Introduction
Neuropathic pain is a well-described side-effect of cancer chemotherapy [88]. The prevalence of painful chemotherapy-induced peripheral neuropathy (CIPN), which is chemotherapy agent-dependent, is highest for platinum-based chemotherapy (70–100%) [12; 56; 88]. Oxaliplatin, a third-generation platinum used to treat solid tumors [9; 14], exerts its antineoplastic effect through the formation of platinum-DNA adducts [39]. Interestingly, oxaliplatin has a side-effect profile that differs from cisplatin and carboplatin [14] in that it causes two phases of neuropathic pain, an early acute-phase of intense pain, which transforms into a long-lasting painful chronic distal sensory neuropathy, the late phase [17; 40; 41; 70]. The early phase of oxaliplatin CIPN, unique among the platinum-based compounds, is predictive of the severity of the chronic or late phase [17; 78].
Stressful life events influence the pathogenesis of a variety of diseases by promoting adaptation through hypothalamic-pituitary-adrenal (HPA) and sympathoadrenal neuroendocrine stress axes [16]. And, unalleviated pain causes stress and, as clinical data suggest, stress and pain interact bi-directionally [8; 37; 59], with stress heightening sensitivity, or exacerbating pain as a result of neuronal plasticity, to produce changes in nociceptor signaling [15; 33; 45; 50]. The diagnosis and treatment of cancer are both stressful and, importantly, the patient’s perceptions of stress and their use of coping strategies impact CIPN [18; 59; 86]. Early-life stress, especially related to preterm birth, is also a risk factor for CIPN in adulthood [24; 27; 59]. Thus, differences in stress responsiveness or in cumulative life stress exposure can impact vulnerability to the neurotoxic effects of cancer chemotherapy [18; 24].
In this study, we employed a preclinical model of painful peripheral neuropathy induced by oxaliplatin, which reproduces both its early and late phases [24; 25; 40; 41], to evaluate the impact of stress on oxaliplatin-induced neuropathic pain (hyperalgesia). Also, given the well-established sex differences in both stress and pain, including in models of CIPN [24; 59], sexual dimorphism in the role of the major neuroendocrine stress axes in oxaliplatin CIPN was addressed.
Methods
Animals
Sprague–Dawley rats were purchased from Charles River Laboratories (Hollister, CA, USA). Primiparous timed-pregnant female rats were used for early-life stress protocols. Dams were housed with their litter in standard cages on postnatal days 0–1. On postnatal day 2, litters were assigned to neonatal limited bedding (NLB), neonatal handling (NH) or standard rearing conditions (control animals). For experimental protocols using adults, male and female Sprague Dawley rats were 8 weeks old. Animals were housed in same sex, 3 per cage, under a 12 h light/dark cycle in a temperature- and humidity-controlled animal care facility at the University of California, San Francisco (UCSF). Food and water were available ad libitum. Experimental protocols were approved by the UCSF Institutional Animal Care and Use Committee (IACUC), adhered to the guidelines of the American Association of Laboratory Animal Care (AALAC), the National Institutes of Health (NIH), and the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP), for the use of animals in research. Every effort was made to minimize the number of animals used and their suffering.
Testing mechanical nociceptive threshold
Mechanical nociceptive threshold was measured using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test; Stoelting, Chicago, IL, USA), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw, as previously described [65; 77]. Rats were placed in cylindrical acrylic restrainers designed to minimize restraint stress, and allow extension of their hind legs from lateral ports in the cylinder during the assessment of nociceptive thresholds. To acclimatize rats to the testing procedure, they were placed in a restrainer for 1 hour prior to starting an experiment and for 30 minutes prior to experimental manipulations. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw. Baseline paw-pressure nociceptive threshold was defined as the mean of 3 readings taken before test agents were injected.
Oxaliplatin CIPN model
To reproduce the two phase model of CIPN [40], oxaliplatin (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline and the volume adjusted to 1 mg/ml for a single intravenous administration of 2 mg/kg [25; 40; 41]. Using this protocol, which recapitulates key clinical features of oxaliplatin CIPN, we have distinguished an initial intense phase, marked by an acute onset that lasted approximately one week (≤7 days) after oxaliplatin administration, followed by a mechanistically distinct chronic phase (≥ 14 days) that persisted for the duration of the 28-day testing period [25; 40; 41]. For the purposes of the present study, we use the terms early (≤7 days) and late (≥ 14 days) to define the two phases.
Adrenalectomy
Under 3% isoflurane anesthesia, rats underwent surgical excision of both adrenal glands. After shaving their abdomen, anesthetized rats were placed on a thermal blanket and their skin swabbed with povidone iodine solution. To provide peri-operative analgesia, rats were given meloxicam (5 mg/kg, s.c.) and bupivacaine (5–8 mg/kg, i.d.) was infiltrated into the skin preoperatively, in the area to be incised. Two flank wall incisions were made, and each adrenal gland visualized and excised; 5–0 silk suture was used to separately close the abdominal wall and skin incisions. To maintain normal plasma corticosterone levels during the experimental period (∼35 days), adrenalectomized rats were supplied with corticosterone (25 μg/ml, ad libitum) and 0.45% NaCl in their drinking water. This procedure simulates the phasic (circadian) corticosterone rhythm; normalizes basal levels of adrenocorticotropic hormone (ACTH) and catecholamines [47; 80]; and, prevents the weight loss observed in adrenalectomized rats that do not receive corticosterone replacement [2]. Control animals had sham adrenalectomy, in which the adrenal glands were located and manipulated, but not excised.
Antisense (AS)-oligodeoxynucleotide (ODN) targeting β2-adrenergic and glucocorticoid receptor mRNAs
To investigate whether catecholamines or glucocorticoids, by acting at β₂-adrenergic (ADRB2) or glucocorticoid (GR) receptors, respectively, on sensory neurons, play a role in oxaliplatin-induced hyperalgesia, an antisense-oligodeoxynucleotide (AS-ODN) for ADRB2, or GR mRNA [21] was administered intrathecally. ODNs were synthesized by Life Technologies (Carlsbad, CA, USA). The AS-ODN sequence for ADRB2, 5′-AAA GGC AGA AGG ATG TGC-3′ is directed against a unique region of rat ADRB2 mRNA (GeneBank accession number NM_012492). The mismatch-ODN (MM-ODN) sequence 5′-ATA GCC TGA TGG AAG TCC-3′ was designed by mismatching six bases (denoted by bold letters) of the antisense sequence. The AS-ODN sequence for GR was 5′-TGG AGT CCA TTG GCA AAT-3′ and its control, a MM-ODN of the same sequence, with five bases switched, denoted by bold letters was 5′-TGA AGT TCA GTG TCA ACT-3′. We have validated ADRB2 and GR AS-ODNs actions by Western blotting, demonstrating a decrease in the expression of ADRB2 and GR in the rat [21; 44]. Before use, antisense and mismatch ODNs were lyophilized and reconstituted to a concentration of 4 μg/μl in 0.9% NaCl, immediately prior to administration. To administer ODNs, rats were briefly anaesthetized with 2.5% isoflurane and a 30-gauge hypodermic needle was inserted into the subarachnoid space, on the midline, between the L4 and L5 vertebrae. ODNs (80 μg/20 μl) were injected intrathecally. The intrathecal site of injection was confirmed by observation of a tail flick, a reflex that is evoked by subarachnoid space access and bolus injection [58]. This robust and reproducible method for intrathecal ODN administration [72], as a direct communication between the subarachnoid space and dorsal root ganglion (DRG) in rats [42] facilitates access of ODN into cell bodies in DRG [48].
Chronic administration of epinephrine and corticosterone
To evaluate whether stress levels of epinephrine and corticosterone affect oxaliplatin-induced hyperalgesia, we administered epinephrine, corticosterone, or their combination, over the testing period. Chronic administration of stress levels of epinephrine was performed by implanting Alzet® mini-osmotic pumps (model 2004; Durect, Cupertino, CA, USA) filled with epinephrine, to deliver epinephrine systemically at a rate of 5.4 μg/0.25 μl/h, which produces plasma levels of 720 ± 67 pg/mL [45; 46] in rats. Corticosterone was administered as a slow-release pellet of 100 mg of fused corticosterone, which produces a plasma corticosterone level of 32.64 ± 2.82 μg⁄dL [74; 75]. The mini-osmotic pumps or pellets were implanted subcutaneously in the interscapular region. Oxaliplatin (2 mg/kg) was injected intravenously one day after rats were submited to hormone implants.
Unpredictable sound stress
Exposure to unpredictable sound stress occurred on days 1, 3, and 4, using a protocol initially developed by Singh and colleagues [69] and used in our laboratory [6; 46; 75]. Briefly, groups of three adult rats were placed in a 12×15×9.5-inch wire mesh cage, 25 cm from a loudspeaker, inside of 22×22×28-inch sound-insulated box. Sound pulses were emitted as pure tones, at three frequencies (11, 15, and 19 kHz) and amplitudes varied from 20 to 110 dB, independently for each frequency. The sound exposure protocol was initiated after placing rats in the wire mesh cage and terminated 30 min later. Over the 30 min period, a 5− or 10−s tone was presented every minute, at random times during the minute. Sham stressed animals were placed in the sound chamber for 30 minutes over 4 days, but without exposure to the sound stimulus. After sound stress or sham treatment, rats were returned to their home cages. To evaluate the neuroplastic changes produced by stress, animals received oxaliplatin 14 days after the last exposure to the sham or sound stress protocol [44]. This protocol does not alone produce changes in cutaneous nociceptive threshold [44–46].
