Abstract
Protein kinases are the second most sought-after G-protein coupled receptors as drug targets because of their overexpression, mutations, and dysregulated catalytic activities in various pathological conditions. Till 2019, 48 protein kinase inhibitors have received FDA approval for the treatment of multiple illnesses, of which the majority of them are indicated for different malignancies. One of the attractive sub-group of protein kinases that has attracted attention for drug development is the family members of MAPKs that are recognized to play significant roles in different cancers. Several inhibitors have been developed against various MAPK members; however, none of them as monotherapy has shown sustainable efficacy. One of the MAPK members, called Mixed Lineage Kinase 3 (MLK3), has attracted considerable attention due to its role in inflammation and neurodegenerative diseases; however, its role in cancer is an emerging area that needs more investigation. Recent advances have shown that MLK3 plays a role in cancer cell survival, migration, drug resistance, cell death, and tumor immunity. This review describes how MLK3 regulates different MAPK pathways, cancer cell growth and survival, apoptosis, and host’s immunity. We also discuss how MLK3 inhibitors can potentially be used along with immunotherapy for different malignancies.
Keywords: Mitogen-activated protein kinase, Mixed lineage kinase 3, Cancer, Innate immunity, Adaptive immunity, T cell, Immunotherapy
Graphical Abstract:
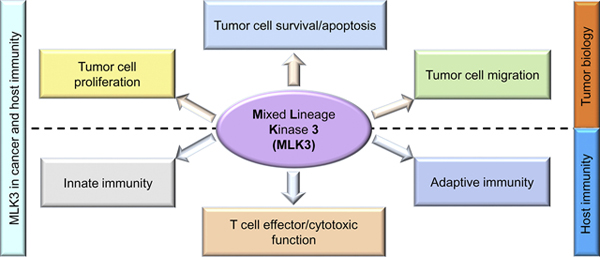
MLK3 in cancer and host immunity.Graphical representation of MLK3 regulatory function in cancer and immune cells. The MLK3 regulates cancer cell proliferation, migration, survival, and apoptosis. The MLK3 also plays a regulatory role in innate and adaptive immunity, including T cell activation, effector function, and survival. Considering the critical role of MLK3 in cancer and host immunity, MLK3 has emerged as a potential therapeutic target in cancer therapy. MLK3, mixed lineage kinase 3.
Graphical Abstract
1. Introduction
Cancer is a global health concern affecting millions of people worldwide (Bray, et al., 2018). Even though with considerable progress made over the last ten years, a total of 9.6 million cancer patients died in the year 2018, signifying that still, new strides have to be made to control the fatality from malignant diseases (Bray, et al., 2018). Despite global efforts to control cancer-associated mortality, only a 1.5% improvement in death rate over the last ten years has been achieved (Siegel, Miller, & Jemal, 2020). Several promising pre-clinical and clinical studies for cancer therapeutics have raised hope for cancer patients. Still, most of these drugs have either been unsuccessful in clinical setup or failed to show long term efficacy due to drug resistance. Several factors have been attributed leading to drug resistance in cancer including genetic and epigenetic factors, metabolic abnormalities, and immunological dysfunctions (Jardim, Groves, Breitfeld, & Kurzrock, 2017; Jo, et al., 2018; Warren, Cartmell, Garrett-Mayer, Salloum, & Cummings, 2019). It is proposed that to overcome drug resistance in cancer patients; it will be more useful to identify the dysregulated molecule(s) and devise the strategy to target the specific molecule (i.e., targeted therapy) to achieve maximum efficacy (Gotwals, et al., 2017). The targeted therapy is defined as a foundation of precision medicine, where specific molecule(s) is targeted in a certain type of cancer (Bedard, Hyman, Davids, & Siu, 2020; Valerio, et al., 2017).
The protein kinases are the second most targeted group after G-protein coupled receptors (GPCRs), and several of their inhibitors are in clinical use for various diseases, including cancer (Bhullar, et al., 2018; Sriram & Insel, 2018). The mitogen-activated protein kinases (MAPKs) is a subgroup of protein kinase family, essential for a broad range of biological functions including tumorigenesis, tumor growth, progression, and survival (Maroufi, et al., 2020; Z. Zhou, et al., 2020). The MAPK members are also involved in the regulation of innate and adaptive immunity (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020; Mercer, et al., 2020). Therefore, MAPK family members have been targeted in various cancers for therapeutic intervention. However, similar to other chemotherapeutic drugs, resistance is a significant problem with MAPKs targeting drugs also (Lu, et al., 2017).
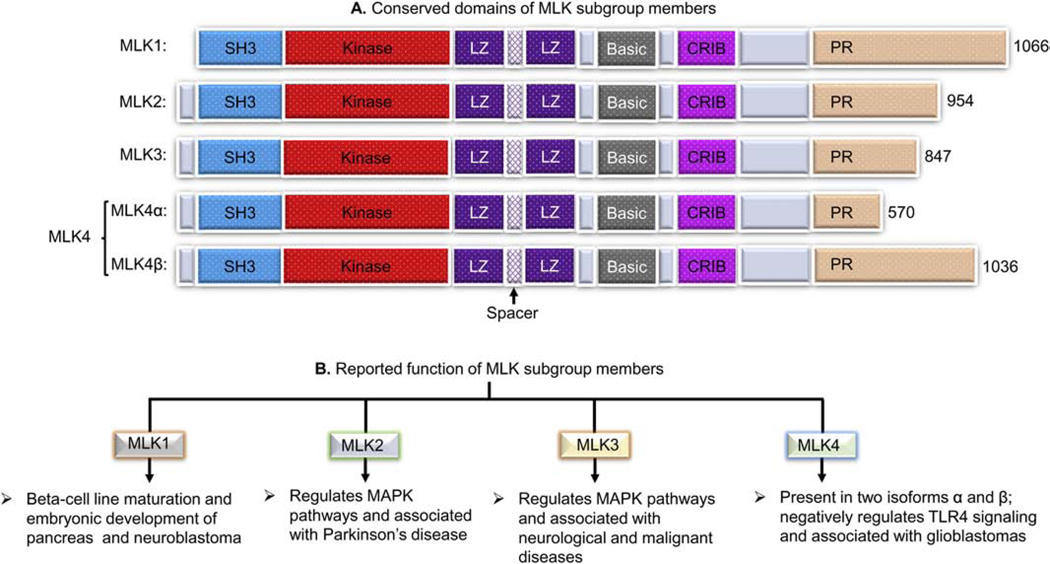
The MAPK pathways signaling are relayed in a sequential manner where mitogen-activated protein kinase kinase kinase kinase (MAP4K) is first activated in response to specific activation signal(s) and transmit the signals sequentially to mitogen-activated protein kinase kinase kinase (MAP3K), mitogen-activated protein kinase kinase (MAP2K), and finally to MAPK, leading to biological response(s) (Crawley, et al., 2017). Mixed lineage kinases (MLKs) are a MAP3K sub-family and act upstream regulators of MAP2Ks and MAPKs (Rana, et al., 1996; Tibbles, et al., 1996). The MLK members possess signature sequences of both serine/threonine and tyrosine kinases within their catalytic domains. Based on the presence of protein domains, MLK subfamily is divided into three subgroups: MLK, dual leucine zipper kinase (DLK), and ZAK groups (Brancho, et al., 2005). The MLK group members are MLK1 (i.e. MAP3K9), MLK2 (i.e. MAP3K10), MLK3 (i.e. MAP3K11), and MLK4 (i.e. MAP3K21), and share similarities in their structural domains (Dorow, Devereux, Dietzsch, & De Kretser, 1993; Dorow, et al., 1995; Ing, Leung, Heng, Tsui, & Lassam, 1994; Kashuba, et al., 2011; Katoh, Hirai, Sugimura, & Terada, 1995; Rana, et al., 1996). The protein domains of MLK1, MLK2, MLK3, and MLK4 consist, a Src homology 3 (SH3) domain, a catalytic domain, two leucine zipper (LZ) domains connected with a spacer, a basic domain, a Cdc42- and Rac-interactive binding (CRIB) domain, and a proline-rich (PR) motif (Burbelo, Drechsel, & Hall, 1995; Hirai, et al., 1997; Katoh, et al., 1995). Figure 1A shows a schematic representation of the protein domains of MLK sub-group members. The MLK1 has been reported to play a regulatory role in the pancreas’ beta-cell maturation and embryonic development and is associated with neuroblastoma’s pathogenesis (DeAizpurua, Cram, Naselli, Devereux, & Dorow, 1997; Durkin, et al., 2004; Tivnan, et al., 2011). The MLK2 is reported to regulate the JNK pathway and is associated with Parkinson’s disease (Dorow, et al., 1995; Katoh, et al., 1995; Nagao & Hayashi, 2009; Pedraza, et al., 2009; Zhong & Kyriakis, 2007). The MLK4 is present in two isoforms, MLK4 alpha (MLK4α) and MLK4 beta (MLK4β). The MLK4 is present in two isoforms, MLK4 alpha (MLK4α) and MLK4 beta (MLK4β). The MLK4 negatively regulates toll-like receptor 4 (TLR4) signaling pathways and associated with diseases like glioblastoma, breast cancer, and hepatocellular carcinoma (Bardelli, et al., 2003; Kim, et al., 2016; Li, Zuo, Wang, & Hu, 2019; Marusiak, et al., 2019; Seit-Nebi, Cheng, Xu, & Han, 2012) (Figure 1B). Among various members of MLKs, MLK3 is the most studied member for its regulatory role in MAPKs activation, neurological disorders, and malignant diseases (Das, et al., 2019; Das, et al., 2015; Lin, et al., 2017; Rattanasinchai, Llewellyn, Conrad, & Gallo, 2017). The MLK3 activates MAP2K family members, MKK4 and MKK7 and their downstream MAPK members, c-Jun N-terminal kinase (JNK), p38 MAPK and extracellular signal-regulated kinase (ERK) leading to various biological function (Chadee & Kyriakis, 2004a; Rana, et al., 1996; Tibbles, et al., 1996). The MLK3 regulates a broad range of cellular functions, including protein trafficking, protein scaffolding, cytoskeleton rearrangement, ion transport, and endocytosis (Amako, et al., 2013; Handley, et al., 2007; Willoughby, Perkins, Collins, & Whitmarsh, 2003; H. Zhang, et al., 2004). In cancer cells, MLK3 plays an essential role in cancer cell proliferation, migration, invasion, survival, and apoptosis (Das, et al., 2019; Rangasamy, et al., 2012; Rattanasinchai, et al., 2017). The MLK3 is also reported to induce apoptosis in cancer cells in a contextual manner; however, in certain cancer cell types, MLK3 expression and activity are associated with apoptosis resistance. In HER2+ breast cancer cell lines and tumors, the diminished MLK3 expression and kinase activity is associated with Herceptin (i.e., Trastuzumab) resistant (Das, et al., 2015). Recently, we have reported that MLK3 kinase activity regulates adaptive immune systems, including activation, effector function, and survival of immune cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). The MLK3 beside regulating peripheral immune cells also regulates tumor-infiltrating cytotoxic T cell (CTL) effector’s function and survival (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). Considering the paradoxical role of MLK3 on cancer cells and host immunity, the inhibitors targeting MLK3 could induce anti-tumor effects and promote host immunity. In this review, we discuss the journey of MLK3 from its discovery to present, highlight regulation of MAPKs by MLK3, and its impact on cellular functions. This review also discusses the regulatory role of MLK3 in cancer cell growth, survival, and apoptosis resistance. We also describe the recently discovered regulatory function of MLK3 in innate and adaptive immunity, specifically its role in T cell activation, effector function, and survival. Moreover, we also discuss the potential of repurposing the MLK3 inhibitors/agonists in targeting the tumor cells and plan to use in cancer immunotherapy.
Figure 1: Conseverd domains of mixed lineage kinases (MLKs) and brief details.
(A) Representative diagram of protein domains of MLK1, MLK2, MLK3 and MLK4. The conserved doamins of MLK subgroup consist of a SH3 domain, a catalytic domain, two LZ domains connected with a spacer, a basic domain, a CRIB domain, and a proline rich (PR) c-terminal tail. (B) Reported function of MLK subgroup members. Where: SH3, Src homology 3; LZ, leucine zipper; CRIB, Cdc42- and Rac-interactive binding; PR, proline-rich.
2. MLK3 journey from its discovery to present
On a comprehensive annotation, the MLK3 journey (from 1994 to 2020) can be separated into three significant advancements (Figure 2). The MLK3, also known as SPRK, PTK1, and MAP3K11, was cloned in 1994 from the human megakaryocytic cell line, CMK11–5 (Gallo, et al., 1994). However, the physiological role of MLK3 was not known until 1996 when it was demonstrated that the MLK3 functions as MAP3K in the JNK pathway (Rana, et al., 1996; Tibbles, et al., 1996). Furthermore, the function of MLK3 was demonstrated in neuronal apoptosis and a small molecule inhibitor of MLK, CEP-1347 was used that prevented neuronal apoptosis (Maroney, et al., 2001). Of late, MLK3 has been implicated as a potential target in several cancers, including breast, ovarian and colorectal cancer (Das, et al., 2019; Rattanasinchai, et al., 2017; Schroyer, Stimes, Abi Saab, & Chadee, 2018). In this section, we will briefly describe some of the significant findings on MLK3 from 1994 up to the present.
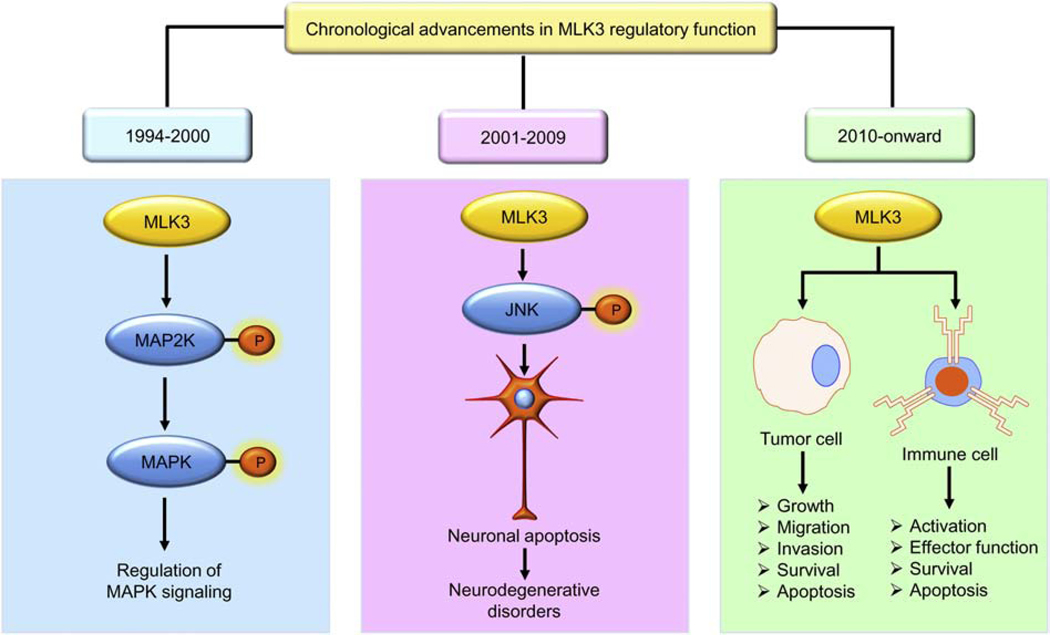
Figure 2: MLK3 regulatory function: Chronological advancements.
Chronologically, progression made in MLK3 biology can be separated into three major advancements. From 1994–2000, MLK3 was established as an activator of MAP2K and MAPK family members; then, from 2001–2009, the pathological function of MLK3 was established in neurodegenerative diseases. From 2010 onward, MLK3’s role in cancer and adaptive immunity, and cancer immunotherapy has been established.
2.1. 1994 to 2000: MLK3 as an activator of MAPKs
The MLK3 was reported for the first time in 1994, in three different research articles describing MLK3, as a novel kinase member with putative signature sequences of both Serine/Threonine and Tyrosine kinases within its kinase domain (Ezoe, Lee, Strunk, & Spritz, 1994; Gallo, et al., 1994; Ing, et al., 1994). The sequence analyses of MLK3 also showed that it contains a well-defined Src homology 3 (SH3) and leucine zipper protein domains (Ing, et al., 1994). The protein domains are evolutionarily conserved regions associated with the functional activity of a given protein. Like other MLK subfamily members, MLK3 also contains multiple interacting domains, including a SH3, two LZs connected with a spacer, a basic domain, a CRIB domain, and a PR region (Burbelo, et al., 1995; Hirai, et al., 1997; Katoh, et al., 1995). The SH3 domain is located at the N-terminus, whereas as the PR region is located at the C-terminus (Figure 1A). The catalytic domain of MLK3 is present, just after the SH3 domain. The CRIB motif of MLK3 interacts with Cdc42 and is reported to induce Cdc42-mediated activation of MLK3 (Bock, Vacratsis, Qamirani, & Gallo, 2000). It is also reported that the SH3 domain plays a role in the auto-inhibition of MLK3 via interacting with the proline containing sequences, which is present between LZ and CRIB domain of MLK3 (Du, Bock, Schachter, Chao, & Gallo, 2005). After that, in in vitro binding assays, it was demonstrated that MLK3 binds with small G-proteins, Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division cycle 42 (Cdc42) via a CRIB domain (Burbelo, et al., 1995). Further study showed that MLK3/SPRK is a potent activator of MAP2K members including SEK1 (i.e., MKK4), MKK3 and MKK6; and SEK1/MKK4 activation by MLK3 potently activates stress-activated protein kinase (SAPK/JNK) and modestly the p38MAPK (Rana, et al., 1996; Tibbles, et al., 1996). Further study demonstrated that the binding of Rac1 and Cdc42, with MLK3, was necessary for MLK3-directed activation of the JNK-c-Jun signaling axis (Teramoto, et al., 1996). Later, it was shown that MLK3 dimerization via tandem leucine zippers (LZ) was one of the mechanisms of MLK3 activation (Leung & Lassam, 1998). However, this report was never confirmed by others, and therefore it is still not clear whether dimerization via LZ indeed has any role in MLK3 activation? The role of MLK3 has also been reported in guanine-nucleotide exchange protein (C3G)-mediated JNK1 activation. The study showed that the co-transfection of MLK3 kinase-deficient and C3G plasmid in HEK-293T cells decreased the C3G-mediated JNK1 activation (Tanaka & Hanafusa, 1998). Interestingly, a mechanistic study conducted in HeLa cells showed that MLK3 kinase activation loop is necessary for its activation and downstream signaling (Leung & Lassam, 2001). These results highlight some of the major findings, specifically defining MLK3 as MAP3K member and its regulatory role on JNK activation and other MAPKs.
