads were recruited prior to surgery either by phone or in-person. Parents who self-identified as the primary caregiver after surgery completed a baseline survey prior to surgery and before receiving routine discharge and medication instruction. After completing the baseline survey, parents were instructed on how to use a semi-structured diary to record all analgesic medications (prescribed or over-the-counter [OTC]) given to the child after discharge to home. Follow-up surveys were completed on days 1–3 (Time 1, early recovery), and days 7–14 (Time 2, late recovery) after discharge. Parents received follow-up emails or phone text messages with links to the electronic follow-up surveys and with reminders to complete diaries contemporaneously with analgesic administration. A final text message reminder was also sent to parents to return their diaries when children were no longer taking analgesics. Parent-child dyads received $50 for completing all surveys.
Parents were randomized based on an a priori computer-generated schema to either the control group (usual or routine instruction) or to routine instruction plus the STOMP educational intervention. Routine instruction included a standardized, computer-generated discharge instruction sheet that included prescription information. Common postoperative adverse effects were listed (e.g., your child may experience nausea, dizziness, sleepiness, pain). These were followed with instructions to call the clinic if unmanageable. This intervention was administered during one session via iPad immediately after completion of the baseline survey in the preoperative area. Technical assistance was provided by the study or healthcare personnel only if needed. The mean duration of time to complete the interactive scenarios with feedback was 7 minutes (range 4 to 17 mins).
STOMP Educational Intervention:
Our tailored messages are designed to provide scenario-specific opioid risk and benefit information meant to promote better decisions toward pain and ADE reduction [47]. The interactive educational program incorporates several descriptive, clinically relevant pain and risk scenarios wherein parents are asked to consider each scenario as if they were caring for their child after surgery. Following each hypothetical scenario description, parents make a decision to give the prescribed opioid analgesic versus other options (e.g., do nothing at this time, give an over-the-counter analgesic). Each decision prompts immediate but brief risk messages combined with advice about what to do to reduce risk and manage pain in that situation. Our messages were based on Fuzzy Trace Theory which supports that risk messages that emphasize gist (i.e., bottom-line or essential) meaning not only improve understanding but also promote risk avoidance behavior.18,19 Presenting risk messages together with actionable advice parsimoniously heightens risk perception while building a sense of behavioral control [25]. As such, combined approaches have been shown to have a synergistic effect on changing perception and behavior [36]. Furthermore, use of electronic platforms that incorporate interactive exercises promote active learning that can improve understanding, skill development and health behavior [2,12,31,33]. A simplified depiction of the intervention that includes example excerpts from STOMP messaging is shown in Figure 1.
Figure 1.
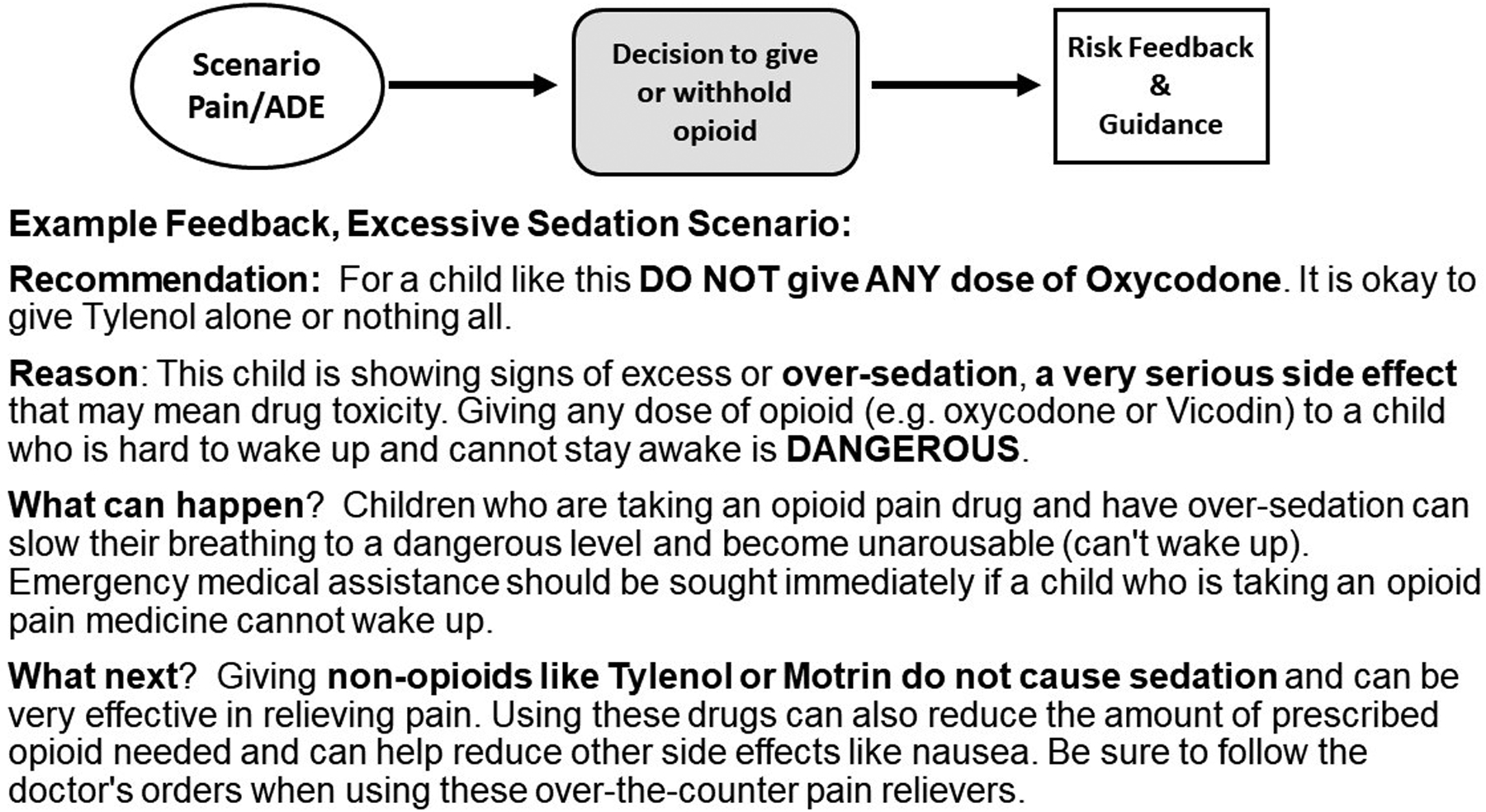
Main Measures (assessed at baseline, Times 1 and 2):
Outcomes for Primary Aims:
Aim 1, Parent ADE knowledge (i.e., awareness) and perceptions:
Parents’ awareness of common (e.g., nausea, constipation) and critical ADEs (i.e., excessive sedation, slowed breathing, addiction/habit) was assessed using the ADE knowledge instrument, where parents used nominal responses to identify whether the ADE was possible during or following prescribed opioid use [46,48]. Thus, awareness variables were dichotomous, Yes/No.
