Abstract
Background:
The cardiovascular (CV) safety of estrogen replacement therapy (ERT) in perimenopausal women remains uncertain. Although exogenous estrogens increase HDL cholesterol (HDL-C), estrogen-mediated effects on alternative metrics of HDL that may better predict CV risk are unknown.
Objective:
To determine the effects of transdermal ERT on HDL composition and cholesterol efflux capacity (CEC), as well as the relationships between these metrics and CV risk factors.
Methods:
Fasting plasma samples were analyzed from 101 healthy, perimenopausal women randomized to receive either transdermal placebo or transdermal estradiol (100 μg/24 hr) with intermittent micronized progesterone. At baseline and after 6 months of treatment, serum HDL CEC, HDL particle concentration, HDL protein composition, insulin resistance and brachial artery flow-mediated dilatation (FMD) were measured.
Results:
No difference between groups was found for change in plasma HDL-C (p=0.69). Between-group differences were found for changes in serum HDL total CEC [median change from baseline −5.4(−17.3,+8.4)% ERT group versus +5.8(−6.3,+16.9)% placebo group, p=0.01] and ABCA1-specific CEC [median change from baseline −5.3(−10.7,+6.7)% ERT group versus +7.4(−1.5,+18.1)% placebo group, p=0.0002]. Relative to placebo, transdermal ERT led to reductions in LDL-C (p<0.0001) and insulin resistance (p=0.0002). An inverse correlation was found between changes in serum HDL total CEC and FMD (β=−0.26, p=0.004).
Conclusions:
Natural menopause leads to an increase in serum HDL CEC, an effect that is abrogated by transdermal ERT. However, transdermal ERT leads to favorable changes in major CV risk factors.
Keywords: estradiol, hormone replacement therapy, menopause, HDL, cholesterol efflux, lipoproteins, cardiovascular disease
Introduction
The impact of postmenopausal estrogen replacement therapy on cardiovascular (CV) health remains an area of substantial controversy. Although epidemiologic data strongly suggested that ERT confers cardiovascular protection (1), the Women’s Health Initiative cast doubt on these protective effects and led to dramatic reductions in the prescription rates of ERT (2,3). Subsequent data, however, have led to refinement of these findings and collectively indicate that transdermal estradiol, delivered during an optimal window of time, may prove a safe and even beneficial regimen with regard to CV health (4–6). Transdermal estradiol is delivered directly to the systemic circulation and therefore circumvents the first-pass gut and liver metabolism that oral estrogens undergo. Consequently, transdermal estradiol does not induce hepatic production of proteins including serum amyloid A, C-reactive protein, and coagulation factors, nor does it raise circulating triglyceride concentrations (7).
Notably, the absence of CV protection found in the WHI occurred despite the anticipated increase in high-density lipoprotein cholesterol (HDL-C) (8), an effect long thought to contribute to estrogen’s purported CV benefit. Nonetheless, abundant clinical data now demonstrate that changes in HDL-C are not uniformly a reliable metric of changes in CVD risk (9,10). Rather, research focus has shifted toward alternative HDL-based metrics that measure HDL’s protein composition, functional capacities, or non-cholesterol lipid cargo in an effort to identify alternative metrics with greater predictive utility (11,12). Measurement of HDL cholesterol efflux capacity – the efficiency with which HDL particles are able to efflux cholesterol from lipid-laden macrophages – captures a pivotal step in reverse cholesterol transport, which is believed to be the most important cardioprotective function of HDL particles (13). In both cross-sectional and longitudinal cohort studies, lower HDL cholesterol efflux capacity (CEC) better predicted greater prevalent and incident CV events than did HDL-C and, importantly, the relationship between HDL CEC and CVD risk was independent of HDL-C (13–15) . In addition to HDL CEC, HDL protein composition and HDL particle concentration and size have been studied as potential predictors of CVD risk in populations including postmenopausal women (16–20). These alternative metrics promise enhanced utility for prediction of CV risk on an individual basis, and their measurement may offer incremental insight into the CV effects of perimenopausal ERT.
Previously, in a longitudinal study of women transitioning through menopause, increases in HDL CEC were observed (21). However, this study had no control group, and the implications of this increase in efflux capacity for CVD risk prognostication remain uncertain in the absence of additional, established CV endpoints in the study. Critically, an inverse association between HDL CEC and CV risk has not been a universal finding (22–24). Consequently, considerable uncertainty persists regarding both the CV safety of ERT and the clinical utility of HDL-based metrics for assessing individual CVD risk.
The Perimenopausal Estrogen Replacement Therapy (PERT) study was a randomized, double-blind, placebo-controlled trial designed to test the effects of transdermal estradiol on depressive symptoms in women undergoing perimenopause and early menopause (25). In addition, cardiometabolic endpoints were measured including flow-mediated dilatation of the brachial artery, an index of vascular endothelial function; insulin resistance; and lipid profiles. We used fasting plasma samples collected at baseline and after 6 months of treatment for a subset of subjects enrolled in the PERT study and measured HDL particle size and concentration, HDL protein composition, and serum HDL CEC. We predicted that these analyses would lend insight into both the relative CV benefits of transdermal ERT and the predictive utility of alternative HDL-based metrics for CVD risk.
Materials and Methods
Study design and subjects
Fasting plasma samples were analyzed from a subset of women enrolled in the PERT study, a randomized, doubled-blind, placebo-controlled trial for which a detailed study design and methods have been previously published (25,26). In brief, 172 healthy women in perimenopause or early menopause were randomized to receive either transdermal placebo or transdermal estradiol (100 mcg/day) with intermittent micronized progesterone (200 mg PO for 12 days every 2–3 months) for a treatment duration of 1 year. Plasma samples were collected 2–3 months following progesterone exposure. Samples from 101 women (n=45 in the ERT group and n=56 in the placebo group) were included in the current analyses and were selected solely on the basis of availability of adequate plasma volumes at the baseline and 6-month timepoints.
Sample collection and processing
Blood samples were collected into pre-chilled, purple-top EDTA tubes and placed in ice cups for transport to the laboratory for processing. Samples were centrifuged at 4°C, and the plasma was separated and aliquoted into cryo-tubes, which were kept on ice until transfer to a −80°C freezer for long-term storage. Maximum total time from blood collection to freezer storage was <60 minutes, and most samples were frozen <30 minutes after collection. Frozen plasma aliquots were shipped to the University of Washington on dry ice without prior freeze-thaw cycles and stored immediately at −80°C until HDL-based assays were performed.
Serum HDL CEC measurement
Serum HDL CEC was measured as previously described (27,28), employing the methods developed by Rothblat and colleagues (13). Plasma was converted to serum, and polyethylene glycol precipitation was used to deplete serum of apolipoprotein B (apoB)-containing particles (serum HDL). Serum HDL was incubated for 4 hours with cells loaded with radiolabeled [3H]-cholesterol. J774 cells were treated with an acyl–coenzyme A:cholesterol acyltransferase inhibitor and stimulated with cAMP to measure serum HDL total CEC (13). BHK-1 cells were transfected with a mifepristone-inducible human ABCA1 transporter to measure CEC specifically mediated by ABCA1 (ABCA1 CEC) (21,29). Samples were measured in duplicate, with mean values presented. For each method of determining CEC, all samples were assayed on the same day. The coefficient of variation for total serum HDL CEC was 7.9% and for ABCA1 CEC 6.7%.