2.2. 2001 to 2009: MLK3 in neurological disorders
The functional role of MLK3 has been well documented in neurodegenerative diseases, including Parkinson’s disease, Alzheimer’s disease, and acquired immunodeficiency syndrome (AIDS) associated dementia (Bodner, et al., 2002; Trotter, et al., 2002). The role of MLK3 was shown in neuronal cell death mediated via JNK activation (Mota, Reeder, Chernoff, & Bazenet, 2001; Z. Xu, Maroney, Dobrzanski, Kukekov, & Greene, 2001). The MLK3 inhibition using CEP-1347 (KT7515) suppressed the MLK3-JNK axis and finally attenuated neuronal apoptosis (Maroney, et al., 2001). Further study demonstrated the role of MLK3 in human immunodeficiency virus type 1 (HIV-1) protein gp120IIIB-induced neuronal cell death. The gp120IIIB-induced neuronal cell death is associated with AIDS-associated dementia. Interestingly, pharmacological inhibition of MLK3 by CEP-1347 attenuated gp120IIIB-induced neuronal cell death that ultimately resulted in improved disease conditions (Bodner, et al., 2002). Further study suggested that HIV-1 proteins, Tat, and gp120 can activate MLK3 kinase activity in primary neurons, and Tat- and gp120- mediated activation of MLK3 was associated with neurotoxicity (Sui, et al., 2006). The role of MLK3 has also been reported in dopaminergic neurons cell death. The dopaminergic neurons release neurotransmitters, dopamine, which is necessary for the number of functions, including body movement. Therefore, lack of dopamine synthesis due to dopaminergic neuronal loss leads to a health condition called Parkinson’s disease (PD) (Wu, et al., 2020). A study carried out in the animal model of PD showed that the neurotoxin 6-hydroxydopamine (6OHDA) was able to activate MLK3 downstream JNK-c-Jun axis and the inhibition by pan-MLK inhibitor attenuated c-Jun activation and dopaminergic neuronal loss (Ganguly, et al., 2004). The potassium ions play an essential role in neurotransmission, and any disturbance in potassium ions flux is known to have significant effects on neuronal excitability (Sibille, Dao Duc, Holcman, & Rouach, 2015). The MLK3 has been implicated in potassium ions-induced neuronal cell death. A study conducted in the rat model showed that MLK3 plays an important role in low potassium ion-induced apoptosis in rat cerebellar granule neurons. The mechanistic study revealed that low potassium promotes MLK3-MKK7-JNK pathway activation and neuronal apoptosis (Trotter, et al., 2002). The Serine/Threonine protein kinase, glycogen synthase kinase 3 beta (GSK3β) is reported to play an essential role in several neurological pathologies (F. Zhang, M. Gannon, et al., 2020). Interestingly, the GSK3β has been reported to act as an upstream activator of MLK3, suggesting that targeting MLK3 might be helpful to block GSK3β-induced neurological disorders (R. Mishra, et al., 2007). These results highlight some of the significant findings related to the role of MLK3 in neurodegenerative diseases from 2001 to 2009.
2.3. 2010 onward: MLK3 in malignancies
Although some initial studies defining the role of MLK3 in cancer were reported before 2010, however, majority of research articles in this area accelerated from the year 2010 (Kraus, Levy, Hanoch, Naor, & Seger, 2004). Since 2010, the role of MLK3 was defined in several cancers, including breast, ovarian, liver, and colorectal cancers. The gene mutations in MAPK members are implicated in oncogenesis. The mutations in MLK3 were reported in gastrointestinal carcinomas with microsatellite instability (MSI) (Velho, et al., 2010). These mutations in MLK3 were associated with mismatch repair deficiency. Interestingly, heterozygous somatic and tumor-specific MLK3 mutations were reported in 14.4% primary gastrointestinal tumors, and most of these mutations caused cell transformation and tumorigenesis in animals (Velho, et al., 2010). The role of MLK3 in metastasis and cell migration via the induction of matrix metalloproteinases (MMPs) was reported in gastric and ovarian cancer (Zhan, et al., 2012). The MMPs are known to promote cancer cell migration and metastasis. The mechanistic study showed that gastric cancer cells treated with amidated gastrin induced MLK3 kinase activity and its downstream JNK1/c-Jun axis, leading to activation of MMP7 and increased cancer cell migration (Mishra, Senthivinayagam, Rangasamy, Sondarva, & Rana, 2010). Similar to migration, the invasion is another oncogenic property of a cancer cell for its expansion to the adjacent area (Ritch, Brandhagen, Goyeneche, & Telleria, 2019). The MLK3 plays a regulatory role in the invasion of cancer cells. The mechanistic analysis showed that oxidative stress in colon cancer cells induces activation of ERK1/2 and MLK3. The activation of MLK3 and ERK1/2 is associated with B-Raf activation, which ultimately increases the invasion potential of cancer cells (Schroyer, et al., 2018). Like migration and invasion, the cancer cells also possess the property of rapid and uncontrolled cellular proliferation. The MLK3 is reported to regulate cancer cell proliferation and cell cycle. The crosstalk between MLK3 and micro RNA (miRNA) was also reported in esophageal and liver cancers (Byrnes, et al., 2016; F. Zhang, Y. Zhu, et al., 2020). In esophageal cancer cells, down-regulation of miR-199–5p induced MLK3-mediated cellular proliferation whereas, in liver cancer cells, miR-520b and MLK3 axis regulates cancer cell migration (Byrnes, et al., 2016; F. Zhang, Y. Zhu, et al., 2020). The majority of studies published in breast cancer showed that MLK3 plays a crucial role in cell survival, migration, and proliferation (Das, et al., 2019; Rattanasinchai, et al., 2017; Schroyer, et al., 2018). In human epidermal growth factor receptor 2 positive (HER2+) breast cancer cells, MLK3 kinase activity was downregulated due to HER2 amplification and provides survival advantage to breast cancer cells (Das, et al., 2015). In estrogen receptor positive (ER+) breast cancer cells, MLK3 kinase activity was downregulated by estrogen and also provides cancer cell survival (Rangasamy, et al., 2010; Viswakarma, et al., 2017). Recently, in triple negative breast cancer cells (TNBC), p21-activated kinase 1 (PAK1) was identified as a downstream target of MLK3, and importantly, MLK3 was able to activate PAK1 and TNBC tumorigenesis (Das, et al., 2019). Recently MLK3 has also been shown to regulate immune cells, including T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). It is reported that MLK3 inhibits T cell activation and effector function, and prevent cluster of differentiation 70 (CD70)-mediated apoptosis in CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). In these very recent reports, authors demonstrated that combined inhibition of MLK3 (by URMC-099) and CD70 (by anti-CD70 monoclonal antibody) was able to significantly reduce TNBC tumor burden in a pre-clinical animal model (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The MLK3 that acts as a potent activator of JNK and shown to promote neurodegeneration, has now emerged as an essential player in cancer and host immunity.
3. Role of MLK3 in cellular physiology
The MLK3 regulates several cellular physiology, including protein scaffolding, cytoskeleton rearrangement, ion transport, and phagocytosis (Amako, et al., 2013; Marker, Tremblay, Puccini, Barbieri, Gantz Marker, et al., 2013; Teramoto, et al., 1996).
3.1. Protein trafficking and scaffolding
Protein trafficking is an essential physiological process required for the transfer of proteins to different cellular organelles or extracellular milieu (Tan, et al., 2020). The protein scaffolding is a cellular process where two or more proteins from a complex to deliver and amplify specific cellular signaling (Greenwald, Redden, Dodge-Kafka, & Saucerman, 2014). The MLK3 involvement in protein scaffolding was demonstrated by its association with heat shock protein 90 (Hsp90). The MLK3 forms a complex with Hsp90, and its kinase-specific co-chaperone, p50 (cdc37) through the MLK3 catalytic domain in an activity-independent manner (H. Zhang, et al., 2004). The JNK-interacting protein (JIP) is an important player in JNK-mediated signaling (Willoughby, et al., 2003). The MLK3 binds with JIP-1 and JIP-2 scaffolds and was reported to be necessary for JNK activation, suggesting that protein scaffolding plays an important role in MLK3 directed activation of JNK (Willoughby, et al., 2003). The cytoplasmic filamentous proteins are a key component of the cytoskeleton, and modulation of these proteins are associated with cytoskeleton rearrangement (Sanghvi-Shah & Weber, 2017). The chemotherapeutics agents-mediated cytoskeletal rearrangement is vital for the downstream anti-tumor responses, including apoptosis in cancer cells (Al Absi, et al., 2018). It was reported that MLK3 modulates the rearrangement of cytoplasmic microtubules during the cell cycle (Swenson, Winkler, & Means, 2003). The MLK3 phosphorylation and activation were seen during the G2/M phase of the cell cycle, and this activation was associated with cytoplasmic microtubules rearrangement. Microtubule rearrangement is crucial for cell cycle progression, and thus, MLK3-mediated cytoskeleton rearrangement is considered necessary for cell cycle progression (Swenson, et al., 2003). These studies showed the important role of MLK3 in cell signaling via protein scaffolding and cell cycle progression via cytoskeleton rearrangement.
3.2. Potassium ion transport
The “ion transporters” which regulates the movement of ions in the cells are the crucial biological process for the proper functioning of cells (Duhm, Gobel, & Beck, 1983; Hahn, et al., 2017; Zou, et al., 2018). The movement of ions through channels is bi-directional. The movement can transpire either inward or outward within the cell or between the cells (Ke, Timin, & Stary-Weinzinger, 2014; Muramatsu, Kumamoto, & Fujiwara, 1978). The ion channels play a significant role in the proper transport of different ions for diverse cellular activities, including neuronal function and apoptosis. Interestingly, MLK3 was reported in regulating the potassium ion (K+) channel (Amako, et al., 2013). The K channel, Kv2.1 present in neuron cells, regulates cellular function like neuronal excitation and apoptosis. The MLK3 increases Kv2.1 channel activity and, thus, potassium channel function (Amako, et al., 2013). The role of MLK3 in K-channel was described in the response of oxidative stress (Mankouri, et al., 2009). The MLK3 was reported to be activated by oxidative stress, and that, in turn, activated downstream target, p38MAPK. In this report, they showed that activated p38MAPK phosphorylates transient receptor potential (TRP) V1 at the C-terminus on Serine 800 (S800) residue. The insertion of phosphorylated channels was seen on the plasma membrane, leading to the efflux of K ion (K+). The outward K+ flux induced apoptosis in cells infected with the hepatitis C virus (HCV) (Mankouri, et al., 2009). The role of MLK3 in regulating ion channels is limited and more study can undoubtedly provide new information about the roles of MLK3 in regulating other than K+ ion channel.
3.3. Phagocytosis
The endocytosis is a cellular process where materials are transported inside the cells via a membranous vesicle. The endocytosis includes two processes, phagocytosis, and pinocytosis (Chambers & Thompson, 1976; Marinovic, et al., 2019). Pinocytosis is a process where cells intake liquids, whereas, in phagocytosis, cells engulf solid substances (Chambers & Thompson, 1976; Dima, Assadpour, Dima, & Jafari, 2020). The MLK3 is reported to regulate phagocytosis in microglia and macrophages (Mφ) (Marker, Tremblay, Puccini, Barbieri, Gantz Marker, et al., 2013; Tomita, Kabashima, et al., 2017; Tomita, Kohli, et al., 2017). In a mouse microglia cell line, BV-2, Tat (a neurotoxic HIV-1 protein) exposure increased the phagocytosis. However, inhibition of MLK3 by using its pharmacological inhibitor, URMC-099, decreased Tat-induced phagocytosis. Furthermore, MLK3 inhibition reduced the release of the inflammatory cytokines by microglia in response to Tat treatment. Most importantly, the MLK3 inhibition protected neurites loss by attenuating microglial phagocytosis (Marker, Tremblay, Puccini, Barbieri, Gantz Marker, et al., 2013). The role of MLK3 in the regulation of microglial phagocytic capacity was also explored in a mouse model of Alzheimer’s disease. The MLK3 inhibitor, URMC-099, increased the expression of scavenger receptors, including cluster of differentiation 36 (CD36) and the cluster of differentiation 47 (CD47) in mouse microglia. The scavenger receptor expression is directly associated with microglial phagocytosis potential, suggesting that MLK3 inhibition could promote microglial phagocytosis in a mouse model of Alzheimer’s disease (Dong, et al., 2016). Apart from microglial phagocytosis, MLK3 was also implicated in phagocytosis in Mφ. In the liver, the Mφ with phagocytosis features was directly correlated with the expression of macrophage galactose-specific lectin (Mac-2) (Idrissova, et al., 2015). The C57BL/6 mice fed with a diet high in saturated fats, fructose, and cholesterol (i.e., FFC) showed increased Mac-2 immuno-positive area in the liver; however, mice fed with FFC and treated with URMC-099 demonstrated diminished Mac-2 immuno-positive area in the liver (Tomita, Kohli, et al., 2017). These results suggest that MLK3 plays a regulatory role during phagocytosis in microglial and Mφ cells.
3.4. ROS generation and mitochondrial function
Reactive oxygen species (ROS) are chemically reactive chemical species comprising oxygen. The cellular ROS are formed as a natural derivative of the normal metabolism of oxygen and have critical roles in cell signaling and homeostasis. However, under environmental stress, ROS levels can increase dramatically and may result in significant damage to cell structure (McCarthy, Somayajulu, Sikorska, Borowy-Borowski, & Pandey, 2004; Roux, et al., 2019). The ROS are recognized to regulate MAPK signaling and thus influence various cellular functions (Y. Liu, et al., 2020). The MAPK members, JNK and ERK are regulated by ROS both in the healthy or neoplastic cells (Changchien, et al., 2019; B. Liu, et al., 2020). It was reported that low ROS activates ERK, however high ROS promotes MLK3-mediated JNK activation suggesting that MLK3 could play a differential regulatory role, depending on cellular ROS levels (Lee, Hwang, Shin, Kwon, & Cho, 2014). In the human prostate cancer cell line, PC-3 treated with genipin, a product derived from Gardenia jasminoides; it was shown that ROS acts as an upstream regulator of MLK3. The genipin treated PC-3 cells showed increased MLK3 phosphorylation/activation, which was associated with alteration in mitochondrial membrane potential and release of cytochrome c that ultimately triggered apoptosis in the PC-3 cells (Hong & Kim, 2007). In pancreatic beta cells, the MLK3 activation by cytokine, IL-1β induced apoptosis by modulating the integrity of the mitochondrial membrane. The MLK3 stabilized TRB3, a pseudokinase, and altered Bax conformation, and increased its permeability through the outer mitochondrial membrane (Humphrey, et al., 2010). In summary, these results suggest an intricate role of MLK3 in ROS signaling and mitochondrial functions in cellular homeostasis.
4. Role of MLK3 in cancer cell growth, survival, and apoptosis
The MLK3 is overexpressed in several malignancies, including breast, cervical, and melanomas, and regulates cancer cell growth and survival (J. Chen, Miller, & Gallo, 2010; Ma, Cheng, & Zeng, 2019). The MLK3 protein expression in human breast cancer cell lines, MCF-7, T47D, SK-BR-3, and MDA-MB-231 were found significantly higher than MLK3 protein expression in non-tumorigenic epithelial cell lines, 184B5, and MCF10A (J. Chen, et al., 2010). In cervical cancer cell lines, HeLa and SiHa, MLK3 gene (i.e., MAP3K11) expression were higher than healthy cervical epithelial squamous cells (Ma, et al., 2019). In human melanoma cell lines, A375, Me1 Ho, Me1 Ju, and Me1 Im, the MLK3 gene and protein expression were more compared to normal immortalized epidermal melanocyte (J. Zhang, et al., 2014). In glioblastoma cell lines, LN229, LN18, and A172, the protein expression of MLK3 were upregulated in comparison to normal human astrocytes (Misek, et al., 2017). To understand the clinical relevance of MLK3 in various cancers, we analyzed MLK3 gene expression in tumor sections from bladder, brain, ovarian, breast, and colorectal cancer patients using publicly available datasets (the cancer genome atlas, TCGA) using Oncomine Platform. (Thermo Fisher, Ann Arbor, MI). Indeed, the MLK3 gene is overexpressed in bladder, brain, ovarian, and breast cancer; however, in colorectal cancer, MLK3 gene expression is downregulated (Figure 3). The TCGA dataset analyses suggest a differential MLK3 gene expression in various malignancies, and perhaps there might be functional heterogeneity of MLK3 in different cancers. In breast cancer subtypes, differential MLK3 activity has been reported in triple negative (i.e., ER−/PR−/HER2−) and HER2 positive (ER−/PR−/HER2+) breast cancer. The Immunohistochemical analyses in human breast tumors showed that MLK3 activity is less in HER2+ tumors in comparison to TNBC (Das, et al., 2015). Furthermore, the protein expression and activity of MLK3 decreases with increase in disease stage of HER2+ breast cancer (Das, et al., 2015). The MLK3 plays an important role in regulation of cancer cell proliferation, migration, apoptosis, and apoptosis resistance. Emerging studies also suggest that MLK3 function is important for host immunity, tumor microenvironment (TME) and tumor infiltrating lymphocytes (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020).