Neonatal limited bedding (NLB)
To generate early life-stress, we used a well-established NLB protocol [31] previously used by us [33]. Briefly, beginning on postnatal day 2, dams and their pups were placed in cages fitted with a stainless steel mesh bottom, raised ~2.5 cm above the home cage floor, to allow collection of urine and feces. The nesting/bedding material consisted of one sheet of paper towel (~25 × 33 cm). Dams and their litters were left undisturbed during postnatal days 2–9. From postnatal day 10 until weaning, dams and their litters were housed in standard cages, with standard bedding. On postnatal day 21, pups were weaned and same sex rats were housed 3 per cage.
Neonatal handling (NH)
To generate resilience in adult rats, we used the well-established NH model developed by Levine and colleagues [49; 51], and previously used by us [5; 7]. The NH protocol involves removing dams from their litters, and gently handling, touching and stroking pups for 15 mins, after which dams and their litters are returned to their home cage. This procedure was conducted daily from postnatal days 2 to 9. On postnatal day 21, pups were weaned and same sex rats housed 3 per cage.
Data analysis
In all experiments, the dependent variable was change in paw-withdrawal threshold, expressed as percentage change from baseline. To compare the hyperalgesia induced by oxaliplatin in the different experimental groups, a two-way repeated-measures analysis of variance (ANOVA) with Bonferroni post hoc contrast was used, as described in the figure legends. Prism 8.0 (GraphPad Software, Inc, San Diego, CA) was used for the graphics and to perform statistical analyses. A p value of < 0.05 was considered statistically significant. Data are presented as means ± standard errors of the mean.
Results
Oxaliplatin induces hyperalgesia in male and female rats
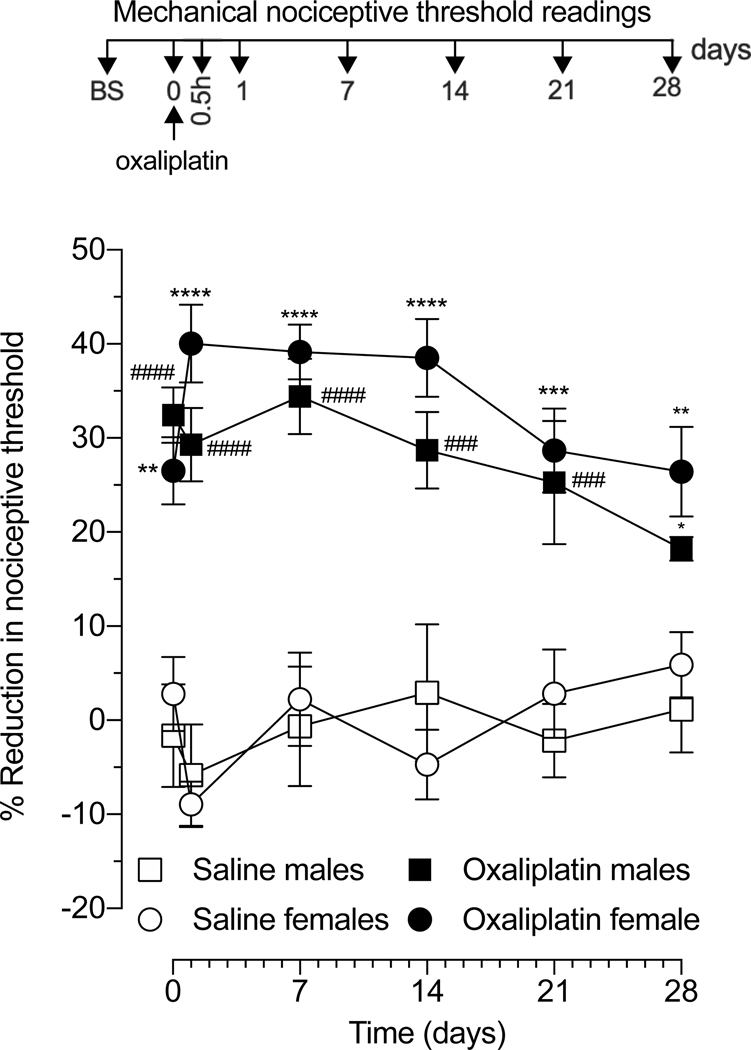
We have previously demonstrated that systemic administration of oxaliplatin produces a decrease in mechanical nociceptive threshold in male rats that manifests in a time-dependent manner [25; 40; 41]. Given the well-described sex differences in pain [13; 24; 35], we evaluated the development and time-course of mechanical hyperalgesia induced by intravenous administration of a single dose of oxaliplatin (2 mg/kg, i.v.), in adult male and female rats (Fig. 1). When compared with vehicle (saline), oxaliplatin produced a rapid onset (detected by 30 minutes) mechanical hyperalgesia, peaking by 24 hours in females and 7 days in males, with both reaching a similar peak level (34–38% decrease in mechanical nociceptive threshold). In both sexes, mechanical hyperalgesia was sustained for at least 4 weeks, with only a small decrease observed in males at day 28 post-oxaliplatin (an 18% and 26% decrease in mechanical threshold in males and females, respectively). Therefore, the development of oxaliplatin CIPN was not dependent on the sex of the rat.
Figure 1. Comparison of oxaliplatin-induced hyperalgesia in male and female rats.
Oxaliplatin (2 mg/kg) or saline (i.v.) was administered to male and female rats, and mechanical nociceptive threshold evaluated before, and 30 min and 1, 7, 14, 21, and 28 days after administration of oxaliplatin. Oxaliplatin was administered on day 0. Results are presented as change in mechanical paw-withdrawal threshold, expressed as percentage change from baseline. In males and females, oxaliplatin decreased mechanical nociceptive threshold (i.e. produced hyperalgesia), observed 30 min after injection and persisting until day 28. Data from male rats is shown as mean ± SEM, ####P<0,0001, ###P<0,001, #P<0,001: oxaliplatin vs saline (n=6 paws per group). Data from female rats is shown as mean ± SEM, ****P<0,0001, ***P<0,001, **P<0,01: oxaliplatin vs saline. Treatment F (3, 120) = 98.00, Time F (5,120) = 0.9645, Interaction F (15,120) = 1.639, using two-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). No difference in magnitude of the hyperalgesia was observed between male and female oxaliplatin-treated rats.
Effect of adrenalectomy
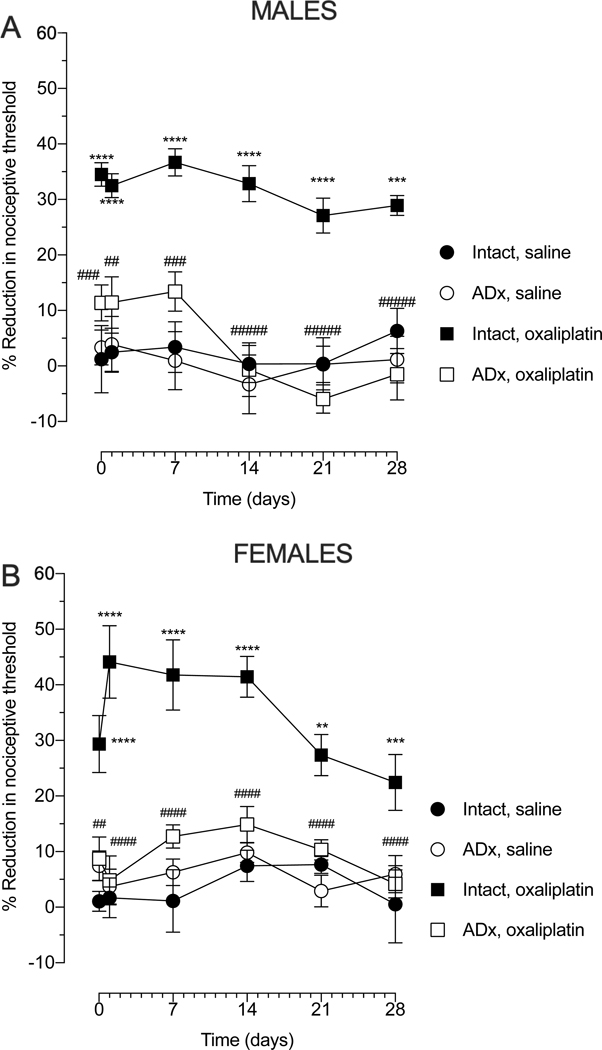
To evaluate the involvement of neuroendocrine stress axes in oxaliplatin-induced hyperalgesia, we administered oxaliplatin to adrenalectomized (AdX) rats, a surgical procedure that eliminates the source of the final common mediators for both the sympathoadrenal and hypothalamic‐pituitary‐adrenal (HPA) stress axes, catecholamines and corticosteroids, respectively. Oxaliplatin did not produce hyperalgesia in ADx male or female rats, at any time-point (Fig. 2A and B), suggesting that the contribution of neuroendocrine stress axes in oxaliplatin CIPN is driven by adrenal-derived stress hormones, epinephrine and corticosterone. Importantly, adrenalectomy alone did not affect mechanical nociceptive threshold in either sex (Fig. 2).