Quantification of HDL particle size and concentration
Calibrated ion mobility analysis (cIMA) was employed to measure total HDL particle concentration and HDL subclasses based on particle size (17,28). As previously described, this method detects 4 species of HDL particles: extra small (xs-HDL-Pima, average diameter 7.8 nm), small (s-HDL-Pima, average diameter 8.4 nm), medium (m-HDL-Pima, average diameter 9.5 nm), and large (l-HDL-Pima, average diameter 10.8 nm). Total HDL particle concentration (t-HDL-Pima) was determined as the sum of all 4 particle subclasses. The average intra-assay coefficient of variation was 11.2% (6.7–18.2%), and average inter-assay value was 11.1% (3.8–18.8%).
Quantification of HDL-associated proteins
HDL protein abundance was measured using a targeted proteomics strategy to measure 34 proteins as described previously (30).
Measurement of sphingosine-1-phosphate
Plasma concentrations of sphingosine-1-phosphate (S1P) were measured using butanol extraction and liquid chromatography-tandem mass spectrometry, as previously described with details including complete mass spectrometric methods (31,32). EDTA-anticoagulated plasma (10 μL) and internal standard (5 μL, 3 μM C17 base D-erythro-sphingosine-1-phosphate in ethanol, Avanti Polar Lipids; Alabaster, AL) were mixed on an orbital shaker for 10 min at 1,400 rpm at 20°C. The sample was acidified with 30mM citric acid/40mM Na2HPO4, pH 4.0 (50 μL) and extracted for 10 min at 1,400 rpm at 20°C with 125 μL water-saturated butanol (Fisher Scientific; Waltham, MA). The butanol layer was isolated and lyophilized in a centrifugal evaporator at 20°C. The residue was resuspended in HPLC buffer and sonicated for 1 min at 20°C. Analytes in 10 μL sample were separated using liquid chromatography (Shimadzu; Kyoto, Japan) and analyzed by tandem mass spectrometry on a 4000 QTRAP mass spectrometer (AB Sciex; Toronto, Canada) in positive ion mode. The between-day coefficient of variation was 7.7%.
Measurement of cardiometabolic endpoints
Insulin resistance was quantified by HOMA-IR using the following formula: (fasting glucose concentration (nmol/L) × fasting insulin concentration (mIU/L))/22.5. Baroreflex sensitivity was measured through 5-minute recording of blood pressure and RR interval with selection of sequences during which systolic blood pressure and RR interval exhibited progressive increases or decreases over 3 consecutive pulses with a difference of at least 1.0 mmHg or 5.0 msec between successive pulses, respectively. The linear regression of RR interval to systolic blood pressure for each sequence was determined, and baroreflex sensitivity was quantified as the mean of the resultant slopes for all selected sequences for an individual participant. Flow-mediated dilatation of the brachial artery was measured using digital, gated ultrasound images and customized software (Vascular Analysis Tools, Medical Imaging Applications, LLC; Coralville, IA). Arterial diameter was defined as the distance between the intima-lumen interfaces of the proximal and distal walls of the brachial artery, with baseline arterial diameter calculated as the average distance captured from ≥10 images. Maximum arterial diameter was determined during reactive hyperemia following 5 minutes of forearm ischemia and calculated as the average measurement taken from 3 consecutive images obtained during peak dilatation. Flow-mediated dilatation was calculated as the % change in arterial diameter during reactive hyperemia relative to baseline diameter.
Statistical analyses
The peak area value for each HDL protein was normalized to apolipoprotein A-I (apoA-I) abundance, log-transformed, and standardized through Z-score calculation. For between-group comparisons, the change in each variable was tested for normality using the D-Agostino & Pearson omnibus test, and Student’s unpaired t-test or Mann Whitney test was used as appropriate. For within-group comparisons, Student’s paired t-test or Wilcoxon matched-pairs signed rank test was used as appropriate. Multistep linear regression was employed to determine associations between changes in HDL-based metrics and endpoints of CV risk. For each CV risk endpoint, independent variables included treatment group and changes in plasma HDL-C concentration, plasma HDL total particle concentration, plasma triglyceride concentration, and serum HDL total and ABCA1 CEC. The final regression models were limited to significant independent variables, and standardized β-coefficients are presented. Statistical significance was defined as a p-value threshold of <0.05 for all endpoints except change in HDL protein abundance, for which a Bonferroni adjusted p-value threshold of <0.0015 was employed to correct for multiple comparisons. All data are shown as median (interquartile range). Statistical analyses were performed using SPSS 25 (IBM Corporation, Armonk, New York), and figures were created with GraphPad Prism 5 (GraphPad Inc., La Jolla, CA).
Results
Study participants
Baseline characteristics of the PERT study participants have been previously published (25). Similar to the overall cohort of women, participants in the subset included for the current analyses were healthy, perimenopausal women with a median body mass index of 25.3 (22,29) kg/m2, median systolic blood pressure of 115.0 (103,120) mmHg, and normal insulin sensitivity [median HOMA-IR 2.4 (1.8,3.3)] (Table 1). None of the participants were on antihypertensive or lipid-lowering therapy. Fasting insulin concentration was higher in the group receiving ERT than in the placebo group at baseline, and no other baseline parameters differed significantly between groups.
Table 1.
Baseline characteristics of study subjects
| Characteristic | Estrogen Replacement | Placebo | P-value |
|---|---|---|---|
| n | 45 | 56 | |
| Age (years) | 51.1 (48,53) | 50.7 (48,53) | 0.99 |
| Body mass index (kg/m2) | 25.7 (22,29) | 25.3 (23,29) | 0.63 |
| Estradiol concentration (pg/mL) | 79.7 (57,133) | 88.7 (60,116) | 0.86 |
| Fasting glucose concentration (mg/dL) | 88.0 (82,96) | 85.5 (81,92) | 0.41 |
| Fasting insulin concentration (mIU/L) | 12.7 (9,15) | 10.6 (8,14) | 0.04 |
| Insulin resistance (HOMA-IR) | 2.8 (1.9,3.4) | 2.3 (1.8,3.0) | 0.05 |
| Systolic blood pressure (mmHg) | 116 (107,121) | 115 (101,120) | 0.19 |
| Diastolic blood pressure (mmHg) | 71 (66,76) | 71 (66,76) | 0.89 |
| Waist circumference (cm) | 87.0 (79,95) | 87.0 (79,93) | 0.67 |
| Total cholesterol (mg/dL) | 214 (178,226) | 199 (181,221) | 0.35 |
| HDL cholesterol (mg/dL) | 63 (56,82) | 70 (58,79.5) | 0.62 |
| LDL cholesterol (mg/dL) | 128 (102,139) | 111 (97,129) | 0.24 |
| Triglycerides (mg/dL) | 73 (56,97) | 69 (58,93) | 0.41 |
Baseline characteristics for study subjects included in the current analyses. Data are shown as median (interquartile range).
