Abstract
Background:
The adoption of low carbohydrate diets can lead to weight loss in many patients. However, these now widespread diets also have the potential to exacerbate hypercholesterolemia.
Objective:
The objective of this study is to display the potentially harmful effects of the ketogenic diet on cholesterol levels in patients with or without underlying hyperlipidemia.
Methods:
We describe five patients who developed marked increases in plasma cholesterol on ketogenic diets and assessed whether they had a well described underlying genetic hyperlipidemia.
Results:
Three out of five patients had extraordinary increases of blood cholesterol levels to over 500 mg/dL. The other two patients more than doubled their LDL-cholesterol levels on a ketogenic diet. One patient had an APOE E2/E2 genotype. A higher burden of common genetic polymorphisms was found in two patients, with no major mutations found. No potential genetic cause was seen in a fourth patient and the fifth patient had no genetic testing. Three patients, including the one who was most hypercholesterolemic, had a marked reduction in cholesterol after reverting to a more liberal diet. One refused to change his diet but had a satisfactory LDL cholesterol reduction on ezetimibe.
Conclusion:
These cases should serve as a caution that high fat low carbohydrate diets have the potential to exacerbate or cause hypercholesterolemia in patients with or without underlying genetic hyperlipidemia.
Keywords: obesity, cholesterol, dysbetalipoproteinemia, hyperlipidemia, ezetimibe, ketogenic diet
Introduction
A cornerstone of the treatment of hypercholesterolemia has been diets lower in cholesterol and saturated fat. Support for these types of diet as a means to lower blood levels of LDL cholesterol has come from randomized controlled diet studies done on outpatients and in metabolic units.1–6 These data have led to widespread advocacy of dietary change as the initial approach to cholesterol management.4, 7–9 Weight loss is also associated with reduced cholesterol, even when this is not driven by a “heart healthy” diet.4, 7, 8 Moreover, because diets that are higher in carbohydrates, especially non-complex carbohydrates, increase triglyceride levels, greater fat intake has been advocated by some.4, 7
The difficulty in achieving weight loss and preventing regain has vexed clinicians for decades. The increasing weight of populations with unlimited access to caloric-rich foods has been attributed to the composition of diet, the microbiome, changes in exercise, and most prominently, the increased intake of calories.10, 11 A number of dietary approaches have been advocated. One approach is based on the “insulin carbohydrate model”, which postulates that foods containing sugars or refined carbohydrates cause a transient rise in insulin levels that trigger hunger and increase eating.4, 8, 12, 13 Advocates of this theory recommend a low carbohydrate diet that drives ketone body production as a method to reduce hunger. However, long term studies have failed to show that low carbohydrates lead to greater weight loss after a year.14
As a caution to those physicians and patients who adopt a low carbohydrate diet, we present a series of five patients for whom a low carbohydrate “ketogenic” diet should have been contra-indicated, although this was not initially appreciated. People vary significantly in their responses to diet; a well-known case described a person who had normal circulating cholesterol despite eating more than 20 eggs each day.15 Clinicians also encounter patients with opposite paradoxical responses. Thus, individual lipid responses to diets need to be monitored, especially in patients with known dyslipidemia.
While our patients’ hyper response to a low-carbohydrate diet is not widely discussed in the medical literature, it appears to have gained significant recognition and attention amongst the general public. There is a Facebook group of almost 6,000 individuals who identify as ‘lean mass hyper-responders’ using the following markers: LDL of 200 mg/dL (5.17 mmol/L) or higher, HDL of 80 mg/dL (2.07 mmol/L) or higher and triglycerides of 70 mg/dL (0.79 mmol/L) or lower.34 Given the apparent pervasiveness of this response to the increasingly popular ketogenic diet amongst the population, a wider discussion of how to address this within clinics is needed.