Figure 2. Effect of adrenalectomy on oxaliplatin-induced hyperalgesia.
Male and female rats were submitted to bilateral adrenalectomy and one week later, oxaliplatin (2 mg/kg, i.v.) was administered (day 0). Mechanical nociceptive threshold was evaluated before oxaliplatin injection and again 30 min, 1, 7, 14, 21 and 28 days after oxaliplatin. (A) When the magnitude of oxaliplatin-induced hyperalgesia was evaluated in adrenalectomized (Adx) male rats, a marked attenuation was observed compared to the adrenal intact oxaliplatin-treated group. Data shown as mean ± SEM, Time F (5, 120) = 2.871, Treatment F (3, 120) = 84.02, Interaction F (15, 120) = 1.042, ****P<0,0001, ***P<0,001: intact oxaliplatin vs intact saline; ####P<0,0001, ###P<0,001, ##P<0,01: Adx oxaliplatin vs intact oxaliplatin, using 2-way repeated measures ANOVA followed by Bonferroni post hoc test, (n=6 paws per group). No differences in the magnitude of the hyperalgesia between the adrenal intact saline-treated group and Adx-saline group was observed in male rats. (B) When the magnitude of oxaliplatin-induced hyperalgesia was evaluated in Adx female rats, a marked attenuation was observed compared to the adrenal intact oxaliplatin-treated group. Data shown as mean ± SEM, Time F (5, 120) = 3.102, Treatment F (3, 120) = 79.29, Interaction F (15, 120) = 1.516, ****P<0,0001, **P<0,001: intact oxaliplatin vs intact saline; ####P<0,0001, ##P<0,001, #P<0,001: Adx oxaliplatin vs intact oxaliplatin, using 2-way repeated measures ANOVA followed by Bonferroni post hoc tests (n=6 paws per group). No differences in the magnitude of the hyperalgesia between intact saline group and Adx-saline group was observed in female rats.
Effect of β₂-adrenergic (ADRB2) receptor knockdown
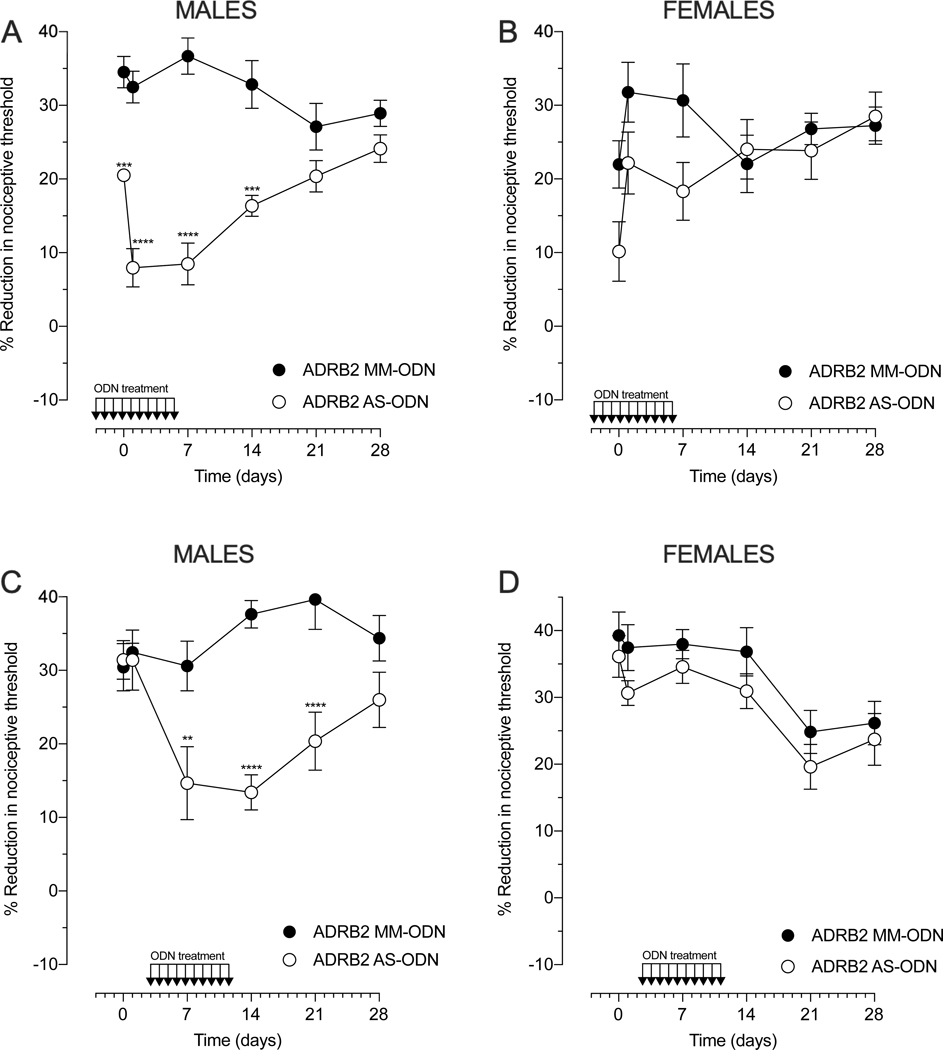
To assess whether catecholamines acting at ADRB2 play a role in oxaliplatin-induced hyperalgesia, AS- or MM-ODN against ADRB2 mRNA (80 μg/20 μl) was administered intrathecally, daily for 10 days (Fig. 3). Two treatment protocols were employed: prevention, to determine whether knock‐down of ADRB2 would delay or prevent the development of oxaliplatin‐induced hyperalgesia, and reversal, to determine whether the AS-ODN could attenuate already established oxaliplatin‐induced hyperalgesia.
Figure 3. Role of β2-adrenergic receptor (ADRB2) in oxaliplatin-induced hyperalgesia.
Male and female rats were treated with intrathecal injections of ODN AS or MM to ADRB2 mRNA, for 10 consecutive days (80 μg/day, 20 μl) in prevention (A and B) or reversal (C and D) protocols. Prevention protocol: oxaliplatin (2 mg/kg, i.v.) was administered approximately 17 hours after the third daily intrathecal injection of ADRB2 ODN (day 0). Mechanical nociceptive threshold was evaluated before ODN treatment was started and again on days 0 (30 min), 1, 7, 14, 21, and 28 days after oxaliplatin. (A) The magnitude of oxaliplatin-induced hyperalgesia was significantly attenuated in males treated with ADRB2 AS-ODN, when it was compared with the ADRB2 MM-ODN-treated group. Data shown as mean ± SEM, Treatment F (1,60) = 139.7, Time F (5, 60) = 2.634, Interaction F (5,60) = 8.180, ****P<0,0001, ***P<0,001: ADRB2 AS-ODN vs ADRB2 AS-MM (n=6 paws per group). (B) The magnitude of oxaliplatin-induced hyperalgesia was not affected by ADRB2 AS-ODN in the prevention protocol, in females. Data shown as mean ± SEM, Treatment F (1,60) = 6.620, Time F (5,60) = 2.452, Interaction F (5,60) = 1.498, ADRB2 AS-ODN vs ADRB2 AS-MM (n=6 paws per group). Reversal protocol: intrathecal treatment with ADRB2 AS- or MM-ODN started 3 days after intravenous administration of oxaliplatin (2 mg/kg). Mechanical nociceptive threshold was evaluated before oxaliplatin administration and again 30 min, 1, 7, 14, 21, and 28 days later (n=6 paws per group). (C) Oxaliplatin-induced hyperalgesia was significantly reversed in males treated with ADRB2 AS-ODN, when compared with the ADRB2 MM-ODN-treated group. Data shown as means ± SEM, Treatment F (1,60) =33.76, Time (5,60) = 2.318, Interaction F (5,60) = 4.256, ****P<0,0001, ***P<0,001, **P<0,01: ADRB2 AS-ODN vs ADRB2 AS-MM (n=6 paws per group). (D) The magnitude of oxaliplatin-induced hyperalgesia was not affected by ADRB2 AS-ODN in the reversal protocol, in females. Data shown as means ± SEM, Treatment F (1,60) = 6.293, Time (5,60) = 8.433, Interaction F (5,60) = 0.1567 (n=6 paws per group). Two-way repeated measures ANOVA followed by Bonferroni post hoc tests were used to compare antisense and mismatch groups over time.
In the prevention protocol (Fig. 3A and 3B), when the intrathecal administration of ODNs was started 3 days before intravenous (i.v.) administration of oxaliplatin (2 mg/kg), ADRB2 AS-ODN markedly attenuated oxaliplatin-induced hyperalgesia during the early (≤ 7 days) and late (≥ 14 days) phase of CIPN, in male rats (Fig. 3A). Even though the last intrathecal injection of ADRB2 AS-ODN was on day 6, on day 14 the hyperalgesia induced by oxaliplatin was still attenuated. In contrast, ADRB2 AS-ODN treatment did not prevent oxaliplatin-induced hyperalgesia in female rats (Fig. 3B).