Transdermal ERT does not increase HDL-C concentration but reduces LDL-C concentration in perimenopausal women
Participants in this cohort were normolipidemic with a baseline median total cholesterol concentration of 209 (181,223) mg/dL, low-density lipoprotein cholesterol (LDL-C) concentration of 118 (100,137) mg/dL, HDL-C concentration of 68 (58,81) mg/dL, and triglyceride concentration of 70 (58,93) mg/dL. Fasting lipid profiles after 6 months of treatment demonstrated a significant difference in the change in total cholesterol concentration between the ERT and placebo groups from baseline [median change −13.0 (−24.5, +1.0) mg/dL in the ERT group versus +4.5 (−11.0, +16.8) mg/dL in the placebo group, p=0.0001] (Figure 1A). Total cholesterol concentrations fell from 214 (178,226) mg/dL at baseline to 196 (169,218) mg/dL in the ERT group (p<0.0001) but exhibited a small, non-significant rise in the placebo group from 199 (181,221) mg/dL to 204 (177,235) mg/dL (p=0.11). The change in plasma HDL-C concentration was negligible and did not differ between the ERT and placebo groups [median change −2.0 (−6.5, +3.0) mg/dL in the ERT group versus −1.0 (−5.8, +4.8) mg/dL in the placebo group, p=0.69] (Figure 1B). In contrast, the change in LDL-C concentration over 6 months of treatment differed between groups [median change −12.0 (−23.5, +1.0) mg/dL in the ERT group versus +3.5 (−7.8, +15.8) mg/dL in the placebo group, p<0.0001] (Figure 1C, Supplemental Table 1). Further, the decrement in LDL-C concentration evident within the ERT group achieved significance [128 (102,139) mg/dL at baseline versus 112 (91,127) mg/dL at 6 months, p<0.0001]. Finally, no between-group difference in the change in fasting plasma triglyceride concentration was found over the treatment period [median change +4.0 (−12.5, +24.0) mg/dL in the ERT group versus +3.5 (−6.0, +14.0) mg/dL in the placebo group, p=0.93] (Figure 1D).
Figure 1.
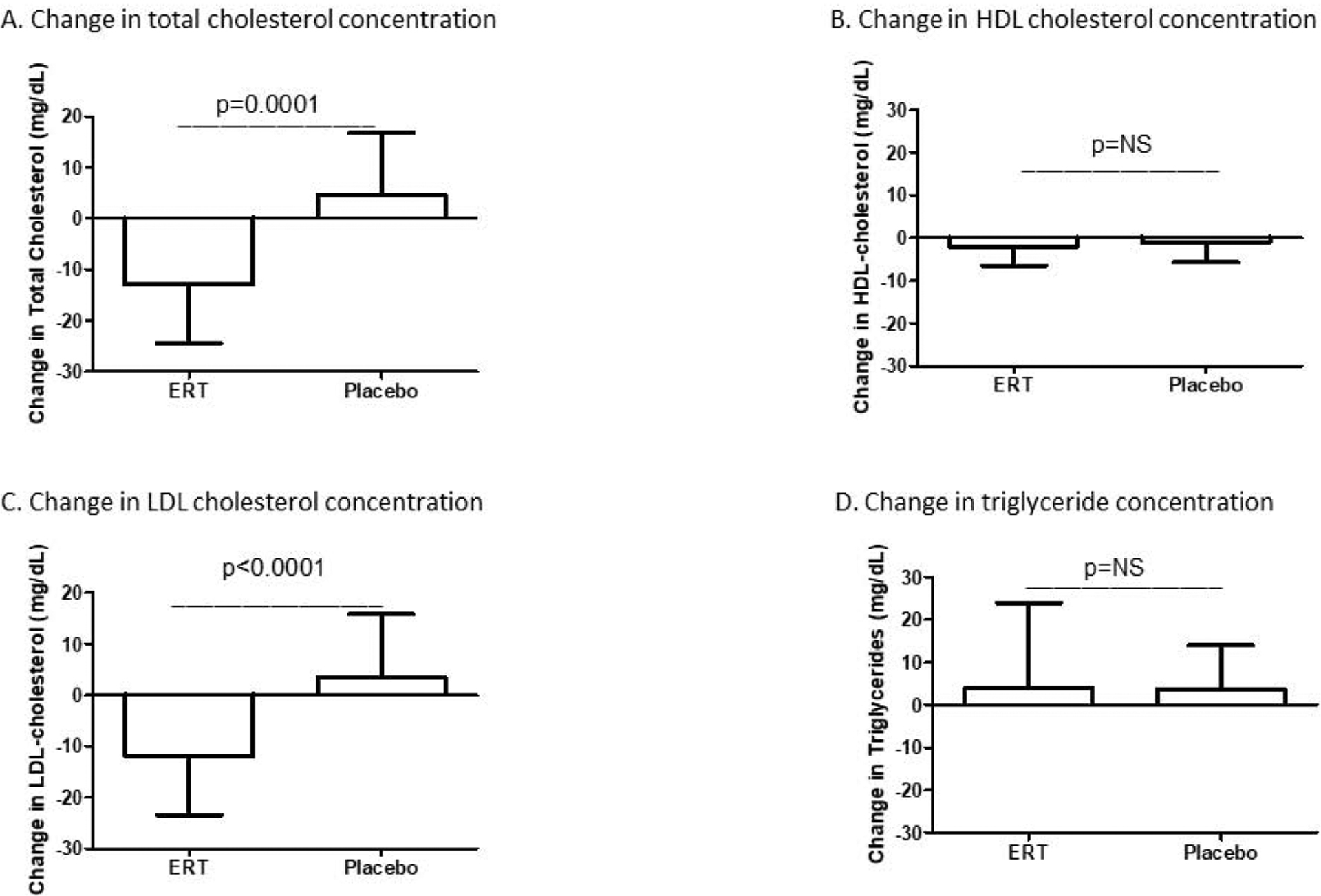
Subjects who received ERT showed a decrease in plasma total cholesterol concentration relative to those who received placebo (A). No between-group difference was found for change in plasma HDL-C concentration (B). Relative to placebo, ERT led to a decrease in plasma LDL-C concentration (C). No difference was found between groups for change in plasma triglyceride concentration (D). ERT=estrogen replacement therapy; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol
Transdermal ERT abrogates perimenopause-associated increases in serum HDL total and ABCA1 CEC
Despite the absence of change in HDL-C concentration in either group, changes in serum HDL CEC differed between groups over 6 months of treatment (Table 2). Relative to the placebo group, the ERT group exhibited a decrease in total serum HDL CEC [median % change from baseline −5.4 (−17.3,+8.4)% in the ERT group versus +5.8 (−6.3,+16.9)% in the placebo group, p=0.01] (Figure 2A). This between-group difference was similarly evident and more pronounced for ABCA1-specific serum HDL CEC [median % change from baseline −5.3 (−10.7,+6.7)% in the ERT group versus +7.4 (−1.5,+18.1)% in the placebo group, p=0.0002] (Figure 2B). Further, the increase in ABCA1-specific CEC within the placebo group achieved significance (p=0.04).
Table 2.
