Abstract
Objectives
To assess second-line antiretroviral therapy (ART) virologic failure and HIV drug resistance-associated mutations (RAMs), in support of third-line regimen planning in Asia.
Methods
Adults >18 years on second-line ART for ≥6 months were eligible. Cross-sectional data on HIV viral load (VL) and genotypic resistance testing were collected or testing was conducted between July 2015 and May 2017 at 12 Asia-Pacific sites. Virologic failure (VF) was defined as VL>1000 copies/mL with a second VL >1000 within 3–6 months. FASTA files were submitted to Stanford HIVdb and RAMs compared to the IAS-USA 2019 mutations list. VF risk factors were analyzed using logistic regression.
Results
Of 1378 patients, 74% were male and 70% acquired HIV through heterosexual exposure. At second-line switch, median age was 37 years (IQR 32–42) and median CD4 count was 103 cells/μL (IQR 43.5–229.5); 93% received regimens with boosted protease inhibitors (PI). Median duration on second-line was 3 years. Amongst 101 patients (7%) with VF, CD4 >200 cells/μL at switch (OR=0.36, 95%CI 0.17–0.77 vs. CD4 ≤50), and HIV exposure through male-male sex (OR=0.32, 95%CI 0.17–0.64 vs. heterosexual) or injecting drug use (OR=0.24, 95%CI 0.12–0.49) were associated with reduced VF. Of 41 (41%) patients with resistance data, 80% had at least one RAM to non-nucleoside reverse transcriptase inhibitors (NNRTI), 63% to NRTIs, and 35% to PIs. Of those with PI RAMs, 71% had ≥2.
Conclusions
There were low proportions with VF and significant RAMs in our cohort, reflecting the durability of current second-line regimens.
Keywords: Second-line antiretroviral therapy, virologic failure, drug resistance, HIV, Asia
Introduction
Efforts to expand antiretroviral therapy (ART) coverage for all people living with HIV (PLHIV) have resulted in an estimated 23.3 million PLHIV on ART globally by the end of 2018, of whom 3.2 million are in the Asia-Pacific region (1). With increasing numbers of PLHIV on ART and longer durations of therapy, first-line treatment failures have become more common, and increasing numbers of PLHIV have initiated second-line ART. Whilst earlier estimations of the proportion of patients on second-line ART in resource-limited settings were between 1–5% (2), in a more recent study of nearly 17,000 HIV-positive adults on ART in seven countries in Asia, 19% were on a second-line regimen (3).
Until recently WHO HIV treatment guidelines recommended a second-line treatment regimen of two nucleoside reverse transcriptase inhibitors (NRTIs) and a boosted protease inhibitor (PI) as part of public health approach (4). Current WHO guidelines recommend two NRTIs and dolutegravir as the preferred second-line regimen (5). Reported virologic failure (VF) rates among adults on second-line therapies vary depending on the definitions of VF used, subpopulations studied, and measurement time points. Rates of VF among adults on second-line ART between 8–41% have been reported in resource-limited settings (6). In addition, previous studies suggest poor adherence rather than resistance as the cause of VF during second-line ART (6, 7).
WHO guidelines recommend routine viral load (VL) monitoring (4) and expanding HIV drug resistance (DR) testing (8). However, VL monitoring is not consistently available across the region, and access to genotype testing to inform optimal ART choice is severely constrained (9, 10). The WHO has also recommended that national programs develop policies for third-line ART that includes antiretroviral drugs (ARVs) such as darunavir, raltegravir, and dolutegravir; however, access remains restricted by high cost and implementation barriers (11–13). Maximizing the durability of second-line regimens, quantifying needs for third-line therapy and reducing the costs of newer antiretroviral regimens are emerging global priorities (14–16).
As increased prevalence of antiretroviral resistance threatens the maintenance of virally suppressive ART, a broader understanding of the durability of second-line ART and HIV drug resistance-associated mutations (RAMs) would facilitate evidence-based regional projections of third-line regimen needs and advocacy efforts around optimizing life-long ART. We therefore conducted a cross-sectional study to assess VF and RAMs among adults living with HIV on second-line ART within the TREAT Asia HIV Observational Database (TAHOD), a regional cohort study of IeDEA Asia-Pacific.
Methods
Study design and study population
A combination of prospective cross-sectional data and retrospective data collection were used to conduct this study. Participating HIV treatment sites were classified as “testing sites” if they did not perform routine viral load (VL) and/or genotypic resistance testing and these tests were obtained for the purposes of the study. “Data sites” were those where routine VL and/or genotypic resistance testing were already conducted, and data could be extracted from medical records. Six testing sites participated in Cambodia (N=1), Indonesia (N=2), Malaysia (N=1), and Vietnam (N=2). Six data sites participated in Hong Kong SAR (N=1), India (N=1), Japan (N=1) Philippines (N=1), Singapore (N=1), and South Korea (N=1). Patients were eligible for inclusion into the study if they were ≥18 years old, had been on second-line ART for at least six months, and had not received mono- or dual-antiretroviral regimens as first-line ART.
Data collection and definitions
At testing sites, VL and genotypic resistance testing was performed prospectively on eligible patients between June 2016 and May 2017. Patients with a first VL >1000 copies/mL were required to have a repeat VL measured within three months. All those with a second VL >1000 copies/mL underwent genotypic resistance testing. Genotyping was performed using Sanger Sequencing. At data sites, available VL and genotypic resistance testing data between July 2015 and December 2016 were retrospectively collected from the medical records of eligible participants. Viral load and genotypic resistance testing at data sites was conducted in line with national or local guidelines. This ranged from viral load testing every 3, 6 or 12 months on virologically suppressed, stable patients. Indications for genotypic resistance testing ranged from a single VL >500 copies/mL, a single VL >1000 copies/mL, or two consecutive VL >1000 copies/mL within 3 months, after ruling out other causes such as poor adherence. Demographic, clinical and laboratory data were captured directly into a study Case Report Form (CRF) at testing sites, and into the study electronic database at data sites.