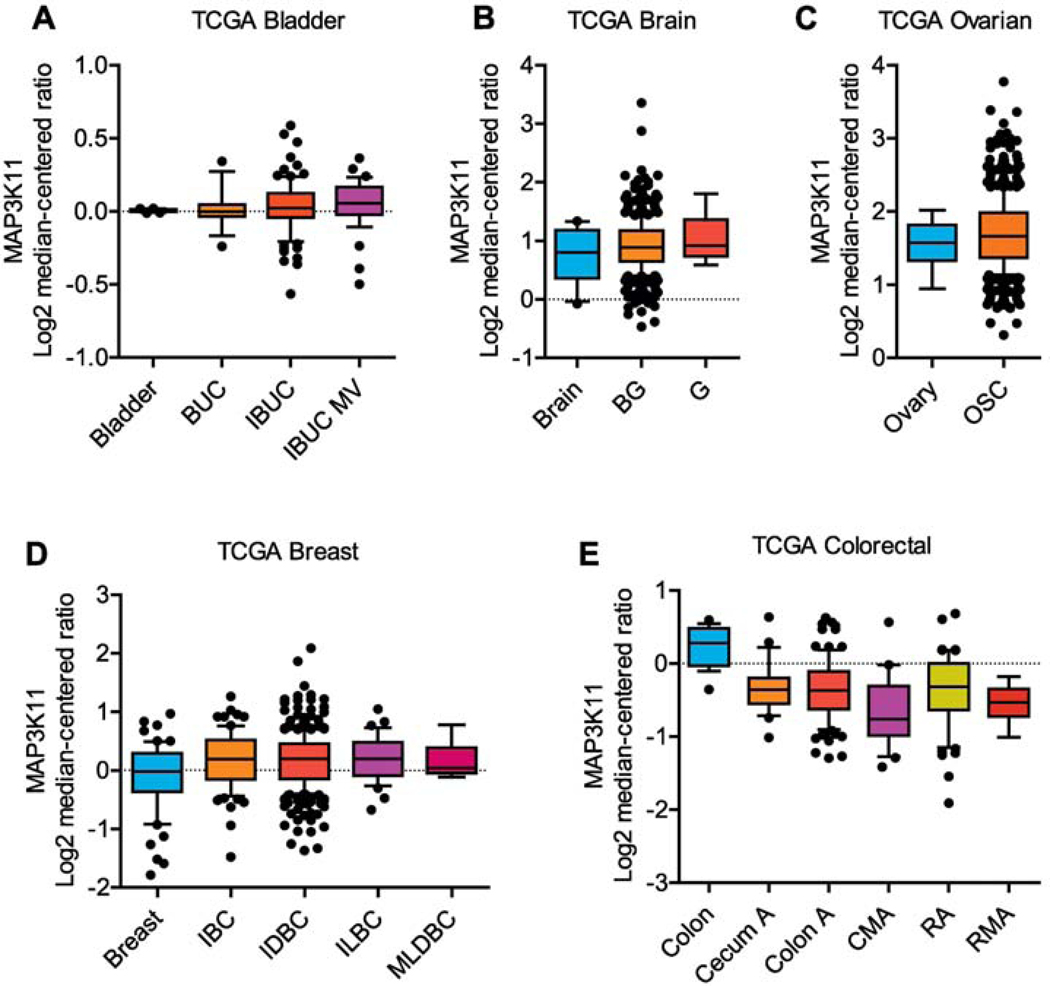
Figure 3: MLK3 gene (i.e. MAP3K11) expression in solid malignancies.
The MAP3K11 expressions were analyzed in bladder, brain, ovarian, breast, and colorectal cancers using the cancer genome atlas (TCGA) with the Oncomine™ Platform (Thermo Fisher, Ann Arbor, MI). (A) The MAP3K11 gene expressions in bladder cancer, where bladder (n=24), BUC (n=14), IBUC (n=99) and IBUC MV (n=38). (B) The MAP3K11 gene expressions in Brain cancer, where brain (n=10), BG (n=542), and G (n=5). (C) The MAP3K11 gene expressions in ovarian cancer, where ovary (n=8) and OSC (n=586). (D) The MAP3K11 gene expressions in breast cancer, where breast (n=61), IBC (n=76), IDBC (n=389), ILBC (n=36) and MLDBC (n=7). (E) The MAP3K11 gene expressions in colorectal cancer, where colon (n=19), cecum A (n=24), colon A (n=102), CMA (n=20), RA (n=60) and RMA (n=6). Where, BUC, urothelial bladder carcinoma; IBUC, infiltrating bladder urothelial carcinoma; MV, micropapillary variant; MLK3, mixed lineage kinase 3; BG, brain glioblastoma; G, glioblastoma; OSC, ovarian serous cystadenocarcinoma; IBC, invasive breast carcinoma; IDBC, invasive ductal breast carcinoma; ILBC, invasive lobular breast carcinoma; MLDBC, mixed lobular and ductal breast carcinoma; Cecum A, cecum adenocarcinoma; Colon A, colon adenocarcinoma; CMA, colon mucinous adenocarcinoma; RA, rectal adenocarcinoma; RMA, rectal mucinous adenocarcinoma.
4.1. Cellular proliferation
Cellular proliferation is a vital biological phenomenon required for cell growth and survival. In normal and healthy cells, cellular proliferation is a controlled phenomenon; however, in cancer cells, it is abnormal and uncontrolled (Gong, Yang, Wang, Wang, & Zhang, 2020). The MAPKs are known to play a crucial role in the regulation of cellular proliferation in various cancer (G. R. Zhou, Huang, Sun, & Zhang, 2020). Being upstream regulator of MAPK members, MLK3 has also been demonstrated a critical role in the regulation of cancer cell proliferation (Chadee & Kyriakis, 2004a; Rangasamy, et al., 2012). The MLK3 is known to regulate B-Raf activation and ERK-mediated cellular proliferation (Chadee & Kyriakis, 2004a). In human melanoma cell lines, miR 125b targets MLK3 to inhibit the JNK pathway and regulates cellular proliferation (J. Zhang, et al., 2014). The MLK3 also regulates PAK1 mediated cellular proliferation in breast cancer (Das, et al., 2019). Apart from breast cancer and melanoma, MLK3 has also been reported to regulate pancreatic, colon, lung, and ovarian cancer cell proliferation (Chadee & Kyriakis, 2004a, 2004b; Chandana, Leece, Gallo, Madhukar, & Conley, 2010; Zhan, et al., 2011). The MLK3 inhibition using K252a or genetic ablation using MLK3 specific siRNA in pancreatic cancer cells (i.e., MiaPACa-2 and Panc-1) decreased cellular proliferation in a dose-dependent manner (Chandana, et al., 2010). The MLK3 has also been reported to be involved in KRAS and neurofibromatosis-1, and −2 (NF-1 and NF-2) mediated cellular proliferation in malignant schwannoma cell line, ST88–14 and murine osteosarcoma cell line, F3439, respectively (Chadee & Kyriakis, 2004a, 2004b). The mechanistic study showed that the genetic ablation of MLK3 attenuates serum-stimulated tumor proliferation in tumors with oncogenic KRAS or tumor where NF-1 is functionally inactive or tumor with NF-2 mutation (Chadee & Kyriakis, 2004a, 2004b). The NF-2 is a tumor suppressor gene, and a decrease of NF-2 is associated with increased tumorigenesis and proliferation in various cancers, including breast, hepatic, skin, clear cell renal cell carcinoma, prostate, and colorectal cancer (Petrilli & Fernandez-Valle, 2016). Moesin-ezrin-radixin-like protein (Merlin, also known as schwannomin) is a protein encoded by the NF-2 gene and associated with MLK3 mediated cellular proliferation (Chadee & Kyriakis, 2004a; Petrilli & Fernandez-Valle, 2016). Studies using pancreatic, lung, and breast cancer cell lines suggest that Merlin inhibition decreases MLK3 activity, MLK3 interaction with Cdc42 and ultimately leads to attenuated cellular proliferation (Zhan, et al., 2011). Collectively, MLK3 plays an essential role in the cellular proliferation of cancer cells. Since MLK3 is differentially expressed in cancers and therefore, an in-depth study is required to explore MLK3 regulated cellular proliferation in various cancer.
4.2. Migration and invasion
The intrinsic properties of tumors decide whether a tumor is benign or malignant (M. L. Chen, Li, Wei, Qi, & Sun, 2019). The benign tumors are localized at their site of origin and usually harmless and lack invading capacity to the neighboring tissues; however, the malignant tumors could migrate from its original location to the neighboring tissue or distal body parts. The invading process of the malignant tumors to the surrounding cells is known as invasion, whereas the journey of the cancer cell to distal locations is known as metastasis. The process of invasion and metastasis is critical for the severity of the malignant diseases, and interestingly, MLK3 was reported to play a critical role in cancer cell invasion and migration (Rattanasinchai, et al., 2017; Schroyer, et al., 2018). It was shown that human breast cancer cell lines MCF-7, T74D, SK-BR-3, and MDA-MB-231 overexpressing MLK3 were associated with increased migratory potential (J. Chen, et al., 2010). The MLK3 has recently been reported to activate oncogenic PAK1 via phosphorylation on the Ser204 site. The overexpression of phospho-deficient PAK1 (i.e., PAK1 S204A) suppressed the migration and invasion of breast cancer cells (Das, et al., 2019). In a comparative study for MLK3 expression in primary and metastatic melanoma, the MLK3 gene expression was significantly higher in metastatic melanomas compared to primary melanomas. Further analysis in human melanoma cell lines suggested that the target of miR 125b, MLK3-cJun axis was necessary for cancer cell migration (J. Zhang, et al., 2014). Similarly, in glioblastoma cells, MLK3 was reported to play an essential role in cancer cell migration. The mechanistic study demonstrated that the epidermal growth factor (EGF)-induced migration and invasion of glioblastoma cells were abrogated upon MLK3 inhibition via downstream JNK (Misek, et al., 2017). These results suggest that the MLK3-JNK axis is crucial for cancer cell migration, and targeting MLK3 might decrease cancer cell metastasis.
4.3. Apoptosis
The biological process that leads to controlled cell death is known as programmed cell death or apoptosis. Programmed cell death can be classified as extrinsic or intrinsic cell death mechanisms. In an extrinsic pathway, the death stimuli received by the receptor, like the TNFR-1 receptor (i.e., FAS), recruits multi-protein complex, which eventually induces caspase-8 activity to promote controlled programmed cell death (Pobezinskaya & Liu, 2012). During intrinsic apoptosis mechanism, the extrinsic signals are relayed to mitochondria and increased pro-apoptotic molecules, Bax and Bak impinge on the mitochondria to ultimately induce cell death (Kuwana, et al., 2020). The Bax, and Bak oligomerize on the mitochondrial membrane and induce the release of cytochrome c and apoptosis-inducing factor (AIF) (Tait & Green, 2013). The released cytochrome c binds with apoptotic protease activating factor 1 (APAF1) in cytosol and form apoptosome, which ultimately induces caspase 9 activation (M. Zhou, et al., 2015). The caspase-8 and caspase-9 induce effector caspase-3, leading to apoptosis (McComb, et al., 2019). In cancer cells, dysregulated extrinsic and intrinsic apoptosis pathways favor cancer cell survival and apoptosis resistance. Importantly, MLK3 was reported to participate both in the extrinsic and intrinsic programmed cell death pathways. The role of MLK3 in apoptosis has been reported in ER+ and ER−, HER2+ and triple negative breast cancers (Das, et al., 2015; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Rangasamy, et al., 2010). The MLK3 activity was significantly higher in ER− compared to ER+ breast tumors, suggesting that perhaps MLK3 activity is suppressed by estrogen. Further analysis revealed that, indeed, estrogen (via ER) downregulated MLK3 kinase activity through PI3K-AKT pathway activation. The inhibition of MLK3 activity by estrogen in ER+ breast cancer was associated with decreased apoptosis (Rangasamy, et al., 2010). In HER2+ breast cancer cells, MLK3 kinase activity was required for apoptosis induction. A study in HER2+ breast cancer cells showed that inhibition of MLK3 kinase activity was due to HER2 activation via HER2-HER3 hetero-dimerization. Additional analyses suggested that HER2-HER3 heterodimer can inhibit MLK3 kinase activity (via PI3K-AKT pathway activation) that ultimately suppressed Trastuzumab-induced cell death in HER2+ breast cancer (Das, et al., 2015). Recently, combined blockade of MLK3 and CD70 has been reported in murine TNBC, 4T1 xenografts. It has been seen that combined inhibition of MLK3 by its pharmacological inhibitor, URMC-099 and anti-CD70 mab induces intrinsic apoptosis, as evidenced by increased Bax and decreased Bcl-2 protein expression in 4T1 tumor (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The role of MAPK family members, JNK1/2 are important in cell death. It was reported that JNK-mediated cell death is a coordinated process that involves regulatory molecules, including MLK3 and plenty of SH3 (POSH). It was observed that endogenous protein expressions of MLK3 and POSH were upregulated during JNK-mediated apoptosis (Z. Xu, Kukekov, & Greene, 2005). In cervical cancer, the MLK3 gene and protein expressions were higher compared to normal cervical epithelial squamous cells. The in vitro studies using HeLa and SiHa cell lines showed that knockdown of MLK3 could attenuate survival and induce apoptosis via pro-apoptotic protein Bax. The Bax is reported to induce intrinsic apoptosis associated with mitochondrial dysfunction (Nutt, et al., 2002). Interestingly, targeting MLK3 promoted mitochondrial apoptosis in cancer cells. An in vitro study showed that upon MLK3 knockdown in HeLa and SiHa cells, the protein expression of Bax increased with the parallel decrease in the anti-apoptotic, Bcl 2 protein expression (Ma, et al., 2019). These results suggest that MLK3 is involved in mitochondrial apoptosis. The MLK3 role has also been explored in etoposide-induced death in the ovarian cancer cell line, SKOV3, and in a murine fibroblast cell line, NIH-3T3. The knockdown of MLK3 decreased inhibitor of kappa B alpha (IκBα) protein expression and partially suppressed the etoposide-induced cell death. The overexpression of MLK3, either kinase dead or kinase active, promoted etoposide-induced apoptosis in ovarian cancer cells (Cole, et al., 2009). These results suggest that MLK3 can mediate pro-apoptotic signals by engaging various signaling pathways in cancer cells.
4.4. Apoptosis resistance
The dysregulation in programmed cell death pathways is one of the mechanisms that provide a survival advantage to cancer cells (Kitada, Pedersen, Schimmer, & Reed, 2002). The apoptosis resistance is the major cause of the failure of several cancer drugs (Mansoori, Mohammadi, Davudian, Shirjang, & Baradaran, 2017). It was reported that MLK3 mediates the Trastuzumab-induced apoptosis in HER2+ breast cancer cells, and loss of MLK3 lead to apoptosis resistance (Das, et al., 2015). Moreover, the MLK3 kinase activity and expression were decreased in Trastuzumab resistant HER2+ breast cancer cell lines compared to sensitive cell lines. Further analysis showed that HER2 amplification activates the PI3K-AKT pathway, which inhibits MLK3 kinase activity. In the same study, the second line of HER2-directed therapy, lapatinib-induced cell death was also blocked upon MLK3 kinase inhibition by PI3K-AKT pathway activation (Das, et al., 2015). Collectively, these studies indicate that heterogeneity in MLK3 kinase activity regulation is associated with apoptosis resistance in cancer cells. Therefore, further research is essential to explore the in-depth mechanism of MLK3-mediated drug resistance in different malignancies.
4.5. Tumor microenvironment (TME)
The efficacy of antineoplastic drugs is regulated by numerous factors, including the tumor microenvironment (TME). The TME can be described as a tumors’ local milieu with various cellular and non-cellular components (B. Zhang, et al., 2020). The MAPK family members are reported to regulate multiple components of TME (Gutjahr, et al., 2018). Our recent report suggests that MLK3 does participate in regulating triple negative breast cancer TME (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The pharmacological inhibition of MLK3 led to hyperactivation in tumor-infiltrating CD8+ T cells and increased cytotoxicity. However, MLK3 inhibition decreased the life span of CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). The analysis of tumors from mice treated with a pharmacological inhibitor of MLK3 showed robust protein expression of TNF-α within the TME. Further investigations suggested that the increase in TNF-α expression was associated with the increased apoptosis in CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The loss of MLK3 induced CD70-TNF-α mediated cell death in CD8+ T cells and therefore the combined inhibition of MLK3 and CD70 was capable of increasing tumor infiltration of cytotoxic T cells significantly and decreased the TNF-α expression in tumors (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The functions of MLK3 in TME have just started to unravel, and more future studies should provide information about the mechanistic role of MLK3 in TME.
5. The regulatory function of MLK3 in innate and adaptive immunity
The immune system is a complex network of cells, organs, and substances that is required to fight against pathogens and other diseases. There are two arms of the immune system, innate and adaptive immunity. The innate immunity is an inherited form of immunity that delivers rapid and non-specific responses against pathogens. Adaptive immunity is another arm of the immune system, mainly associated with antigen-based immune responses. The significant components of the adaptive immune system are B and T cells. The MLK3 has been reported to play crucial roles in regulating innate and adaptive components of the immune system (Figure 4).
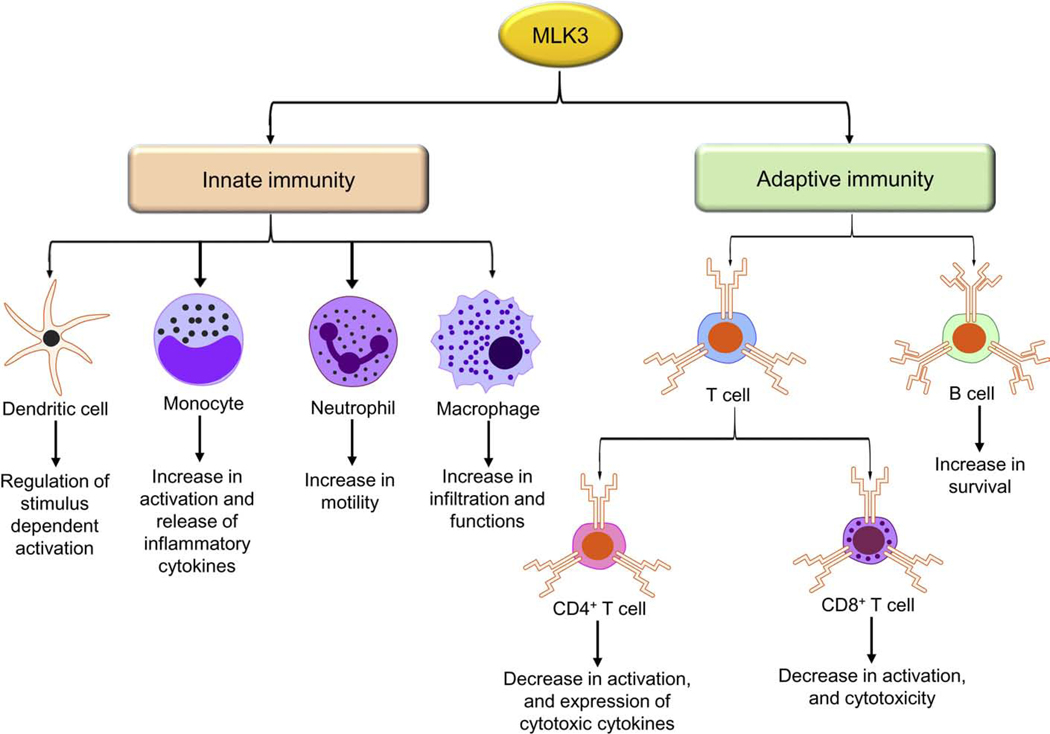
Figure 4: MLK3 regulation in innate and adaptive immune cells.
The MLK3 contributes in the regulation of various innate immune system functions, including dendritic cells, monocytes, neutrophils, and macrophages. In the adaptive immune system, MLK3 regulates T cell activation, effector function, and survival. Where, MLK3, mixed lineage kinase 3.