In the reversal protocol, when ODNs were administered intrathecally, starting 3 days after i.v. oxaliplatin, ADRB2 AS-ODN was able to reverse oxaliplatin-induced hyperalgesia, measured on days 7, 14 and 21, in males (Fig. 3C). Thus, the knockdown of ADRB2 reverses the early and late phase of oxaliplatin-induced CIPN in males. Of note, no difference between ADRB2 AS- and MM-treated groups was observed on day 28, presumably due to loss of the effect of the ADRB2 AS-ODN, since the last administration of ODN was on day 12. Furthermore, ADRB2 AS-ODN did not reverse already established oxaliplatin‐induced hyperalgesia in females, suggesting that the catecholamine driven ADRB2 pathway does not contribute to oxaliplatin-induced CIPN in females (Fig. 3D). These results support the suggestion of a sexual dimorphism in the involvement of the sympathoadrenal stress axis in oxaliplatin CIPN.
Effect of glucocorticoid receptor (GR) knockdown
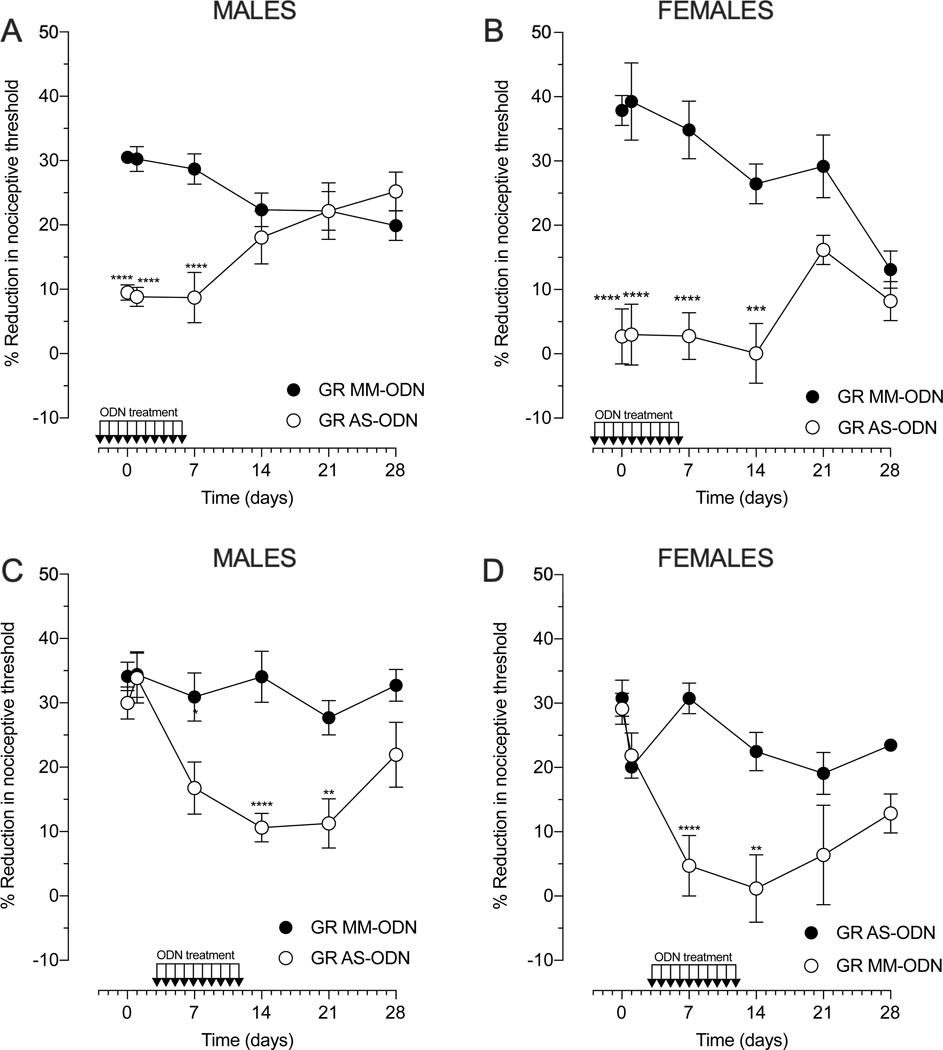
To evaluate the contribution of glucocorticoids, via action at GR, in oxaliplatin-induced hyperalgesia, AS- or MM-ODN against GR mRNA (80 μg/20 μl) was administered intrathecally, in prevention and reversal protocols, daily for 10 days (Fig. 4). In the prevention protocol (Fig. 4A and 4B), AS- or MM-ODN targeting GR mRNA was started 3 days prior to intravenous oxaliplatin (2 mg/kg) and the treatment continued for a week. Using this protocol, oxaliplatin-induced hyperalgesia was significantly attenuated in both sexes. In males (Fig. 4A), the involvement of GR was evident in the early phase (≤7 days) of oxaliplatin-induced CIPN, since the reduction in hyperalgesia was observed at 30 min, 24 hours, and 7 days after oxaliplatin injection. In contrast, in females, the GR AS-ODN effect was observed in both early (≤7 days) and late (≥ 14 days) phases of oxaliplatin-induced hyperalgesia (Fig. 4B). AS-ODN-treatment markedly attenuated the magnitude of oxaliplatin-induced hyperalgesia, until day 21. In addition to a role in the initiation of oxaliplatin-induced CIPN, GR activity was required to maintain oxaliplatin-induced hyperalgesia, in both sexes (Fig. 4C and 4D). To assess this role of GR, the AS- or MM-ODN targeting GR mRNA was injected in a reversal protocol, when the oxaliplatin-induced hyperalgesia was already established (day 3). Using this protocol (ODN administered daily, from day 3 to day 12), oxaliplatin-induced hyperalgesia was significantly attenuated in both sexes. Hyperalgesia returned by day 28 in males, and by day 21 in females, likely due to return of GR expression. Thus, ongoing GR signaling is essential to maintain, as well as initiate oxaliplatin CIPN, in box sexes.
Figure 4. Role of glucocorticoid receptor (GR) in oxaliplatin-induced hyperalgesia.
Male and female rats were treated with intrathecal injections of AS-ODN or MM-ODN against GR mRNA, for 10 consecutive days (80 μg/day, 20 μl) in the prevention or reversal protocol. Prevention protocol (A and B): oxaliplatin (2 mg/kg, i.v.) was administered approximately 17 hours after the third intrathecal injection of GR ODN (day 0). Mechanical nociceptive threshold was evaluated before ODN treatment was started and again 30 min and 1, 7, 14, 21, and 28 days after administration of oxaliplatin. (A) The magnitude of oxaliplatin-induced hyperalgesia was significantly attenuated in males treated with ADRB2 AS-ODN, compared with the ADRB2 MM-ODN-treated group. Data shown as mean ± SEM, Treatment F (1,60) = 39.75, Time F (5,60) = 0.5798, Interaction F (5,60) = 9.021, ****P<0,0001: GR AS-ODN vs GR AS-MM (n=6 paws per group). (B) The magnitude of oxaliplatin-induced hyperalgesia was significantly attenuated in females treated with ADRB2 AS-ODN, compared with the ADRB2 MM-ODN-treated group. Data shown as mean ± SEM, Treatment F (1,60) = 112.9, Time F (5,60) = 2.813, Interaction F (6.70) = 5.133, ****P<0,0001, ***P<0,001: GR AS-ODN vs GR AS-MM (n=6 paws per group). Reversal protocol (C and D): intrathecal treatment with GR AS- or MM-ODN started 3 days after intravenous administration of oxaliplatin (2 mg/kg). Mechanical nociceptive threshold was evaluated before oxaliplatin administration, and again 30 min and 1, 7, 14, 21 and 28 days later. (C) The magnitude of oxaliplatin-induced hyperalgesia was significantly reversed in males treated with GR AS-ODN, compared to the GR MM-ODN-treated group. Data shown as mean ± SEM, Treatment F (1,60) = 33.83, Time F (5,60)= 5.483, Interaction F (5,60) = 2.929, ****P<0,0001, **P<0,01, *P<0,05: GR AS-ODN vs ADRB2 GR-MM (n=6 paws per group). (D) The magnitude of oxaliplatin-induced hyperalgesia was significantly reversed in males treated with GR AS-ODN, compared to the GR MM-ODN-treated group. Data shown as mean ± SEM, Treatment F (1,60) = 28.94, Time (5,60), Interaction (5,60) = 4.069, ****P<0,0001, ***P<0,001: GR AS-ODN vs GR AS-MM (n=6 paws per group). Two-way repeated measures ANOVA followed by Bonferroni post hoc tests were used to compare antisense and mismatch groups over time.