For this analysis, virologic failure was defined as (i) testing sites: VL >1000 copies/mL with a second confirmed VL measurement of >1000 copies/mL within three months; (ii) data sites: VL >1000 copies/mL with a second confirmed VL measurement of >1000 copies/mL within six months or a single VL >1000 copies/mL with evidence of at least one RAM on subsequent genotype resistance testing. Genotypic sequence (FASTA) files were submitted to the Stanford University HIV Drug Resistance Database (Stanford HIVdb) version 8.8 for genotyping (17) and the Rega HIV-1 Subtyping Tool version 3 for subtyping (18, 19). RAMs were defined according to the IAS-USA 2019 mutations list (20), excluding minor protease inhibitor (PI) mutations. Evidence of HIV drug resistance on genotypic resistance testing was interpreted as being the presence of any RAM from this list.
Statistical analysis
Factors associated with VF were analyzed using logistic regression. World Bank country income group was adjusted a priori. Variables with p <0.1 in the univariate analysis were included in the multivariate model using backward stepwise selection process. Variables with p <0.05 in the final multivariate model were considered statistically significant. The proportions and patterns of RAMs were reported descriptively with confidence intervals calculated using the exact binomial method. To account for variations in VL testing patterns and potential bias in the definitions of VF used, we conducted a sensitivity analysis defining virologic failure as a single VL >1000 copies/mL, irrespective of subsequent viral load or genotype resistance testing, for both testing and data sites. Data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata software version 14.2 (Stata Corp., College Station, TX, USA).
Ethical considerations
Ethics approvals were obtained from respective local institutional review boards of all participating sites, the data management and biostatistical center (The Kirby Institute, UNSW Sydney), and the coordinating center (TREAT Asia/amfAR). Written informed consent was obtained from testing site participants prior to enrolment. For data sites where anonymized data were collected, written informed consent was obtained only if required by the local ethics committee.
Results
A total of 642 patients from six testing sites and 736 patients from six data sites were eligible for inclusion in the study. Three patients from testing sites were lost to follow up during the study. Of the total 1378 patients included, there were 1023 males (74%), and 964 (70%) acquired HIV through heterosexual contact (Table 1). The median age at switch to second-line ART was 37 years (IQR 32–42), median CD4 cell count at switch was 103 cells/μL (IQR 43.5–229.5), and 1281 (93%) were on second-line regimens with nucleoside reverse transcriptase inhibitors (NRTI) and boosted protease inhibitors (PI). The main NRTIs used during second-line were lamivudine (1016/1336, 76%), tenofovir (972/1336, 73%), and emtricitabine (243/1336, 18%). The main boosted PIs used in second-line were lopinavir (735/1345, 55%) and atazanavir (529/1345, 39%). The main integrase inhibitors used in second-line were raltegravir (65/78, 83%) and dolutegravir (8/78, 10%). Of the 1378 study participants, 873 (63%) had switched to second-line ART due to virologic failure, 221 (16%) due to virologic and immunologic failure, and 233 (17%) due to clinical failure alone or in combination with virologic and/or immunologic failure. The median duration on second-line ART up to the time of the first VL test during the study period was 3 years (IQR 1–5).
Table 1:
Patient characteristics
| Total patients (%) | Data site patients (%) | Testing site patients (%) | |
|---|---|---|---|
| N =1378 | N=736 | N=642 | |
| Age at switch to second-line ART (years) | Median =37, IQR (32–42) | Median =39, IQR (34–44.5) | Median =44, IQR (30–39) |
| ≤30 | 290 (21) | 113 (15) | 177 (28) |
| 31–40 | 645 (47) | 316 (43) | 329 (51) |
| 41–50 | 337 (24) | 231 (31) | 106 (17) |
| >50 | 106 (8) | 76 (10) | 30 (5) |
| Duration on second line ART (years) | Median = 3, IQR (1–5) | Median = 2, IQR (1–5) | Median = 3, IQR (2–6) |
| Sex | |||
| Male | 1023 (74) | 570 (77) | 453 (71) |
| Female | 355 (26) | 166 (23) | 189 (29) |
| HIV mode of exposure | |||
| Heterosexual contact | 964 (70) | 570 (77) | 394 (61) |
| Male-male sex | 134 (10) | 91 (12) | 43 (7) |
| Injecting drug use | 172 (12) | 5 (1) | 167 (26) |
| Other/Unknown | 108 (8) | 70 (10) | 38 (6) |
| CD4 at switch to second-line (cells/μL) | Median =103, IQR (43.5–229.5) | Median =178.5, IQR (70–306) | Median =67.5, IQR (27–149) |
| ≤50 | 298 (22) | 81 (11) | 217 (34) |
| 51–100 | 209 (15) | 73 (10) | 136 (21) |
| 101–200 | 217 (16) | 108 (15) | 109 (17) |
| >200 | 304 (22) | 210 (29) | 94 (15) |
| Not reported | 350 (25) | 264 (36) | 86 (13) |
| Second-line ART Regimen | |||
| NRTI+PI | 1281 (93) | 653 (89) | 628 (98) |
| Integrase inhibitor combination | 78 (6) | 74 (10) | 4 (1) |
| Other combination | 19 (1) | 9 (1) | 10 (2) |
| Reason for switching to second-line ART | |||
| Virologic failure only | 873 (63) | 687 (93) | 186 (29) |
| Immunologic failure only | 51 (4) | 11 (1) | 40 (6) |
| Virologic and immunologic failure | 221 (16) | 17 (2) | 204 (32) |
| *Other reasons | 233 (17) | 21 (3) | 212 (33) |
| World Bank country income group | |||
| Lower + upper middle | 1267 (92) | 625 (85) | 642 (100) |
| High | 111 (8) | 111 (16) | 0 (0) |
NRTI: Nucleoside Reverse Transcriptase Inhibitor; PI: Protease Inhibitor
Other reasons include clinical failure only; virologic and clinical failure; immunologic and clinical failure; and virologic, immunologic and clinical failure
Virologic failure
Confirmed VF occurred in 101 (7%) patients, 26 (26%) were from testing sites, and 75 (74%) were from data sites. Factors associated with VF are shown in Table 2. In the multivariate analysis, adjusting for Word Bank country income group, those with HIV exposure through male-to-male sex (OR=0.29, 95%CI 0.10–0.83, p=0.020) and injecting drug use (IDU) (OR=0.22, 95%CI 0.08–0.60, p=0.003) were less likely to have VF compared those exposed to HIV through heterosexual sex. Patients with CD4 cell count >200 cells/μL at the time of switching to second-line ART were less likely to fail compared to those with CD4 ≤50 cells/μL (OR=0.36, 95%CI 0.17–0.77, p=0.008).