Serum HDL cholesterol efflux capacity and HDL particle concentration as baseline and 6 months
| Estrogen Replacement | Placebo | |||
|---|---|---|---|---|
| HDL-based Metric | Baseline | 6 months | Baseline | 6 months |
| Serum HDL total cholesterol efflux capacity (%) | 12.0 (10.4,14.3) | 11.4 (10.4,13.7) | 11.3 (9.9,12.9) | 11.9 (10.9,12.6) |
| Serum HDL ABCA1 cholesterol efflux capacity (%) | 12.2 (10.8,13.6) | 11.7 (102,13.3) | 11.0 (9.5,12.9) | 12.4 (10.8,13.6)* |
| Total HDL particle concentration (μmol/L) | 23.2 (20.4,26.8) | 23.0 (21.0,26.2) | 22.6 (19.9,25.9) | 22.8 (19.8,25.2) |
| Small HDL particle concentration (μmol/L) | 4.5 (3.2,5.5) | 4.2 (3.0,5.8) | 3.9 (2.9,5.0) | 3.9 (3.2,5.3) |
| Medium HDL particle concentration (μmol/L) | 8.4 (7.6,10.8) | 8.1 (7.2,10.7) | 8.3 (6.9,9.4) | 8.4 (7.1,9.7) |
| Large HDL particle concentration (μmol/L) | 6.1 (4.5,10.3) | 7.1 (5.0,9.9) | 7.9 (5.5,10.5) | 7.3 (4.9,9.5) |
Serum HDL total and ABCA1 cholesterol efflux capacity and HDL total particle concentration and size distribution at baseline and after 6 months of treatment. Data are shown as median (interquartile range).
p<0.05 for within-group change
Figure 2.
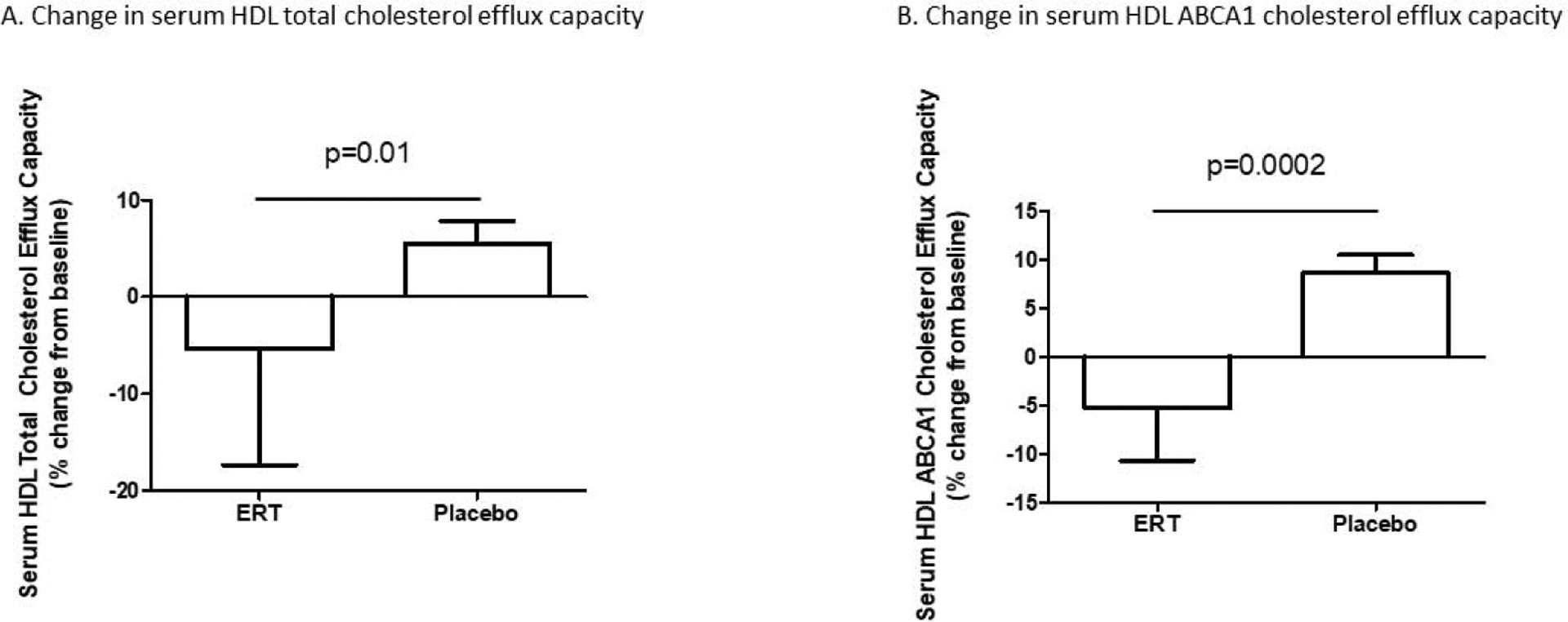
Relative to placebo, ERT led to reductions in serum HDL total (A) and ABCA1 (B) cholesterol efflux capacity.
Transdermal ERT leads to a shift toward larger HDL particle size but does not influence HDL protein composition
During the 6-month treatment period, change in total HDL particle concentration was similarly insignificant in both groups [median % change from baseline −1.2 (−5.9, +3.3)% in the ERT group versus −1.5 (−8.4, +7.3)% in the placebo group, p=0.78] (Figure 3A). However, differential changes in HDL particle subclasses were evident between groups, as subjects in the placebo group exhibited enrichment in small HDL particles, whereas relative depletion of small HDL particles was found in the ERT group [median % change from baseline −8.1 (−21.1, +10.9)% in the ERT group versus +4.2 (−8.1, +25.7)% in the placebo group, p=0.01] (Figure 3B). Further, reciprocal changes were found for the large HDL particle subclass, as placebo treatment resulted in a decrease in large HDL particle concentration relative to ERT [median % change from baseline +4.5 (−7.0, +19.3)% in the ERT group versus −5.2 (−14.9, +12.3)% in the placebo group, p=0.02] (Figure 3C). In contrast, change in the medium HDL subclass did not differ between groups [median % change from baseline −3.6 (−19.0, +14.1)% in the ERT group versus +1.9 (−11.0, +13.8)% in the placebo group, p=0.42] (Figure 3D).
Figure 3.