Perceived seriousness of these ADEs was assessed using a 6 point Likert scale where 0=Not serious to 5 = Extremely serious [46,48,49]. These items were developed with established content and face validity and have been found to have predictive validity toward scenario-based analgesic decision-making [46,48]. These variables were treated as continuous.
Aim 2, Parent analgesic self-efficacy:
This 7-item measure was derived from medication self-efficacy tools that have been shown to predict medication use and outcomes [35]. Self-efficacy is a context driven concept meant to measure the perceived confidence that one can successfully perform behaviors to achieve a desired health outcome [1,6,20,23]. The context of analgesic use differs from that of chronic (i.e., regularly scheduled) medication use, in that analgesics are prescribed as needed for pain management. We therefore modified self-efficacy items to reflect the desired outcomes of analgesic use, that is, effective pain management and minimization of their ADEs. Similar to previous tools, parents rated each item from 0 to 5, where 0= not at all sure and 5 = extremely sure; e.g., “I am sure that I can … keep my child safe while giving pain relievers”, “…manage my child’s pain effectively”, “…recognize and reduce the most important risks of pain relievers,”…”manage pain well enough so that my child can resume regular activity”. Scores on this instrument ranged from 0–35 (treated as continuous), where 35 reflects high confidence in administering analgesics to achieve the dual outcomes for the child.
Outcomes for Secondary Aims:
Aim 3, Scenario-based opioid decisions:
For this aim, we used parents’ decisional responses to the hypothetical scenarios from the STOMP as a proxy measure of their decisions under a variety of situations (parents in the control group completed decisions, but received no risk messaging feedback). The first scenario depicts the child with high pain (7 out of 10 on the faces pain scale - the commonest threshold parents use to treat pain) [8],[49] and no signs of ADEs since the last opioid dose. This exercise was used to measure parents’ willingness to give a prescribed opioid to the child with no evident risk (i.e., safe and effective use). Two additional scenarios depicted the child with the same degree of pain but also with signs of a potentially serious ADE (i.e., excessive sedation) or a common, but less serious ADE (i.e., nausea/vomiting). For each scenario, parents made the decision to give the prescribed dose of opioid, a lower dose, an OTC drug, or nothing. Opioid decisions were coded as binary, i.e., give an opioid vs. withhold the dose. Scenario-based decisions were assessed at baseline, Times 1 and 2.
Aim 4, Total Opioid Doses Administered:
Parent contemporaneous diary recordings and survey data were used to summarize the analgesics administered to children after discharge home. Our main outcome was the number of opioid doses given. We also report total oral morphine equivalents ([meq]/kg), the total number of OTC doses given, and the number of days opioid and OTC analgesics were used (all treated as continuous variables). These outcomes were tallied after analgesics were discontinued at Time 2.
Co-variate measures:
Parent pain relief preference (PR Pref):
This instrument assesses the parents’ desire to provide pain relief relative to their desire to minimize ADE risks for their children [49]. The instrument includes 6 risk–benefit items similar to those used to assess the importance patients place on chronic medication benefits versus their concern for adverse effects [5,19,42]. Agreement with each statement is ranked on a 5 point Likert scale from strongly disagree (−2) to strongly agree (+2), to yield a total score ranging from −12 to +12 (treated as continuous data). Lower numbers indicate a preference for risk avoidance, higher numbers pain relief, and the middle range, ambivalence [5,42]. We previously demonstrated the instrument’s internal consistency, construct and predictive validity [45,49].
Subject characteristics:
Parent and child demographics and their history of pain and analgesic use were documented on their baseline surveys.
Parent health literacy:
The Health Literacy BRIEF was used to assess parent’s self-assessed ability to understand medical advice or instruction at baseline [3]. The four-item instrument assesses the degree to which the individual needs assistance with reading, understanding, interpreting medical information. The measure yields scores from 0 to 16 (treated as continuous) which we reversed so that higher scores indicate higher literacy.
Child postoperative pain:
The PROMIS pain interference 8-item short-form (parent version) was used to capture the degree to which postoperative pain interfered with the child’s daily functioning at their final survey (Time 2) [44]. Parents also documented the child’s self-reported 0–10 pain intensity ratings using the revised faces scale [18] at least once per day prior to administering prescribed opioids or OTC analgesics. Average pain scores were calculated from the early and later recovery periods (days 0–3, 4–7 and 8–14).
Surgical covariates:
Surgical service, perioperative duration (induction of anesthesia to discharge from the postoperative recovery unit) and opioid usage (meq/kg) were recorded from the electronic medical record. Prescription details were also documented from the records.
Analgesic-related adverse events:
Parents were asked to record all ADEs, including nausea/vomiting (rated as none-mild or needing treatment), constipation (rated as none-mild or requiring laxative), and excessive sedation (ranked as none-mild or moderate-severe). We also report any opioid-related ADE that prompted a call or non-scheduled return visit to the clinic or emergency room, as documented on the medical record.
Statistical analyses
All data were analyzed with SPSS (v. 24). Frequency data are presented as n (%), and descriptive data, as mean ± standard deviations. Missing data were not imputed and were sparse due to survey prompts requesting completion. Percentages shown are calculated from complete responses for each variable.
Primary Outcomes Analyses:
Chi-square tests were conducted to examine group differences in ADE awareness (Aim 1). Odds ratios (OR) with 95% confidence intervals [CI] are presented. Repeated measures analysis of variance (ANOVA) with post-hoc tests were used to examine group differences in perceived seriousness of opioid-related risks and in analgesic efficacy over time (Aims 1 & 2). Bonferroni’s corrections were applied.
Secondary Outcomes Analyses:
Mixed effects logistic regression models were used to test our hypotheses that STOMP would affect scenario-based opioid use decisions over time (Aim 3). Each decision model (i.e., no ADE, excess sedation, and nausea/vomiting) was adjusted for the random effect of subject, repeated measures over time, and covariates. Adjusted odds ratios (adj.OR) with [95% CI] are shown for all variables included in the models.
We compared postoperative pain and adverse event data in univariate analyses, and then used a generalized linear regression model to examine the effect of STOMP, parent and child factors on the total number of opioid doses administered at home (Aim 4). We present data with adj.OR or mean difference (MD) with (95% CI) as appropriate. Estimated coefficients are adjusted for the effects of all covariates.