Table 2:
Factors associated with confirmed virologic failure in patients on second-line antiretroviral therapy
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Total patients | Total virologic failures(%) | OR | 95% CI | P | OR | 95% CI | p | |
| Total | 1378 | 101 | ||||||
| Age at switch to second-line ART (years) | 0.547 | |||||||
| ≤30 | 290 | 21(21) | 1 | |||||
| 31–40 | 645 | 50(50) | 1.08 | (0.63, 1.83) | 0.785 | |||
| 41–50 | 337 | 26(26) | 1.07 | (0.59, 1.95) | 0.822 | |||
| >50 | 106 | 4(4) | 0.50 | (0.17, 1.50) | 0.217 | |||
| Sex | ||||||||
| Male | 1023 | 69(69) | 1 | |||||
| Female | 355 | 32(32) | 1.37 | (0.88, 2.12) | 0.159 | |||
| HIV mode of exposure | 0.002 | 0.001 | ||||||
| Heterosexual contact | 964 | 89(89) | 1 | 1 | ||||
| Male-male sex | 134 | 4(4) | 0.30 | (0.11, 0.84) | 0.021 | 0.29 | (0.10, 0.83) | 0.020 |
| Injecting drug use | 172 | 4(4) | 0.23 | (0.08, 0.65) | 0.005 | 0.22 | (0.08, 0.60) | 0.003 |
| Other/Unknown | 108 | 4(4) | 0.38 | (0.14, 1.05) | 0.062 | 0.39 | (0.14, 1.09) | 0.073 |
| CD4 at switch to second-line (cells/μL) | 0.061 | 0.020 | ||||||
| ≤50 | 298 | 24(24) | 1 | 1 | ||||
| 51–100 | 209 | 17(17) | 1.01 | (0.53, 1.93) | 0.974 | 0.97 | (0.50, 1.86) | 0.923 |
| 101–200 | 217 | 10(10) | 0.55 | (0.26, 1.18) | 0.125 | 0.48 | (0.22, 1.03) | 0.058 |
| >200 | 304 | 11(11) | 0.43 | (0.21, 0.89) | 0.023 | 0.36 | (0.17, 0.77) | 0.008 |
| Not reported | 350 | 39(39) | ||||||
| Second-line ART Regimen | 0.200 | |||||||
| NRTI+PI | 1281 | 95(95) | 1 | |||||
| Integrase inhibitor combination | 78 | 3(3) | 0.50 | (0.15, 1.61) | 0.246 | |||
| Other combination | 19 | 3(3) | 2.34 | (0.67, 8.18) | 0.183 | |||
| Reason for switching to second-line ART | 0.075 | |||||||
| Virologic failure only | 873 | 75(75) | 1 | |||||
| Immunologic failure only | 51 | 1(1) | 0.21 | (0.03, 1.56) | 0.128 | |||
| Virologic and immunologic failure | 221 | 15(15) | 0.77 | (0.44, 1.38) | 0.384 | |||
| *Other reasons | 233 | 10(10) | 0.48 | (0.24, 0.94) | 0.032 | |||
| World Bank country income group | ||||||||
| Lower + upper middle | 1267 | 95(95) | 1 | 1 | ||||
| High | 111 | 6(6) | 0.70 | (0.30, 1.65) | 0.420 | 1.34 | (0.54, 3.29) | 0.526 |
P-values in bold represent significant covariates in the final model. Global p-values are test for heterogeneity excluding missing values.
World Bank country income group was included in the multivariate model a priori.
NRTI: Nucleoside Reverse Transcriptase Inhibitor; PI: Protease Inhibitor.
Other reasons include clinical failure only; virologic and clinical failure; immunologic and clinical failure; and virologic, immunologic and clinical failure
In our sensitivity analysis on the 248 (18%) of patients with only a single VL >1000 copies/mL we found that HIV exposure through male-male sex (OR=0.32, 95%CI 0.17–0.64, p=0.001), IDU (OR=0.24, 95%CI 0.12–0.49, p <0.001), and other or unknown HIV exposure (OR=0.50, 95%CI 0.27–0.91, p=0.024) were all less likely to have a single VL >1000 copies/mL compared to heterosexual mode of exposure (Table 3). Patients who lived in high-income countries were less likely to experience a single VL >1000 copies/mL compared to those who lived in low and upper-middle income countries (OR=0.38, 95%CI 0.17–0.84, p=0.017). CD4 cell count at time of switch to second-line was not associated with a single VL >1000 copies/mL in this analysis.