5.1. Innate immunity
The major components of innate immunity are Mφ, DCs, mast cells, natural killer (NK) cells, basophils, eosinophils, neutrophils, granulocytes, and complement proteins (Gajewski, Schreiber, & Fu, 2013). The cells involved in innate immunity, including DCs, Mφ, and basophils, possess pattern recognition receptors (PRRs). The PRRs recognize pathogen-associated molecular patterns (PAMPs) present on pathogens, including bacteria and viruses (Pfannkuch, et al., 2019). The interaction between PRRs and PAMPs is crucial for innate immunity responses. The detailed functions of MLK3 have been reported in Mφ, DCs, monocytes, and neutrophils (Handley, et al., 2007; Polesskaya, et al., 2014; Sui, et al., 2006; Tomita, Kohli, et al., 2017).
5.1.1. Macrophage
The Mφs are components of innate immunity involved in several immunological functions, including phagocytosis and antigen presentation (Kurynina, et al., 2018). The MLK3 was reported to regulate Mφ functions in diet-induced nonalcoholic steatohepatitis (NASH), a serious health concern worldwide. The MLK3 pharmacological inhibitor, URMC-099, was able to decrease Mφ infiltration in the liver of the NASH animal model. The MLK3 inhibition suppressed Mφ activation and pro-inflammatory signals in the NASH animal model, which ultimately lead to improved disease conditions (Tomita, Kohli, et al., 2017). The toll-like receptors (TLRs) play a major role in innate immunity and are generally expressed on DCs and Mφ (Grassin-Delyle, et al., 2020). The MLK3 was implicated in TLR adaptor protein, MyD88-mediated p38 MAPK signaling. The mechanistic study suggests that MLK3 is downstream of MyD88, and an upstream regulator MKK3 and p38 that regulate MyD88dependent transcripts stabilization (Sun & Ding, 2006). There are very few reports related to role of MLK3 in Mφ. It is expected more reports will emerge in future especially defining interrelation between MLK3 and Mφ in malignant diseases.
5.1.2. Dendritic cells
Like Mφ, the dendritic cells (DCs) are another component of the innate immune system and are involved in various immune functions, including antigen presentation to T cells (Medel, et al., 2018). The regulatory functions of MLK3 in DCs are reported (Handley, et al., 2007). The MLK members, gene expressions including MLK1, MLK2, MLK3, MLK4, and DLK were determined in DCs where all MLK members’ expressions were detected in DCs. The MLK2, MLK3, and DLK proteins were also present in DCs (Handley, et al., 2007). The MLK3 phosphorylation was also determined in DCs upon treatment with lipopolysaccharide (LPS). The LPS induced MLK3 phosphorylation on Thr277 and Ser281 sites, necessary for MLK3 activation (Handley, et al., 2007). The role of MLK3 has also been examined in p38α MAPK-regulated JNK activation in DCs upon LPS treatment. The mechanistic analyses showed that activities of JNK upstream regulators, including TAK1 MKK4 and MKK7 were significantly upregulated in p38α MAPK deficient DCs. However, MLK3 activation was not altered due to the loss of p38α MAPK, suggesting p38α MAPK is downstream of MLK3 in DCs (Zheng, et al., 2018). These studies indicate that MLK3 is present in DCs and plays regulatory roles in a stimulus-dependent DCs activation. Since DCs are considered as classical antigen-presenting cells (APCs) and therefore, further study is required to understand how MLK3 regulates antigen presentation properties of DCs.
5.1.3. Monocytes and neutrophils
The monocytes are another component of innate immunity system, which is a leukocyte or white blood cells (WBCs) that participate in the immune response against pathogens, including bacteria and viruses (Germic, Frangez, Yousefi, & Simon, 2019). The MLK3 is reported to participate in monocyte activation during HIV-1 infection. The HIV-1 proteins, Tat, and gp120 activated MLK3 kinase activity, leading to monocytes activation and release of inflammatory cytokines (Sui, et al., 2006). Similar to monocytes, neutrophils are another WBCs involved in repairing damaged tissue and mitigation of infections (Kantari, Pederzoli-Ribeil, & Witko-Sarsat, 2008). It was reported that MLK3 inhibition or loss attenuated N-formylmethionyl-leucyl-phenylalanine (fMLP)-induced motility in neutrophils in in vitro and in vivo (Polesskaya, et al., 2014). The dysregulated motility of neutrophils is a serious problem in several diseases, including leukocyte adhesion deficiencies and autosomal dominant, hyper IgE syndrome (Leiding, 2017). The infiltration of neutrophils in the tumors was reported to promote tumor survival and metastasis (Spiegel, et al., 2016), and therefore, targeting MLK3 could provide a novel insight on its role in neutrophil migration in cancer.
5.2. Adaptive immunity
The significant components of adaptive immunity are B- and T- lymphocytes. The B and T cells recognize a range of antigens to deliver immune responses against pathogens and antigens present on cancer cells (Sutti & Albano, 2020). Once an antigen is recognized by B cells, the B cells produce antibodies-mediated immune responses; however, antigen recognition by T cells lead to immune responses in conjunction with a helper (Th) and effector T (Teff) cells. The B cells serve as a defender against micro-organisms in the humoral system, like blood and lymphatic fluids (Ferrara, et al., 2018). The B cells are originated from bone marrow- or bursa-derived cells. The pre-B cells are converted into mature B cells following several developmental stages, regulated by various factors, including micro RNA. The miR-125b is reported to regulate the survival of transformed precursor B cells, which is mediated by MLK3 (Knackmuss, et al., 2016). Our recent study with MLK3 knockout mice showed an increased splenic B cell population compared to wild type mice (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The B cells are primarily responsible for antibodies production but also acts as classical APCs. Since the genetic ablation of MLK3 increased B cell numbers and therefore, the loss or inhibition of MLK3 could increase the total numbers of antigen-presenting B cells. However, further study is required to understand the in-depth function of MLK3 in B cell biology. The T cells are a major component of the adaptive immune system and deliver immune responses against pathogens and tumor cells. There are several subsets of T cells, including CD4+ and CD8+ T cells, and regulatory T cells (Tregs). The T cells develop into the thymus and, upon maturation, are distributed to the peripheral system. The regulatory function of MLK3 has recently been established in T cells, where MLK3 negatively regulates T cell effector function (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). In systemic lupus erythematosus (SLE), MLK3 is reported to regulate the infiltration of CD4+ T cells into the kidney, which is essential for the initiation of immune reactions against autoantigens (Okamoto, Fujio, Tsuno, Takahashi, & Yamamoto, 2012). In prenephritic mouse model, the adoptive transfer of MLK3-expressing CD4+ T cells accelerates nephritis progression (Okamoto, et al., 2012). The functional roles of MLK3 in regulating T cell activation, effector function and survival are discussed in section 6.
5.3. Cytokines/chemokines
The cytokines are secreted proteins produced by immune cells like helper T cells and Mφ, and non-immune cells, including tumor cells. Based on the inflammatory responses by the cytokines, they can be classified as pro-inflammatory or anti-inflammatory cytokines. The typical examples of pro-inflammatory cytokines are: IL-1β, IL-6, and TNF-α, whereas IL-4, IL-13, IL-19, IL-27, and IL-37 are members of anti-inflammatory cytokines (Dong, et al., 2016; Moideen, et al., 2020). The Zika virus infection is detrimental for human health, and interestingly MLK3 is reported to play an essential role in Zika pathogenesis by regulating cytokines levels. In the neonatal mouse brain, it has been shown that MLK3 suppresses Zika virus replication by increasing cytokines IL-6, IL-8, MCP-1, and TNF-α levels (H. Xu, et al., 2019). The MLK3 has been reported to upregulate interferon-gamma (IFNγ) in an infection model or upon TCR activation. The stimulation of the IFNγ receptor has been correlated with activation of Unc-51-like kinase 1 (ULK1). Interestingly, the ULK1 interacts with MLK3, and this interaction promotes activation of MLK3. Upon activation, MLK3 triggers downstream ERK5-mediated signaling leading to antiviral response (Saleiro, et al., 2018). In one of our recent studies, we have demonstrated that intracellular IFNγ expression was higher in activated CD4+ and CD8+ T cells isolated from MLK3 knockout mice (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). The pro-inflammatory cytokines TNF-α and IL-1β have been reported to regulate MLK3 kinase activation (Humphrey, et al., 2010; Korchnak, Zhan, Aguilar, & Chadee, 2009; Sathyanarayana, et al., 2002). The MLK3 has also been reported to regulate anti-inflammatory cytokine production in amyloid-β stimulated microglia. The inhibition of MLK3 by using the pharmacological inhibitor, URMC-099 in amyloid-β stimulated microglia upregulated the gene expressions of IL-4 and IL-13 (Dong, et al., 2016). We have recently seen that genetic loss of MLK3 in mice increased IL-4, IL-5, and IL-13 levels in the serum (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). Similar to cytokines, MLK3 is also reported to regulate chemokines. The chemokines are low molecular weight (6–14 kDa) cytokines, which play a crucial role in chemotaxis (Gao, et al., 2020; Godiska, et al., 1997). Interestingly, MLK3 regulates chemokines CXCL3, CXCL4, CXCL8, and CXCL10 and the chemokine receptor CXCR3. Upon TCR activation, genetic loss of MLK3 increased the release of CCL3 and CCL4 and surface expression of CXCR3 on CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). Importantly, CCL3 and CCL4 are known to regulate CD8+ T cell migration (Castellino, et al., 2006). The MLK3 mediated regulation of IL-8 (i.e., CXCL8) gene expression has been reported in LPS treated Jurkat cells (Zhong & Kyriakis, 2007). Another chemokine C-X-C motif chemokine 10 (CXCL10), also known as interferon gamma-induced protein 10, has also been reported to be regulated by MLK3 in lipotoxic diseases. It has been reported that in lipotoxic hepatocytes, MLK3 induces signal transducer and activator of transcription 1 (STAT1) phosphorylation, leading to increased expression of chemokine, CXCL10 (Tomita, Kabashima, et al., 2017). The recent and past reports suggest that MLK3 plays a broad range of regulatory functions in innate and adaptive immunity and modulates inflammatory pathways in various diseases.
6. Role of MLK3 in T cell activation, effector function, and survival
The T lymphocytes are one of the significant components of the adaptive immune system. The functions of T cells include cytokines production, activation of other immune cells, and direct cancer or infected host cells killing (Bracq, et al., 2017; Spranger, Dai, Horton, & Gajewski, 2017). Recently, we have reported that MLK3 is present in T cells and regulates T cell activation, effector function, and survival (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). In the following subsection, we will discuss role of MLK3 in T cell development, activation, effector function, survival, and exhaustion.
6.1. T cell development
The T cells originate from hematopoietic stem cells present in the bone marrow (Famili, Wiekmeijer, & Staal, 2017). We have recently reported that genetic loss or pharmacological inhibition of MLK3 increases the c-Kit+Lin−SCA-1+CD34dim hematopoietic cell population in C57BL/6 mice (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). Some of these hematopoietic cells travel to the thymus via the circulatory system (Zlotoff & Bhandoola, 2011). The T cell development in the thymus encompasses several phases, including DN1 (CD25−CD44high), DN2 (CD25+CD44high), DN3 (CD25−CD44low) and DN4 (CD25−CD44−) (Misslitz, et al., 2004). Interestingly, loss of MLK3 does not affect any of these T cell developmental phases in the thymus (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). Neither double-positive (i.e., CD4+CD8+) nor CD4 and CD8 single-positive thymocytes is affected due to loss of MLK3 in mice (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). The recent results collectively suggest that MLK3 does regulate hematopoietic stem cell generation; however, it does not affect T cell developmental phases in the thymus.
6.2. T cell activation
The mature T cells travel from the thymus to peripheral lymphoid organs and await for receiving antigenic signals. Once a T cell recognizes antigen present on APCs, it gets activated and undergoes expansion and effector function. The T cell activation is a coordinated process involving primarily three critical steps. As a first step, T cells recognize antigens presented on MHC-I of APCs by its TCR. In the second step, co-stimulatory molecules like cluster of differentiation 28 (CD28), cluster of differentiation 80 (CD80), and the cluster of differentiation 86 (CD86) decrease the threshold of T cell activation (Mikami, et al., 2020). The other costimulatory molecules are cluster of differentiation 40 ligands (CD40L), inducible T cell costimulator (ICOS), TNF receptor superfamily member 4 (TNFRSF4 or OX40), and TNFRSF9 (also known as 4–1BB). In addition to costimulatory molecules, there are some coinhibitory signaling molecules also expressed on T cells. The coinhibitory signaling molecules, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), are reported to increases the threshold of T cell activation. The third phase of T cell activation is determined by the presence of cytokines milieu that controls the nature of immune responses generated by the activated T cells. T cell activation is an important immunological process that regulates T cell proliferation, expansion, and effector function. We have reported recently that genetic loss or pharmacological inhibition of MLK3 induces T cell activation as determined by upregulated expressions of activation markers cluster of differentiation 25 (CD25), the cluster of differentiation 38 (CD38) and the cluster of differentiation 69 (CD69) on activated CD4+ and CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). The T cell activation, due to genetic loss of MLK3 is associated with increased expression of costimulatory molecules CD28 and CD40L on the surface of T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). It was also observed that the MLK3 knockout mouse treated of LPS or anti-CD3e mab had a higher population of activated CD4+ and CD8+ T cells. Furthermore, in a complementary in vitro experiment in Jurkat T cells, expressing MLK3, the activation was reduced upon PMA/Ionomycin, or TCR mediated activation (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). These results suggest that loss of MLK3 promotes T cell activation by increasing T cell costimulatory molecules. However, further study will be required to appreciate the full functions of MLK3 in T cell biology.
6.3. T cell effector function
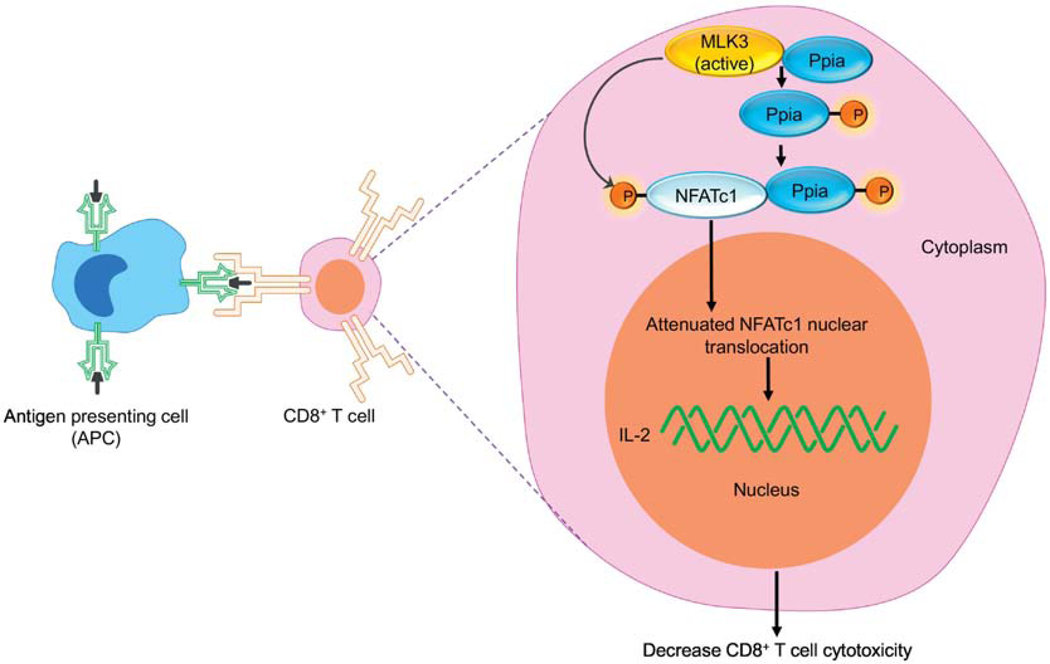
The T cells perform their effector function, including the release of cytokines and cytotoxicity upon activation (Balanca, et al., 2020; Yang, et al., 2020). It is reported that cytotoxicity of T cells is regulated via nuclear translocation of nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) (Klein-Hessling, et al., 2017). Recently, we have reported that MLK3 regulates nuclear translocation of NFATc1. The MLK3 binds with and activates cis-trans isomerase, peptidyl-prolyl isomerase A (Ppia), and increases its cis-trans isomerase activity. The activated Ppia binds with phosphorylated NFATc1, and the Ppia-NFATc1 complex is retained in the cytoplasm. The attenuated NFATc1 nuclear translocation leads to decreased IL-2 expression and, thus, down-regulates T cell effector function (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). Interestingly, genetic loss of MLK3 increased NFATc1 nuclear translocation and induced granzyme B protein expression in CD8+ T cells, isolated from breast cancer patients (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020), suggesting that MLK3 inhibition could increase T cell activation in cancer patients. We also observed in a murine model of breast cancer that pharmacological inhibition of MLK3 increased the tumor infiltration of cytotoxic T cells. Besides improving the cytotoxic function of T cells, genetic loss of MLK3 in CD8+ T cells also increased the chemotaxis as determined by increased release of chemokines, CCL3 and CCL4 in TCR activated CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). These recent results suggest an exciting role of MLK3 in T cell effector function via NFATc1 nuclear translocation and opens-up novel avenues to increase T cell cytotoxicity in cancer patients for therapeutic interventions (Figure 5).
Figure 5: MLK3 mediated regulation of CD8+ T cell cytotoxicity.
Diagram showing the role of MLK3 in CD8+ T cells’ effector functions. The MLK3 phosphorylates Ppia and activates its PPIase activity. The Ppia binds with NFATc1 and Ppia-NFATc1 complex retains in the cytosol. The decreased NFATc1 nuclear translocation attenuates T cell cytotoxicity. Where, MLK3, mixed lineage kinase 3; NFATc1, nuclear factor of activated T cells 1; Ppia, peptidyl-prolyl isomerase A.
6.4. T cell survival or apoptosis
The program cell death is a process where unwanted cells are eliminated from the system in a controlled manner. The T cells also go through the process of program cell death; however, aberrations in T cell apoptosis are frequent during infections and malignant diseases. The MLK3 has recently been reported to regulate CD8+ T cell apoptosis via CD70, both under basal and activated conditions (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). However, under basal or TCR-activated conditions, the other T cell subset, CD4+ T cell apoptosis, is not regulated by MLK3. Moreover, we observed that among three CD8+ T cells activated phenotypes (i.e. CD8+CD25+, CD8+CD69+ and CD8+CD38+ T cells), the apoptosis in CD8+CD38+ T cell population was more prevalent in absences of MLK3. Mechanistic studies suggested that loss of MLK3 increased TNFRSF1a expression on CD8+ T cells. The TNFRSF1a is a soluble receptor for TNF-α (Yue, et al., 2020), and we observed that CD70 regulated MLK3-mediated TNF-α expression in the tumor. Therefore the combined inhibition of MLK3 and CD70 showed enhanced cytotoxic T cells infiltration leading to an anti-tumor response in TNBC (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020). These recent interesting results suggest that MLK3 indeed plays a regulatory role in peripheral and tumor-infiltrating T cells apoptosis.