Materials and Methods
Case 1 – Exacerbation of dysbetalipoproteinemia
A 69-year-old female was referred to a Lipid Clinic for marked hypercholesterolemia and hypertriglyceridemia while consuming a weight loss diet. She had a history of coronary atherosclerosis, prediabetes, obesity and acquired hypothyroidism following a thyroidectomy for benign multinodular goiter. She reports a personal history of long-standing dyslipidemia, although she does not recall specific family history. She was treated with statin therapy for many years and was rendered biochemically euthyroid on levothyroxine. She does not consume alcohol.
At the time of first presentation, she had initiated a very high fat diet (which she termed the Atkins diet) to lose weight. She had remained on this diet for approximately 6 weeks, consuming less than 20 grams of carbohydrate/day. Thereafter, she developed extreme elevations in her lipids and orange colored deposits in the creases of her hands and wrists that limited her ability to open door knobs and wear a watch. Laboratory assessment revealed both triglyceride and cholesterol levels exceeding 600 mg/dL: total cholesterol was 947 mg/dL and triglycerides were 1109 mg/dL (Table 1). Physical examination was remarkable for palmar xanthomas (Fig. 1A). The diagnosis of dysbetalipoproteinemia was suspected on the basis of total cholesterol/triglyceride ratio ~1, the triglyceride/cholesterol ratio <0.33 mg/dL in her VLDL isolated by ultracentrifugation, together with the pathognomonic physical findings. DNA analysis was performed using a targeted next generation sequencing platform designed to detect rare variants causing all known monogenic dyslipidemias together with common variants that contribute to polygenic risk of dyslipidemia16. The diagnosis was verified by the presence of APOE E2/E2 homozygosity.
Table 1.
Patient Characteristics
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Gender | Female | Female | Male | Female |