Table 3:
Factors associated with a single VL >1000 copies/mL in patients on second-line antiretroviral therapy
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Total patients | Total with VL >1000(%) | OR | 95% CI | p | OR | 95% CI | p | |
| Total | 1378 | 248 | ||||||
| Age at switch to second-line ART (years) | 0.009 | |||||||
| ≤30 | 290 | 39(16) | 1 | |||||
| 31–40 | 645 | 117(47) | 1.43 | (0.96, 2.11) | 0.076 | |||
| 41–50 | 337 | 78(31) | 1.94 | (1.27, 2.96) | 0.002 | |||
| >50 | 106 | 14(6) | 0.98 | (0.51, 1.89) | 0.950 | |||
| Sex | ||||||||
| Male | 1023 | 183(74) | 1 | |||||
| Female | 355 | 65(26) | 1.03 | (0.75, 1.41) | 0.859 | |||
| HIV mode of exposure | <0.001 | <0.001 | ||||||
| Heterosexual contact | 964 | 216(87) | 1 | 1 | ||||
| Male-male sex | 134 | 10(4) | 0.28 | (0.14, 0.54) | <0.001 | 0.32 | (0.17, 0.64) | 0.001 |
| Injecting drug use | 172 | 9(4) | 0.19 | (0.10, 0.38) | <0.001 | 0.24 | (0.12, 0.49) | <0.001 |
| Other/Unknown | 108 | 13(5) | 0.47 | (0.26, 0.86) | 0.015 | 0.50 | (0.27, 0.91) | 0.024 |
| CD4 at switch to second-line (cells/μL) | 0.446 | |||||||
| ≤50 | 298 | 39(16) | 1 | |||||
| 51–100 | 209 | 32(13) | 1.20 | (0.72, 1.99) | 0.478 | |||
| 101–200 | 217 | 37(15) | 1.37 | (0.84, 2.22) | 0.212 | |||
| >200 | 304 | 38(15) | 0.95 | (0.59, 1.53) | 0.829 | |||
| Not reported | 350 | 102(41) | ||||||
| Second-line ART Regimen | 0.623 | |||||||
| NRTI+PI | 1281 | 233(94) | 1 | |||||
| Integrase inhibitor combination | 78 | 11(4) | 0.74 | (0.38, 1.42) | 0.363 | |||
| Other combination | 19 | 4(2) | 1.20 | (0.39, 3.65) | 0.749 | |||
| Reason for switching to second-line ART | <0.001 | <0.001 | ||||||
| Virologic failure only | 873 | 203(82) | 1 | 1 | ||||
| Immunologic failure only | 51 | 1(0) | 0.07 | (0.01, 0.48) | 0.007 | 0.09 | (0.01, 0.66) | 0.018 |
| Virologic and immunologic failure only | 221 | 27(11) | 0.46 | (0.30, 0.71) | <0.001 | 0.55 | (0.35, 0.86) | 0.009 |
| *Other reasons | 233 | 17(7) | 0.26 | (0.15, 0.44) | <0.001 | 0.28 | (0.17, 0.47) | <0.001 |
| World Bank country income group | ||||||||
| Lower + upper middle | 1267 | 241(97) | 1 | 1 | ||||
| High | 111 | 7(3) | 0.29 | (0.13, 0.62) | 0.002 | 0.38 | (0.17, 0.84) | 0.017 |
P-values in bold represent significant covariates in the final model. Global p-values are test for heterogeneity excluding missing values.
World Bank country income group was included in the multivariate model a priori.
NRTI: Nucleoside Reverse Transcriptase Inhibitor; PI: Protease Inhibitor
Other reasons include clinical failure only; virologic and clinical failure; immunologic and clinical failure; and virologic, immunologic and clinical failure
HIV subtypes and drug resistance-associated mutations of patients with virologic failure
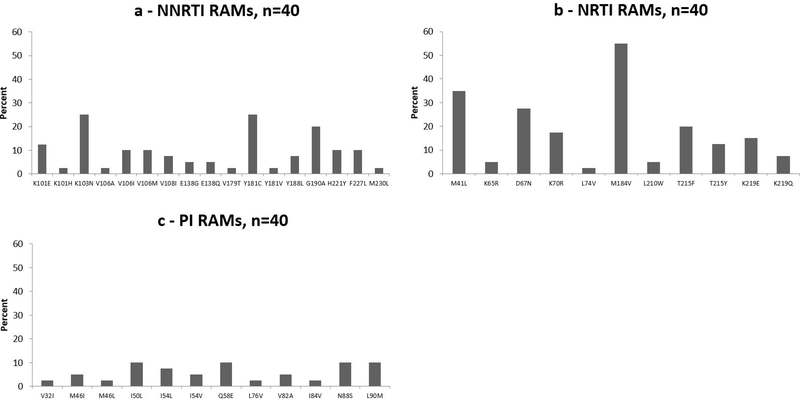
Of the 101 patients with confirmed VF, 41 (41%) patients had a FASTA file available: 17/41 (41%) were from testing sites, and 24/41 (59%) were from data sites. Of these, 39 (95%) had genotypic resistance test results for both reverse transcriptase (RT) and protease (PR) gene regions, with four (4.0%) with integrase gene results. Overall, there were 40 RT-gene regions, 40 PR-gene regions, and 4 integrase-gene regions available for genotyping. Predominant HIV-1 subtypes consisted of subtype C (20/41, 49%), A1 (9/41, 22%), and CRF01_AE (7/41, 17%).