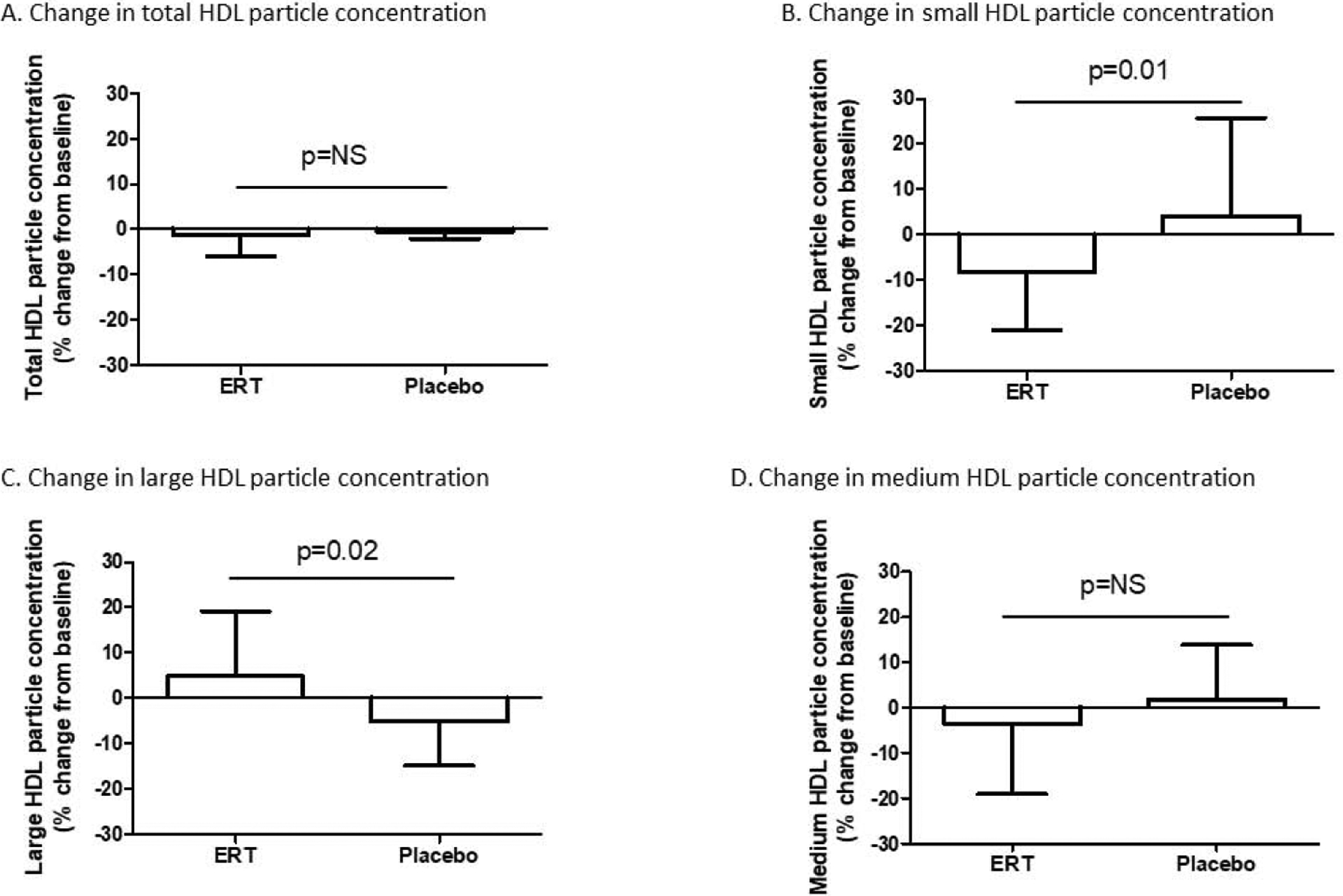
Changes in total HDL particle concentration were comparable in the ERT and placebo groups (A). Subjects in the ERT group demonstrated a decrease in small HDL particles relative to subjects in the placebo group (B). No difference in change in medium HDL particle concentration was evident between groups (C), but an increase in large HDL particle concentration was seen in the ERT group relative to the placebo group (D).
The relative abundance of 31 HDL-associated proteins was measured at baseline and after 6 months of treatment. Only change in apoliproprotein D (apoD) abundance differed between groups using a p-value threshold of <0.05 for significance [median change +0.004 (−0.01, +0.02) in the ERT group versus −0.003 (−0.02, +0.1) in the placebo group, p=0.049]; however, this between-group difference was no longer significant after correction for multiple comparisons.
Transdermal ERT improves major CV risk factors
To better understand both the CV effects of transdermal ERT and the relevance of the HDL-based metrics to CV risk, insulin resistance, baroreflex sensitivity, and flow-mediated dilatation (FMD) of the brachial artery were measured at baseline and after 6 months of treatment. A highly significant difference was found between groups for change in insulin resistance as quantified by HOMA-IR [median change −0.6 (−1.0, −0.1) in the ERT group versus +0.2 (−0.3, +0.9) in the placebo group, p=0.0002] (Figure 4A). Further, the decrease in HOMA-IR observed within the ERT group achieved significance [2.8 (1.9,3.4) at baseline versus 2.1 (1.6, 3.0) at 6 months, p=0.0008]. The increase in HOMA-IR among subjects in the placebo group did not achieve significance [2.3 (1.8, 3.0) at baseline versus 2.5 (1.5,3.1), p=0.10]. Change in baroreflex sensitivity did not differ significantly between groups [median change +0.6 (−0.2, +1.0) in the ERT group versus 0 (−1.1, +1.1) in the placebo group, p=0.14] (Figure 4B). However, an increase in baroreflex sensitivity was found among subjects in the ERT group [4.7 (3.2, 6.2) at baseline versus 5.7 (4.1, 6.8) at 6 months, p=0.008]. Change in brachial artery FMD was not different between groups (p=0.76) (Figure 4C). As an additional metric of endothelial cell function, plasma concentrations of sphingosine-1-phosphate (S1P) were measured. Change in S1P from baseline to 6 months was negligible and did not differ between groups [+0.1 (−0.1, +0.2) μM in the ERT group versus 0 (−0.1, +0.1) in the placebo group, p=0.56). A significant, positive correlation was found between changes in brachial artery FMD and plasma S1P concentration (r=0.23, p=0.03).
Figure 4.
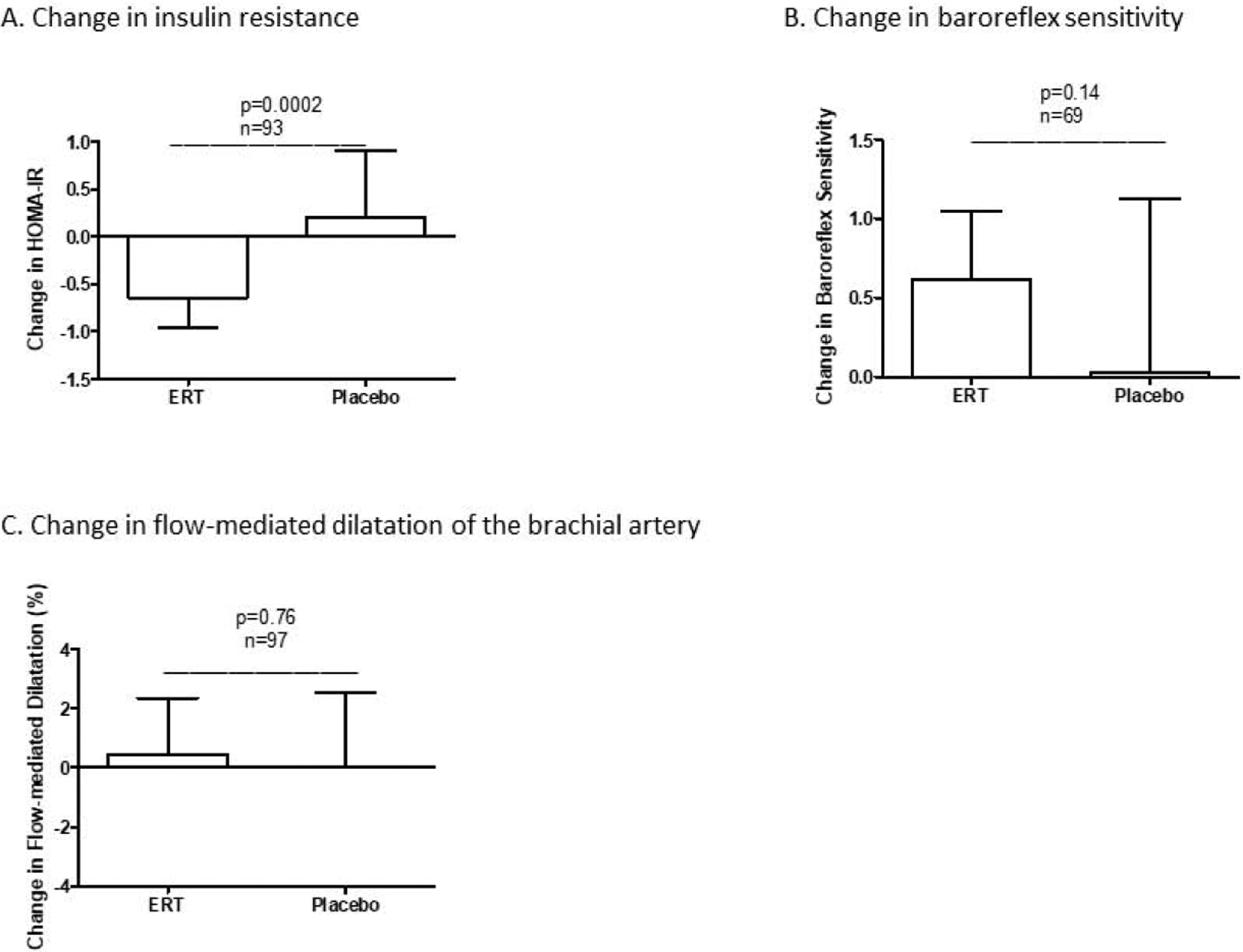
Relative to the placebo group, the ERT group exhibited significant decreases in LDL-C concentration (A) and insulin resistance (B) but an increase in baroreflex sensitivity (C). LDL-C=low-density lipoprotein cholesterol; HOMA-IR=homeostasis model of assessment of insulin resistance
Change in total serum HDL CEC exhibits an inverse correlation with change in brachial artery FMD
In order to gain additional insight into the predictive relevance of alternative HDL-based metrics for CVD risk, associations between HDL-based metrics and established endpoints of CVD risk were determined using multiple linear regression analyses. Change in HOMA-IR did not correlate with changes in any of the HDL-based metrics but showed a significant correlation with treatment group (β=0.26, p=0.006). Change in baroreflex sensitivity similarly did not correlate with change in any HDL-based metric. Notably, however, a negative correlation was found between change in brachial artery FMD and change in total serum HDL CEC (β=−0.26, p=0.004).
Discussion
We show that transdermal estradiol delivered during perimenopause does not affect plasma HDL-C concentration but reduces total and ABCA1-specific serum HDL cholesterol efflux capacity and leads to a shift toward larger HDL particle size. Whereas lower HDL CEC has been associated with increased CVD risk in other studies, transdermal estradiol in our study also led to significant decreases in LDL-C and insulin resistance and a nonsignificant increase in baroreflex sensitivity. These findings are consistent with those observed in the full study sample (26) and indicate that transdermal estradiol overall may be beneficial with regard to CVD risk. Moreover, change in brachial artery FMD, an index of vascular endothelial function that is predictive of cardiovascular events (33), exhibited a negative correlation with change in total serum HDL CEC. These data suggest that an increase in HDL CEC may not be a reliable index of attenuated CV risk in perimenopausal women.