| Age, yrs. | 69 | 36 | 31 | 52 |
| BMI, kg/m2 | 35.6 | 18.1 | 20.4 | 24.6 |
| Genetic Testing | APOE E2/E2 | No mutations; LDL polygenic score 50th percentile; APOE E3/E3 | LDL polygenic score 92nd percentile; APOE E3/E3 | LDL polygenic score 75th percentile; APOE E4/E3 |
Figure 1.
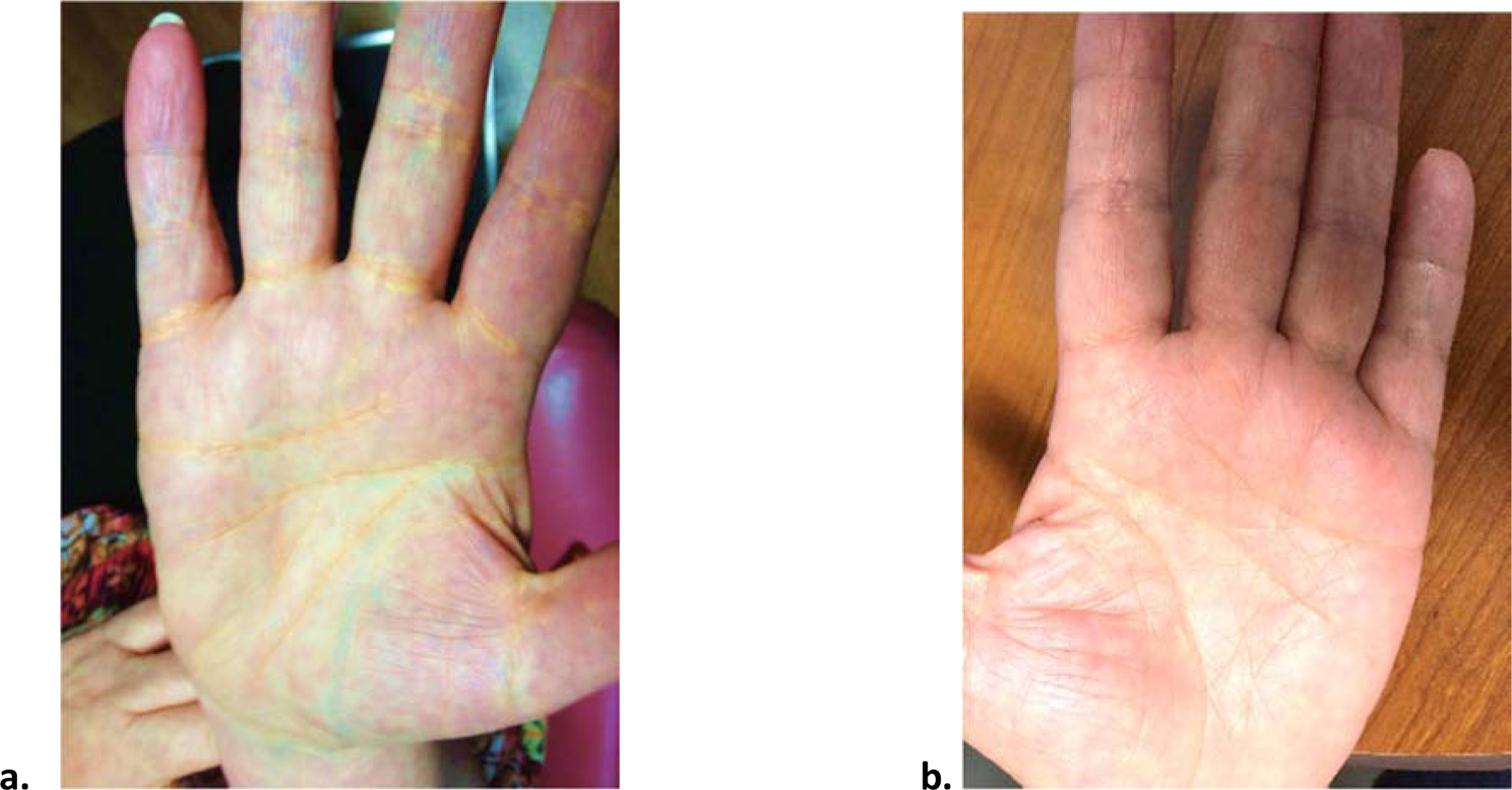
Regression of palmar xanthomas after reduction in fat consumption
(a) Palmar xanthomas while on a high fat, ketogenic diet
(b) Resolution of the palmar xanthomas following discontinuation of the very high fat diet
A diet low in both fat and refined carbohydrates was recommended. She was also treated with rosuvastatin 40 mg/day, fenofibrate 160 mg/day and eicosapentaenoic acid 4 g/day. The lipid panel improved: total and LDL cholesterol were 273 mg/dL and 170 mg/dL, respectively, and triglyceride was 256 mg/dL. Furthermore, her palmar xanthomas disappeared (Fig. 1B). Several years later, she underwent bariatric surgery, which resulted in an approximate 25-pound weight loss. While adhering to the same diet and medication regimen, her total and LDL cholesterol decreased further to 139 mg/dL and 65 mg/dL, respectively, and her HDL cholesterol to 50 mg/dL and triglyceride to 158 mg/dL.
Case 2 - Exacerbation of underlying hypercholesterolemia
A 36 year-old woman presented with concern regarding an alarmingly high total cholesterol. The patient was not aware of a personal history of hyperlipidemia, but had a family history of hyperlipidemia; her mother and brother had total cholesterol in the 300 to 400 mg/dL range by her recollection. Her paternal grandmother had a myocardial infarction at the age of 50. She had been on a ketogenic diet for six months prior to presentation and was content that within that period of time she had lost weight, dropping from 124 to 102 lbs (her body mass index [BMI] was 18.07 kg/m2). She consumed less than 20 g of carbohydrates a day and her diet was high in animal protein and fats such as whole fat cheese and butter.