7. Regulation of cis-trans isomerase activity by MLK3 in cancer and T cells
Protein dynamics are crucial for living cell activities and function. Several proteins’ function is regulated via post-translational modifications, like phosphorylation, acetylation, and glycosylation, and by foldases catalytic activity (Lin, et al., 2019). One of the foldases family members, peptidyl-prolyl cis/trans isomerases (PPIase), regulates the isomerization of “cis” to “trans” form of peptide bonds. The PPIases target prolyl bond and linked with 180° rotation in a polypeptide (Kawagoe, Nakagawa, Kumeta, Ishimori, & Saio, 2018; Lin, et al., 2019). The post-translational modification by PPIase is reported to regulate protein functions, including the enzymatic activity of the target protein. The peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) is reported to regulate breast cancer cell proliferation. The peptidyl-prolyl Isomerase A (Ppia) induces nuclear translocation of NFATc1 in T cells (Figure 6) (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020; Rangasamy, et al., 2012). Interestingly, both Pin1 and Ppia have been reported to be regulated by MLK3 (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020; Rangasamy, et al., 2012). The biological functions of various proteins, including retinoblastoma protein (Rb), cyclin D1, cyclin E, p27, Cdc25C, and Wee1, are regulated by Pin1 (Cheng & Tse, 2018). The defects in Pin1 function and expression are common in malignant diseases, including breast cancer, where Pin1 is reported to be overexpressed (Rangasamy, et al., 2012). Interestingly, MLK3 phosphorylates Pin1 at S138 site and increases Pin1’s cis-trans isomerase activity. Furthermore, phosphorylated Pin1 translocates to the nucleus and participates in transcriptional activities and regulates biological function, including cellular proliferation (Rangasamy, et al., 2012). The Pin1 also regulates nuclear translocation of nuclear factor of activated T-cells (NFAT) by forming a Pin1-NFAT complex in T cells. The WW domain of Pin1 binds with Ser/Pro-rich domains of NFAT, and this interaction regulates the nuclear translocation of NFAT (W. F. Liu, Youn, Zhou, Lu, & Liu, 2001). The immunosuppressive drug, cyclosporine, along with Ppia inhibits TCR-induced cellular proliferation in CD4+ T cells (Colgan, Asmal, Yu, & Luban, 2005). The genetic loss of Ppia in mice induces IgE-mediated allergic responses and increased Th2 cytokines by CD4+ helper T cells. Interestingly, genetic loss of Ppia increased the TCR-mediated activation of Th2 cells (Colgan, et al., 2004). The role of MLK3 in regulating Ppia’s function in T cells has recently been reported. It has been shown that MLK3 transcriptionally regulates ppia via AP-1/c-Fos activation. The MLK3 phosphorylates Ppia and regulates its cis-trans isomerase activity. Further analyses suggest that Ppia binds with cytosolic NFATc1 and restricts the NFATc1 nuclear translocation in T cells, which results in decreased T cell effector function (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). These results suggest, coordinated action of MLK3 in tumors as well as in T cells via regulating PPIase members, Pin1, and Ppia.
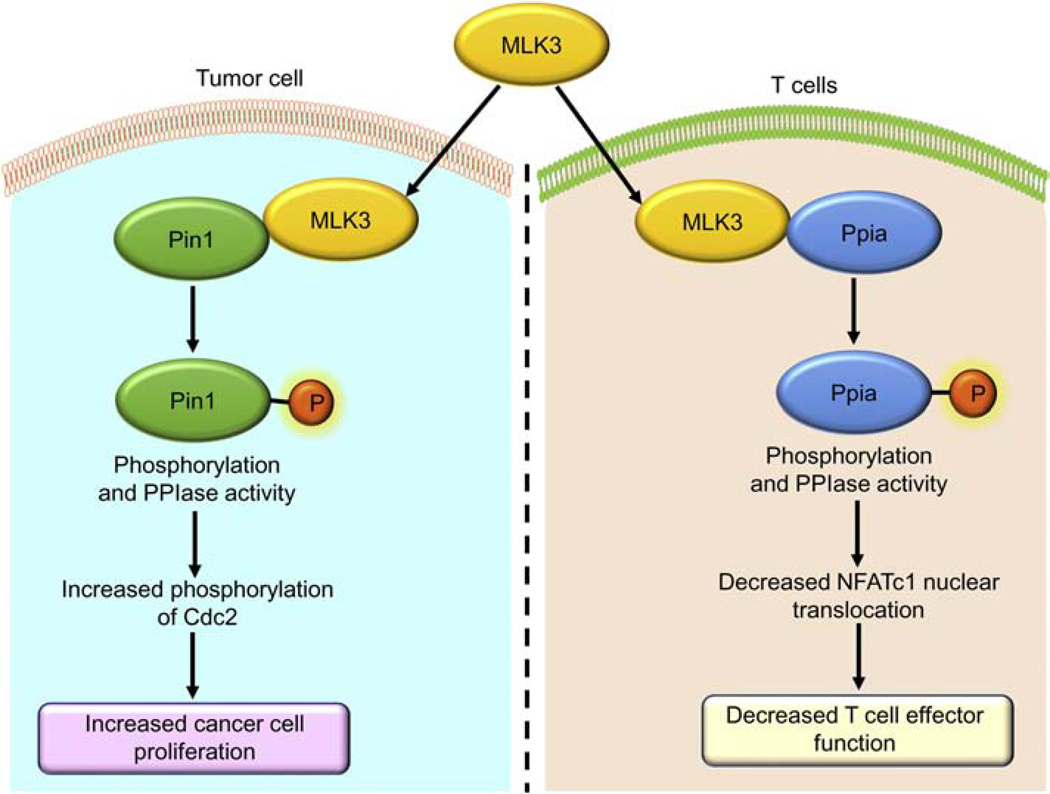
Figure 6: MLK3 regulates PPIase activity in tumor and T cells.
MLK3 phosphorylates Pin1 and Ppia and induce PPIase activities. In breast cancer, MLK3 phosphorylates Pin1 and increases PPIase activity, and promotes Cdc2 mediated cancer cell proliferation. In T cells, MLK3 phosphorylates Ppia and increases PPIase activity and attenuates T cell effector function. Where, Cdc2, cell division cycle 2; MLK3, mixed lineage kinase 3; Pin1, peptidylprolyl cis/trans isomerase, NIMA-Interacting 1; Ppia, peptidylprolyl isomerase A.
8. Future of MLK3 inhibitors in T cell-dependent cancer immunotherapy
The role of MLK3 in various cancers is apparent; primarily, MLK3 is involved in cancer cell survival and growth. Similarly, MLK3 has also been studied in host immunity and have provided very promising results. Although the immune function of MLK3 is an emerging area, however, it is clear that several immune surface molecules are regulated by MLK3, including splenic surface molecules CD28 and CD70 (Table 1). So far, MLK3 association with T cells costimulatory molecules like CD28, CD40L, and CD70 and co-inhibitory molecule CTLA-4 have just been reported (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020; Kumar, Singh, Viswakarma, Sondarva, Nair, Thatcher, et al., 2020). The MLK3 regulates the protein expression of co-stimulatory molecules, CD28 and CD40L, which are known to participate in T cell activation. The primary issue with tumor-infiltrating T cells is its activation status. Since loss or inhibition of MLK3 promotes T cell activation, and therefore, it is conceivable that inhibitors of MLK3 might be useful to enhance T cell activation.
Table 1:
Protein expressions on splenocytes of WT and MLK3−/− mice under basal condition
| Surface markers | Change in MLK3−/− compared to WT (Increased) a, b | Change in MLK3−/− compared to WT (Decreased) b | Change in MLK3−/− compared to WT (Non-significant) a, b |
|---|---|---|---|
| CD1d | ✓ | ||
| CD11b | ✓ | ||
| CD11c | ✓ | ||
| CD14 | ✓ | ||
| CD25 | ✓ | ||
| CD27 | ✓ | ||
| CD28 | ✓ | ||
| CD38 | ✓ | ||
| CD40 | ✓ | ||
| CD44 | ✓ | ||
| CD68 | ✓ | ||
| CD69 | ✓ | ||
| CD70 | ✓ | ||
| CD80 | ✓ | ||
| CD86 | ✓ | ||
| CD98 | ✓ | ||
| CD127 | ✓ | ||
| CD152 | ✓ | ||
| CD154 | ✓ | ||
| CD197 | ✓ | ||
| CD205 | ✓ | ||
| CD273 | ✓ | ||
| CD274 | ✓ | ||
| CD279 | ✓ | ||
| F4/80 | ✓ | ||
| H-2kb | ✓ | ||
Where, CD, cluster of differentiation; MLK3−/−, mixed lineage kinase 3 knockout; WT, wild type. The data compiled from
(Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020) and
(Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020).
8.1. CD28
The CD28 is a T cell co-stimulatory molecule present on the surface of T cells, bone marrow stromal cells, plasma cells, eosinophils, and neutrophils (Esensten, Helou, Chopra, Weiss, & Bluestone, 2016). The CD28 binds with B7 molecules, and CD28-B7 axis provides co-stimulatory signals for T cell activation (Esensten, et al., 2016). However, the aberrant expression of B7 is associated with decreased immunity against tumors and promote tumor growth (Cai, et al., 2020). The decreased CD28 expression in sentinel lymph nodes is reported to be associated with immunosuppression in breast cancer (Schule, Bergkvist, Hakansson, Gustafsson, & Hakansson, 2004). Considering the importance of CD28 in tumor immunotherapy, CD28 based bispecific activator “TSAxCD28” has been used with a checkpoint inhibitor for cancer immunotherapy. Interestingly, the combined treatment of TSAxCD28 and PD-1 blockade induced a robust and synergistic anti-tumor response (Waite, et al., 2020). We have reported that MLK3 diminishes CD28 expression on CD4+ and CD8+ T cells, and therefore pharmacological inhibitor of MLK3 could promote T cells activation via increased CD28 expression (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020).
8.2. CD40L
The CD40L is a costimulatory surface molecule, primarily expressed on activated T cells (Deng, et al., 2020). The CD40L receptor, CD40, is a TNF family member present on B cells, myeloid cells, and dendritic cells, and the CD40L-CD40 axis regulates T cell activation (Piechutta & Berghoff, 2019). Emerging studies suggest that the CD40L-CD40 axis is very important for T cell-mediated anti-tumor immunity (Vonderheide, 2020). The MLK3 plays an important role in CD40L-CD40 axis mediated signaling (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020; Ramirez, et al., 2010). In brain microvascular endothelial cells (BMVECs), treatment with soluble CD40L increases phosphorylation and kinase activity of MLK3 and stimulates downstream JNK (Ramirez, et al., 2010). Recently, we reported that genetic loss of MLK3 in mice induces splenic CD40L expression (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Sinha, et al., 2020). Considering the importance of the CD40L-CD40 axis, several agonistic antibodies have been formulated, which are under clinical trials (Vonderheide, 2020).
8.3. CD70
The CD70 is another co-stimulatory molecule present on immune and non-immune cells, including cancer cells (Inaguma, et al., 2020). In immune cells, CD70 is present on APCs and binds with the CD27 receptor, present on T cells. The binding between CD27 and CD70 induces stimulatory signals to increase T cell activation. The CD70 expression is also reported to be upregulated in various cancers, including colorectal, osteosarcoma, and breast cancer (Jacobs, et al., 2018; L. Liu, et al., 2018; Pahl, et al., 2015). A paradoxical role of CD70 present on tumor cells is reported, where it transmits negative signals to T cells via their CD27 that ultimately results in T cell dysfunction. It is believed this could be one of the mechanisms by which tumors escape from host immune surveillance (Pahl, et al., 2015). The CD70 has also been implicated in tumor and Treg cells survival (Jacobs, et al., 2015). The breast cancer cells with overexpression of CD70 is associated with lung specific metastasis (L. Liu, et al., 2018). The “ARGX-110,” a CD70 targeting drug, has shown promising outcomes in cancers with increased CD70 expression (Aftimos, et al., 2017). We have reported recently that genetic loss or the pharmacological inhibitor of MLK3 prompts CD70 protein expression on T cells and subsequently promotes apoptosis in CD8+ T cells via TNF-α-TNFRSF1a axis. Therefore, combined blockade of MLK3 and CD70 together was seen to have anti-tumor immunity in an animal model of TNBC (Kumar, Singh, Viswakarma, Sondarva, Nair, Sethupathi, Dorman, et al., 2020).
8.4. CTLA-4
The CTLA-4 is one of the co-inhibitory molecules primarily present on activated T cells, and its expression is higher on regulatory T cells. The CTLA-4 expression has also been reported in several cancers (H. Zhang, et al., 2019). There is a Food and Drug Administration (FDA) approved CTLA-4 antagonist, Ipilimumab, which is in clinical use to treat melanoma. Limited knowledge is available about MLK3 relation with CTLA-4; however, our recent data suggest a paradoxical role of MLK3-CTLA-4 axis in TNBC and CD8+ T cells (Kumar, Singh, Viswakarma, Sondarva, Nair, Thatcher, et al., 2020). We have observed that pharmacological inhibition of MLK3 increases CTLA-4 protein expression on tumor and CD8+ T cells. The increased CTLA4 protein expression due to MLK3 inhibition promotes tumor cell proliferation; however, increased CTLA-4 protein expression on CD8+ T cells was associated with decreased CD8+ T cells’ proliferation. These observations suggest that combined inhibition of MLK3 and CTLA-4 can be harnessed to increase CD8+ T cell proliferation and decrease tumor proliferation, desirable for cancer therapy.
9. Repurposing of MLK3 inhibitors/agonists in malignant diseases
The emerging discoveries on the role of MLK3 in cancer and host immune system, have raised hope for MLK3 targeted therapy in cancer. Various antagonistic or agonistic approaches can be designed to target MLK3 functions in cancer. There are several MLKs/MLK3 inhibitors, including CEP-1347, CEP-11004, URMC-099, and CLFB-1134, whereas we have reported earlier that ceramides and TNF-α are agonists of MLK3 that increase its kinase activity.
9.1. MLK3 Inhibitors
In 1997, CEP-1347 was reported as a neurotrophic agent, derived by synthetic modification of a natural product hit identified in a phenotypic screen (Kaneko, et al., 1997). Later identification of JNK pathway inhibition (Saporito, Brown, Miller, & Carswell, 1999) by CEP-1347 was subsequently ascribed to direct inhibition of MLKs (Maroney, et al., 2001). CEP-1347 demonstrated anti-apoptotic actions and efficacy in preventing dopaminergic neuronal loss in animal models of Parkinson’s disease (PD) (Saporito, et al., 1999), which was replicated by derivatives that selectively inhibited MLK1 and MLK3 (Hudkins, et al., 2008). These observations led to CEP-1347 advancing to clinical trials (PRECEPT trial) as a disease-modifying therapeutics in Parkinson’s disease, after proving its safety in a 4 week trial in PD patients (Parkinson Study Group, 2007). As in all clinical trials of neuroprotective agents in that period (Waldmeier, Bozyczko-Coyne, Williams, & Vaught, 2006), the trial failed to reach its primary endpoint. It was terminated within three years because of no significant improvement compared to the placebo arm (Sweeney & Lewcock, 2011). Unfortunately, from this study, the direct effect of CEP-1347 administration in inhibiting the JNK pathway, and specifically inhibiting MLKs, in human subjects was not determined. In 2013, URMC-099 was reported as an MLK3 inhibitor with improved pharmacokinetic properties over CEP-1347 and brain bioavailability (Goodfellow, et al., 2013), with potential use in treating HIV-associated dementia, based, in part, on anti-inflammatory actions (Marker, Tremblay, Puccini, Barbieri, Marker, et al., 2013). More recently, URMC-099 has been reported to have potential in diseases, including Alzheimer’s (Kiyota, et al., 2018) and heart failure (Calamaras, et al., 2019); and in multiple sclerosis (Bellizzi, et al., 2018). Since MLKs play a pro-apoptotic function in neurons, we were interested to discover any probable functions of MLKs in proliferative disease such as cancer, which are discussed above in detail. The K252a, the backbone of the current pharmacological inhibitor of MLK3 (i.e., CEP-1347), has shown anti-neoplastic efficacy in pancreatic cancer (Chandana, et al., 2010). In vitro study showed that inhibition of MLK3 using K252a in Panc-1 and MiaPaCa-2 cells decreased cellular proliferation and increased apoptosis (Chandana, et al., 2010). The epidermal growth factor receptor (EGFR) is a crucial player in pancreatic tumorigenesis and targeted for pancreatic cancer therapy (Ueda, et al., 2006). It is reported that the therapeutic efficacy of the EGFR inhibitor can be further boosted along with inhibition of MLK3 (by K252a) in pancreatic cancer (Chandana, et al., 2010). The combined anti-tumor efficacy of EGFR and MLK3 inhibition has been tested in human pancreatic cancer cells. The results suggest that combined inhibition of MLK3 and EGFR decreases AKT phosphorylation and increases poly (ADP-ribose) polymerase (PARP) cleavage leading to increased cancer cell apoptosis (Chandana, et al., 2010). The positive outcomes due to the combined treatment of MLK3 and EGFR inhibitors suggest that MLK3 might be involved in survival and apoptosis resistance in pancreatic cancer cells. The apoptosis resistance is one of the properties of cancer stem cells (CSCs) and could lead to failure of anticancer regimens. In ovarian cancer, survivin, an anti-apoptotic protein, plays a critical role in the apoptosis resistance of CSCs (Ambrosini, Adida, & Altieri, 1997; Togashi, et al., 2018). Interestingly, MLK3 plays a role in regulating survivin function (Togashi, et al., 2018). A study in ovarian cancer showed that CEP-1347 targets survivin, decreases drug resistance, and increases chemotherapeutic agents’ sensitivity in ovarian cancer (Togashi, et al., 2018). Similar to ovarian cancer, CEP-1347 treatment also reduces differentiation and tumorigenic potential of glioblastoma and pancreatic cancer stem cells (Okada, et al., 2017). Most importantly, there was no adverse effect on the growth and survival of neural stem cells and normal fibroblasts at clinically relevant doses, suggesting that CEP-1347 specifically targets cancer stem cells (Okada, et al., 2017). These findings suggest that targeting MLK3 alone or in combination, could have robust anticancer efficacy in various malignancies. However, in-depth mechanistic studies for the anticancer efficacy of MLK3-targeted therapy in multiple types of malignancies will open a novel direction for cancer treatment.