In contrast to oral exogenous estrogens, which increase plasma HDL-C concentration, transdermal estradiol has been shown to confer negligible effect on HDL-C concentration (8,34,35). Our data are consistent with these previous findings, as HDL-C concentration remained similar to baseline in both the transdermal estradiol and placebo groups. Despite this absence of change in HDL-C, significant differences between groups were evident for both serum HDL total and ABCA1 CEC, indicating a dissociation between HDL-C and HDL CEC. We previously observed a similar dissociation between HDL-C and HDL CEC in response to changes in sex steroid exposure in men, as medical castration led to an increase in HDL-C concentration without attendant change in HDL CEC (28). Similar to our current findings, a prior, observational study showed that natural menopause was characterized by increases in both HDL total and ABCA1 CEC in the absence of change in HDL-C (21). These findings collectively indicate that change in HDL-C concentration due to differential sex steroid exposure is not a reliable index for inferring changes in HDL function. Importantly, HDL CEC is only one index of HDL function, and additional work is needed to establish how differential sex steroid exposure, including the menopausal transition, affects other HDL-mediated functions, including regulation of endothelial and immune cell activity as well as antioxidant and anti-thrombotic functions (12).
The basis of the observed changes in HDL CEC is unknown but may be explained, in part, by changes in the size distribution of HDL particles. HDL particle size has been established as a key determinant of HDL CEC, as small HDL particles have been shown to efflux cholesterol more efficiently and preferentially through the ABCA1-mediated pathway (36,37). Therefore, the shift toward smaller HDL particle size evident in the placebo group may underlie the increases in serum HDL total and ABCA1 CEC relative to the ERT group. It is also possible that the increased ABCA1-specific CEC of serum HDL is mediated by increased levels of pre-β1 HDL, which is known to be a major acceptor of cholesterol (38,39). It has been shown that increased levels of pre-β1 HDL may associate with dyslipidemia, and, in at least one study, higher level of pre-β1 HDL was associated with increased risk of CVD (40,41). Recent studies also have suggested that in addition to apoA-I-containing particles, other proteins present in serum HDL (i.e. apoB-depleted serum) may mediate cholesterol efflux. Plasminogen, for example, has been shown to efficiently mediate ABCA1-specific cholesterol efflux from ABCA1 expressing BHK cells (42). ABCA1-specific cholesterol efflux may be further modulated by Lp(a) (43). Thus, both HDL remodeling and change in HDL particle distribution, as well as changes in other serum mediators of efflux, may underlie the observed changes in ABCA1-specific serum HDL CEC.
Prior studies of hormone replacement therapy utilizing conjugated equine estrogens (CEE) similarly have demonstrated increases in large HDL particles (44,45). The specific mechanisms underlying this HDL particle remodeling are unclear but may implicate, in part, the differential changes in insulin resistance observed in the ERT and placebo groups. Insulin resistance can promote smaller HDL size through multiple mechanisms, including increased lipid exchange with triglyceride-rich lipoproteins and accelerated HDL particle metabolism through increased hepatic triglyceride lipase activity (46). An increase in plasma triglyceride concentration might be expected in association with these changes but was not observed in the current study. However, although fasting triglyceride concentrations were similar between groups and unchanged from baseline, exaggerated postprandial triglyceride excursions have been observed in peri- and postmenopausal women and may be attenuated by ERT (47,48). The ERT-mediated increase in large HDL particles also may be attributable to estrogen-induced suppression of scavenger receptor, class B, type I (SR-BI) expression in liver (49), but suppression of hepatic SR-BI expression was demonstrated in rodents administered pharmacologic doses of ethinyl estradiol (50) and has not been shown for physiologic replacement of 17β-estradiol. Therefore, enhanced understanding of the observed changes in HDL particle size requires further study, ideally with dynamic measurement of lipids in the postprandial state rather than exclusive focus on fasting measurements.