On this ketogenic diet, her total and LDL cholesterol were 658 mg/dL and >500 mg/dL, respectively (Table 1). She was a thin woman with no stigmata of hypercholesterolemia (i.e. no xanthomas, corneal arcus, or xanthelasmas). The patient agreed to stop the ketogenic diet and to begin a low cholesterol diet, but refused statin therapy. Although this woman had no evidence of liver disease and her LDL isolated by ultracentrifugation at density between 1.02 and 1.063 mg/ml, we excluded this increase being Lp(x) or Lp(a). Shown in Table 3 are the free cholesterol/total cholesterol, and Lp(a) levels at this initial visit.
Table 3.
Patient Lp(a) profiles and Free Cholesterol/Total Cholesterol (FC/TC)
| Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|
| Lp(a) | <6 | <6 | 31 |
| Free Cholesterol/Total Cholesterol (FC/TC) | .238 | .254 | .232 |
After this first visit, the patient initiated a low cholesterol diet focused on lean protein (primarily chicken), vegetables, egg whites and ad libitum carbohydrates (greater than 45 g per meal). After one month on the low cholesterol diet, her total cholesterol decreased to 426 mg/dL and over time was subsequently even further reduced. After changing to a low-fat vegan diet, her weight was 98 pounds (BMI 17.39 kg/m2), her total and LDL cholesterol decreased to 128 mg/dL, and 48 mg/dL, respectively, and HDL cholesterol and TG to 109 mg/dL.
Genetic analysis by targeted sequencing did not reveal any clear definitive pathogenic mutation in either the LDLR, APOB or PCSK9 genes; the LDL polygenic score16 was not increased (Table 1). Her APOE genotype was E3/E3.
Case 3 – Increased LDL without underlying dyslipoproteinemia
A 31-year-old man was referred for evaluation of hypercholesterolemia on a ketogenic diet. Neither the patient nor his family had a history of dyslipidemia or coronary artery disease. He had tried to lose weight for many years and reported that the ketogenic diet led to reduced food intake along with more satiety. Prior to this diet his lipid profile was remarkable for an LDL cholesterol <100 mg/dL.
On the ketogenic diet, he lost over 50 pounds in 6 months. His diet consisted of mostly chicken and fish, with little consumption of eggs and red meat. While on this diet, his total and LDL cholesterol were 317 mg/dL and 243 mg/dL, respectively (Table 1). He did not have evidence of Lp(a) elevations (Table 3). Because he had successfully reduced his weight, he refused to change his diet, despite his high cholesterol levels. He refused statins but agreed to take ezetimibe, which reduced his total and LDL cholesterol to 181 mg/dL and 105 mg/dL, respectively within 6 weeks.
Although the clinical course and response to ezetimibe suggested cholesterol hyper absorption, no genetic abnormalities were found in the NPC1L1 gene. He did have a series of minor allelic mutations consistent with increased polygenic risk for elevated LDL cholesterol (Table 1). It is possible that this increased burden of small effect alleles combined to contribute to an abnormal response to a high fat/high cholesterol diet similar to that of patients with a single major mutation. His APOE genotype was E3/E3.
Case 4 - Exacerbated hyperlipidemia
A 52-year-old woman presented with hyperlipidemia while on a high-fat, ketogenic diet. She had been on the ketogenic diet for almost 5 months and had lost about 30 pounds. She reports a significant family history on her maternal side of hyperlipidemia, but no sudden cardiac death, coronary artery disease, or stroke. She had never been on any cholesterol medications.
Prior to starting the ketogenic diet, the patient’s initial lipid levels were total, LDL and HDL cholesterol were 213 mg/dL, 142 mg/dL and 43 mg/dL, respectively, and triglyceride was 141 mg/dL. Her high fat diet consisted of 70% fat, 20% protein and ~5% carbohydrates. The sources of fat included coconut oil, bacon fat, heavy cream, medium chain triglycerides (MCTs), butter (infrequently), and olive oil (infrequently). Her protein sources were beef, chicken and nuts.