Power analysis
We based our sample size on the most conservative estimate needed to detect a small effect of the STOMP educational intervention on parents’ opioid administration. To obtain a small effect (Cohen’s d=.26) of our intervention on opioid use in a model with up to 15 covariates (α = 0.05; β = 0.20), we needed a minimum of 233 participants per group. This sample size was deemed more than sufficient, in a factorial design, to test for the expected larger effects (Cohen’s d=.5) of our interventions on parents’ decisions to safely withhold (i.e., sample needed = 147). We over-recruited in order to account for loss-to-follow-up.
Results
Six hundred fifty-five parent-child dyads met inclusion criteria for this study; 317 were randomized to STOMP, 338 to Controls. Of these, 21 (6.6%) and 30 (8.9%) received no opioid prescription after surgery and were therefore, excluded. This left 604 parents who completed the baseline and at least one follow-up survey. Ninety-three percent (n=563) of the sample reported follow-up pain outcome data (either by survey or diary). See Figure 2 Consort Diagram for Details of the analytic sample.
Figure 2.
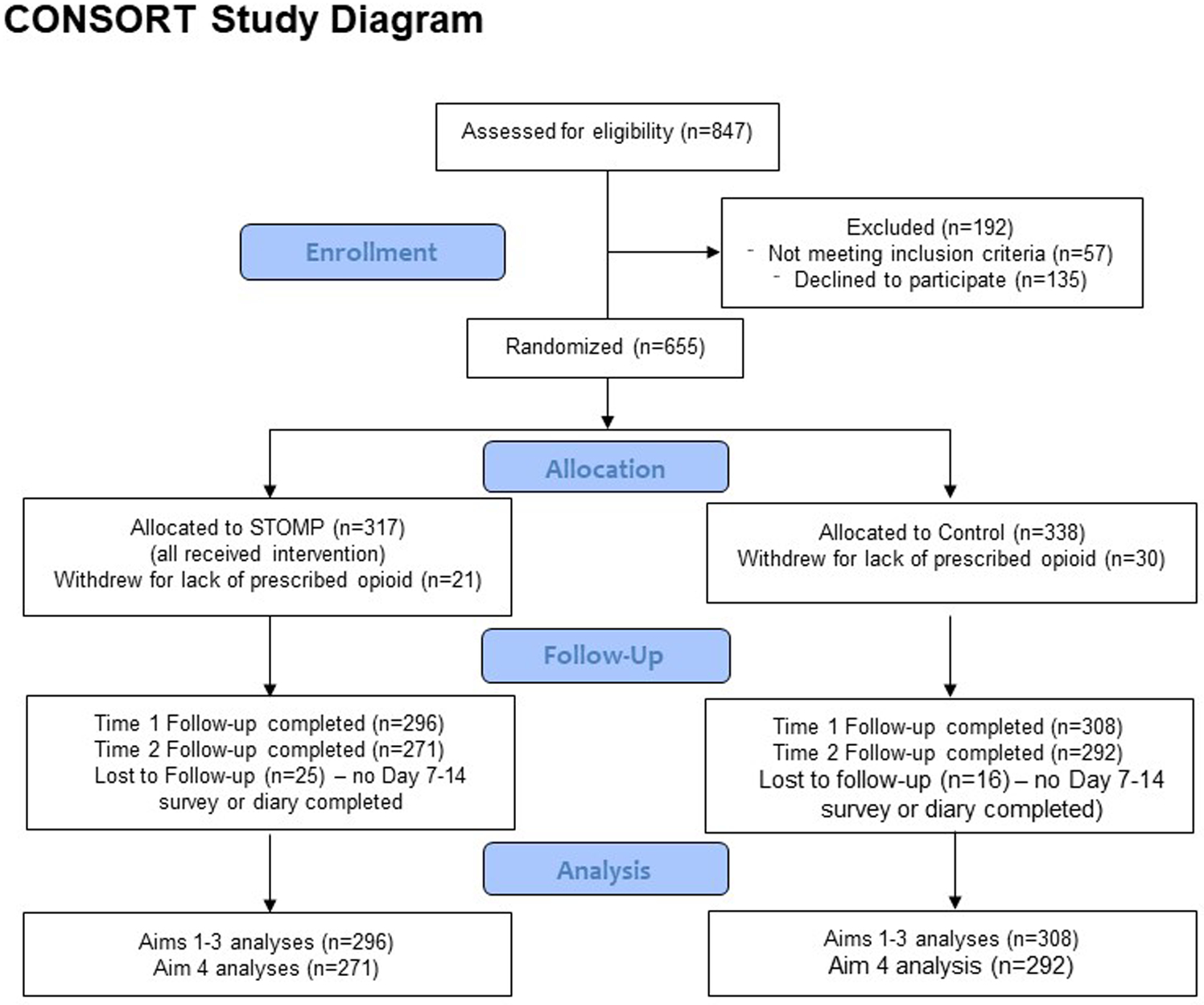
Characteristics of the Sample:
Table 1 presents the baseline characteristics of the groups. As shown, parents and children in the groups were similar in characteristics except that parents in the STOMP group were more likely to have taken an opioid previously. STOMP parents were also slightly more pain relief preferent only at baseline (i.e., higher PR Pref scores; see Table 2, row 2, column 2). Repeated measures ANOVA showed a significant effect of time and group*time on PR Pref scores, with post hoc tests showing how parents in both groups became more risk averse at each follow-up assessment (i.e., decreasing PR Pref Scores; F=74.15; df2; p<.001; Table 2).
Table 1.