9.2. Agonists
As we learn more about the biological functions of MLK3 in different cancers and immune cells, it is becoming more apparent that in some specific types of cancer, the MLK3 agonist will be desirable to induce cell death in cancer cells (Figure 2). For instance, in HER2+ breast cancer, Trastuzumab activates MLK3 kinase activity to induce cancer cell apoptosis (Das, et al., 2015). Therefore, it will be desirable to increase the MLK3 activity in cancers where MLK3 kinase activation is necessary to induce cancer cell death. There are two reported agonists of MLK3, i.e., ceramide and TNF-α (Sathyanarayana, et al., 2002). The TNF-α is a pleiotropic cytokine with multiple functions, including immune modulation and cellular survival (Parameswaran & Patial, 2010). Although TNF-α showed anti-tumor activity by inducing apoptosis in cancer cells, however the systemic toxicity induced by TNF-α is a serious health concern (Montfort, et al., 2019). Therefore it will be undesirable to use TNF-α, where MLK3 activation is necessary for any therapeutic intervention. Ceramides, bioactive lipids, which are generated by both chemo- and radiotherapies, play critical roles in inducing apoptosis of cancer cells (Herr, Wilhelm, Bohler, Angel, & Debatin, 1997). Any cellular process that affects ceramide levels also lowers the efficacy of these therapies. For example, ceramide glycosylation through glucosylceramide synthase (GCS) allows cellular escape from ceramide-induced programmed cell death, which confers cancer cell resistance to cytotoxic anticancer agents leading to a trait called multidrug resistance (Lavie, et al., 1997). Since ceramide activates MLK3, and many anti-cancer drugs like daunorubicin and Ara-C are known to generate ceramide for causing cytotoxicity (Grazide, et al., 2002), it is possible that MLK3 might mediate chemotherapeutic drug-induced cell death in cancers. Taken together, MLK3 antagonists and agonists have enormous possibility in targeted therapy of cancer and warrants further investigations.
10. Conclusions
In summary, the MAP3K family member, MLK3, regulates a broad range of biological functions in tumor and host immunity. Chronologically, research on MLK3 could be separated into three significant advancements. First, MLK3 was verified as an activator of MAP2K family members; second, the pathological function of MLK3 was shown in neurodegenerative diseases, and lately, MLK3’s role in cancer immunotherapy has been established. It is interesting to note that MLK3 promotes proliferation, migration, invasion, and apoptosis in cancer cells; however, in immune cells, especially in T cells, MLK3 regulates activation, effector function, and survival of immune cells. The paradoxical functions of MLK3 in cancer and immune cells make it an attractive target because, ultimately, the goal of any anti-neoplastic drug is to induce cell death in cancer cells and preferably augment anti-tumor immunity. MLK3 inhibitors meet both the characteristics, with only one limitation of parallel activation of the cell death pathway in T cells. However, with more understanding of the biology of MLK3 in immune cells, corrective measures can be devised to harness the beneficial effects of MLK3 inhibitor in cancer. As an example, we identified that MLK3 inhibitor-induced CD-70 to cause cell death in T cells, and upon using CD70 inhibitor along with MLK3 inhibitor, the breast cancer tumorigenesis was significantly reduced. A new MLK3 selective inhibitor, CLFB-1134, has shown promising outcomes in preclinical models of neurological diseases (Kline, et al., 2019). More studies are required to understand the role of CLFB-1134 in cancer cells’ survival, apoptosis, and immune functions. The past and emerging research on the MLK3 regulatory functions in tumor and immune cells suggest that MLK3 and other members of this family warrant an in-depth study to consider them potential therapeutic targets in cancer immunotherapy.
Acknowledgments
The authors acknowledge Dr. Enrico Benedetti MD, Chair Department of Surgery, for providing access to the departmental resources and financial support.
Funding
The authors acknowledge funding supports from the Department of Surgery, Veterans Affairs Career Scientist Award (BX004855), and National Cancer Institute Award (CA 216410) to A.R. B.R. is supported by NCI Award (CA 219764 and CA178063), Veterans Affairs Merit Award (BX003296) and UI-Cancer Center Pilot Grant Award.
Abbreviations:
- APC
antigen-presenting cell
- CD4
cluster of differentiation 4
- CD8
cluster of differentiation 8
- CD70
cluster of differentiation 70
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DC
dendritic cell
- ER
estrogen receptor
- ERK
extracellular-signal-regulated kinase
- HER2
human epidermal growth factor receptor 2
- JNK
c-Jun N-terminal Kinase
- MAP3K
mitogen-activated protein kinase kinase kinase
- MAP2K
mitogen-activated protein kinase kinase
- MAPK
mitogen-activated protein kinase
- MLK3
mixed lineage kinase 3
- Mφ
macrophage
- NFATc1
nuclear factor of activated T-cells, cytoplasmic 1 TNBC triple-negative breast cancer
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftimos P, Rolfo C, Rottey S, Offner F, Bron D, Maerevoet M, Soria JC, Moshir M, Dreier T, Van Rompaey L, Michot JM, Silence K, Hultberg A, Gandini D, de Haard H, Ribrag V, Peeters M, Thibault A, Leupin N, & Awada A (2017). Phase I Dose-Escalation Study of the Anti-CD70 Antibody ARGX-110 in Advanced Malignancies. Clinical Cancer Research, 23, 6411–6420. [DOI] [PubMed] [Google Scholar]
- Al Absi A, Wurzer H, Guerin C, Hoffmann C, Moreau F, Mao X, Brown-Clay J, Petrolli R, Casellas CP, Dieterle M, Thiery JP, Chouaib S, Berchem G, Janji B, & Thomas C. (2018). Actin Cytoskeleton Remodeling Drives Breast Cancer Cell Escape from Natural Killer-Mediated Cytotoxicity. Cancer Research, 78, 5631–5643. [DOI] [PubMed] [Google Scholar]
- Amako Y, Igloi Z, Mankouri J, Kazlauskas A, Saksela K, Dallas M, Peers C, & Harris M. (2013). Hepatitis C virus NS5A inhibits mixed lineage kinase 3 to block apoptosis. Journal of Biological Chemistry, 288, 24753–24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, & Altieri DC (1997). A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Medicine, 3, 917–921. [DOI] [PubMed] [Google Scholar]
- Balanca CC, Scarlata CM, Michelas M, Devaud C, Sarradin V, Franchet C, Martinez Gomez C, Gomez-Roca C, Tosolini M, Heaugwane D, Lauzeral-Vizcaino F, Mir-Mesnier L, Feliu V, Valle C, Pont F, Ferron G, Gladieff L, Motton S, Tanguy Le Gac Y, Dupret-Bories A, Sarini J, Vairel B, Illac C, Siegfried-Vergnon A, Mery E, Fournie JJ, Vergez S, Delord JP, Rochaix P, Martinez A, & Ayyoub M. (2020). Dual Relief of T-lymphocyte Proliferation and Effector Function Underlies Response to PD-1 Blockade in Epithelial Malignancies. Cancer Immunology Research, 8, 869–882. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, Markowitz S, Willson JK, Parmigiani G, Kinzler KW, Vogelstein B, & Velculescu VE (2003). Mutational analysis of the tyrosine kinome in colorectal cancers. Science, 300, 949. [DOI] [PubMed] [Google Scholar]
- Bedard PL, Hyman DM, Davids MS, & Siu LL (2020). Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet, 395, 1078–1088. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Hammond JW, Li H, Gantz Marker MA, Marker DF, Freeman RS, & Gelbard HA (2018). The Mixed-Lineage Kinase Inhibitor URMC-099 Protects Hippocampal Synapses in Experimental Autoimmune Encephalomyelitis. eNeuro, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, & Rupasinghe HPV (2018). Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer, 17, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Vacratsis PO, Qamirani E, & Gallo KA (2000). Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. Journal of Biological Chemistry, 275, 14231–14241. [DOI] [PubMed] [Google Scholar]
- Bodner A, Maroney AC, Finn JP, Ghadge G, Roos R, & Miller RJ (2002). Mixed lineage kinase 3 mediates gp120IIIB-induced neurotoxicity. J Neurochem, 82, 1424–1434. [DOI] [PubMed] [Google Scholar]
- Bracq L, Xie M, Lambele M, Vu LT, Matz J, Schmitt A, Delon J, Zhou P, Randriamampita C, Bouchet J, & Benichou S. (2017). T Cell-Macrophage Fusion Triggers Multinucleated Giant Cell Formation for HIV-1 Spreading. J Virol, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, & Davis RJ (2005). Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol, 25, 3670–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, & Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68, 394–424. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, & Hall A. (1995). A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. Journal of Biological Chemistry, 270, 29071–29074. [DOI] [PubMed] [Google Scholar]
- Byrnes KA, Phatak P, Mansour D, Xiao L, Zou T, Rao JN, Turner DJ, Wang JY, & Donahue JM (2016). Overexpression of miR-199a-5p decreases esophageal cancer cell proliferation through repression of mitogenactivated protein kinase kinase kinase-11 (MAP3K11). Oncotarget, 7, 8756–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, Li P, Lyu N, Sun T, Xie S, Guo L, Ni L, Jin L, & Dong C. (2020). Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol, 17, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamaras TD, Baumgartner RA, Aronovitz MJ, McLaughlin AL, Tam K, Richards DA, Cooper CW, Li N, Baur WE, Qiao X, Wang GR, Davis RJ, Kapur NK, Karas RH, & Blanton RM (2019). Mixed lineage kinase-3 prevents cardiac dysfunction and structural remodeling with pressure overload. Am J Physiol Heart Circ Physiol, 316, H145–H159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, & Germain RN (2006). Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature, 440, 890–895. [DOI] [PubMed] [Google Scholar]
- Chadee DN, & Kyriakis JM (2004a). MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol, 6, 770–776. [DOI] [PubMed] [Google Scholar]
- Chadee DN, & Kyriakis JM (2004b). A novel role for mixed lineage kinase 3 (MLK3) in B-Raf activation and cell proliferation. Cell Cycle, 3, 1227–1229. [DOI] [PubMed] [Google Scholar]
- Chambers JA, & Thompson JE (1976). Phagocytosis and pinocytosis in Acanthamoeba castellanii. J Gen Microbiol, 92, 246–250. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Leece CM, Gallo KA, Madhukar BV, & Conley BA (2010). Inhibition of MLK3 Decreases Proliferation and Increases Antiproliferative Activity of Epidermal Growth Factor Receptor (EGFR) Inhibitor in pancreatic cancer cell Lines. Cancer Growth and Metastasis, 3, CGM.S2824. [Google Scholar]
- Changchien CY, Lin YH, Cheng YC, Chang HH, Peng YS, & Chen Y. (2019). Indoxyl sulfate induces myotube atrophy by ROS-ERK and JNK-MAFbx cascades. Chem Biol Interact, 304, 43–51. [DOI] [PubMed] [Google Scholar]
- Chen J, Miller EM, & Gallo KA (2010). MLK3 is critical for breast cancer cell migration and promotes a malignant phenotype in mammary epithelial cells. Oncogene, 29, 4399–4411. [DOI] [PubMed] [Google Scholar]
- Chen ML, Li XT, Wei YY, Qi LP, & Sun YS (2019). Can spectral computed tomography imaging improve the differentiation between malignant and benign pulmonary lesions manifesting as solitary pure ground glass, mixed ground glass, and solid nodules? Thorac Cancer, 10, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, & Tse E. (2018). PIN1 in Cell Cycle Control and Cancer. Front Pharmacol, 9, 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ET, Zhan Y, Abi Saab WF, Korchnak AC, Ashburner BP, & Chadee DN (2009). Mixed lineage kinase 3 negatively regulates IKK activity and enhances etoposide-induced cell death. Biochim Biophys Acta, 1793, 1811–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, & Luban J. (2004). Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity, 21, 189–201. [DOI] [PubMed] [Google Scholar]
- Colgan J, Asmal M, Yu B, & Luban J. (2005). Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. J Immunol, 174, 6030–6038. [DOI] [PubMed] [Google Scholar]
- Crawley O, Giles AC, Desbois M, Kashyap S, Birnbaum R, & Grill B. (2017). A MIG-15/JNK-1 MAP kinase cascade opposes RPM-1 signaling in synapse formation and learning. PLoS Genet, 13, e1007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Nair RS, Mishra R, Sondarva G, Viswakarma N, Abdelkarim H, Gaponenko V, Rana B, & Rana A. (2019). Mixed lineage kinase 3 promotes breast tumorigenesis via phosphorylation and activation of p21-activated kinase 1. Oncogene, 38, 3569–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sondarva G, Viswakarma N, Nair RS, Osipo C, Tzivion G, Rana B, & Rana A. (2015). Human Epidermal Growth Factor Receptor 2 (HER2) Impedes MLK3 Kinase Activity to Support Breast Cancer Cell Survival. Journal of Biological Chemistry, 290, 21705–21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAizpurua HJ, Cram DS, Naselli G, Devereux L, & Dorow DS (1997). Expression of mixed lineage kinase-1 in pancreatic beta-cell lines at different stages of maturation and during embryonic pancreas development. Journal of Biological Chemistry, 272, 16364–16373. [DOI] [PubMed] [Google Scholar]
- Deng N, Chen Q, Guo X, Liu L, Chen S, Wang A, Li R, Huang Y, Ding X, Yu H, Hu S, Zhao Y, Chen X, & Nie H. (2020). Blockade of CD40L inhibits immunogenic maturation of lung dendritic cells: Implications for the role of lung iNKT cells in mouse models of asthma. Mol Immunol, 121, 167–185. [DOI] [PubMed] [Google Scholar]
- Dima C, Assadpour E, Dima S, & Jafari SM (2020). Nutraceutical nanodelivery; an insight into the bioaccessibility/bioavailability of different bioactive compounds loaded within nanocarriers. Crit Rev Food Sci Nutr, 1–35. [DOI] [PubMed] [Google Scholar]
- Dong W, Embury CM, Lu Y, Whitmire SM, Dyavarshetty B, Gelbard HA, Gendelman HE, & Kiyota T. (2016). The mixed-lineage kinase 3 inhibitor URMC-099 facilitates microglial amyloid-beta degradation. J Neuroinflammation, 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorow DS, Devereux L, Dietzsch E, & De Kretser T. (1993). Identification of a new family of human epithelial protein kinases containing two leucine/isoleucine-zipper domains. Eur J Biochem, 213, 701–710. [DOI] [PubMed] [Google Scholar]
- Dorow DS, Devereux L, Tu GF, Price G, Nicholl JK, Sutherland GR, & Simpson RJ (1995). Complete nucleotide sequence, expression, and chromosomal localisation of human mixed-lineage kinase 2. Eur J Biochem, 234, 492–500. [DOI] [PubMed] [Google Scholar]
- Du Y, Bock BC, Schachter KA, Chao M, & Gallo KA (2005). Cdc42 induces activation loop phosphorylation and membrane targeting of mixed lineage kinase 3. Journal of Biological Chemistry, 280, 42984–42993. [DOI] [PubMed] [Google Scholar]
- Duhm J, Gobel BO, & Beck FX (1983). Sodium and potassium ion transport accelerations in erythrocytes of DOC, DOC-salt, two-kidney, one clip, and spontaneously hypertensive rats. Role of hypokalemia and cell volume. Hypertension, 5, 642–652. [DOI] [PubMed] [Google Scholar]
- Durkin JT, Holskin BP, Kopec KK, Reed MS, Spais CM, Steffy BM, Gessner G, Angeles TS, Pohl J, Ator MA, & Meyer SL (2004). Phosphoregulation of mixed-lineage kinase 1 activity by multiple phosphorylation in the activation loop. Biochemistry, 43, 16348–16355. [DOI] [PubMed] [Google Scholar]
- Esensten JH, Helou YA, Chopra G, Weiss A, & Bluestone JA (2016). CD28 Costimulation: From Mechanism to Therapy. Immunity, 44, 973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoe K, Lee ST, Strunk KM, & Spritz RA (1994). PTK1, a novel protein kinase required for proliferation of human melanocytes. Oncogene, 9, 935–938. [PubMed] [Google Scholar]
- Famili F, Wiekmeijer AS, & Staal FJ (2017). The development of T cells from stem cells in mice and humans. Future Sci OA, 3, FSO186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Kolnik M, D’Angelo S, Erasmus FM, Vorholt D, & Bradbury ARM (2018). Rapid purification of billions of circulating CD19+ B cells directly from leukophoresis samples. N Biotechnol, 46, 14–21. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Schreiber H, & Fu YX (2013). Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol, 14, 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo KA, Mark MR, Scadden DT, Wang Z, Gu Q, & Godowski PJ (1994). Identification and characterization of SPRK, a novel src-homology 3 domain-containing proline-rich kinase with serine/threonine kinase activity. Journal of Biological Chemistry, 269, 15092–15100. [PubMed] [Google Scholar]
- Ganguly A, Oo TF, Rzhetskaya M, Pratt R, Yarygina O, Momoi T, Kholodilov N, & Burke RE (2004). CEP11004, a novel inhibitor of the mixed lineage kinases, suppresses apoptotic death in dopamine neurons of the substantia nigra induced by 6-hydroxydopamine. J Neurochem, 88, 469–480. [DOI] [PubMed] [Google Scholar]
- Gao A, Yan F, Zhou E, Wu L, Li L, Chen J, Lei Y, & Ye J. (2020). Molecular characterization and expression analysis of chemokine (CXCL12) from Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol, 104, 314–323. [DOI] [PubMed] [Google Scholar]
- Germic N, Frangez Z, Yousefi S, & Simon HU (2019). Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ, 26, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R, Chantry D, Raport CJ, Schweickart VL, Trong HL, & Gray PW (1997). Monocyte chemotactic protein-4: tissue-specific expression and signaling through CC chemokine receptor-2. J Leukoc Biol, 61, 353–360. [DOI] [PubMed] [Google Scholar]
- Gong Y, Yang G, Wang Q, Wang Y, & Zhang X. (2020). NME2 Is a Master Suppressor of Apoptosis in Gastric Cancer Cells via Transcriptional Regulation of miR-100 and Other Survival Factors. Mol Cancer Res, 18, 287–299. [DOI] [PubMed] [Google Scholar]
- Goodfellow VS, Loweth CJ, Ravula SB, Wiemann T, Nguyen T, Xu Y, Todd DE, Sheppard D, Pollack S, Polesskaya O, Marker DF, Dewhurst S, & Gelbard HA (2013). Discovery, synthesis, and characterization of an orally bioavailable, brain penetrant inhibitor of mixed lineage kinase 3. J Med Chem, 56, 8032–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S, Sabatos-Peyton C, Petruzzelli L, Engelman JA, & Dranoff G. (2017). Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer, 17, 286–301. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S, Abrial C, Salvator H, Brollo M, Naline E, & Devillier P. (2020). The Role of Toll-Like Receptors in the Production of Cytokines by Human Lung Macrophages. J Innate Immun, 12, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazide S, Maestre N, Veldman RJ, Bezombes C, Maddens S, Levade T, Laurent G, & Jaffrezou JP (2002). Ara-C- and daunorubicin-induced recruitment of Lyn in sphingomyelinase-enriched membrane rafts. Faseb Journal, 16, 1685–1687. [DOI] [PubMed] [Google Scholar]