After 5 months on the diet, her total, LDL and HDL cholesterol were was 676 mg/dL, 533 mg/dL and 60 mg/dL, respectively, and triglyceride was 86 mg/dL (Table 1). The LDL was also isolated by ultracentrifugation without evidence of Lp(a) (Table 3). Her weight had decreased from 176 pounds (BMI 29.3 kg/m2) to 148 pounds (BMI 24.6 kg/m2).
On genetic testing, patient 4 had no mutations in genes related to hypercholesterolemia. Similarly, to patient 3, she was found to have a series of common polymorphisms consistent with increased polygenic risk for elevated LDL cholesterol. Her APOE genotype was E4/E3.
Case 5 - Elevated lipid levels in a family
A 46-year-old woman presented with hyperlipidemia 8 months after starting a higher fat, ketogenic diet. On the diet, she lost 19 pounds, going from 136 (BMI 24.9 kg/m2) to 117 pounds (BMI 21.4 kg/m2). There is no history of familial hypercholesterolemia, but there is a history of high LDL/low HDL cholesterol ratio. Her brother—despite a normal BMI, running >20 miles per week, and eating a low-fat diet—had LDL cholesterol >150 mg/dL. Her father recently had a myocardial infarction at the age of 76 despite having both a normal BMI and a regular vigorous exercise program. Her father’s 3 brothers and sister all had significant coronary artery disease.
Prior to the diet, her total, LDL and HDL cholesterol were 146 mg/dL, 89 mg/dL and 49 mg/dL, respectively, and triglyceride was 42 mg/dL. Her high fat diet consisted of 70% fat, 25% protein and 5% carbohydrates, with total carbohydrates <20 g/day. The sources of fat included bacon fat, heavy cream, butter, and olive oil. Her protein sources were beef, chicken and nuts. After 8 months on the diet, her total, LDL and HDL cholesterol were 349 mg/dL, 270 mg/dL and 60 mg/dL, respectively, and triglyceride was 95 mg/dL.
She began to substitute light cream for heavy cream and increased her daily carbohydrate intake to 50 g/day. On repeat testing 4 months later, her total, LDL and HDL cholesterol were 214 mg/dL, 137 mg/dL and 65 mg/dL, respectively, and triglyceride was 59 mg/dL.
Discussion
Finding a diet that leads to significant weight loss and that can subsequently be maintained is a difficult clinical problem. Moreover, weight loss followed by weight gain, “yo-yoing,” appears to be an independent risk for cardiovascular disease.17 Each of our patients had tried a number of methods to achieve weight loss, and each succeeded using a ketogenic diet. However, in each case, this diet exacerbated or created a hyperlipidemia, although except for the first patient, none had a defined underlying genetic hyperlipidemia. This patient developed palmar xanthomas, a rare finding that led to a diagnosis of genetic dysbetalipoproteinemia. The second patient had no obvious genetic basis for hyperlipidemia. The third patient had normal LDL cholesterol level until losing weight on the ketogenic diet, so there was no way to know beforehand that he would have such a marked LDL elevation. He had polygenic susceptibility to hyperlipidemia, which only became manifest after the dietary stress. The fourth patient was similarly found to have no significant large effect genetic mutations, but may have had polygenic susceptibility underlying a marked response to the ketogenic diet. There was no genetic testing performed for Patient 5.
Apo E is a ligand for the several LDL receptor family members. Although APOE E2/E2 homozygosity leads to dysbetalipoproteinemia associated with a defect in clearance of remnant lipoproteins, the genotype is associated with an abnormal lipoprotein pattern in only a minority of people. For this reason, numerous studies have conjectured that a second lipoprotein altering mutation is required for the phenotypic expression of the lipid disorder, namely high cholesterol and high triglyceride with cholesterol enriched VLDL.18, 19 In the presented case, several risk factors associated with expression of the APOE E2/E2 phenotype were identified, including female gender, menopause, prediabetes and obesity. Hypothyroidism, when uncontrolled, can also contribute to the phenotype. Our patient shows that diet can be responsible for expression of dyslipoproteinemia in an individual with E2/E2 genotype, which was readily apparent by the patient’s presentation with palmar xanthomas.