Characteristics of the Groups
| Control (n=308) | STOMP (n=296) | OR or MD [95% CI] | |
|---|---|---|---|
| Female Sex | 241 (78.2%) | 250 (84.5) | 1.51 [1.00, 2.29] |
| Other | 11 (3.5) | 4 (1.3) | |
| Bachelor degree or higher | 164 (53.1) | 139 (46.9) | |
| Health Literacy | 8.03 ± 1.26 | 8.09 ± 1.16 | 0.06 [−0.14, 0.25] |
| Parent past pain condition | 290 (94.2) | 275 (92.9) | 0.81 [0.42, 1.56] |
| Parent past opioid use | 137 (44.5) | 160 (54.1) | 1.47 [1.07, 2.02] |
| Parent past non-pharm use | 280 (90.9) | 260 (87.8) | 0.72 [0.43, 1.22] |
| Child female | 143 (46.4) | 115 (38.9) | 0.73 [0.53, 1.01] |
| Child age | 12.81 ± 3.68 | 13.19 ± 3.59 | −0.38 [−0.96, 0.20] |
| ASA status 1–2 | 296 (96.1) | 285 (96.3) | 1.05 [0.46, 2.42] |
| Child previous surgery | 187 (60.7) | 188 (63.5) | 1.13 [0.81, 1.57] |
| Child past opioid use | 119 (38.6) | 113 (38.2) | 0.98 [0.71, 1.36] |
| Child past OTC pain treatment | 249 (80.8) | 225 (76) | 0.75 [0.51, 1.11] |
| Child co-morbidity | 176 (49.4) | 184 (52.3) | 1.12 [0.83, 1.50] |
| Respiratory (including OSA) | 100 (32.5) | 103 (34.8) | 1.11 [0.79, 1.56] |
| Depression/mood/anxiety | 25 (8.1) | 24 (8.1) | 0.99 [0.56, 1.79] |
| General surgery & other | 34 (11) | 49 (13.8) | |
| Perioperative duration (mins) | 288.2 ± 100.8 | 292.7 ± 95.3 | 4.55 [−11.12, 20.23] |
| Perioperative meq/kg | 0.20 ± 0.12 | 0.19 ± 0.11 | −0.01 [−0.03, 0.01] |
Data presented as n (%) or mean ± standard deviation. Univariate comparisons made with Chi-square or unpaired t-tests as appropriate; odds ratios (OR) or mean differences (MD) and 95% confidence intervals [CI], where appropriate. OTC = over-the-counter; OSA=Obstructive sleep apnea; meq= morphine equivalents. Bolding indicates significant difference.
Table 2.
Parent Pain Relief Preferences, Analgesic Efficacy and Perceived Seriousness of Opioid Adverse Effects over Time
| Control (C) | Stomp | Control (C) | Stomp | Control (C) | Stomp | |
|---|---|---|---|---|---|---|
| Pain Relief Preference Scores (range −12 to +12) | −1.19 (3.11) | −0.48 (3.25) | −1.73 (3.51) | −1.78 (3.64) | −2.51 (3.59) | −2.67 (3.98) |
| Perceived seriousness of opioid-related adverse drug effects (each measured from 0–5) | ||||||
| Excessive-sedation | 3.38 (1.35) | 3.22 (1.35) | 3.46 (1.33) | 3.70 (1.21) | 3.63 (1.32) | 3.79 (1.22) |
| Slowed breathing | 4.21 (1.21) | 4.09 (1.26) | 4.33 (1.04) | 4.48 (0.90) | 4.45 (0.97) | 4.56 (0.87) |
| Addiction/Habit | 4.69 (0.88) | 4.65 (1.07) | 4.72 (0.83) | 4.86 (0.48) | 4.70 (0.85) | 4.80 (0.41) |
| Nausea/vomiting | 2.63 (1.33) | 2.41 (1.37) | 2.77 (1.39) | 2.77 (1.22) | 2.88 (1.44) | 2.97 (1.39) |
| Constipation | 2.91 (1.28) | 2.89 (1.28) | 3.07 (1.23) | 3.03 (1.15) | 3.12 (1.28) |
3.15 (1.23) p<.034 v B |
| Analgesic self-efficacy (potential range in score from 0 to 35) | ||||||
| Analgesic efficacy | 30.07 (4.39) | 30.10 (4.54) | 30.9 (3.66) | 31.2 (3.68) | 32.2 (3.25) | 32.5 (3.22) |
Data presented as mean (SD). Comparisons were made with repeated measures analyses of variance and post-hoc tests.
Bonferroni’s corrections were applied and bolded scores are significantly different compared to either Baseline (B) or Control Group (C) Scores.
Aim 1. Effect of STOMP on ADE Awareness and Perceptions:
A majority of parents in both groups were aware at baseline of the common and more serious ADEs associated with prescribed opioids (range 72% to 95%). Fewer were aware of the potential for slowed breathing (72.4% of Controls and 72.6% STOMP) while most were aware of the possibility of addiction (93.2% Controls & 95.3% STOMP). While parents in both groups became more aware of these at the follow-up assessments, by Time 1, slightly more STOMP parents knew about the possibility of the critical ADEs including excessive-sedation (97% vs. 94%; OR 2.97 [95% CI 1.16, 7.59] and slowed breathing (89.2% vs. 82.5%; OR 1.75 [95% CI 1.10, 2.81]).
Table 2 depicts parental perceptions of ADE seriousness over time. STOMP parents rated the seriousness of nausea/vomiting higher, but were otherwise similar at baseline. Repeated measures ANOVA demonstrated significant effects of time, and time*group on risk perceptions; Post hoc comparisons showed that parents in the STOMP group (but not controls) gained an increased perceived seriousness of the ADEs excessive-sedation, slowed breathing, addiction, and nausea vomiting, but not constipation. Between group differences were established only for the perceived risk of addiction, which was slightly higher for STOMP parents.
Aim 2. Effect of STOMP on parental analgesic self-efficacy:
As shown in the final row of Table 2, parents in both groups indicated a similar and fairly high degree of analgesic efficacy at baseline. On average, parents in the STOMP group exhibited marginally higher efficacy scores at the follow-up assessments (MD 0.58 [95% CI 0.08, 1.09], p=.023). However, there was a significant effect of time and time*group on efficacy for both groups; with post hoc tests showing how parents became more confident in their use of analgesics at every follow-up assessment (F=84.02; df3; p<0.001).
Aim 3. Effect of STOMP on Parents’ Scenario-Based Opioid Decisions:
Decisions to give the prescribed opioid differed across the hypothetical scenarios and between groups at baseline and at the follow-up assessments. A small majority of both groups were willing to give an opioid for the no ADE scenario at baseline (63.9% STOMP vs. 60.4% Control; OR 1.16 [0.83, 1.61], p=.381). This willingness lessened by Time 2, with no difference between groups (56.3% STOMP vs. 49.1% Control, OR 1.34 [0.95, 1.87], p=.093). STOMP parents were more willing to give the opioid for the excessive sedation scenario at baseline (40.2% vs. 31.8%; OR 1.44 [1.03, 2.01, p=0.032], but became 37% and 53% less likely to do so at the follow-up assessments (Time 1: 14.3% vs. 20.9%; OR 0.63 [0.41, 0.98], p=.040; Time 2: 9.6% vs. 18.5%; OR 0.47 [0.28, 0.78], p=.003). Interestingly, STOMP parents were less likely to give the opioid for the nausea and vomiting scenario at baseline (17.2% vs. 35.4%; OR 0.38 [0.26, 0.57], p<.001), but were similarly likely to do so at follow-up (Time 1: 22.4% vs. 26%; OR 0.82 [0.55, 1.20], p=.307; Time 2: 14.9% vs. 19.6%; OR 0.72 [0.46, 1.13], p=.155).