- Greenwald EC, Redden JM, Dodge-Kafka KL, & Saucerman JJ (2014). Scaffold state switching amplifies, accelerates, and insulates protein kinase C signaling. Journal of Biological Chemistry, 289, 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr JC, Szenes E, Tschech L, Asslaber D, Schlederer M, Roos S, Yu X, Girbl T, Sternberg C, Egle A, Aberger F, Alon R, Kenner L, Greil R, Orian-Rousseau V, & Hartmann TN (2018). Microenvironment-induced CD44v6 promotes early disease progression in chronic lymphocytic leukemia. Blood, 131, 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Faulhaber J, Srisawang L, Stortz A, Salomon JJ, Mall MA, Frings S, & Mohrlen F. (2017). Cellular distribution and function of ion channels involved in transport processes in rat tracheal epithelium. Physiol Rep, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley ME, Rasaiyaah J, Barnett J, Thakker M, Pollara G, Katz DR, & Chain BM (2007). Expression and function of mixed lineage kinases in dendritic cells. Int Immunol, 19, 923–933. [DOI] [PubMed] [Google Scholar]
- Herr I, Wilhelm D, Bohler T, Angel P, & Debatin KM (1997). Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J, 16, 6200–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Katoh M, Terada M, Kyriakis JM, Zon LI, Rana A, Avruch J, & Ohno S. (1997). MST/MLK2, a member of the mixed lineage kinase family, directly phosphorylates and activates SEK1, an activator of c-Jun N-terminal kinase/stress-activated protein kinase. Journal of Biological Chemistry, 272, 15167–15173. [DOI] [PubMed] [Google Scholar]
- Hong HY, & Kim BC (2007). Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun, 362, 307–312. [DOI] [PubMed] [Google Scholar]
- Hudkins RL, Diebold JL, Tao M, Josef KA, Park CH, Angeles TS, Aimone LD, Husten J, Ator MA, Meyer SL, Holskin BP, Durkin JT, Fedorov AA, Fedorov EV, Almo SC, Mathiasen JR, Bozyczko-Coyne D, Saporito MS, Scott RW, & Mallamo JP (2008). Mixed-Lineage Kinase 1 and Mixed-Lineage Kinase 3 Subtype-Selective Dihydronaphthyl[3,4-a]pyrrolo[3,4-c]carbazole-5-ones: Optimization, Mixed-Lineage Kinase 1 Crystallography, and Oral in Vivo Activity in 1-Methyl-4-phenyltetrahydropyridine Models. J Med Chem. [DOI] [PubMed] [Google Scholar]
- Humphrey RK, Newcomb CJ, Yu SM, Hao E, Yu D, Krajewski S, Du K, & Jhala US (2010). Mixed lineage kinase-3 stabilizes and functionally cooperates with TRIBBLES-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells. Journal of Biological Chemistry, 285, 22426–22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrissova L, Malhi H, Werneburg NW, LeBrasseur NK, Bronk SF, Fingas C, Tchkonia T, Pirtskhalava T, White TA, Stout MB, Hirsova P, Krishnan A, Liedtke C, Trautwein C, Finnberg N, El-Deiry WS, Kirkland JL, & Gores GJ (2015). TRAIL receptor deletion in mice suppresses the inflammation of nutrient excess. J Hepatol, 62, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaguma S, Lasota J, Czapiewski P, Langfort R, Rys J, Szpor J, Waloszczyk P, Okon K, Biernat W, Schrump DS, Hassan R, Kasai K, Miettinen M, & Ikeda H. (2020). CD70 expression correlates with a worse prognosis in malignant pleural mesothelioma patients via immune evasion and enhanced invasiveness. J Pathol, 250, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing YL, Leung IW, Heng HH, Tsui LC, & Lassam NJ (1994). MLK-3: identification of a widely-expressed protein kinase bearing an SH3 domain and a leucine zipper-basic region domain. Oncogene, 9, 1745–1750. [PubMed] [Google Scholar]
- Jacobs J, Deschoolmeester V, Zwaenepoel K, Flieswasser T, Deben C, Van den Bossche J, Hermans C, Rolfo C, Peeters M, De Wever O, Lardon F, Siozopoulou V, Smits E, & Pauwels P. (2018). Unveiling a CD70-positive subset of cancer-associated fibroblasts marked by pro-migratory activity and thriving regulatory T cell accumulation. Oncoimmunology, 7, e1440167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Deschoolmeester V, Zwaenepoel K, Rolfo C, Silence K, Rottey S, Lardon F, Smits E, & Pauwels P. (2015). CD70: An emerging target in cancer immunotherapy. Pharmacol Ther, 155, 1–10. [DOI] [PubMed] [Google Scholar]
- Jardim DL, Groves ES, Breitfeld PP, & Kurzrock R. (2017). Factors associated with failure of oncology drugs in late-stage clinical development: A systematic review. Cancer Treat Rev, 52, 12–21. [DOI] [PubMed] [Google Scholar]
- Jo Y, Choi N, Kim K, Koo HJ, Choi J, & Kim HN (2018). Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development. Theranostics, 8, 5259–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Saito Y, Saito H, Matsumoto T, Matsuda Y, Vaught JL, Dionne CA, Angeles TS, Glicksman MA, Neff NT, Rotella DP, Kauer JC, Mallamo JP, Hudkins RL, & Murakata C. (1997). Neurotrophic 3,9-bis[(alkylthio)methyl]-and-bis(alkoxymethyl)-K-252a derivatives. J Med Chem, 40, 1863–1869. [DOI] [PubMed] [Google Scholar]
- Kantari C, Pederzoli-Ribeil M, & Witko-Sarsat V. (2008). The role of neutrophils and monocytes in innate immunity. Contrib Microbiol, 15, 118–146. [DOI] [PubMed] [Google Scholar]
- Kashuba VI, Grigorieva EV, Kvasha SM, Pavlova TV, Grigoriev V, Protopopov A, Kharchenko O, Gizatullin R, Rynditch AV, & Zabarovsky ER (2011). Cloning and Initial Functional Characterization of Mlk4alpha and Mlk4beta. Genomics Insights, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Hirai M, Sugimura T, & Terada M. (1995). Cloning and characterization of MST, a novel (putative) serine/threonine kinase with SH3 domain. Oncogene, 10, 1447–1451. [PubMed] [Google Scholar]
- Kawagoe S, Nakagawa H, Kumeta H, Ishimori K, & Saio T. (2018). Structural insight into proline cis/trans isomerization of unfolded proteins catalyzed by the trigger factor chaperone. Journal of Biological Chemistry, 293, 15095–15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Timin EN, & Stary-Weinzinger A. (2014). Different inward and outward conduction mechanisms in NaVMs suggested by molecular dynamics simulations. PLoS Comput Biol, 10, e1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ezhilarasan R, Phillips E, Gallego-Perez D, Sparks A, Taylor D, Ladner K, Furuta T, Sabit H, Chhipa R, Cho JH, Mohyeldin A, Beck S, Kurozumi K, Kuroiwa T, Iwata R, Asai A, Kim J, Sulman EP, Cheng SY, Lee LJ, Nakada M, Guttridge D, DasGupta B, Goidts V, Bhat KP, & Nakano I. (2016). Serine/Threonine Kinase MLK4 Determines Mesenchymal Identity in Glioma Stem Cells in an NF-kappaB-dependent Manner. Cancer Cell, 29, 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada S, Pedersen IM, Schimmer AD, & Reed JC (2002). Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene, 21, 3459–3474. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Machhi J, Lu Y, Dyavarshetty B, Nemati M, Zhang G, Mosley RL, Gelbard HA, & Gendelman HE (2018). URMC-099 facilitates amyloid-beta clearance in a murine model of Alzheimer’s disease. J Neuroinflammation, 15, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hessling S, Muhammad K, Klein M, Pusch T, Rudolf R, Floter J, Qureischi M, Beilhack A, Vaeth M, Kummerow C, Backes C, Schoppmeyer R, Hahn U, Hoth M, Bopp T, Berberich-Siebelt F, Patra A, Avots A, Muller N, Schulze A, & Serfling E. (2017). NFATc1 controls the cytotoxicity of CD8+ T cells. Nat Commun, 8, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline EM, Butkovich LM, Bradner JM, Chang J, Gelbard H, Goodfellow V, Caudle WM, & Tansey MG (2019). The second generation mixed lineage kinase-3 (MLK3) inhibitor CLFB-1134 protects against neurotoxin-induced nigral dopaminergic neuron loss. Exp Neurol, 318, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss U, Lindner SE, Aneichyk T, Kotkamp B, Knust Z, Villunger A, & Herzog S. (2016). MAP3K11 is a tumor suppressor targeted by the oncomiR miR-125b in early B cells. Cell Death Differ, 23, 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchnak AC, Zhan Y, Aguilar MT, & Chadee DN (2009). Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell Signal, 21, 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S, Levy G, Hanoch T, Naor Z, & Seger R. (2004). Gonadotropin-releasing hormone induces apoptosis of prostate cancer cells: role of c-Jun NH2-terminal kinase, protein kinase B, and extracellular signal-regulated kinase pathways. Cancer Research, 64, 5736–5744. [DOI] [PubMed] [Google Scholar]
- Kumar S, Singh SK, Viswakarma N, Sondarva G, Nair RS, Sethupathi P, Dorman M, Sinha SC, Hoskins K, Thatcher G, Rana B, & Rana A. (2020). Rationalized inhibition of mixed lineage kinase 3 and CD70 enhances life span and antitumor efficacy of CD8(+) T cells. J Immunother Cancer, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Singh SK, Viswakarma N, Sondarva G, Nair RS, Sethupathi P, Sinha SC, Emmadi R, Hoskins K, Danciu O, Thatcher GRJ, Rana B, & Rana A. (2020). Mixed lineage kinase 3 inhibition induces T cell activation and cytotoxicity. Proc Natl Acad Sci U S A, 117, 7961–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Singh SK, Viswakarma N, Sondarva G, Nair RS, Thatcher G, Rana B, & Rana A. (2020). Paradoxical Role of MLK3-CTLA-4 axis in Triple-negative Breast Cancer and CD8+ T cells. In Annual Meeting of the American Association of Immunologists (Vol. 204, pp. 241.247–241.247): The Journal of Immunology. [Google Scholar]
- Kurynina AV, Erokhina MV, Makarevich OA, Sysoeva VY, Lepekha LN, Kuznetsov SA, & Onishchenko GE (2018). Plasticity of Human THP-1 Cell Phagocytic Activity during Macrophagic Differentiation. Biochemistry (Mosc), 83, 200–214. [DOI] [PubMed] [Google Scholar]
- Kuwana T, King LE, Cosentino K, Suess J, Garcia-Saez AJ, Gilmore AP, & Newmeyer DD (2020). Mitochondrial residence of the apoptosis inducer BAX is more important than BAX oligomerization in promoting membrane permeabilization. Journal of Biological Chemistry, 295, 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, & Cabot MC (1997). Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. Journal of Biological Chemistry, 272, 1682–1687. [DOI] [PubMed] [Google Scholar]
- Lee HS, Hwang CY, Shin SY, Kwon KS, & Cho KH (2014). MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species. Sci Signal, 7, ra52. [DOI] [PubMed] [Google Scholar]
- Leiding JW (2017). Neutrophil Evolution and Their Diseases in Humans. Frontiers in Immunology, 8, 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung IW, & Lassam N. (1998). Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. Journal of Biological Chemistry, 273, 32408–32415. [DOI] [PubMed] [Google Scholar]
- Leung IW, & Lassam N. (2001). The kinase activation loop is the key to mixed lineage kinase-3 activation via both autophosphorylation and hematopoietic progenitor kinase 1 phosphorylation. Journal of Biological Chemistry, 276, 1961–1967. [DOI] [PubMed] [Google Scholar]
- Li Y, Zuo H, Wang H, & Hu A. (2019). Decrease of MLK4 prevents hepatocellular carcinoma (HCC) through reducing metastasis and inducing apoptosis regulated by ROS/MAPKs signaling. Biomed Pharmacother, 116, 108749. [DOI] [PubMed] [Google Scholar]
- Lin W, Bonin M, Boden A, Wieduwild R, Murawala P, Wermke M, Andrade H, Bornhauser M, & Zhang Y. (2019). Peptidyl prolyl cis/trans isomerase activity on the cell surface correlates with extracellular matrix development. Commun Biol, 2, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wang S, Yang Z, Lin J, Ke Q, Lan W, Shi J, Wu S, & Cai B. (2017). Heme Oxygenase-1 Inhibits Neuronal Apoptosis in Spinal Cord Injury through Down-Regulation of Cdc42-MLK3-MKK7-JNK3 Axis. J Neurotrauma, 34, 695–706. [DOI] [PubMed] [Google Scholar]
- Liu B, Shen LJ, Zhao TX, Sun M, Wang JK, Long CL, He DW, Lin T, Wu SD, & Wei GH (2020). Automobile exhaust-derived PM2.5 induces blood-testis barrier damage through ROS-MAPK-Nrf2 pathway in sertoli cells of rats. Ecotoxicol Environ Saf, 189, 110053. [DOI] [PubMed] [Google Scholar]
- Liu L, Yin B, Yi Z, Liu X, Hu Z, Gao W, Yu H, & Li Q. (2018). Breast cancer stem cells characterized by CD70 expression preferentially metastasize to the lungs. Breast Cancer, 25, 706–716. [DOI] [PubMed] [Google Scholar]
- Liu WF, Youn HD, Zhou XZ, Lu KP, & Liu JO (2001). Binding and regulation of the transcription factor NFAT by the peptidyl prolyl cis-trans isomerase Pin1. FEBS Lett, 496, 105–108. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei H, Tang J, Yuan J, Wu M, Yao C, Hosoi K, Yu S, Zhao X, Han Y, & Chen G. (2020). Dysfunction of pulmonary epithelial tight junction induced by silicon dioxide nanoparticles via the ROS/ERK pathway and protein degradation. Chemosphere, 255, 126954. [DOI] [PubMed] [Google Scholar]
- Lu H, Liu S, Zhang G, Bin W, Zhu Y, Frederick DT, Hu Y, Zhong W, Randell S, Sadek N, Zhang W, Chen G, Cheng C, Zeng J, Wu LW, Zhang J, Liu X, Xu W, Krepler C, Sproesser K, Xiao M, Miao B, Liu J, Song CD, Liu JY, Karakousis GC, Schuchter LM, Lu Y, Mills G, Cong Y, Chernoff J, Guo J, Boland GM, Sullivan RJ, Wei Z, Field J, Amaravadi RK, Flaherty KT, Herlyn M, Xu X, & Guo W. (2017). PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature, 550, 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Cheng Y, & Zeng J. (2019). MLK3 silence induces cervical cancer cell apoptosis via the Notch-1/autophagy network. Clin Exp Pharmacol Physiol, 46, 854–860. [DOI] [PubMed] [Google Scholar]
- Mankouri J, Dallas ML, Hughes ME, Griffin SD, Macdonald A, Peers C, & Harris M. (2009). Suppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistence. Proc Natl Acad Sci U S A, 106, 15903–15908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoori B, Mohammadi A, Davudian S, Shirjang S, & Baradaran B. (2017). The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv Pharm Bull, 7, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovic M, Mijanovic L, Sostar M, Vizovisek M, Junemann A, Fonovic M, Turk B, Weber I, Faix J, & Filic V. (2019). IQGAP-related protein IqgC suppresses Ras signaling during large-scale endocytosis. Proc Natl Acad Sci U S A, 116, 1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker DF, Tremblay ME, Puccini JM, Barbieri J, Gantz Marker MA, Loweth CJ, Muly EC, Lu SM, Goodfellow VS, Dewhurst S, & Gelbard HA (2013). The new small-molecule mixed-lineage kinase 3 inhibitor URMC-099 is neuroprotective and anti-inflammatory in models of human immunodeficiency virus-associated neurocognitive disorders. J Neurosci, 33, 9998–10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, Xu Z, Meyer SL, Savage MJ, Greene LA, Scott RW, & Vaught J. L. g. (2001). Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. Journal of Biological Chemistry, 276, 25302–25308. [DOI] [PubMed] [Google Scholar]
- Maroufi NF, Vahedian V, Akbarzadeh M, Mohammadian M, Zahedi M, Isazadeh A, Pouremamali F, Taefehshokr S, Heidari M, Rashidi M, & Nouri M. (2020). The apatinib inhibits breast cancer cell line MDA-MB-231 in vitro by inducing apoptosis, cell cycle arrest, and regulating nuclear factor-kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) signaling pathways. Breast Cancer, 27, 613–620. [DOI] [PubMed] [Google Scholar]
- Marusiak AA, Prelowska MK, Mehlich D, Lazniewski M, Kaminska K, Gorczynski A, Korwat A, Sokolowska O, Kedzierska H, Golab J, Biernat W, Plewczynski D, Brognard J, & Nowis D. (2019). Upregulation of MLK4 promotes migratory and invasive potential of breast cancer cells. Oncogene, 38, 2860–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, & Pandey S. (2004). Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol, 201, 21–31. [DOI] [PubMed] [Google Scholar]
- McComb S, Chan PK, Guinot A, Hartmannsdottir H, Jenni S, Dobay MP, Bourquin JP, & Bornhauser BC (2019). Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or −7. Sci Adv, 5, eaau9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medel B, Costoya C, Fernandez D, Pereda C, Lladser A, Sauma D, Pacheco R, Iwawaki T, Salazar-Onfray F, & Osorio F. (2018). IRE1alpha Activation in Bone Marrow-Derived Dendritic Cells Modulates Innate Recognition of Melanoma Cells and Favors CD8(+) T Cell Priming. Frontiers in Immunology, 9, 3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer HL, Snyder LM, Doherty CM, Fox BA, Bzik DJ, & Denkers EY (2020). Toxoplasma gondii dense granule protein GRA24 drives MyD88independent p38 MAPK activation, IL-12 production and induction of protective immunity. PLoS Pathog, 16, e1008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami N, Kawakami R, Chen KY, Sugimoto A, Ohkura N, & Sakaguchi S. (2020). Epigenetic conversion of conventional T cells into regulatory T cells by CD28 signal deprivation. Proc Natl Acad Sci U S A, 117, 12258–12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misek SA, Chen J, Schroeder L, Rattanasinchai C, Sample A, Sarkaria JN, & Gallo KA (2017). EGFR Signals through a DOCK180-MLK3 Axis to Drive Glioblastoma Cell Invasion. Mol Cancer Res, 15, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Mishra, Senthivinayagam S, Rangasamy V, Sondarva G, & Rana B. (2010). Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol Endocrinol, 24, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, Woodgett JR, & Rana A. (2007). Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. Journal of Biological Chemistry, 282, 30393–30405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, & Forster R. (2004). Thymic T cell development and progenitor localization depend on CCR7. Journal of Experimental Medicine, 200, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moideen K, Kumar NP, Bethunaickan R, Banurekha VV, Nair D, & Babu S. (2020). Heightened systemic levels of anti-inflammatory cytokines in pulmonary tuberculosis and alterations following anti-tuberculosis treatment. Cytokine, 127, 154929. [DOI] [PubMed] [Google Scholar]
- Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, & Segui B. (2019). The TNF Paradox in Cancer Progression and Immunotherapy. Frontiers in Immunology, 10, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota M, Reeder M, Chernoff J, & Bazenet CE (2001). Evidence for a role of mixed lineage kinases in neuronal apoptosis. J Neurosci, 21, 4949–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I, Kumamoto M, & Fujiwara M. (1978). Effects of conditioning polarization on the membrane ionic currents in rat myometrium. J Membr Biol, 44, 331–352. [DOI] [PubMed] [Google Scholar]
- Nagao M, & Hayashi H. (2009). Mixed lineage kinase 2 and hippocalcin are localized in Lewy bodies of Parkinson’s disease. J Neurol Sci, 281, 51–54. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, & Swisher SG (2002). Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. Journal of Biological Chemistry, 277, 9219–9225. [DOI] [PubMed] [Google Scholar]
- Okada M, Takeda H, Sakaki H, Kuramoto K, Suzuki S, Sanomachi T, Togashi K, Seino S, & Kitanaka C. (2017). Repositioning CEP-1347, a chemical agent originally developed for the treatment of Parkinson’s disease, as an anti-cancer stem cell drug. Oncotarget, 8, 94872–94882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Fujio K, Tsuno NH, Takahashi K, & Yamamoto K. (2012). Kidney-infiltrating CD4+ T-cell clones promote nephritis in lupus-prone mice. Kidney Int, 82, 969–979. [DOI] [PubMed] [Google Scholar]
- Pahl JH, Santos SJ, Kuijjer ML, Boerman GH, Sand LG, Szuhai K, Cleton-Jansen A, Egeler RM, Bovee JV, Schilham MW, & Lankester AC (2015). Expression of the immune regulation antigen CD70 in osteosarcoma. Cancer Cell Int, 15, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N, & Patial S. (2010). Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr, 20, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group PI (2007). Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology, 69, 1480–1490. [DOI] [PubMed] [Google Scholar]
- Pedraza N, Rafel M, Navarro I, Encinas M, Aldea M, & Gallego C. (2009). Mixed lineage kinase phosphorylates transcription factor E47 and inhibits TrkB expression to link neuronal death and survival pathways. Journal of Biological Chemistry, 284, 32980–32988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli AM, & Fernandez-Valle C. (2016). Role of Merlin/NF2 inactivation in tumor biology. Oncogene, 35, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuch L, Hurwitz R, Traulsen J, Sigulla J, Poeschke M, Matzner L, Kosma P, Schmid M, & Meyer TF (2019). ADP heptose, a novel pathogen-associated molecular pattern identified in Helicobacter pylori. Faseb Journal, 33, 9087–9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechutta M, & Berghoff AS (2019). New emerging targets in cancer immunotherapy: the role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open, 4, e000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinskaya YL, & Liu Z. (2012). The role of TRADD in death receptor signaling. Cell Cycle, 11, 871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya O, Wong C, Lebron L, Chamberlain JM, Gelbard HA, Goodfellow V, Kim M, Daiss JL, & Dewhurst S. (2014). MLK3 regulates fMLP-stimulated neutrophil motility. Mol Immunol, 58, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Fan S, Dykstra H, Reichenbach N, Del Valle L, Potula R, Phipps RP, Maggirwar SB, & Persidsky Y. (2010). Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J Neurosci, 30, 9454–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis JM, & Avruch J. (1996). The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. Journal of Biological Chemistry, 271, 19025–19028. [DOI] [PubMed] [Google Scholar]
- Rangasamy V, Mishra R, Mehrotra S, Sondarva G, Ray RS, Rao A, Chatterjee M, Rana B, & Rana A. (2010). Estrogen suppresses MLK3-mediated apoptosis sensitivity in ER+ breast cancer cells. Cancer Research, 70, 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy V, Mishra R, Sondarva G, Das S, Lee TH, Bakowska JC, Tzivion G, Malter JS, Rana B, Lu KP, Kanthasamy A, & Rana A. (2012). Mixed-lineage kinase 3 phosphorylates prolyl-isomerase Pin1 to regulate its nuclear translocation and cellular function. Proc Natl Acad Sci U S A, 109, 8149–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanasinchai C, Llewellyn BJ, Conrad SE, & Gallo KA (2017). MLK3 regulates FRA-1 and MMPs to drive invasion and transendothelial migration in triple-negative breast cancer cells. Oncogenesis, 6, e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritch SJ, Brandhagen BN, Goyeneche AA, & Telleria CM (2019). Advanced assessment of migration and invasion of cancer cells in response to mifepristone therapy using double fluorescence cytochemical labeling. Bmc Cancer, 19, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Jafari SM, Shinde R, Duncan G, Cescon DW, Silvester J, Chu MF, Hodgson K, Berger T, Wakeham A, Palomero L, Garcia-Valero M, Pujana MA, Mak TW, McGaha TL, Cappello P, & Gorrini C. (2019). Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci U S A, 116, 4326–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleiro D, Blyth GT, Kosciuczuk EM, Ozark PA, Majchrzak-Kita B, Arslan AD, Fischietti M, Reddy NK, Horvath CM, Davis RJ, Fish EN, & Platanias LC (2018). IFN-gamma-inducible antiviral responses require ULK1-mediated activation of MLK3 and ERK5. Sci Signal, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghvi-Shah R, & Weber GF (2017). Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front Cell Dev Biol, 5, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, & Carswell S. (1999). CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J Pharmacol Exp Ther, 288, 421–427. [PubMed] [Google Scholar]
- Sathyanarayana P, Barthwal MK, Kundu CN, Lane ME, Bergmann A, Tzivion G, & Rana A. (2002). Activation of the Drosophila MLK by Ceramide Reveals TNF-alpha and Ceramide as Agonists of Mammalian MLK3. Mol Cell, 10, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Schroyer AL, Stimes NW, Abi Saab WF, & Chadee DN (2018). MLK3 phosphorylation by ERK1/2 is required for oxidative stress-induced invasion of colorectal cancer cells. Oncogene, 37, 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule JM, Bergkvist L, Hakansson L, Gustafsson B, & Hakansson A. (2004). CD28 expression in sentinel node biopsies from breast cancer patients in comparison with CD3-zeta chain expression. J Transl Med, 2, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seit-Nebi A, Cheng W, Xu H, & Han J. (2012). MLK4 has negative effect on TLR4 signaling. Cell Mol Immunol, 9, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille J, Dao Duc K, Holcman D, & Rouach N. (2015). The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLoS Comput Biol, 11, e1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A. (2020). Cancer statistics, 2020. CA Cancer J Clin, 70, 7–30. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, & Weinberg RA (2016). Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov, 6, 630–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Dai D, Horton B, & Gajewski TF (2017). Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell, 31, 711–723 e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, & Insel PA (2018). G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol, 93, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Fan S, Sniderhan L, Reisinger E, Litzburg A, Schifitto G, Gelbard HA, Dewhurst S, & Maggirwar SB (2006). Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol, 177, 702–711. [DOI] [PubMed] [Google Scholar]
- Sun D, & Ding A. (2006). MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol, 7, 375–381. [DOI] [PubMed] [Google Scholar]
- Sutti S, & Albano E. (2020). Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol, 17, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ZK, & Lewcock JW (2011). ACS chemical neuroscience spotlight on CEP-1347. ACS Chem Neurosci, 2, 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson KI, Winkler KE, & Means AR (2003). A New Identity for MLK3 as an NIMA-related, Cell Cycle-regulated Kinase That Is Localized near Centrosomes and Influences Microtubule Organization. Molecular Biology of the Cell, 14, 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, & Green DR (2013). Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JZA, Fourriere L, Wang J, Perez F, Boncompain G, & Gleeson PA (2020). Distinct anterograde trafficking pathways of BACE1 and amyloid precursor protein from the TGN and the regulation of amyloid-beta production. Molecular Biology of the Cell, 31, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, & Hanafusa H. (1998). Guanine-nucleotide exchange protein C3G activates JNK1 by a ras-independent mechanism. JNK1 activation inhibited by kinase negative forms of MLK3 and DLK mixed lineage kinases. Journal of Biological Chemistry, 273, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, & Gutkind JS (1996). Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. Journal of Biological Chemistry, 271, 27225–27228. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, & Lassam NJ (1996). MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J, 15, 7026–7035. [PMC free article] [PubMed] [Google Scholar]
- Tivnan A, Tracey L, Buckley PG, Alcock LC, Davidoff AM, & Stallings RL (2011). MicroRNA-34a is a potent tumor suppressor molecule in vivo in neuroblastoma. Bmc Cancer, 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K, Okada M, Yamamoto M, Suzuki S, Sanomachi T, Seino S, Yamashita H, & Kitanaka C. (2018). A Small-molecule Kinase Inhibitor, CEP-1347, Inhibits Survivin Expression and Sensitizes Ovarian Cancer Stem Cells to Paclitaxel. Anticancer Res, 38, 4535–4542. [DOI] [PubMed] [Google Scholar]
- Tomita K, Kabashima A, Freeman BL, Bronk SF, Hirsova P, & Ibrahim SH (2017). Mixed Lineage Kinase 3 Mediates the Induction of CXCL10 by a STAT1-Dependent Mechanism During Hepatocyte Lipotoxicity. J Cell Biochem, 118, 3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Kohli R, MacLaurin BL, Hirsova P, Guo Q, Sanchez LHG, Gelbard HA, Blaxall BC, & Ibrahim SH (2017). Mixed-lineage kinase 3 pharmacological inhibition attenuates murine nonalcoholic steatohepatitis. JCI Insight, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter L, Panton W, Hajimohamadreza I, Petalidis L, Ward R, Fleming Y, Armstrong CG, Cohen P, Karran EH, & Kinloch RA (2002). Mitogen-activated protein kinase kinase 7 is activated during low potassium-induced apoptosis in rat cerebellar granule neurons. Neurosci Lett, 320, 29–32. [DOI] [PubMed] [Google Scholar]
- Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, Einama T, Morita D, Fukatsu K, Sugiura Y, Matsubara O, & Mochizuki H. (2006). Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol, 19, 788–796. [DOI] [PubMed] [Google Scholar]
- Valerio L, Pieruzzi L, Giani C, Agate L, Bottici V, Lorusso L, Cappagli V, Puleo L, Matrone A, Viola D, Romei C, Ciampi R, Molinaro E, & Elisei R. (2017). Targeted Therapy in Thyroid Cancer: State of the Art. Clin Oncol (R Coll Radiol), 29, 316–324. [DOI] [PubMed] [Google Scholar]
- Velho S, Oliveira C, Paredes J, Sousa S, Leite M, Matos P, Milanezi F, Ribeiro AS, Mendes N, Licastro D, Karhu A, Oliveira MJ, Ligtenberg M, Hamelin R, Carneiro F, Lindblom A, Peltomaki P, Castedo S, Schwartz S Jr., Jordan P, Aaltonen LA, Hofstra RM, Suriano G, Stupka E, Fialho AM, & Seruca R. (2010). Mixed lineage kinase 3 gene mutations in mismatch repair deficient gastrointestinal tumours. Hum Mol Genet, 19, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswakarma N, Nair RS, Sondarva G, Das S, Ibrahimi L, Chen Z, Sinha S, Rana B, & Rana A. (2017). Transcriptional regulation of mixed lineage kinase 3 by estrogen and its implication in ER-positive breast cancer pathogenesis. Oncotarget, 8, 33172–33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH (2020). CD40 Agonist Antibodies in Cancer Immunotherapy . Annu Rev Med, 71, 47–58. [DOI] [PubMed] [Google Scholar]
- Waite JC, Wang B, Haber L, Hermann A, Ullman E, Ye X, Dudgeon D, Slim R, Ajithdoss DK, Godin SJ, Ramos I, Wu Q, Oswald E, Poon P, Golubov J, Grote D, Stella J, Pawashe A, Finney J, Herlihy E, Ahmed H, Kamat V, Dorvilliers A, Navarro E, Xiao J, Kim J, Yang SN, Warsaw J, Lett C, Canova L, Schulenburg T, Foster R, Krueger P, Garnova E, Rafique A, Babb R, Chen G, Stokes Oristian N, Siao CJ, Daly C, Gurer C, Martin J, Macdonald L, MacDonald D, Poueymirou W, Smith E, Lowy I, Thurston G, Olson W, Lin JC, Sleeman MA, Yancopoulos GD, Murphy AJ, & Skokos D. (2020). Tumor-targeted CD28 bispecific antibodies enhance the antitumor efficacy of PD-1 immunotherapy. Sci Transl Med, 12. [DOI] [PubMed] [Google Scholar]
- Waldmeier P, Bozyczko-Coyne D, Williams M, & Vaught JL (2006). Recent clinical failures in Parkinson’s disease with apoptosis inhibitors underline the need for a paradigm shift in drug discovery for neurodegenerative diseases. Biochem Pharmacol, 72, 1197–1206. [DOI] [PubMed] [Google Scholar]
- Warren GW, Cartmell KB, Garrett-Mayer E, Salloum RG, & Cummings KM (2019). Attributable Failure of First-line Cancer Treatment and Incremental Costs Associated With Smoking by Patients With Cancer. JAMA Netw Open, 2, e191703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby EA, Perkins GR, Collins MK, & Whitmarsh AJ (2003). The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. Journal of Biological Chemistry, 278, 10731–10736. [DOI] [PubMed] [Google Scholar]
- Wu Z, Xia Y, Wang Z, Su Kang S, Lei K, Liu X, Jin L, Wang X, Cheng L, & Ye K. (2020). C/EBPbeta/delta-secretase signaling mediates Parkinson’s disease pathogenesis via regulating transcription and proteolytic cleavage of alpha-synuclein and MAOB. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Xu H, Cheng M, Chi X, Liu X, Zhou J, Lin T, & Yang W. (2019). High-Throughput Screening Identifies Mixed-Lineage Kinase 3 as a Key Host Regulatory Factor in Zika Virus Infection. J Virol, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Kukekov NV, & Greene LA (2005). Regulation of apoptotic c-Jun N-terminal kinase signaling by a stabilization-based feed-forward loop. Mol Cell Biol, 25, 9949–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, & Greene LA (2001). The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol, 21, 4713–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Gibson SA, Yan Z, Wei H, Tao J, Sha B, Qin H, & Benveniste EN (2020). Protein kinase 2 (CK2) controls CD4(+) T cell effector function in the pathogenesis of colitis. Mucosal Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue M, Huang P, Wang C, Fan H, Tian T, Wu J, Luo F, Fu Z, Xia X, Zhu P, Li J, Han Y, Zhang Y, & Hou W. (2020). Genetic Variation on TNF/LTA and TNFRSF1A Genes is Associated with Outcomes of Hepatitis C Virus Infection. Immunol Invest, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Modi N, Stewart AM, Hieronimus RI, Liu J, Gutmann DH, & Chadee DN (2011). Regulation of mixed lineage kinase 3 is required for Neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene, 30, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Saab WFA, Modi N, Stewart AM, Liu JS, & Chadee DN (2012). Mixed lineage kinase 3 is required for matrix metalloproteinase expression and invasion in ovarian cancer cells. Experimental Cell Research, 318, 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wu Q, Li B, Wang D, Wang L, & Zhou YL (2020). m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer, 19, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gannon M, Chen Y, Yan S, Zhang S, Feng W, Tao J, Sha B, Liu Z, Saito T, Saido T, Keene CD, Jiao K, Roberson ED, Xu H, & Wang Q. (2020). beta-amyloid redirects norepinephrine signaling to activate the pathogenic GSK3beta/tau cascade. Sci Transl Med, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhu Y, Wu S, Hou G, Wu N, Qian L, & Yang D. (2020). MLK3 is a newly identified microRNA-520b target that regulates liver cancer cell migration. PLoS One, 15, e0230716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dutta P, Liu J, Sabri N, Song Y, Li WX, & Li J. (2019). Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J Cell Mol Med, 23, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu W, Du Y, Santos SJ, Conrad SE, Watson JT, Grammatikakis N, & Gallo KA (2004). Hsp90/p50cdc37 is required for mixed-lineage kinase (MLK) 3 signaling. Journal of Biological Chemistry, 279, 19457–19463. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lu L, Xiong Y, Qin W, Zhang Y, Qian Y, Jiang H, & Liu W. (2014). MLK3 promotes melanoma proliferation and invasion and is a target of microRNA-125b. Clin Exp Dermatol, 39, 376–384. [DOI] [PubMed] [Google Scholar]
- Zheng T, Zhang B, Chen C, Ma J, Meng D, Huang J, Hu R, Liu X, Otsu K, Liu AC, Li H, Yin Z, & Huang G. (2018). Protein kinase p38alpha signaling in dendritic cells regulates colon inflammation and tumorigenesis. Proc Natl Acad Sci U S A, 115, E12313-E12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, & Kyriakis JM (2007). Dissection of a signaling pathway by which pathogen-associated molecular patterns recruit the JNK and p38 MAPKs and trigger cytokine release. Journal of Biological Chemistry, 282, 24246–24254. [DOI] [PubMed] [Google Scholar]
- Zhou GR, Huang DP, Sun ZF, & Zhang XF (2020). Long non-coding RNA BCAR4 accelerates cell proliferation and suppresses cell apoptosis in gastric cancer via regulating MAPK/ERK signaling. Eur Rev Med Pharmacol Sci, 24, 3657–3664. [DOI] [PubMed] [Google Scholar]
- Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan C, Scheres SH, & Shi Y. (2015). Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev, 29, 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhou Q, Wu X, Xu S, Hu X, Tao X, Li B, Peng J, Li D, Shen L, Cao Y, & Yang L. (2020). VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett, 473, 62–73. [DOI] [PubMed] [Google Scholar]
- Zlotoff DA, & Bhandoola A. (2011). Hematopoietic progenitor migration to the adult thymus. Ann N Y Acad Sci, 1217, 122–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Linck V, Zhai YJ, Galarza-Paez L, Li L, Yue Q, Al-Khalili O, Bao HF, Ma HP, Thai TL, Jiao J, & Eaton DC (2018). Knockout of mitochondrial voltage-dependent anion channel type 3 increases reactive oxygen species (ROS) levels and alters renal sodium transport. Journal of Biological Chemistry, 293, 1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]