Of the 41 patients with FASTA files available, 40 were on an NRTI+PI-based second-line regimen. A total of 34/41 (83%) patients had at least one RAM. Including only patients with the specific gene region available in the denominator, 32 of 40 (80%) had a non-nucleoside reverse transcriptase inhibitor (NNRTI) RAM, 25 of 40 (63%) had an NRTI RAM, 14 of 40 (35%) had a PI RAM, and 0 of 4 (0%) had an integrase strand transfer inhibitor (INSTI) RAM. There were 13/39 (33%) patients with both NRTI and major PI RAMs. Among patients with major PI RAMs, 4/14 (29%) had one RAM, 5/14 (36%) had two RAMs, and 5/14 (36%) had three or more RAMs.
The most common NNRTI RAMs were K103N (10/40, 25%) and Y181C (10/40, 25%). The most common NRTI RAMs were M184V (22/40, 55%), M41L (14/40, 35%), and D67N (11/40, 28%) (Figure 1). The most common PI RAMs were I50L (4/40, 10%), Q58E (4/40, 10%), N88S (4/40, 10%), and L90M (4/40, 10%).
Figure 1:
Resistance-associated mutations among those with confirmed virologic failure on second-line antiretroviral therapy
Of the 60 patients with confirmed VF for whom FASTA files were not available, 51 were from data sites. These FASTA files were not available as sites had difficulty retrieving historical FASTA files due to the retrospective nature of the data collection from the data sites
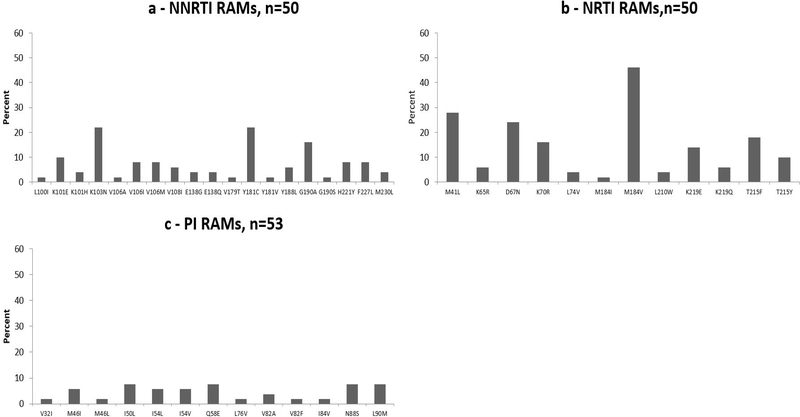
When we allowed for inclusion of FASTA files from those with a single VL >1000 copies/mL, an additional 13 patients had FASTA files available. As with those with confirmed VF, the most common HIV-1 subtypes were subtype C, CRF01_AE and A1. Compared to those with confirmed VF, similar proportions of those with a single VL >1000 copies/mL had at least one RAM (70% vs. 83%), an NNRTI RAM (70% vs. 80%), an NRTI RAM (54% vs. 60%), a PI RAM (30% vs. 35%), and an INSTI RAM (0% vs. 0%). Patterns of RAMs were also similar to those with confirmed VF and are presented in Figure 2.
Figure 2:
Resistance-associated mutations among those with a single VL >1000 copies/mL on second-line antiretroviral therapy
Discussion
In our study of adults on second-line ART in the Asia-Pacific region, the rate of virologic failure was 7%. A CD4 cell count >200 cells/μL at switch to second-line ART, and MSM and IDU HIV exposure were associated with a reduced odds of VF. Of the 41% of VF patients with FASTA files available, almost all (40/41) were on an NRTI + PI second line regimen and 62% had NRTI RAMs, 36% had PI RAMs, and 33% had both NRTI and PI RAMs. Of those with PI RAMs, 72% had ≥2. The most common NNRTI RAMs were K103N and Y181C. The most common NRTI RAMs were M184V, M41L, and D67N. The most common PI RAMs were I50L, Q58E, N88S, and L90M.
The 7% virologic failure rate amongst adults on second line ART for a median of 3 years in our cohort is lower than that documented by other studies in Asia using similar definitions of VF, for example recent studies of adults living with HIV on second line ART in India and Vietnam found rates of VF of 15.8% and 9.5% respectively (21, 22). The relatively low rate of VF we observed is encouraging and likely a reflection of the level of care available at our participating sites, which are primarily tertiary care or referral centers with the resources to support VL monitoring and drug resistance testing, as well as support for treatment adherence. The substantially higher proportion of patients with VF from data sites might be a reflection of a more complicated case mix of patients seen at data sites, ascertainment bias, or sociodemographic or clinical differences between data site and testing site study populations, such as age, duration on second line ART, proportion on second line INSTI regimens, and the proportion on second line ART due to VF.
Other studies in the Asia region have identified increasing age, higher baseline VL, poorer ART adherence, and specific second-line ART regimens such as non-lopinavir, non-atazanavir PI regimens, as risk factors for second-line failure (21–25). However, our observation of associations between HIV exposure through male-male sex and IDU with reduced odds of second-line VF has not been frequently reported (14, 26). This association may reflect the increasing focus of regional HIV services and ART programs on key populations in more recent years.