Mixed effect logistic regression models supported our hypothesis that STOMP had a significant effect on parents’ follow-up decisions to give an opioid for the excessive-sedation scenario, but no effect on giving an opioid for the other scenario (See Table 3). Controlled for mean health literacy, PR Pref, perceived seriousness and efficacy scores and other factors shown, STOMP parents were 7% less likely to give an opioid for the scenario, excessive sedation (estimated marginal mean 14% of STOMP parents vs. 21% Controls; adj.β −0.07 [95% CI −0.12, −0.02], p=.006.
Table 3.
Impact of STOMP on Parents’ Scenario-Based (Hypothetical) Decisions to Administer the Prescribed Opioid
| Factor | Decision No Adverse Event | Decision Excess-sedation | Decision Nausea Vomiting |
|---|---|---|---|
| Female sex (v. male) | 0.78 [0.56, 1.08], .133 | 0.47 [0.32, 0.69], <.001 | 0.63 [0.44, 0.92], .017 |
| White race (v. minority) | 1.36 [0.96, 1.94], .087 | 0.96 [0.60, 1.54], .860 | 1.83 [1.11, 3.02], .019 |
| Health Literacy Score | 1.13 [1.02, 1.26], .020 | 1.06 [0.92, 1.22], .444 | 0.98 [0.86, 1.11], .756 |
| Child female | 1.18 [0.92, 1.52], .200 | 1.16 [0.82, 1.63], .409 | 1.11 [0.81, 1.51], .514 |
| Child past opioid use | 0.80 [0.62, 1.04], .092 | 1.16 [0.82, 1.65], .408 | 0.94 [0.68, 1.30], .705 |
| Parent past opioid use | 1.26 [0.98, 1.62], .076 | 0.81 [0.57, 1.14], .220 | 0.85 [0.62, 1.16], .297 |
| Pain Relief Preference Score | 1.05 [1.02, 1.09], .005 | 1.10 [1.05, 1.16], <.001 | 1.13 [1.08, 1.19], <.001 |
| Analgesic Efficacy Score | 0.95 [0.92, 0.99], .010 | 0.95 [0.91, 0.99], .018 | 0.93 [0.89, 0.97], <.001 |
| Perceived serious addiction | 1.04 [0.87, 1.25], .631 | 1.13 [0.90, 1.41], .288 | 1.02 [0.83, 1.25], .847 |
| Perceived serious sedation | 1.07 [0.95, 1.20], .295 | 0.73 [0.62, 0.86], <.001 | 1.19 [1.04, 1.38], .015 |
| Perceived serious nausea | 0.94 [0.84, 1.05], .263 | 1.15 [0.98, 1.34], .079 | 0.74 [0.65, 0.85], <.001 |
| STOMP Group | 1.13 [0.88, 1.45], .349 | 0.73 [0.62, 0.86], <.001 | 0.77 [0.57, 1.05], .100 |
Data reflect results from our mixed effect logistic regression models – one for each of the situational decisions.
All models accounted for the random effect of subject and the repeated measures of risk perceptions.
Data for all covariates in the model are shown and are presented as Adjusted Odds Ratio [95% Confidence Interval], p value.
We have bolded significant comparisons for ease of interpretation.
Aim 4. Effect of STOMP on Opioid Usage after Surgery:
Table 4 depicts the analgesic and pain outcomes in the groups, showing no significant differences between STOMP and Control groups by univariate analyses. Linear regression demonstrated that the number of opioid doses administered was not influenced by the STOMP educational intervention. Rather, parent factors (including PR Pref and Efficacy) predicted 7% of the variance and child and surgical factors predicted an additional 20% of the variance in the number of doses administered (Table 5).
Table 4.
Child Pain and Analgesic Outcomes in the Groups (tallied from daily diaries and surveys)
| Control n=292 | STOMP n=271 | Odds Ratio or Mean Difference [95% CI] | |
|---|---|---|---|
| Opioid given at home (yes vs. no) | 240 (81.1%) | 216 (79.1%) | 0.88 [0.59, 1.34] |
| Opioid doses dispensed | 21.5 ± 13.76 | 22.0 ± 16.48 | 0.50 [−1.95, 2.96] |
| Opioid doses given at home (total) | 7.9 ± 8.6 | 8.6 ± 10.3 | 0.71 [−0.87, 2.28 |
| Opioid meq/kg given | 0.20 ± 0.23 | 0.21 ± 0.26 | 0.01, [−0.33, 0.05] |
| Days opioid given | 3.31 ± 3.2 | 3.80 ± 4.2 | 0.49 [−0.13, 1.12] |
| OTC doses given | 20.2 ± 16.0 | 19.7 ± 15.3 | −0.45 [−3.06, 2.16] |
| Days OTC given | 7.6 ± 4.6 | 7.8 ± 5.2 | 0.18 [−0.63, 1.00] |
| Days 8 to14 | 3.2 ± 1.9 | 3.5 ± 2.3 | 0.30 [−0.23, 0.83] |
| Parent-reported Pain Interference Score Day 14 | 8.06 ± 8.06 | 8.63 ± 8.39 | 0.57 [−0.68, 1.82] |
| Adverse events (yes vs. no) | |||
| Nausea (requiring intervention) | 34 (14.2%) | 23 (9.9%) | 0.66 [0.38, 1.17] |
| Constipation (requiring intervention) | 105 (46.3%) | 106 (47.5%) | 1.05 [0.73, 1.52] |
| Moderate to severe sedation | 45 (19.1%) | 37 (15.9%) | 0.80 [0.49, 1.29] |
| Serious adverse events* | 10 (3.4%) | 9 (3.3%) | 0.93 [0.37, 2.33] |
Data shown as n (%) or mean ± standard deviation. Univariate comparisons were made using Chi-square or unpaired t-tests. Unadjusted odds ratios are shown.
Meq/kg=Morphine equivalents per kilogram; OTC=Over-the-counter;
serious adverse events include those that led to a call or unplanned return visit to the clinic or hospital setting as determined by medical record review. Other adverse effects were reported by parents on their daily diaries; percentages were calculated from those who reported events.
Table 5.