Effect of unpredictable sound stress and administration of stress hormones
To examine whether stress hormones in adult rats affects oxaliplatin CIPN, rats were submitted to the continuous exposure of stress levels of epinephrine, corticosterone, or their combination (Fig. 5A and 5B). Epinephrine-containing osmotic minipumps, as well as corticosterone fused pellets, were implanted in the interscapular region of rats 24h prior to the administration of oxaliplatin. Due to a rapid attainment of stress levels of epinephrine and corticosterone in implanted rats, these experiments were carried out 24h after the minipumps and/or pellets being implanted [44]. Male rats exposed to stress plasma levels of epinephrine or the combination of epinephrine and corticosterone, exhibited enhanced oxaliplatin-induced hyperalgesia in both phases of oxaliplatin CIPN (Fig. 5A). A further increase in oxaliplatin-induced hyperalgesia, albeit not statistically significant, occurred in the group exposed to the combination of hormones. Moreover, in corticosterone-exposed rats, the exacerbation of oxaliplatin-induced hyperalgesia was more robust in the early phase of oxaliplatin CIPN, in males (Fig. 5A). In females, only the continuous exposure to systemic corticosterone exacerbated oxaliplatin CIPN, since the combination of corticosterone and epinephrine similarly enhanced hyperalgesia (Fig. 5B). These effects were observed in both phases. Thus, enhancement of oxaliplatin-induced hyperalgesia by stress is glucocorticoid dependent in females (Fig. 5B). The exposure of control animals to stress hormones alone did not affect nociceptive threshold at any time point (data not show).
Figure 5. Effect of chronic administration of stress hormones and unpredictable sound stress on oxaliplatin-induced hyperalgesia.
Male and female rats were submitted to stress levels of epinephrine (osmotic minipumps filled with 5.4 μg/0.25 μL/h of epinephrine), corticosterone (100 mg in pellets), or their combination or exposed to unpredictable sound stress on days 1, 3, and 4. Stress hormone exposure protocol: (A and B): surgery for the implantation of epinephrine-containing osmotic minipumps or corticosterone fused pellets in the interscapular space was performed 24h before intravenous administration of oxaliplatin (2 mg/kg) (day 0). (A) In male rats, exposed to epinephrine or the combination of epinephrine and corticosterone, the magnitude of oxaliplatin-induced hyperalgesia was increased in both phases of oxaliplatin CIPN. Rats exposed to corticosterone alone, exhibited an increase in the magnitude of oxaliplatin-induced hyperalgesia at 30 min and 1 (early phase) and 21 (late phase) days after oxaliplatin administration. Data shown as mean ± SEM Treatment F (3,120) = 86.39; Time (5, 120) = 13.42; Interaction (15, 120) =1.221, ****P<0,0001: epinephrine or epinephrine +corticosterone vs oxaliplatin; **P<0,01, *P<0,05: epinephrine+ corticosterone vs oxaliplatin; ####P<0,0001, #P<0,05: corticosterone vs oxaliplatin, using two-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). (B) When female rats were exposed to corticosterone alone or the combination of corticosterone and epinephrine, an increase in the magnitude of oxaliplatin-induced hyperalgesia was observed. The magnitude of oxaliplatin-induced hyperalgesia was not affected by epinephrine exposure. Data shown as mean ± SEM Treatment F (3,120) = 51.27; Time (5, 120) = 3.397; Interaction (15, 120) =1.007, ####P<0,0001, ##P<0,01: corticosterone vs oxaliplatin or corticosterone plus epinephrine vs oxaliplatin; #P<0,05: corticosterone plus epinephrine vs oxaliplatin, using two-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). For the sound stress protocol (C and D): oxaliplatin (2 mg/kg, i.v.) was administered (day 0) 14 days after the last exposure to sound stress. Mechanical nociceptive threshold was evaluated before and again 30 min and 1, 7, 14, 21 and 28 days after oxaliplatin. (C) In male rats exposed to sound stress, the magnitude of oxaliplatin-induced hyperalgesia was increased at 30 min and 1, 7, 14 and 21 days after oxaliplatin administration. Data shown as mean ± SEM, Treatment F (1,48) = 81, Time F (5,48) = 18.05, Interaction (5,48) = 6.293, ****P<0,0001, ***P<0,001, **P<0,001: sound stress oxaliplatin vs sham stress oxaliplatin, (n=6 paws per group), using two-way repeated measures ANOVA followed by Bonferroni post hoc tests (n=6 paws per group). (D) The magnitude of oxaliplatin-induced hyperalgesia was increased in female rats exposed to sound stress at 30 min and 1, 7 and 14 days after oxaliplatin administration. Data shown as mean ± SEM, Treatment F (1,60) = 20.6, Time F (5,60) = 15.71, Interaction (5,60) = 2.367, *P<0,05: sound stress oxaliplatin vs sham stress oxaliplatin, using two-way repeated measures ANOVA followed by Bonferroni post hoc tests (n=6 paws per group).
To support the suggestion that stress enhances oxaliplatin CIPN, we next used unpredictable sound stress, a protocol that triggers the adrenal glands to release stress hormones [44; 45; 75]. Because activation of neuroendocrine stress axes and neuroplastic changes in primary afferent nociceptors are fully established 14 days after the last exposure to the sound stress [6; 44; 45], we injected oxaliplatin in male and female rats, at this time-point. When males were exposed to sound stress, oxaliplatin-induced hyperalgesia was markedly increased in the early (≤7 days) and late (≥ 14 days) phases of oxaliplatin CIPN (Fig. 5C). By contrast, we observed a small, albeit significant, increase in oxaliplatin-induced hyperalgesia in females, only in the early phase (≤7 days) of oxaliplatin CIPN (Fig. 5D). Together, our data support the suggestion that stress is able to aggravate oxaliplatin CIPN, with a sexual dimorphism in the neuroendocrine stress axes mediating the exacerbation.
Effect of early life stress
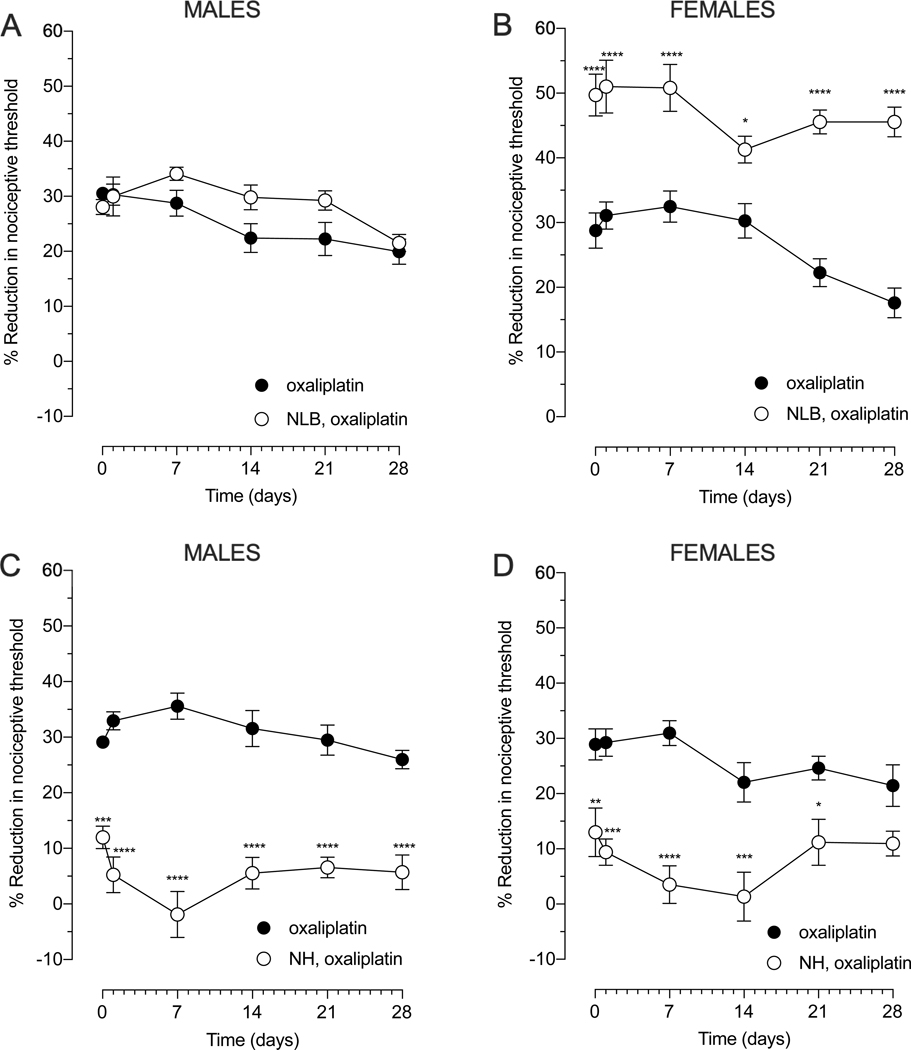
Early postnatal life is a critical period for setting neuroendocrine stress axis responsiveness in adults [26; 52] and the exposure to early-life adversity determines the vulnerability to later stressful events [4; 33]. To understand how early life stress, a clinical risk factor for CIPN [59], affects oxaliplatin CIPN, we used two well-established, contrasting early-life interventions that produce a stress-sensitive (NLB protocol) and a stress-resilient (NH protocol) phenotype in adult rats. While the NLB protocol did not affect the magnitude of oxaliplatin-induced hyperalgesia in adult males (Fig. 6A), in females it significantly enhanced the magnitude of oxaliplatin-induced hyperalgesia in both the early (≤7 days) and late (≥ 14 days) phase of oxaliplatin CIPN (Fig. 6B). Whereas the NH protocol fully attenuated oxaliplatin-induced hyperalgesia in males, in both phases, in females, its protective effect was marked in the early phase and, albeit statistically significant, small in the late phase of oxaliplatin CIPN (Fig. 6C and 6D). Thus, NH-induced protection exhibits sex differences in the late phase of oxaliplatin CIPN with females having less protection. These data provide evidence that early life stress impacts the development of oxaliplatin CIPN in adult rats, in a sex-dependent manner.