Notably, the relative decreases in serum HDL total and ABCA CEC evident in the ERT group were accompanied by improvement in key CVD risk factors, including insulin resistance and plasma LDL-C concentration. Whereas an LDL-C lowering effect has been found reproducibly with both oral and estradiol replacement regimens (7), the respective effects of these ERT formulations on systemic insulin sensitivity have been inconsistent with studies variably showing either neutral or beneficial effects of transdermal ERT (51–54). In the present study, the directional change in baroreflex sensitivity also favored the ERT group, although this finding did not achieve statistical significance. Further, a negative association was found between total serum HDL CEC and brachial artery FMD. Thus, these findings collectively suggest that the increase in HDL CEC that occurs during the menopausal transition may occur in the context of worsening of other CVD risk factors. This unexpected relationship between HDL CEC and CVD risk is not unprecedented; a prior study, for example, observed a positive association between higher HDL CEC and incident CV events (55), increased HDL CEC after HDL-raising therapy was not associated with clinical benefit (56,57), and HDL CEC was not found to associate with CV events among patients with chronic kidney disease undergoing hemodialysis (22). A larger study specifically designed to address the effects of transdermal ERT on cardiovascular outcomes will be necessary to conclusively answer these questions.
Our study has several key limitations. Most importantly, measurements of lipids and HDL-based metrics were made solely in the fasted state and do not capture dynamic, postprandial changes in lipoprotein remodeling that play a critical role in atherogenesis. Participants in the ERT group also received micronized progesterone, so the extent to which progesterone treatment contributed to study results cannot be determined. However, in contrast to medroxyprogesterone acetate, which clearly has been shown to counter ERT-mediated effects on HDL (49,58), micronized progesterone appears to exert neutral effects on HDL and other lipoproteins (6,35). Plasma samples further were collected 2–3 months after the most recent progesterone exposure in an effort to minimize the impact of progesterone-mediated effects. Nonetheless, as transdermal estradiol is commonly prescribed in conjunction with micronized progesterone, the study results are clinically relevant despite the inability to definitively identify the respective effects of estradiol and progesterone exposure. Participants in this study were healthy women, and changes in HDL particle size and function - both during the natural menopausal transition and in response to ERT - may vary considerably in women with underlying cardiometabolic disorders (45,49). Additional work is also necessary to delineate the interactions between the menopausal transition and clinical variables including age, race/ethnicity, and dietary habits on HDL composition and function. Importantly, our analyses examined changes in study endpoints after only 6 months of treatment with placebo or ERT. This short time frame may account for some of our negative findings, including absence of change in brachial artery FMD. Notably, in the parent study, brachial artery FMD was shown to decline in age-dependent fashion over 1 year of follow-up among women receiving placebo, whereas no decline in FMD was found among women receiving ERT irrespective of age (26). Our study entailed secondary analyses of samples collected during a previously conducted randomized controlled study. Finally, our analyses may be limited by multiple comparisons with statistical correction applied only to the HDL protein composition data.
Conclusions
In summary, our study corroborates previous findings of an increase in serum HDL CEC during the menopausal transition and, further, demonstrates that this increase in CEC is abrogated by ERT in concert with a shift toward larger HDL particle size. Notably, this relative decrease in efflux capacity conferred by ERT was accompanied by significant improvements in well-established CVD risk factors. Therefore, our findings suggest cardiometabolic benefit from transdermal ERT in perimenopausal women and highlight the need to assess the predictive utility of HDL CEC as a metric of CVD risk specifically in this population.
Supplementary Material
Highlights.
The cardiovascular effects of hormone replacement therapy are uncertain.
The impact of transdermal estradiol on alternative HDL-based metrics is unknown.
Transdermal estradiol reduced HDL cholesterol efflux capacity.
Transdermal estradiol also led to a shift toward increased HDL particle size.
Transdermal estradiol improved major cardiovascular risk factors including LDL-C.
Acknowledgements:
This work was supported by the American Heart Association [grant number 16GRNT30700006]; the North Carolina Technology Center [Institutional Development Grant number 2013-IDG-1023]; and the National Institutes of Health [grant number R01-MH087619].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
N/A (The parent study was registered at ClinicalTrials.gov: ID NCT01308814).
References
- 1.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325(11):756–762. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Investigators Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 3.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. [DOI] [PubMed] [Google Scholar]
- 4.Miller VM, Manson JE. Women’s Health Initiative Hormone Therapy Trials: New insights on Cardiovascular Disease from Additional Years of Follow up. Curr Cardiovasc Risk Rep. 2013;7(3):196–202.23682305 [Google Scholar]
- 5.Wild RA, Wu C, Curb JD, Martin LW, Phillips L, Stefanick M, Trevisan M, Manson JE. Coronary heart disease events in the Women’s Health Initiative hormone trials: effect modification by metabolic syndrome: a nested case-control study within the Women’s Health Initiative randomized clinical trials. Menopause. 2013;20(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L’Hermite M HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric. 2013;16 Suppl 1:44–53. [DOI] [PubMed] [Google Scholar]
- 7.Kopper NW, Gudeman J, Thompson DJ. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug design, development and therapy. 2009;2:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, Robinson JG, Lund B, Kuller LH, Group WsHIR. Lipoprotein particle concentrations may explain the absence of coronary protection in the women’s health initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28(9):1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. [DOI] [PubMed] [Google Scholar]
- 10.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W, Investigators A-H. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. [DOI] [PubMed] [Google Scholar]
- 11.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino RB, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7(5):484–525. [DOI] [PubMed] [Google Scholar]
- 12.Karathanasis SK, Freeman LA, Gordon SM, Remaley AT. The Changing Face of HDL and the Best Way to Measure It. Clin Chem. 2017;63(1):196–210. [DOI] [PubMed] [Google Scholar]
- 13.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Superko HR, Pendyala L, Williams PT, Momary KM, King SB, Garrett BC. High-density ipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012;6(6):496–523. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins PM, Ronsein GE, Monette JS, Pamir N, Wimberger J, He Y, Anantharamaiah GM, Kim DS, Ranchalis JE, Jarvik GP, Vaisar T, Heinecke JW. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem. 2014;60(11):1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation. 2017;135(25):2494–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117(3):746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamon-Fava S, Herrington DM, Reboussin DM, Sherman M, Horvath KV, Cupples LA, White C, Demissie S, Schaefer EJ, Asztalos BF. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol. 2008;28(3):575–579. [DOI] [PubMed] [Google Scholar]