Effect of STOMP on Total Opioid Doses Administered at Home
| Factors | Step 1-Parent Factors R2= .067 (df7), p<.001 | Step 2-Child Factors R2=.329 (r2 change=.20; df9); p<.001 |
|---|---|---|
| STOMP (v. controls) | 0.88 [−0.91, 2.67], .333 | 0.79 [−0.75, 2.33], .313 |
| Parent female (v. male) | 1.47 [−0.89, 3.82], .221 | 0.62 [−1.41, 2.65], .549 |
| Parent race (White v. minority) | −1.85 [−4.61, 0.91], .189 | 0.12 [−2.26, 2.50], .921 |
| Health literacy | 0.43 [−0.33, 1.18], .270 | 0.03 [−0.62, 0.68], .937 |
| Parent past opioid use (v. none) | 0.29 [−1.52, 2.09], .756 | 0.95 [−0.62, 2.52], .234 |
| Parent Pain Relief Preference | 0.68 [0.42, 0.94], <.001 | 0.49 [0.26, 0.71], <.001 |
| Parent Analgesic Efficacy | −0.37 [−0.67, −0.08], .013 | −0.40 [−0.65, −0.15], .002 |
| Child female (v. male) | 1.18 [−0.43, 2.79], .149 | |
| Child age | 0.28 [0.05, 0.50], .017 | |
| Child past opioid use (v. none) | −0.57 [−2.22, 1.07], .494 | |
| Child depression or mood disorder (v. none) | 0.98 [−1.93, 3.90], .508 | |
| Orthopedic/Sports Medicine (v. other services) | 3.24 [1.64, 4.84], <.001 | |
| Perioperative minutes | 0.01 [0.002, 0.02], .020 | |
| Opioid doses dispensed | 0.14 [0.09, 0.19], <.001 | |
| Average pain intensity (child self-reported) | 1.45 [1.04, 1.86], <.001 | |
| Post-discharge ADE (v. none) | 0.12 [−4.42, 4.66], 0.959 |
Results derived from linear regression. Data presented as adjusted β [95% confidence interval], p value; ADE=Adverse drug event (y/n). Bolded items are those that are significant.
Discussion
Findings from our study demonstrated that providing scenario-tailored opioid risk information effectively enhanced parents’ ADE knowledge, perceptions and their scenario-based opioid decision-making, but did not influence the total number of opioid doses they gave their children after surgery. Specifically, we showed that parents who received STOMP gained enhanced awareness and perceived seriousness of the critical opioid-related ADEs, excessive sedation and slowed breathing. They made safer scenario-based decisions to withhold opioids for the hypothetical child who exhibited excessive sedation, but were equally likely to give an opioid to the hypothetical child with no ADE or with nausea. STOMP parents showed a slightly higher gain in analgesic efficacy, even as parents in both groups became more risk averse and more confident. The total analgesic doses administered and pain outcomes for children were not influenced by the STOMP educational intervention. Instead, parents’ pain relief preferences (PR Pref) and efficacy contributed marginally, while child and surgical factors mattered most toward to the total number of opioid doses administered.
Knowledge and perceptions of ADEs are important determinants of adherent and safe medication use. Heightened perception of risks may lead to hesitancy or underutilization of medications, while low risk perceptions may lead to unsafe decisions that contribute to ADEs or potential harm. Parental decisions to give prescription opioids to their children may be particularly challenging, since most parents want to minimize harm for their children which can arise from pain or opioid-related ADEs. The risk messaging used in our educational intervention was intended to enhance parental recognition of situations when opioids could be safely administered, and when they should be withheld. Our findings suggest that the program effectively heightened parents’ perceived seriousness of critical ADEs and decreased their administration of opioids for the scenario depicting an excessively sedated child. Our finding that parents were similarly likely to give the opioid for other scenarios suggest that our tailored messages effectively targeted the high risk situation. Our finding here, is similar to our findings when we tested an abbreviated form of STOMP in a non-clinical sample of parents [47]. Tailoring risk messages through content-matched feedback has been previously shown to have a significant effect on changing health behaviors and lower risk [16,22,24]. Our feedback combined important ADE riskiness information with advice on how to manage pain while reducing the risk. Thus, our STOMP educational intervention may have yielded a synergistic effect on parental decision-making.
We further demonstrated how parents’ preference for analgesic benefit (i.e., PR Pref scores) strongly persuaded their scenario-based opioid decision-making as well as their overall opioid administration to their children. This finding is similar to previous studies showing how parental motivation to relieve their child’s pain and their worry about unrelieved pain are highly associated with the number of prescribed opioid analgesic doses they give their children [32,49]. Similar to our previous studies CITE, we found that baseline PR Pref scores at baseline largely reflected ambivalence about the importance of pain relief versus risk avoidance (mean scores −0.48 to −1.19 for STOMP and control parents, respectively). Parents in the STOMP group leaned significantly more pain-relief preferent at baseline. Yet, parents in both groups became more risk averse (i.e., lower PR Pref scores) over time. This shift toward analgesic risk aversion may have been influenced not only by patient education, but by the child’s decreasing pain (and, thus less need to relieve it) or the presence of and/or increased worry about opioid-related ADEs. These findings suggest that medication preferences can be shaped by information and/or experience.
Notably, parents’ analgesic efficacy, which was relatively high at baseline, increased significantly for both groups over time. However, STOMP was associated with a slightly improved parental confidence, overall, which may be, in part, explained by the advice component of the intervention (i.e., “how to manage”). Perceived efficacy has been described as being “malleable” by a variety of influences, including persuasion and experience (both personal and vicarious) [30]. Although self-efficacy has been shown to influence health behavior and self-management, lack of skill or motivation can limit the contribution of self-efficacy [30]. In our study we found that higher parental self-efficacy scores were associated with a lower likelihood of giving opioids across scenarios and with lower total opioid doses given after surgery. Our efficacy instrument assessed parents’ perceived confidence in managing pain while recognizing and minimizing ADEs. The negative association between efficacy and opioid administration suggests that higher parental confidence in managing pain and ADEs may contribute to a more nuanced approach to pain management with less opioid dosing. On the other hand, parental confidence may also have evolved as children’s pain lessened over time with less need for opioids.