Figure 6. Effect of neonatal limited bedding (NLB) and neonatal handling (NH) on oxaliplatin-induced hyperalgesia.
Rats were exposed neonatally to either NLB (stress, upper panels, A and B) or NH (resilience, lower panels, C and D) protocols and, approximately 8 weeks later oxaliplatin (2 mg/kg, i.v.) (day 0) was administered. Mechanical nociceptive threshold was evaluated before and again 30 min and 1, 7, 14, 21 and 28 days after oxaliplatin. (A) The magnitude of oxaliplatin-induced hyperalgesia in NLB male rats did not differ when it was compared with control adult rats that received oxaliplatin. Data shown as mean ± SEM, Treatment F (1,60) = 5.97, Time F (5,60) = 6.306, Interaction F (5,60) =1.747, using 2-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). (B) The magnitude of oxaliplatin-induced hyperalgesia was significantly enhanced in female rats submitted to the NLB protocol, when compared with control adult rats that received oxaliplatin. Data shown as mean ± SEM, Treatment F (1,60) = 167.6, Time F (5,60) = 4.55, Interaction F (5,60) = 2.159, ****P<0,0001, *P<0,05: NLB, oxaliplatin vs oxaliplatin, using two-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). (C) Male rats submitted to the NH protocol showed significant attenuation in oxaliplatin-induced hyperalgesia when compared with control adult rats that received oxaliplatin. Data shown as mean ± SEM, Treatment (1,60) = 279.7, Time F (5,60) = 0.8117, Interaction F (5,60) = 3.681, ****P<0,0001, ***P<0,001: NH, oxaliplatin vs oxaliplatin, using 2-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group). (D) Female rats submitted to the NH protocol showed a marked attenuation in oxaliplatin-induced hyperalgesia when compared with control adult rats that received oxaliplatin. Data shown as mean ± SEM, Treatment (1,60) = 89.65, Time F (5,60) = 1.862, Interaction F (5,60) = 1.68, ****P<0,0001, ***P<0,001, **P<0,01, *P<0,05 : NH, oxaliplatin vs oxaliplatin, using 2-way repeated measures ANOVA followed by Bonferroni post hoc test (n=6 paws per group).
Discussion
Given that CIPN occurs in 38 – 90% [59; 64] of the ~16.9 million cancer survivors in United States [60], there is an urgent need to better understand its underlying mechanisms. Due to the link between psychological stress and CIPN [24; 59; 64], we evaluated the contribution of the sympathoadrenal and HPA neuroendocrine stress axes to oxaliplatin CIPN.
Stress plays a substantial role in increasing the symptom burden of oncology patients [59]. A diagnosis of cancer and associated treatments can lead to symptoms of stress in up to a third of patients receiving chemotherapy [63], due to illness, prospect of early death and financial burden arising from cost of treatment and potential inability to work [3; 59; 54; 59; 86]. Using a preclinical model of oxaliplatin CIPN to provide insights into neurotoxic mechanisms that initiate and maintain CIPN, we first compared the magnitude and time-course of oxaliplatin-induced hyperalgesia, between male and female rats, to further allow us to explore the effect of stress on CIPN. With regard to sex differences in oxaliplatin CIPN, our results are consistent with previous preclinical and clinical studies supporting the suggestion that while female rats exhibited faster onset to peak hyperalgesia than males, the overall severity of oxaliplatin CIPN is not sexually dimorphic [10; 34; 38; 81; 82].
As the adrenal gland is the major source of stress hormones and the role of neuroendocrine stress axes in models of neuropathic pain is supported by our prior studies, [21; 24; 76] we administered oxaliplatin to adrenalectomized rats. Oxaliplatin-induced hyperalgesia was markedly attenuated in both male and female adrenalectomized rats. To further address the action of key neuroendocrine stress mediators, catecholamines (epinephrine and norepinephrine) and glucocorticoids, at their cognate receptors on nociceptors, we downregulated expression of ADRB2 and GR in sensory neurons by administering AS-ODN intrathecally in both prevention and reversal protocols. Knockdown of ADRB2 markedly attenuated oxaliplatin-induced hyperalgesia in males, but not in females, in both prevention and reversal protocols. On the other hand, AS-ODN targeting GR markedly attenuated oxaliplatin-induced hyperalgesia in both sexes, in both protocols. The mechanisms underlying the sexual dimorphism in the contribution of ADRB2 and GR receptors in CIPN are currently unknown. However, with respect to the sympathoadrenal stress axis, male rats normally exhibit greater epinephrine-induced hyperalgesia, even at lower doses [20; 46]. Furthermore, in males, but not in females, epinephrine acts on ADRB2 to stimulate protein kinase C-epsilon and ERK 1/2, kinases that modulate nociceptor function in cultured DRG neurons [36]. This sexual dimorphism in ADRB2 signaling could contribute to the sexual dimorphism in the role of ADRB2 in CIPN. Also, the release of glucocorticoids in females is more rapid and intense, while de-escalation of HPA axis drive is slower [45]. In this regard, even residual receptor expression after antisense knockdown may be sufficient to maintain oxaliplatin-induced hyperalgesia in females, if mediated by increased corticosterone levels. Of note, while the nociceptor is the only cell that is exposed both to mechanical stimulation and to intrathecal antisense ODN, and CIPN pain is thought to be due to nociceptor hyperexcitability [40], we cannot exclude a contribution of an effect of antisense on cells in the spinal cord.
To evaluate the effect of stress on oxaliplatin CIPN, we submitted adult rats to intense unpredictable sound stress, which produces a persistent increase in epinephrine and corticosterone [44]. Males and females were differentially affected by sound stress, with CIPN markedly increased in males with only a small increase in females. Our findings are in agreement with a previous report showing that males are more sensitive to acoustic stress, measured by its greater corticosterone response [11], cardiovascular changes [71] and brain c-Fos expression [23], a molecule involved in the signal transduction of sympathetic activity [64; 74] and linked to sexual dimorphism [36].
Stress resilience differs between the sexes [32], and these differences are affected by early postnatal experience [27]. Human and rodent studies have reported that adults who experienced early-life stress, such as being born prematurely, have altered neuroendocrine stress reactivity [27] and pain threshold [33; 84], and have an increased risk for developing CIPN [59]. In fact, disruption of rodent maternal behavior, by limiting available bedding material [neonatal limited bedding (NLB) protocol] to disrupt dams’ nesting and maternal care, produces a life-long enhanced neuroendocrine response to stress [61], as well as enhanced hyperalgesia induced by inflammatory mediators, in male rats [33]. We observed that the NLB protocol increases oxaliplatin-induced hyperalgesia, only in females, supporting the suggestion that the sex dependent differences in oxaliplatin CIPN phenotype may result, at least in part, from different neuroendocrine stress pathways impacted by early-life stress. These long-lasting changes in neuroendocrine stress axis function may be triggered by epigenetic mechanisms that translate early-life stress conditions into long-lasting changes in gene expression, particularly in key neuronal genes of HPA axis function such FKBP5 [53], NR3C1, NR4C1 and BNDF [19; 68], leading to changes underpinning stress-related behaviors in the adult [68]. The smaller HPA axis response of males could be due to its active suppression by the sympathoadrenal axis during the early postnatal period [33; 83]. Alternatively, male- and female-typical brain circuitry is normally initiated during tightly regulated hormone-sensitive periods of development, when the sexes are exposed to different patterns of gonadal hormones secretion [85]. In rats and mice, the postnatal testosterone surge plays a critical role in programming the development of HPA axis function [85], with the HPA axis being less responsive to androgens in adulthood [5; 55]. Indeed, neonatal orchiectomy increases corticosterone response to stress, whereas sex hormone replacement in adults does not reverse this change [55]. In line with this, rat pups submitted to the NH protocol had significantly less oxaliplatin-induced hyperalgesia, in both sexes, with a greater effect of NH in male rats. Interestingly, the NH protocol increases the capacity of rodent’s neuroendocrine response to adapt to adversity, trauma, threat, or other stressors [27; 28; 66], and produces a protective effect against chronic pain in the adult, especially in male rats, probably due to the need of androgens for the expression of NH-induced effects [5; 24; 29]. Sexual dimorphism in NH rats may be related to the finding that it promotes increased maternal care, thought to be responsible for the development of resilience, and that greater maternal behavior is directed to male pups, which may explain why NH attenuates stress-induced increases in corticosterone levels in male but not female rats [57].