The proportion of second-line VF patients with NNRTI RAMs in our study was comparable to levels documented in other studies in Asia; however, we found substantially lower proportions of patients with NRTI and PI RAMs (21, 22, 27–29). With approximately one-third of genotyped patients who had confirmed viral failure on second-line ART having no NRTI RAMs, and nearly two-thirds having no major PI RAMs, our study also emphasizes that VL elevation alone is not enough to switch to a new regimen. It also highlights the importance of adherence strengthening prior to considering costly alternative treatment options and a potential need for broader access to HIV DR testing in the region in order to identify those patients that truly require switching to third-line ART regimens (30–32). A recent study of an adult South African cohort found that many patients with VF on a boosted PI resuppressed after a period of intense adherence counseling (33). Although cost and logistical challenges mean that individualized genotype resistance testing in support of treatment regimen optimization remains unfeasible in the region, an analysis of strategies for patients failing second-line ART found that genotype assays and an appropriate third-line regimen were cost-effective in resource-limited settings, compared to a population-based approach that included no genotyping (34). A South African study of adults on lopinavir-based second-line ART regimen that found the majority failed due to poor drug exposure, highlights the potential value of using hair and plasma lopinavir concentrations in diagnosing the cause of VF, and of targeted genotypic resistance testing in patients where VF is not explained by poor drug exposure (35).
As documented in other resource-limited settings, the high prevalence of NNRTI mutations amongst those genotyped in our cohort may be an indication of extensive drug resistance prior to the start of second-line ART (36). K103N is associated with ongoing resistance to nevirapine and efavirenz (37), and its high frequency amongst those genotyped in our cohort suggests recycling these widely used NNRTIs in third-line regimens in Asia might be limited. In addition, the high prevalence of etravirine-associated mutations such as Y181C, G190A, and A98G raise concerns around continued susceptibility to this newer generation NNRTI (30) and potential limitations to its use in third-line therapy.
The most common NRTI and PI RAMs amongst those genotyped in our cohort are consistent with the RAMs identified in other studies from the region (22, 27, 38). M184V, the predominant NRTI mutation found in our study, has been shown to confer high-level resistance to lamivudine and emtricitabine (39). The high prevalence of thymidine analogue mutations we observed supports the need for better access to routine viral load testing in the region as these mutations suggest delayed detection of treatment failure and a patient remaining on a failing first-line ART regimen for some time (36, 40). Whilst found in a lower proportion than documented in other cohorts, the small proportion of those genotyped with a K65R mutation is of note as it is associated with multi-NRTI resistance and a high level of resistance to tenofovir (41, 42).
Maintaining patients with virologic failure on a failing second-line PI/r-based regimen raises concerns over the development of resistance to third-line PI options such as darunavir. Whilst data from Asia is limited, a recent study in South Africa found that 57% of participants in a third-line antiretroviral therapy program had some degree of resistance to darunavir at third-line initiation (43), and a study of patients failing second-line ART in Nigeria estimated that patients developed a median of 0.6 PR mutations for every 6 months on a failing second-line regimen (44). Common PI mutations among our cohort, such as L90M, M46I and V82A have been found to be associated with resistance to nelfinavir and sanquinavir, indinavir, and lopinavir, respectively (45), a concern given 53% of our cohort were on second-line lopinavir. In addition, the L76V mutation found in a small proportion of our cohort is of concern as it can confer cross resistance to PIs such as darunavir that can be used for third-line therapy in resource limited settings (45–47).
Discussion and interpretation of our results should take account of a number of limitations. The study sites are mostly tertiary care and referral centers, and therefore not necessarily representative of all HIV-related clinical care centers within a country. A substantial number of FASTA files were not available for analysis, raising concerns around the generalizability of our findings. Data on adherence and the results of previous HIV DR tests were not available, limiting the interpretation of study results because the contribution of suboptimal adherence to virologic failure could not be explored and we were not able to determine which of the prevalent RAMs might have been present prior to the start of second-line ART. It should also be noted that prediction of clinical outcomes from genotypic resistance testing is challenging, and the utility of resistance testing in the public health approach to ART management remains uncertain (48). Nevertheless, because of the number of sites involved, countries represented, and the strict definition of second-line ART virologic failure applied, we believe our results are a reasonable reflection of second-line VF rates, associated factors, and RAMs in the Asia-Pacific region.
In conclusion, only 7% of adults on second-line ART in our Asia-Pacific regional cohort had confirmed virologic failure, but our study suggests that between one-third to over half of them had some level of resistance to the medicines they were being treated with. Broader implementation of routine viral load monitoring would identify those in need of enhanced adherence support before the emergence of substantial HIV drug resistance and treatment failure necessitated the use of costly resistance testing and third-line ART regimens.
Acknowledgements
The authors are grateful to the study participants and all site staff for their commitment to the study. The TASER-2 Study Group: PS Ly, V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; MP Lee, PCK Li, W Lam, YT Chan, Queen Elizabeth Hospital, Hong Kong SAR; N Kumarasamy, C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India; TP Merati, DN Wirawan, F Yuliana, KT Amijaya, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; E Yunihastuti, D Imran, A Widhani, A Citra, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; J Tanuma, S Oka, T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; A Kamarulzaman, I Azwa, Tan Chin Heng, N Raman, University Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco, MK Pasayan, ML Mationg, Research Institute for Tropical Medicine, Muntinlupa City, Philippines; OT Ng, PL Lim, LS Lee, PS Ohnmar, Tan Tock Seng Hospital, Singapore (note: OT Ng was also supported by the NMRC Clinician Scientist Award (MOH-000276), which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.); JY Choi, Na S, JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; KV Nguyen, HV Bui, DTH Nguyen, DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam; CD Do, AV Ngo, LT Nguyen, Bach Mai Hospital, Hanoi, Vietnam; AH Sohn, JL Ross, B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand; MG Law, A Jiamsakul, R Bijker, D Rupasinghe, The Kirby Institute, UNSW Sydney, NSW, Australia.