Findings from this study are strengthened by the prospective, randomized design that included a large sample of parent-child dyads. We had minimal loss-to-follow-up and used two methods to examine the effect of STOMP on parental opioid decision-making. First, our use of hypothetical scenarios provided an efficient way to assess scenario-based decision-making over time (particularly for low prevalence situations such as excessive sedation). Such scenario-based decisions are critical toward the safe use of opioids. We and others have previously demonstrated that hypothetical decisions are associated with real healthcare decisions and behaviors [41,50]. As a secondary aim, we used parental opioid dose administration to evaluate the effect of STOMP and other decisional factors on the child’s actual outcomes. Our STOMP messaging emphasizes the importance of using OTC analgesics to enhance pain relief, reduce the amount of opioids needed and promote rapid and effective opioid weaning shortly after surgery. Our inability to demonstrate an effect of STOMP on the child’s total opioid dosing after surgery may have been, in part, limited by our inclusion criteria. We included only children who had ambulatory and short-stay surgical procedures – perhaps with little variability in opioid use. Thus, we may have been underpowered to test the effect on opioid use. Instead, we showed that surgery, the number of opioid doses prescribed, and child factors were the primary drivers for total opioid use in this sample. Even though we found no independent effect of STOMP on this outcome, there may have been an indirect effect through parental efficacy which was associated with less opioid use. Given recent policy changes that have led to decreases in opioid prescribing, parental efficacy in managing pain via non-opioid methods may be even more important toward effective pain relief. Additionally, the lack of STOMP effect on short-term opioid use does not diminish the impact of the intervention on parental knowledge and perceptions. Indeed, our finding suggests that heightening risk perception did not diminish parental ability to manage pain, but may enhance their response to serious but rare ADEs (not measured in real-time in this research). Importantly, we administered the STOMP education prior to surgery in a preparatory fashion. Testing STOMP administered via a smartphone App may be important to determine the impact on real-time opioid administration decisions. Finally, the ability to generalize our findings to other settings may also be limited given our sample and setting that largely represented well-educated, white parents from a state that has been heavily impacted by the opioid crisis.
In summary, we have demonstrated that scenario-tailored messages can effectively enhance several of the knowledge, perception and skill factors that are important for safe administration of prescription opioids by parents. Compared to routine clinic education, our scenario-tailored educational intervention was more effective in heightening risk perceptions and promoting safe scenario-based decisions. Such skills are critical for parents whose children and teens are prescribed opioids for pain management. Finally, educating parents may have an indirect effect on teens whose analgesic usage is largely influenced by parental practices [37,38]. Parental education is critically important given that many adolescents who admit to misusing opioids do so while still under the supervision of a parent.
Acknowledgments:
The authors are very grateful for the assistance of our research assistants, Monica Weber, Emily Currier, Trevor de Sebour, and Elizabeth Loescher.
Funding: This research was fully supported by the National Institute on Drug Addiction (NIDA) (Grant #R01DA044245).
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest related to this manuscript.
References
- [1].Bandura A Guide for constructing self-efficacy scales In: Pajares F, Urdan T, editors. Self-efficacy beliefs of adolescents. Charlotte: Information Age Publishing, 2006. pp. 307–337. [Google Scholar]
- [2].Cairncross S, Mannion M. Interactive multimedia and learning: Realizing the benefits. Innovations in Education and Teaching International 2001;38(2):156–164. [Google Scholar]
- [3].Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23(5):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chung CP, Callahan ST, Cooper WO, Dupont WD, Murray KT, Franklin AD, Hall K, Dudley JA, Stein CM, Ray WA. Outpatient Opioid Prescriptions for Children and Opioid-Related Adverse Events. Pediatrics 2018;142(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. J Psychosom Res 2008;64(1):41–46. [DOI] [PubMed] [Google Scholar]
- [6].Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, Ariagno JE, Price N, Schwend R. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine 2014;39(3):E174–181. [DOI] [PubMed] [Google Scholar]
- [7].Coté CJ, Posner KL, Domino KB. Death or Neurologic Injury after Tonsillectomy in Children with a Focus on Obstructive Sleep Apnea: Houston, We Have a Problem! Anesth Analg 2014;118(6):1276–1283. [DOI] [PubMed] [Google Scholar]
- [8].Demyttenaere S, Finley GA, Johnston CC, McGrath PJ. Pain treatment thresholds in children after major surgery. Clinical J Pain 2001;17(2):173–177. [DOI] [PubMed] [Google Scholar]
- [9].Fortuna RJ, Robbins BW, Caiola E, Joynt M, Halterman JS. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics 2010;126(6):1108–1116. [DOI] [PubMed] [Google Scholar]
- [10].Fotiou A, Kanavou E, Richardson CR, Ploumpidis D, Kokkevi A. Misuse of prescription opioid analgesics among adolescents in Greece: The importance of peer use and past prescriptions. Drugs: Education, Prevention and Policy 2014;21(5):357–369. [Google Scholar]
- [11].Garcia MC, Heilig CM, Lee SH, Faul M, Guy G, Iademarco MF, Hempstead K, Raymond D, Gray J. Opioid Prescribing Rates in Nonmetropolitan and Metropolitan Counties Among Primary Care Providers Using an Electronic Health Record System - United States, 2014–2017. MMWR Morb Mortal Wkly Rep 2019;68(2):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Granger BB, Locke SC, Bowers M, Sawyer T, Shang H, Abernethy AP, Bloomfield RA Jr., Gilliss CL. The Digital Drag and Drop Pillbox: Design and Feasibility of a Skill-based Education Model to Improve Medication Management. J Cardiovasc Nurs 2017;32(5):E14–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Groenewald CB. Opioid-prescribing Patterns for Pediatric Patients in the United States. Clin J Pain 2019;35(6):515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Groenewald CB, Rabbitts JA, Gebert JT, Palermo TM. Trends in opioid prescriptions among children and adolescents in the United States: a nationally representative study from 1996 to 2012. Pain 2016;157(5):1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamers JP, Abu-Saad HH. Children’s pain at home following (adeno) tonsillectomy. Eur J Pain 2002;6(3):213–219. [DOI] [PubMed] [Google Scholar]
- [16].Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res 2008;23(3):454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hegarty M, Calder A, Davies K, Shave M, Christiansen E, Meyer T, von Ungern-Sternberg BS. Does take-home analgesia improve postoperative pain after elective day case surgery? A comparison of hospital vs parent-supplied analgesia. Paediatr Anaesth 2012;23:385–389. [DOI] [PubMed] [Google Scholar]
- [18].Hockenberrry MJ, Wilson D editors|. Wong’s essentials of Pediatric Nursing, 7th Edition, St. Louis MO: Mosby. [Google Scholar]
- [19].Johnson FR, Hauber AB. Quantifying patient benefit-risk tradeoff preferences: A brief introduction. Research Triangle Park: RTI Health Solutions, 2008.