Familial hypercholesterolemia is a common genetic disorder estimated to occur in one in 250–500 people.20 Most commonly, it is due to a defect in the LDL receptor or its ligand ApoB.20–23 It was surprising that none of our patients had genetic familial hypercholesterolemia. However, it is likely that some patients with this genetic disorder would also develop marked hypercholesterolemia on a ketogenic diet. Furthermore, hypercholesterolemia is often polygenic in nature rather than the result of a single dramatic mutation, which appears to be the case in at least one patient in our series.
The extraordinary response of the third patient to ezetimibe, which normally reduces LDL-cholesterol by 15–20%, suggested that he was a hyper-absorber of dietary cholesterol. This could result from a gain of function of the cholesterol transporter, Niemann Pick C1 like protein 1 (NPC1L1), although this was not detected by DNA sequencing. It is possible that he, like some of the other patients, may have had a mutation in a novel gene not known to be associated with dyslipidemia. Understanding the basic physiology leading to the exuberant LDL elevation in this patient and others will require metabolic studies to assess cholesterol uptake, lipoprotein uptake, and intestinal assessment of NPCL1 expression.
The ketogenic diet is a carbohydrate restrictive diet that has been traditionally used to treat intractable epilepsy since the 1920’s, but is also a popular weight-loss strategy.24 There is also a rise in interest in carbohydrate restrictive diets to improve glycemic control and other cardiometabolic risk factors, such as high blood pressure in patients with prediabetes or type 2 diabetes.1, 4–7, 24, 25 While there are several types of carbohydrate-restrictive diets, which vary in limitations set on carbohydrate and protein intake, the current, most popular version of the ketogenic diet restricts carbohydrate intake to about 20–50 g/day; the amount of carbohydrate needed to induce ketosis in most individuals.24, 26–28 Consequently, most carbohydrates in the diet are replaced with fat, with no emphasis on the particular types of dietary fats consumed.24 As a result, some diets become heavy in saturated fats and cholesterol.24
Effects of the ketogenic diet on circulating lipid levels have differed across studies and trials. Several studies have shown no significant change in cholesterol levels.29, 30 In contrast, a study designed to assess the effect of the ketogenic diet on adults with atherogenic dyslipidemia reported significant increases in ApoB and LDL cholesterol after 90 days.30 In children with epilepsy, one study also reported significant increases in ApoB-containing lipoproteins and decreases in HDL cholesterol at 6 months on the diet.31 Other reports did not corroborate this.31–33 These variations in the changes in lipid levels on the ketogenic diet amongst adults and children could be due to the length of time on the diet, the types of fats ingested29, or a balance of the benefits of weight loss versus the detrimental effects of dietary composition.
There is a scarcity of medical literature on a hyper response to a ketogenic diets. The patients who had LDL levels over 300 were specifically referred because their endocrinologists, cardiologists or primary care doctors had told them that they had been unable to find reports of such elevations occurring except with development of thyroid, liver or autoimmune diseases. In contrast, within a general population, there is no shortage of information available on the internet for the public. The Cholesterol Code Team, led by a software engineer and entrepreneur, created a Facebook group for ‘lean mass hyper-responders.’34 The Cholesterol Code Team defines hyper-responders as those “who have a very dramatic increase in their cholesterol after adopting a low carb diet.”35 Furthermore, “the increase can be anywhere from 50% to 100% or more of original, pre-diet cholesterol numbers.”35 The Cholesterol Code Team claims ‘we don’t know’ whether having high LDL-C on a low-carbohydrate diet is dangerous; it further presents a number of ‘good reasons’ why LDL-C could be so high.35 These include that the “body is transporting more fat for energy to cells due to being on a high-fat diet.”35 Given the weight such statements could have on a significant number of individuals seeking guidance on the internet, it is important to identify and advise patients with similar presentations.