- 21.El Khoudary SR, Hutchins PM, Matthews KA, Brooks MM, Orchard TJ, Ronsein GE, Heinecke JW. Cholesterol Efflux Capacity and Subclasses of HDL Particles in Healthy Women Transitioning Through Menopause. J Clin Endocrinol Metab. 2016;101(9):3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopecky C, Ebtehaj S, Genser B, Drechsler C, Krane V, Antlanger M, Kovarik JJ, Kaltenecker CC, Parvizi M, Wanner C, Weichhart T, Säemann MD, Tietge UJ. HDL Cholesterol Efflux Does Not Predict Cardiovascular Risk in Hemodialysis Patients. J Am Soc Nephrol. 2017;28(3):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yassine HN, Belopolskaya A, Schall C, Stump CS, Lau SS, Reaven PD. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism. 2014;63(5):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attia N, Ramaharo A, Paul JL, Cambillau M, Beaune P, Grynberg A, Simon A, Fournier N. Enhanced removal of cholesterol from macrophage foam cells to serum from type IV hypertriglyceridemic subjects. Atherosclerosis. 2008;198(1):49–56. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS. Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA Psychiatry. 2018;75(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon JL, Rubinow DR, Watkins L, Hinderliter AL, Caughey MC, Girdler SS. The Effect of Perimenopausal Transdermal Estradiol and Micronized Progesterone on Markers of Risk for Arterial Disease. J Clin Endocrinol Metab. 2020;105(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinow KB, Tang C, Hoofnagle AN, Snyder CN, Amory JK, Heinecke JW, Page ST. Acute sex steroid withdrawal increases cholesterol efflux capacity and HDL-associated clusterin in men. Steroids. 2012;77(5):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinow KB, Vaisar T, Chao JH, Heinecke JW, Page ST. Sex steroids mediate discrete effects on HDL cholesterol efflux capacity and particle concentration in healthy men. J Clin Lipidol. 2018;12(4):1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101(35):13032–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoofnagle AN, Becker JO, Oda MN, Cavigiolio G, Mayer P, Vaisar T. Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin Chem. 2012;58(4):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaisar T, Couzens E, Hwang A, Russell M, Barlow CE, DeFina LF, Hoofnagle AN, Kim F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS One. 2018;13(3):e0192616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ieronimakis N, Pantoja M, Hays AL, Dosey TL, Qi J, Fischer KA, Hoofnagle AN, Sadilek M, Chamberlain JS, Ruohola-Baker H, Reyes M. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet Muscle. 2013;3(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vrablik M, Fait T, Kovar J, Poledne R, Ceska R. Oral but not transdermal estrogen replacement therapy changes the composition of plasma lipoproteins. Metabolism. 2008;57(8):1088–1092. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Hyun HS, Park HG, Seo JH, Lee EY, Lee JS, Lee DY, Choi DS, Yoon BK. Effects of Hormone Therapy on Serum Lipid Levels in Postmenopausal Korean Women. J Menopausal Med. 2015;21(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133–1142. [DOI] [PubMed] [Google Scholar]
- 37.Heinecke JW. Small HDL promotes cholesterol efflux by the ABCA1 pathway in macrophages: implications for therapies targeted to HDL. Circ Res. 2015;116(7):1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asztalos BF, Horvath KV, Mehan M, Yokota Y, Schaefer EJ. Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. Journal of Lipid Research. 2017;58(6):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, Adelman SJ, Nissen SE, Rader DJ. Cholesterol Efflux Capacity and Pre-Beta-1 HDL Concentrations Are Increased in Dyslipidemic Patients Treated With Evacetrapib. J Am Coll Cardiol. 2015;66(20):2201–2210. [DOI] [PubMed] [Google Scholar]
- 40.Miida T, Nakamura Y, Inano K, Matsuto T, Yamaguchi T, Tsuda T, Okada M. Pre beta 1-high-density lipoprotein increases in coronary artery disease. Clin Chem. 1996;42(12):1992–1995. [PubMed] [Google Scholar]
- 41.Guey LT, Pullinger CR, Ishida BY, O’Connor PM, Zellner C, Francone OL, Laramie JM, Naya-Vigne JM, Siradze KA, Deedwania P, Redberg RF, Frost PH, Seymour AB, Kane JP, Malloy MJ. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. The American journal of cardiology. 2011;108(3):360–366. [DOI] [PubMed] [Google Scholar]
- 42.Pamir N, Hutchins PM, Ronsein GE, Wei H, Tang C, Das R, Vaisar T, Plow E, Schuster V, Reardon CA, Weinberg R, Dichek DA, Marcovina S, Getz GS, Heinecke JW, Koschinsky ML. Plasminogen promotes cholesterol efflux by the ABCA1 pathway. JCI Insight. 2017;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavori H, Fenton AM, Plubell DL, Rosario S, Yerkes E, Gasik R, Miles J, Bergstrom P, Minnier J, Fazio S, Pamir N. Elevated lipoprotein(a) levels lower ABCA1 cholesterol efflux capacity. J Clin Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamon-Fava S, Posfai B, Asztalos BF, Horvath KV, Dallal GE, Schaefer EJ. Effects of estrogen and medroxyprogesterone acetate on subpopulations of triglyceride-rich lipoproteins and high-density lipoproteins. Metabolism. 2003;52(10):1330–1336. [DOI] [PubMed] [Google Scholar]
- 45.Lamon-Fava S, Herrington DM, Horvath KV, Schaefer EJ, Asztalos BF. Effect of hormone replacement therapy on plasma lipoprotein levels and coronary atherosclerosis progression in postmenopausal women according to type 2 diabetes mellitus status. Metabolism. 2010;59(12):1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjornstad P, Eckel RH. Pathogenesis of Lipid Disorders in Insulin Resistance: a Brief Review. Curr Diab Rep. 2018;18(12):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bessesen DH, Cox-York KA, Hernandez TL, Erickson CB, Wang H, Jackman MR, Van Pelt RE. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: impact of menopause and estradiol. Obesity (Silver Spring). 2015;23(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikata R, Miyahara Y, Onoe Y, Okano H, Ohta H. Possible risk factor for postmenopausal women: postprandial hypertriglyceridemia. J Obstet Gynaecol Res. 2008;34(6):1032–1036. [DOI] [PubMed] [Google Scholar]
- 49.Lamon-Fava S, Herrington DM, Reboussin DM, Sherman M, Horvath K, Schaefer EJ, Asztalos BF. Changes in remnant and high-density lipoproteins associated with hormone therapy and progression of coronary artery disease in postmenopausal women. Atherosclerosis. 2009;205(1):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98(4):984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raudaskoski T, Tomás C, Laatikainen T. Insulin sensitivity during postmenopausal hormone replacement with transdermal estradiol and intrauterine levonorgestrel. Acta Obstet Gynecol Scand. 1999;78(6):540–545. [PubMed] [Google Scholar]
- 52.Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC. Effects of oral and transdermal 17beta-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism. 2000;49(6):742–747. [DOI] [PubMed] [Google Scholar]
- 53.O’Sullivan AJ, Ho KK. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80(6):1783–1788. [DOI] [PubMed] [Google Scholar]
- 54.Os I, Os A, Abdelnoor M, Larsen A, Birkeland K, Westheim A. Insulin sensitivity in women with coronary heart disease during hormone replacement therapy. J Womens Health (Larchmt). 2005;14(2):137–145. [DOI] [PubMed] [Google Scholar]
- 55.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33(7):1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE, Investigators A. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med. 2017;376(20):1933–1942. [DOI] [PubMed] [Google Scholar]
- 57.Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, Adelman SJ, Nissen SE, Rader DJ. Cholesterol Efflux Capacity and Pre-Beta-1 HDL Concentrations Are Increased in Dyslipidemic Patients Treated With Evacetrapib. J Am Coll Cardiol. 2015;66(20):2201–2210. [DOI] [PubMed] [Google Scholar]
- 58.Lamon-Fava S, Postfai B, Diffenderfer M, DeLuca C, O’Connor J, Welty FK, Dolnikowski GG, Barrett PH, Schaefer EJ. Role of the estrogen and progestin in hormonal replacement therapy on apolipoprotein A-I kinetics in postmenopausal women. Arterioscler Thromb Vasc Biol. 2006;26(2):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