- [20].Kalichman SC, Cain D, Fuhrel A, Eaton L, Di Fonzo K, Ertl T. Assessing medication adherence self-efficacy among low-literacy patients: development of a pictographic visual analogue scale. Health Educ Res 2005;20(1):24–35. [DOI] [PubMed] [Google Scholar]
- [21].Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, Carleton B, Hayden MR, Madadi P, Koren G. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012;129(5):e1343–1347. [DOI] [PubMed] [Google Scholar]
- [22].Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med 2010;51(3–4):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lamarche L, Tejpal A, Mangin D. Self-efficacy for medication management: a systematic review of instruments. Patient Prefer Adherence 2018;12:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lustria ML, Noar SM, Cortese J, Van Stee SK, Glueckauf RL, Lee J. A meta-analysis of web-delivered tailored health behavior change interventions. J health comm 2013;18(9):1039–1069. [DOI] [PubMed] [Google Scholar]
- [25].Luszczynska A, Tryburcy M, Schwarzer R. Improving fruit and vegetable consumption: a self-efficacy intervention compared with a combined self-efficacy and planning intervention. Health Educ Res 2007;22(5):630–638. [DOI] [PubMed] [Google Scholar]
- [26].Madadi P, Hildebrandt D, Gong IY, Schwarz UI, Ciszkowski C, Ross CJ, Sistonen J, Carleton BC, Hayden MR, Lauwers AE, Koren G. Fatal hydrocodone overdose in a child: pharmacogenetics and drug interactions. Pediatrics 2010;126(4):e986–989. [DOI] [PubMed] [Google Scholar]
- [27].McCabe SE, West BT, Cranford JA, Ross-Durow P, Young A, Teter CJ, Boyd CJ. Medical misuse of controlled medications among adolescents. Arch Pediatr Adolesc Med 2011;165(8):729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McCabe SE, West BT, Veliz P, McCabe VV, Stoddard SA, Boyd CJ. Trends in Medical and Nonmedical Use of Prescription Opioids Among US Adolescents: 1976–2015. Pediatrics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015;136(5):e1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nafradi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One 2017;12(10):e0186458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Price EL, Mackenzie TD, Metlay JP, Camargo CA Jr., Gonzales R. A computerized education module improves patient knowledge and attitudes about appropriate antibiotic use for acute respiratory tract infections. Patient Educ Couns 2011;85(3):493–498. [DOI] [PubMed] [Google Scholar]
- [32].Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and Health-Related Quality of Life After Pediatric Inpatient Surgery. J Pain 2015;16(12):1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riiser K, Londal K, Ommundsen Y, Smastuen MC, Misvaer N, Helseth S. The outcomes of a 12-week Internet intervention aimed at improving fitness and health-related quality of life in overweight adolescents: the Young & Active controlled trial. PLoS One 2014;9(12):e114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rony RY, Fortier MA, Chorney JM, Perret D, Kain ZN. Parental postoperative pain management: attitudes, assessment, and management. Pediatrics 2010;125(6):e1372–1378. [DOI] [PubMed] [Google Scholar]
- [35].Schwarzer R editor. Self-efficacy: thought control of action. Routledge, Taylor & Francis Group: New York, 1992. [Google Scholar]
- [36].Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull 2014;140(2):511–543. [DOI] [PubMed] [Google Scholar]
- [37].Shehnaz SI, Khan N, Sreedharan J, Arifulla M. Drug knowledge of expatriate adolescents in the United Arab Emirates and their attitudes towards self-medication. Int J Adolesc Med Health 2014;26(3):423–431. [DOI] [PubMed] [Google Scholar]
- [38].Skarstein S, Lagerlov P, Kvarme LG, Helseth S. High use of over-the-counter analgesic; possible warnings of reduced quality of life in adolescents - a qualitative study. BMC Nurs 2016;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sutters KA, Miaskowski C, Holdridge-Zeuner D, Waite S, Paul SM, Savedra MC, Lanier B. A randomized clinical trial of the effectiveness of a scheduled oral analgesic dosing regimen for the management of postoperative pain in children following tonsillectomy. Pain 2004;110(1–2):49–55. [DOI] [PubMed] [Google Scholar]
- [40].Sutters KA, Miaskowski C, Holdridge-Zeuner D, Waite S, Paul SM, Savedra MC, Lanier B, Mahoney K. A randomized clinical trial of the efficacy of scheduled dosing of acetaminophen and hydrocodone for the management of postoperative pain in children after tonsillectomy. Clin J Pain 2010;26(2):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tait AR, Zikmund-Fisher BJ, Fagerlin A, Voepel-Lewis T. Effect of various risk/benefit trade-offs on parents’ understanding of a pediatric research study. Pediatrics 2010;125(6):e1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tibaldi G, Clatworthy J, Torchio E, Argentero P, Munizza C, Horne R. The utility of the Necessity--Concerns Framework in explaining treatment non-adherence in four chronic illness groups in Italy. Chronic Illn 2009;5(2):129–133. [DOI] [PubMed] [Google Scholar]
- [43].Unsworth V, Franck LS, Choonara I. Parental assessment and management of children’s postoperative pain: a randomized clinical trial. J Child Health Care 2007;11(3):186–194. [DOI] [PubMed] [Google Scholar]
- [44].Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, Yeatts K, Dewalt DA. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain 2010;11(11):1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Voepel-Lewis T, Boyd CJ, McCabe SE, Zikmund-Fisher BJ, Malviya S, Grant J, Weber M, Tait AR. Deliberative Prescription Opioid Misuse Among Adolescents andEmerging Adults: Opportunities for Targeted Interventions. J Adolesc Health 2018;63(5):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Voepel-Lewis T, Zikmund-Fisher B, Smith EL, Zyzanski S, Tait AR. Opioid-related adverse drug events: do parents recognize the signals? Clin J Pain 2015;31(3):198–205. [DOI] [PubMed] [Google Scholar]
- [47].Voepel-Lewis T, Zikmund-Fisher BJ, Boyd CJ, Veliz PT, McCabe SE, Weber MJ, Tait AR. Effect of a Scenario-tailored Opioid Messaging Program on Parents’ Risk Perceptions and Opioid Decision-making. Clin J Pain 2018;34(6):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, Redman RW, Zyzanski S, Tait AR. Parents’ Analgesic Trade-Off Dilemmas: How Analgesic Knowledge Influences Their Decisions to Give Opioids. Clin J Pain 2016;32(3):187–195. [DOI] [PubMed] [Google Scholar]
- [49].Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, Zyzanski S, Tait AR. Parents’ preferences strongly influence their decisions to withhold prescribed opioids when faced with analgesic trade-off dilemmas for children: a prospective observational study. Int J Nurs Stud 2015;52(8):1343–1353. [DOI] [PubMed] [Google Scholar]
- [50].Waters EA, Weinstein ND, Colditz GA, Emmons K. Formats for improving risk communication in medical tradeoff decisions. Journal of health communication 2006;11(2):167–182. [DOI] [PubMed] [Google Scholar]