In view of the fact that oxaliplatin CIPN can ultimately progress to sensory neuron loss, secondary to the neuronal accumulation of platinum [73], and pain may be an early manifestation of a process that ultimately leads to cell death [41], our findings raise the intriguing possibility that mechanisms involved in cell death may be involved in painful CIPN. In support of this hypothesis, concentration of oxaliplatin in DRG is correlated with the magnitude of mechanical hyperalgesia [62]. Organic cation transporter 2 (OCT2), present in DRGs, serves as an initial regulator for accumulation of platinum-based agents [73]. Of note, OCT transporters are corticosterone and epinephrine sensitive [30]. Therefore, the regulation of OCT2 function by neuroendocrine stress axis mediators may increase the amount of oxaliplatin in DRG, thereby increasing the severity of CIPN.
Despite shared mechanisms by cytostatic chemotherapeutic agents to sensitize nociceptors [17], we previously demonstrated a distinct mechanism for the impact of stress in exacerbating paclitaxel CIPN in rats [24]. In contrast to our present findings with oxaliplatin, the sympathoadrenal axis has a critical role for paclitaxel CIPN in both sexes, while the HPA axis contributes only in male rats [24]. Mechanisms underlying differences in the role of neuroendocrine stress axis in oxaliplatin vs paclitaxel CIPN, in male and female rats, have yet to be elucidated. Differences in administration schedule (oxaliplatin, single injection, vs paclitaxel 4 injections over 8 days) may play a role, but there are important differences in mechanisms of action of these two chemotherapeutic agents. While both oxaliplatin and paclitaxel produce bilaterally symmetric, painful sensory peripheral neuropathy and mechanical hyperalgesia [87], acute paclitaxel increases [43], while oxaliplatin decreases [67], voltage-gated calcium channel current in DRG neurons. Furthermore, paclitaxel, but not oxaliplatin, increases substance P release from DRGs [79]. And, although HPA function and corticosterone [22], as well as epinephrine, [1] affect nociceptor function in a calcium channel-dependent manner, future studies are needed to determine whether these chemotherapeutic agents activate the HPA and/or sympathoadrenal neuroendocrine stress axes and the mechanistic differences in their activation in male and female rats.
Together, our findings raise the possibility that assessment and management of stress is critically important to the development of patient-specific therapeutic approaches to prevent or treat CIPN, which may differ between specific classes of chemotherapeutic agents and sex, including by platinum- and taxane-based [24] chemotherapy agents.
Acknowledgments
This work was supported by a grant from National Cancer Institute (CA250017).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- [1].Abdulla FA, Smith PA. Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J Neurosci 1997;17(5):1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol 1985;249(5 Pt 2):R527–532. [DOI] [PubMed] [Google Scholar]
- [3].Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst 2017;109(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiatry 2013;74(9):688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alvarez P, Green PG, Levine JD. Neonatal Handling Produces Sex Hormone-Dependent Resilience to Stress-Induced Muscle Hyperalgesia in Rats. J Pain 2018;19(6):670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alvarez P, Green PG, Levine JD. Unpredictable stress delays recovery from exercise-induced muscle pain: contribution of the sympathoadrenal axis. Pain Rep 2019;4(5):e782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alvarez P, Levine JD, Green PG. Neonatal handling (resilience) attenuates water-avoidance stress induced enhancement of chronic mechanical hyperalgesia in the rat. Neurosci Lett 2015;591:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ameringer S, Elswick RK, , Shockey DP, Dillon R. A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nurs 2013;36(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Argyriou AA. Updates on Oxaliplatin-Induced Peripheral Neurotoxicity (OXAIPN). Toxics 2015;3(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Attal N, Bouhassira D, Gautron M, Vaillant JN, Mitry E, Lepere C, Rougier P, Guirimand F. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain 2009;144(3):245–252. [DOI] [PubMed] [Google Scholar]
- [11].Babb JA, Masini CV, Day HE, Campeau S. Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress 2014;17(3):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 2017;7(1):e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boerner KE, Chambers CT, Gahagan J, Keogh E, Fillingim RB, Mogil JS. Conceptual complexity of gender and its relevance to pain. Pain 2018;159(11):2137–2141. [DOI] [PubMed] [Google Scholar]
- [14].Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, Pritchard JR, Pommier Y, Lippard SJ, Hemann MT. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 2017;23(4):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res 2017;95(6):1257–1270. [DOI] [PubMed] [Google Scholar]
- [16].Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA 2007;298(14):1685–1687. [DOI] [PubMed] [Google Scholar]
- [17].Colvin LA. Chemotherapy-induced peripheral neuropathy: where are we now? Pain 2019;160 Suppl 1:S1–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 2017;4(4):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM. Early Life Stress Effects on Glucocorticoid-BDNF Interplay in the Hippocampus. Front Mol Neurosci 2015;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci 2001;13(12):2227–2233. [DOI] [PubMed] [Google Scholar]
- [21].Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci 2008;27(1):83–92. [DOI] [PubMed] [Google Scholar]
- [22].Dolatshahi-Somehsofla M, Esmaeili-Mahani S, Motamedi F, Haeri A, Ahmadiani A. Adrenalectomy potentiates the antinociceptive effects of calcium channel blockers. Pharmacol Biochem Behav 2009;92(2):327–334. [DOI] [PubMed] [Google Scholar]
- [23].Fernandez-Quezada D, Garcia-Zamudio A, Ruvalcaba-Delgadillo Y, Luquin S, Garcia-Estrada J, Jauregui Huerta F. Male rats exhibit higher pro-BDNF, c-Fos and dendritic tree changes after chronic acoustic stress. Biosci Trends 2019;13(6):546–555. [DOI] [PubMed] [Google Scholar]
- [24].Ferrari LF, Araldi D, Green PG, Levine JD. Marked sexual dimorphism in neuroendocrine mechanisms for the exacerbation of paclitaxel-induced painful peripheral neuropathy by stress. Pain 2020;161(4):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferrari LF, Gear RW, Levine JD. Attenuation of activity in an endogenous analgesia circuit by ongoing pain in the rat. J Neurosci 2010;30(41):13699–13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fogelman N, Canli T. Early Life Stress, Physiology, and Genetics: A Review. Front Psychol 2019;10:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron 2012;75(5):747–761. [DOI] [PubMed] [Google Scholar]
- [28].Freitas D, Antoniazzi CT, Segat HJ, Metz VG, Vey LT, Barcelos RC, Duarte T, Duarte MM, Burger ME. Neonatal tactile stimulation decreases depression-like and anxiety-like behaviors and potentiates sertraline action in young rats. Int J Dev Neurosci 2015;47 (Pt B):192–197. [DOI] [PubMed] [Google Scholar]
- [29].Friborg O, Hjemdal O, Rosenvinge JH, Martinussen M, Aslaksen PM, Flaten MA. Resilience as a moderator of pain and stress. J Psychosom Res 2006;61(2):213–219. [DOI] [PubMed] [Google Scholar]
- [30].Gasser PJ, Lowry CA. Organic cation transporter 3: A cellular mechanism underlying rapid, non-genomic glucocorticoid regulation of monoaminergic neurotransmission, physiology, and behavior. Horm Behav 2018;104:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 1996;15(2):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goldfarb EV, Seo D, Sinha R. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress 2019;11:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain 2011;152(11):2549–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Griffith KA, Zhu S, Johantgen M, Kessler MD, Renn C, Beutler AS, Kanwar R, Ambulos N, Cavaletti G, Bruna J, Briani C, Argyriou AA, Kalofonos HP, Yerges-Armstrong LM, Dorsey SG. Oxaliplatin-Induced Peripheral Neuropathy and Identification of Unique Severity Groups in Colorectal Cancer. J Pain Symptom Manage 2017;54(5):701–706 e701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hendrich J, Alvarez P, Joseph EK, Ferrari LF, Chen X, Levine JD. In vivo and in vitro comparison of female and male nociceptors. J Pain 2012;13(12):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci 2006;24(2):527–534. [DOI] [PubMed] [Google Scholar]
- [37].Hwang KH, Cho OH, Yoo YS. Symptom clusters of ovarian cancer patients undergoing chemotherapy, and their emotional status and quality of life. Eur J Oncol Nurs 2016;21:215–222. [DOI] [PubMed] [Google Scholar]
- [38].Johnston IN, Tan M, Cao J, Matsos A, Forrest DRL, Si E, Fardell JE, Hutchinson MR. Ibudilast reduces oxaliplatin-induced tactile allodynia and cognitive impairments in rats. Behav Brain Res 2017;334:109–118. [DOI] [PubMed] [Google Scholar]
- [39].Johnstone TC, Suntharalingam K, Lippard SJ. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem Rev 2016;116(5):3436–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain 2008;9(5):463–472. [DOI] [PubMed] [Google Scholar]