Conclusions
We advise practicing physicians to exercise caution when prescribing low carbohydrate diets, especially if they lead to increased consumption of saturated fats and cholesterol. The significant and potentially harmful increases in lipid levels in all patients reported here illustrate the peril in assuming that an individual’s lipid levels would change favorably when weight is reduced on the ketogenic diet. We recommend that patients with known dyslipidemias should avoid ketogenic diets. Furthermore, as discussed here, underlying dyslipidemias may only become manifest as a result of a high fat diet, which might warrant the reassessment of circulating lipids in all patients on a ketogenic diet. The suggestion that the marked elevations of LDL with these diets might not be harmful is chilling and has the potential to create premature CVD in uninformed patients seeking to control their body weight.
Table 2.
Patient lipid and lipoprotein levels on higher fat diet (HFD) and lower fat diet (LFD)/medication
| Parameter (mg/dL) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HFD | LFD* | HFD | LFD | HFD | LFD** | HFD | LFD*** | HFD | LFD | |
| TC | 947 | 139 | 658 | 245 | 317 | 181 | 629 | 213 | 349 | 214 |
| TG | 1109 | 158 | 112 | 139 | 92 | 41 | 69 | 141 | 95 | 59 |
| HDL-C | 42 | 50 | 75 | 74 | 56 | 68 | 62 | 43 | 60 | 65 |
| LDL-C | 683 | 65 | 563 | 143 | 243 | 105 | 553 | 142 | 270 | 137 |
denotes patient 1’s lipid levels on LFD, post bariatric surgery.
denotes patient 3’s lipid levels on ezetimibe, with no change in diet.
denotes patient 4’s lipid levels on lower fat diet prior to starting the ketogenic diet, as opposed to post (patients 1, 2, 5).
Highlights.
Low carb diets can lead to weight loss, but can exacerbate hypercholesterolemia.
Hypercholesterolemia can develop with or without underlying genetic hyperlipidemia.
This paper describes patients who developed marked increases in plasma cholesterol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
IJG is supported by grants HL45095, 73029, and 135987; has received consulting fees from Arrowhead, Esperion, and Amgen; and funds for preclinical laboratory studies from Ionis, Bristol-Meyers, and Arrowhead. RAH reports grants and personal fees from Acasti and Akcea/Ionis; personal fees from Aegerion, Amgen, Gemphire, and Sanofi; and grants from Regeneron and Boston Heart Diagnostics. All other authors declare no conflicts of interest.
References
- 1.Abbasi J. Interest in the Ketogenic Diet Grows for Weight Loss and Type 2 Diabetes. Jama. 2018;319:215–217. [DOI] [PubMed] [Google Scholar]
- 2.Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. The British journal of nutrition. 2013;110:1178–1187. [DOI] [PubMed] [Google Scholar]
- 3.Tay J, Luscombe-Marsh ND, Thompson CH, et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. The American journal of clinical nutrition. 2015;102:780–790. [DOI] [PubMed] [Google Scholar]
- 4.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European journal of clinical nutrition. 2013;67:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey C, Schofield GM, Zinn C, Thornley SJ, Crofts C, Merien FLR. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: A randomised clinical trial. PeerJ. 2019;7:e6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyde PN, Sapper TN, Crabtree CD, et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paoli A. Ketogenic diet for obesity: friend or foe? International journal of environmental research and public health. 2014;11:2092–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obesity reviews : an official journal of the International Association for the Study of Obesity.2006;7:49–58. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. Journal of clinical lipidology. 2015;9:S1–122.e121. [DOI] [PubMed] [Google Scholar]
- 10.Bray GA, Heisel WE, Afshin A, et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocrine reviews. 2018;39:79–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet (London, England). 2011;378:804–814. [DOI] [PubMed] [Google Scholar]