Funding support was provided through a grant to amfAR, The Foundation for AIDS Research from ViiV Healthcare, and a grant from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, and the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Conflicts of Interest
AHS has received grants to her institution from ViiV Healthcare. ML has received grants to his institution from Gilead Sciences, Janssen Cilag and ViiV Healthcare. The other authors declare no conflicts of interest related to this study.
References
- 1.UNAIDS. 2018 Global HIV Statistics Fact Sheet. 2019.
- 2.Patrikar S, Subramaniam S, Vasudevan B, Bhatti V, Kotwal A, Basannar D, et al. Profile of HIV Patients on Second Line Antiretroviral Therapy: The Indian Experience. Journal of AIDS and Clinical Research 2015;6(5). [Google Scholar]
- 3.Martinez-Vega R, De La Mata NL, Kumarasamy N, Ly PS, Van Nguyen K, Merati TP, et al. Durability of antiretroviral therapy regimens and determinants for change in HIV-1-infected patients in the TREAT Asia HIV Observational Database (TAHOD-LITE). Antivir Ther. 2018;23(2):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Second edition 2016. [PubMed]
- 5.WHO. Update of recommendations on first- and second-line antiretroviral regimens. Policy Brief; 2019. [Google Scholar]
- 6.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26(8):929–38. [DOI] [PubMed] [Google Scholar]
- 7.Johnston V, Cohen K, Wiesner L, Morris L, Ledwaba J, Fielding KL, et al. Viral suppression following switch to second-line antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. The Journal of infectious diseases. 2014;209(5):711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global Action Plan on HIV Drug Resistance 2017–2021: 2018 progress report. 2018.
- 9.Kumarasamy N, Krishnan S. Beyond first-line HIV treatment regimens: the current state of antiretroviral regimens, viral load monitoring, and resistance testing in resource-limited settings. Current opinion in HIV and AIDS. 2013;8(6):586–90. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Progress Report on HIV in the WHO South-East Asia Region. 2016.
- 11.Evans D, Hirasen K, Berhanu R, Malete G, Ive P, Spencer D, et al. Predictors of switch to and early outcomes on third-line antiretroviral therapy at a large public-sector clinic in Johannesburg, South Africa. AIDS Res Ther. 2018;15(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. WHO HIV Update: global epidemic, progress in scale up and policy uptake. 2018.
- 13.UNAIDS. Global AIDS Update 2018: Miles to Go. Closing Gaps, breaking barriers, righting injustices 2018.
- 14.Pujades-Rodriguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. Jama. 2010;304(3):303–12. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Juneja S, Vitoria M, Habiyambere V, Nguimfack BD, Doherty M, et al. Projected Uptake of New Antiretroviral (ARV) Medicines in Adults in Low- and Middle-Income Countries: A Forecast Analysis 2015–2025. PloS one. 2016;11(10):e0164619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinsztejn B, Hughes MD, Ritz J, Salata R, Mugyenyi P, Hogg E, et al. Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): a prospective strategy study. The lancet HIV. 2019;6(9):e588–e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pineda-Pena AC, Faria NR, Imbrechts S, Libin P, Abecasis AB, Deforche K, et al. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol. 2013;19:337–48. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira T, Deforche K, Cassol S, Salminen M, Paraskevis D, Seebregts C, et al. An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics. 2005;21(19):3797–800. [DOI] [PubMed] [Google Scholar]
- 20.Annemarie M Wensing VC, Francesca Ceccherini-Silberstein, Charlotte Charpentier, Günthard Huldrych F., Roger Paredes, Shafer Robert W., Richman Douglas D.. Update of the Drug Resistance Mutations in HIV-1. 2019 Resistance Mutations Update 2019;27(3). [PMC free article] [PubMed] [Google Scholar]
- 21.Thao VP, Quang VM, Day JN, Chinh NT, Shikuma CM, Farrar J, et al. High prevalence of PI resistance in patients failing second-line ART in Vietnam. The Journal of antimicrobial chemotherapy. 2016;71(3):762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarty J, Sundar S, Chourasia A, Singh PN, Kurle S, Tripathy SP, et al. Outcome of patients on second line antiretroviral therapy under programmatic condition in India. BMC infectious diseases. 2015;15:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boettiger DC, Nguyen VK, Durier N, Bui HV, Heng Sim BL, Azwa I, et al. Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: results from the TREAT Asia HIV observational database. Journal of acquired immune deficiency syndromes. 2015;68(2):186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Wang Z, Liu J, Yue Y, Yang S, Huang H, et al. Efficacy and HIV drug resistance profile of second-line ART among patients having received long-term first-line regimens in rural China. Scientific reports. 2015;5:14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Zhang M, Shang M, Yang W, Wang Z, Shang H. Research on the treatment effects and drug resistances of long-term second-line antiretroviral therapy among HIV-infected patients from Henan Province in China. BMC infectious diseases. 2018;18(1):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thao VP, Quang VM, Wolbers M, Anh ND, Shikuma C, Farrar J, et al. Second-Line HIV Therapy Outcomes and Determinants of Mortality at the Largest HIV Referral Center in Southern Vietnam. Medicine. 2015;94(43):e1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saravanan S, Vidya M, Balakrishnan P, Kantor R, Solomon SS, Katzenstein D, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern India. Clinical infectious diseases. 