- [41].Joseph EK, Levine JD. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J Pain 2009;10(5):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Joukal M, Klusakova I, Dubovy P. Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. Ann Anat 2016;205:9–15. [DOI] [PubMed] [Google Scholar]
- [43].Kawakami K, Chiba T, Katagiri N, Saduka M, Abe K, Utsunomiya I, Hama T, Taguchi K. Paclitaxel increases high voltage-dependent calcium channel current in dorsal root ganglion neurons of the rat. J Pharmacol Sci 2012;120(3):187–195. [DOI] [PubMed] [Google Scholar]
- [44].Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci 2008;28(22):5721–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain 2009;10(10):1073–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain 2005;116(1–2):79–86. [DOI] [PubMed] [Google Scholar]
- [47].Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein DS, Kopin IJ. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology 1993;133(3):1411–1419. [DOI] [PubMed] [Google Scholar]
- [48].Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 2002;95(1–2):143–152. [DOI] [PubMed] [Google Scholar]
- [49].Levine S, Alpert M, Lewis GW. Infantile experience and the maturation of the pituitary adrenal axis. Science 1957;126(3287):1347. [DOI] [PubMed] [Google Scholar]
- [50].Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011;67(9):942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav 2006;50(5):667–680. [DOI] [PubMed] [Google Scholar]
- [52].Maniam J, Antoniadis C, Morris MJ. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front Endocrinol (Lausanne) 2014;5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Matosin N, Halldorsdottir T, Binder EB. Understanding the Molecular Mechanisms Underpinning Gene by Environment Interactions in Psychiatric Disorders: The FKBP5 Model. Biol Psychiatry 2018;83(10):821–830. [DOI] [PubMed] [Google Scholar]
- [54].Mazor M, Paul SM, Chesney MA, Chen LM, Smoot B, Topp K, Conley YP, Levine JD, Miaskowski C. Perceived stress is associated with a higher symptom burden in cancer survivors. Cancer 2019;125(24):4509–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res 1998;105(2):295–307. [DOI] [PubMed] [Google Scholar]
- [56].McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 2009;8(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Melniczek JR, Ward IL. Patterns of ano-genital licking mother rats exhibit toward prenatally stressed neonates. Physiol Behav 1994;56(3):457–461. [DOI] [PubMed] [Google Scholar]
- [58].Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 1994;32(4):197–200. [DOI] [PubMed] [Google Scholar]
- [59].Miaskowski C, Paul SM, Mastick J, Abrams G, Topp K, Smoot B, Kober KM, Chesney M, Mazor M, Mausisa G, Schumacher M, Conley YP, Sabes JH, Cheung S, Wallhagen M, Levine JD. Associations Between Perceived Stress and Chemotherapy-Induced Peripheral Neuropathy and Otoxicity in Adult Cancer Survivors. J Pain Symptom Manage 2018;56(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- [61].Naninck EF, Oosterink JE, Yam KY, de Vries LP, Schierbeek H, van Goudoever JB, Verkaik-Schakel RN, Plantinga JA, Plosch T, Lucassen PJ, Korosi A. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J 2017;31(2):505–518. [DOI] [PubMed] [Google Scholar]
- [62].Nishida K, Takeuchi K, Hosoda A, Sugano S, Morisaki E, Ohishi A, Nagasawa K. Ergothioneine ameliorates oxaliplatin-induced peripheral neuropathy in rats. Life Sci 2018;207:516–524. [DOI] [PubMed] [Google Scholar]
- [63].Parikh D, De Ieso P, Garvey G, Thachil T, Ramamoorthi R, Penniment M, Jayaraj R. Post-traumatic stress disorder and post-traumatic growth in breast cancer patients--a systematic review. Asian Pac J Cancer Prev 2015;16(2):641–646. [DOI] [PubMed] [Google Scholar]
- [64].Petrovchich I, Kober KM, Wagner L, Paul SM, Abrams G, Chesney MA, Topp K, Smoot B, Schumacher M, Conley YP, Hammer M, Levine JD, Miaskowski C. Deleterious Effects of Higher Body Mass Index on Subjective and Objective Measures of Chemotherapy-Induced Peripheral Neuropathy in Cancer Survivors. J Pain Symptom Manage 2019;58(2):252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 1957;111(4):409–419. [PubMed] [Google Scholar]
- [66].Rio-Alamos C, Oliveras I, Piludu MA, Gerboles C, Canete T, Blazquez G, Lope-Piedrafita S, Martinez-Membrives E, Torrubia R, Tobena A, Fernandez-Teruel A. Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in a genetic model of differential anxiety: Behavioral-volumetric associations in the Roman rat strains. Eur Neuropsychopharmacol 2017;27(2):146–158. [DOI] [PubMed] [Google Scholar]
- [67].Schmitt LI, Leo M, Kleinschnitz C, Hagenacker T. Oxaliplatin Modulates the Characteristics of Voltage-Gated Calcium Channels and Action Potentials in Small Dorsal Root Ganglion Neurons of Rats. Mol Neurobiol 2018;55(12):8842–8855. [DOI] [PubMed] [Google Scholar]
- [68].Silberman DM, Acosta GB, Zorrilla Zubilete MA. Long-term effects of early life stress exposure: Role of epigenetic mechanisms. Pharmacol Res 2016;109:64–73. [DOI] [PubMed] [Google Scholar]
- [69].Singh VB, Corley KC, Phan TH, Boadle-Biber MC. Increases in the activity of tryptophan hydroxylase from rat cortex and midbrain in response to acute or repeated sound stress are blocked by adrenalectomy and restored by dexamethasone treatment. Brain Res 1990;516(1):66–76. [DOI] [PubMed] [Google Scholar]
- [70].Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 2014;10(12):694–707. [DOI] [PubMed] [Google Scholar]
- [71].Soldani P, Pellegrini A, Gesi M, Natale G, Lenzi P, Martini F, Paparelli A. Gender difference in noise stress-induced ultrastructural changes in rat myocardium. J Submicrosc Cytol Pathol 1997;29(4):527–536. [PubMed] [Google Scholar]
- [72].Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull 2009;78(6):335–341. [DOI] [PubMed] [Google Scholar]
- [73].Sprowl JA, Ciarimboli G, Lancaster CS, Giovinazzo H, Gibson AA, Du G, Janke LJ, Cavaletti G, Shields AF, Sparreboom A. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc Natl Acad Sci U S A 2013;110(27):11199–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Strausbaugh HJ, Dallman MF, Levine JD. Repeated, but not acute, stress suppresses inflammatory plasma extravasation. Proc Natl Acad Sci U S A 1999;96(25):14629–14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci 2003;17(4):805–812. [DOI] [PubMed] [Google Scholar]
- [76].Tachibana Y, Kita H, Chiken S, Takada M, Nambu A. Motor cortical control of internal pallidal activity through glutamatergic and GABAergic inputs in awake monkeys. Eur J Neurosci 2008;27(1):238–253. [DOI] [PubMed] [Google Scholar]
- [77].Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience 1989;32(3):577–580. [DOI] [PubMed] [Google Scholar]
- [78].Tanishima H, Tominaga T, Kimura M, Maeda T, Shirai Y, Horiuchi T. Hyperacute peripheral neuropathy is a predictor of oxaliplatin-induced persistent peripheral neuropathy. Support Care Cancer 2017;25(5):1383–1389. [DOI] [PubMed] [Google Scholar]
- [79].Tatsushima Y, Egashira N, Kawashiri T, Mihara Y, Yano T, Mishima K, Oishi R. Involvement of substance P in peripheral neuropathy induced by paclitaxel but not oxaliplatin. J Pharmacol Exp Ther 2011;337(1):226–235. [DOI] [PubMed] [Google Scholar]
- [80].Taylor BK, Akana SF, Peterson MA, Dallman MF, Basbaum AI. Pituitary-adrenocortical responses to persistent noxious stimuli in the awake rat: endogenous corticosterone does not reduce nociception in the formalin test. Endocrinology 1998;139(5):2407–2413. [DOI] [PubMed] [Google Scholar]
- [81].Terrazzino S, Argyriou AA, Cargnin S, Antonacopoulou AG, Briani C, Bruna J, Velasco R, Alberti P, Campagnolo M, Lonardi S, Cortinovis D, Cazzaniga M, Santos C, Kalofonos HP, Canonico PL, Genazzani AA, Cavaletti G. Genetic determinants of chronic oxaliplatin-induced peripheral neurotoxicity: a genome-wide study replication and meta-analysis. J Peripher Nerv Syst 2015;20(1):15–23. [DOI] [PubMed] [Google Scholar]
- [82].Vincenzi B, Frezza AM, Schiavon G, Spoto C, Silvestris N, Addeo R, Catalano V, Graziano F, Santini D, Tonini G. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer 2013;21(5):1313–1319. [DOI] [PubMed] [Google Scholar]
- [83].Walker CD. Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am J Physiol 1995;268(5 Pt 2):R1281–1288. [DOI] [PubMed] [Google Scholar]
- [84].Walker SM, O’Reilly H, Beckmann J, Marlow N, Group EPS. Conditioned pain modulation identifies altered sensitivity in extremely preterm young adult males and females. Br J Anaesth 2018;121(3):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wilson HA, Martin ER, Howes C, Wasson CS, Newman AEM, Choleris E, MacLusky NJ. Low dose prenatal testosterone exposure decreases the corticosterone response to stress in adult male, but not female, mice. Brain Res 2020;1729:146613. [DOI] [PubMed] [Google Scholar]
- [86].Wright F, Kober KM, Cooper BA, Paul SM, Conley YP, Hammer M, Levine JD, Miaskowski C. Higher levels of stress and different coping strategies are associated with greater morning and evening fatigue severity in oncology patients receiving chemotherapy. Support Care Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012;203:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci 2019;20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]