- 12.Gibson AA, Seimon RV, Lee CM, et al. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16:64–76. [DOI] [PubMed] [Google Scholar]
- 13.Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Frontiers in psychology. 2015;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. The New England journal of medicine. 2003;348:2082–2090. [DOI] [PubMed] [Google Scholar]
- 15.Kern F Jr. Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day. Mechanisms of adaptation. The New England journal of medicine. 1991;324:896–899. [DOI] [PubMed] [Google Scholar]
- 16.Dron JS, Wang J, McIntyre AD, et al. Six years’ experience with LipidSeq: clinical and research learnings from a hybrid, targeted sequencing panel for dyslipidemias. BMC Med Genomics. 2020;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-Weight Fluctuations and Outcomes in Coronary Disease. The New England journal of medicine. 2017;376:1332–1340. [DOI] [PubMed] [Google Scholar]
- 18.Henneman P, van der Sman-de Beer F, Moghaddam PH, et al. The expression of type III hyperlipoproteinemia: involvement of lipolysis genes. European journal of human genetics : EJHG. 2009;17:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sijbrands EJ, Hoffer MJ, Meinders AE, et al. Severe hyperlipidemia in apolipoprotein E2 homozygotes due to a combined effect of hyperinsulinemia and an SstI polymorphism. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2722–2729. [DOI] [PubMed] [Google Scholar]
- 20.Bouhairie VE, Goldberg AC. Familial hypercholesterolemia. Cardiology clinics. 2015;33:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gidding SS, Champagne MA, de Ferranti SD, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2167–2192. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JL, Brown MS. The LDL receptor. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. European heart journal. 2016;37:1384–1394. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. Journal of clinical lipidology. 2019;13:689–711. [DOI] [PubMed] [Google Scholar]
- 25.Brouns F. Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable? European journal of nutrition. 2018;57:1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke C, Best T. Food choice motivations: Profiling low-carbohydrate, high-fat dieters. Appetite. 2019;141:104324. [DOI] [PubMed] [Google Scholar]
- 27.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition (Burbank, Los Angeles County, Calif.) 2015;31:1–13. [DOI] [PubMed] [Google Scholar]
- 28.Evans M. Keto diets: good, bad or ugly? The Journal of physiology. 2018;596:4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cervenka MC, Patton K, Eloyan A, Henry B, Kossoff EH. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutritional neuroscience. 2016;19:131–137. [DOI] [PubMed] [Google Scholar]
- 30.Walton CM, Perry K, Hart RH, Berry SL, Bikman BT. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. Journal of diabetes research. 2019;2019:8681959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapetanakis M, Liuba P, Odermarsky M, Lundgren J, Hallbook T. Effects of ketogenic diet on vascular function. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2014;18:489–494. [DOI] [PubMed] [Google Scholar]
- 32.Heussinger N, Della Marina A, Beyerlein A, et al. 10 patients, 10 years - Long term follow-up of cardiovascular risk factors in Glut1 deficiency treated with ketogenic diet therapies: A prospective, multicenter case series. Clinical nutrition (Edinburgh, Scotland). 2018;37:2246–2251. [DOI] [PubMed] [Google Scholar]
- 33.Liu YM, Lowe H, Zak MM, Kobayashi J, Chan VW, Donner EJ. Can children with hyperlipidemia receive ketogenic diet for medication-resistant epilepsy? Journal of child neurology. 2013;28:479–483. [DOI] [PubMed] [Google Scholar]
- 34.Cholesterol Code Team. In Facebook [LMHR]. https://www.facebook.com/groups/LeanMassHyperResponder/
- 35.Cholesterol Code Team. Lean Mass Hyper-responders. Cholesterol Code Team website https://cholesterolcode.com/lmhr/