2012;54(7):995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. The Journal of infectious diseases. 2013;207 Suppl 2:S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferradini L, Ouk V, Segeral O, Nouhin J, Dulioust A, Hak C, et al. High efficacy of lopinavir/r-based second-line antiretroviral treatment after 24 months of follow up at ESTHER/Calmette Hospital in Phnom Penh, Cambodia. Journal of the International AIDS Society. 2011;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levison JH, Orrell C, Gallien S, Kuritzkes DR, Fu N, Losina E, et al. Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa. PloS one. 2012;7(3):e32144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barennes H, Guillet S, Limsreng S, Him S, Nouhin J, Hak C, et al. Virological failure and HIV-1 drug resistance mutations among naive and antiretroviral pre-treated patients entering the ESTHER program of Calmette Hospital in Cambodia. PloS one. 2014;9(8):e105736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eric Nerrienet JN, Sopheak Ngin, Olivier Segeral, Sreymom Ken, Kerya Phon, Chanroeurn Hak, Vara Ouk, Vonthanak Saphonn, Laurent Ferradini and Jean-Francois Delfraissy,. HIV-1 Protease Inhibitors Resistance Profiles in Patients with Virological Failure on LPV/r-based 2nd Line Regimen in Cambodia. J AIDS Clinic Res. 2012;S5:003. [Google Scholar]
- 33.Collier D, Iwuji C, Derache A, de Oliveira T, Okesola N, Calmy A, et al. Virological Outcomes of Second-line Protease Inhibitor-Based Treatment for Human Immunodeficiency Virus Type 1 in a High-Prevalence Rural South African Setting: A Competing-Risks Prospective Cohort Analysis. Clinical infectious diseases. 2017;64(8):1006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzana SB, Hughes MD, Grinsztejn B, Collier AC, Luz PM, Freedberg KA, et al. Genotype assays and third-line ART in resource-limited settings: a simulation and cost-effectiveness analysis of a planned clinical trial. AIDS. 2012;26(9):1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. Journal of acquired immune deficiency syndromes. 2011;56(4):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chimbetete C, Katzenstein D, Shamu T, Spoerri A, Estill J, Egger M, et al. HIV-1 Drug Resistance and Third-Line Therapy Outcomes in Patients Failing Second-Line Therapy in Zimbabwe. Open Forum Infect Dis. 2018;5(2):ofy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koigi P, Ngayo MO, Khamadi S, Ngugi C, Nyamache AK. HIV type 1 drug resistance patterns among patients failing first and second line antiretroviral therapy in Nairobi, Kenya. BMC research notes. 2014;7:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin B, Sun X, Su S, Lv C, Zhang X, Lin L, et al. HIV drug resistance in HIV positive individuals under antiretroviral treatment in Shandong Province, China. PloS one. 2017;12(7):e0181997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ssemwanga D, Lihana RW, Ugoji C, Abimiku A, Nkengasong J, Dakum P, et al. Update on HIV-1 acquired and transmitted drug resistance in Africa. AIDS Rev. 2015;17(1):3–20. [PubMed] [Google Scholar]
- 40.Cozzi-Lepri A, Phillips AN, Martinez-Picado J, Monforte A, Katlama C, Eg Hansen AB, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. The Journal of infectious diseases. 2009;200(5):687–97. [DOI] [PubMed] [Google Scholar]
- 41.Melikian GL, Rhee SY, Taylor J, Fessel WJ, Kaufman D, Towner W, et al. Standardized comparison of the relative impacts of HIV-1 reverse transcriptase (RT) mutations on nucleoside RT inhibitor susceptibility. Antimicrob Agents Chemother. 2012;56(5):2305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karade SK, Ghate MV, Chaturbhuj DN, Kadam DB, Shankar S, Gaikwad N, et al. Cross-sectional study of virological failure and multinucleoside reverse transcriptase inhibitor resistance at 12 months of antiretroviral therapy in Western India. Medicine. 2016;95(37):e4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moorhouse M, Maartens G, Venter WDF, Moosa MY, Steegen K, Jamaloodien K, et al. Third-Line Antiretroviral Therapy Program in the South African Public Sector: Cohort Description and Virological Outcomes. Journal of acquired immune deficiency syndromes. 2019;80(1):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawizza HE, Chaplin B, Meloni ST, Darin KM, Olaitan O, Scarsi KK, et al. Accumulation of protease mutations among patients failing second-line antiretroviral therapy and response to salvage therapy in Nigeria. PloS one. 2013;8(9):e73582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boender TS, Hamers RL, Ondoa P, Wellington M, Chimbetete C, Siwale M, et al. Protease Inhibitor Resistance in the First 3 Years of Second-Line Antiretroviral Therapy for HIV-1 in Sub-Saharan Africa. The Journal of infectious diseases. 2016;214(6):873–83. [DOI] [PubMed] [Google Scholar]
- 46.Rhee SY, Taylor J, Fessel WJ, Kaufman D, Towner W, Troia P, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54(10):4253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiga AI, Fofana DB, Cisse M, Diallo F, Maiga MY, Traore HA, et al. Characterization of HIV-1 antiretroviral drug resistance after second-line treatment failure in Mali, a limited-resources setting. The Journal of antimicrobial chemotherapy. 2012;67(12):2943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paton NI, Kityo C, Thompson J, Nankya I, Bagenda L, Hoppe A, et al. Nucleoside reverse-transcriptase inhibitor cross-resistance and outcomes from second-line antiretroviral therapy in the public health approach: an observational analysis within the randomised, open-label, EARNEST trial. The lancet HIV. 2017;4(8):e341–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]