Abstract
Purpose:
Low-dose tamoxifen reduces breast cancer risk, but remains untested in chest-irradiated cancer survivors – a population with breast cancer risk comparable to BRCA mutation carriers. We hypothesized that low-dose tamoxifen would be safe and efficacious in reducing radiation-related breast cancer risk.
Experimental Design:
We conducted an investigator-initiated, randomized, phase IIb, double-blinded, placebo-controlled trial (FDA IND107367) between 2010 and 2016 at 15 US sites. Eligibility included ≥12Gy of chest radiation by age 40y and age at enrollment ≥25y. Patients were randomized 1:1 to low-dose tamoxifen (5mg/day) or identical placebo tablets for 2y. The primary endpoint was mammographic dense area at baseline, 1y and 2y. Insulin growth factor-1 (IGF-1) plays a role in breast carcinogenesis; circulating IGF-1 and IGF-BP3 levels at baseline, 1y and 2y served as secondary endpoints.
Results:
Seventy-two participants (low-dose tamoxifen: n=34, placebo: n=38) enrolled at a median age of 43.8y (35-49) were evaluable. They had received chest radiation at a median dose of 30.3 Gy. Compared with the placebo arm, the low-dose tamoxifen arm participants had significantly lower mammographic dense area (P=0.02) and IGF1 levels (P<0.0001), and higher IGFBP-3 levels (P=0.02). There was no difference in toxicity biomarkers (serum bone-specific alkaline phosphatase, lipids, and anti-thrombin III; urine N-telopeptide crosslinks) between the treatment arms. We did not identify any grade 3-4 adverse events related to low-dose tamoxifen.
Conclusions:
In this randomized trial in chest-irradiated cancer survivors, we find that low-dose tamoxifen is effective in reducing established biomarkers of breast cancer risk and could serve as a risk-reduction strategy.
INTRODUCTION
Adolescent and young adult females treated with chest radiation for their primary cancer are at risk for breast cancer; the cumulative incidence of radiation-related breast cancer exceeds 35% by age 50.(1) This risk is as high as that observed in BRCA mutation carrier.(1) Mortality rates are higher after radiation-related breast cancer than after primary breast cancer.(2) These findings present an urgent yet unmet need to develop breast cancer risk-reduction strategies for radiation-exposed cancer survivors.
The risk of radiation-related breast cancer is lower in survivors who also received ovarian radiation.(3) These findings suggest a role for endogenous estrogens in radiation-related breast carcinogenesis, making tamoxifen a viable risk-reducing option for pre- and post-menopausal cancer survivors.(4) While effective in reducing the risk of primary breast cancer, the possibility of severe adverse events (AEs), such as venous thromboembolism and endometrial cancer, have contributed to the low uptake of tamoxifen at 20mg/d.(4) Low-dose tamoxifen (5mg/d) appears to retain the efficacy in reducing breast cancer risk, but with a safer AE profile.(5) These findings make low-dose tamoxifen an attractive breast cancer risk-reducing strategy for chest-irradiated cancer survivors.
Radiation-related breast cancer has a latency of 8-10y after exposure, necessitating use of a biomarker as a surrogate endpoint for assessing efficacy of breast cancer prevention. Mammographic density(6) and serum Insulin Growth Factors (IGF-1 and IGF-BP3)(7) are established biomarkers of breast cancer risk. We hypothesized that tamoxifen at a dose of 5mg/d for 2y would be an efficacious and safe option for reducing mammographic dense area and serum IGF-1 levels and increasing IGFBP-3 levels, in young female cancer survivors at risk for radiation-related breast cancer.
PATIENTS AND METHODS
Study Participants
We conducted a multi-center, investigator-initiated, randomized phase IIb, double-blinded, placebo-controlled trial of tamoxifen 5mg/d vs. placebo administered for 2y (FDA IND 107367; NCT01196936; protocol in Appendix).
Study Design
Women who were ≥25yo at enrollment, with a history of chest radiation at ≥12Gy at age ≤40 for a primary cancer, and were off-therapy for ≥6mo, were eligible. Patients with a prior history of breast cancer or ductal carcinoma in situ in both breasts, and those with baseline mammographic dense area <25% were excluded (eligibility in Appendix Table A1). The trial was conducted in accordance with the Declaration of Helsinki, and approval was received from institutional review boards at all participating sites. Participants provided written informed consent and enrolled between October 2010 and September 2016; last patient follow-up occurred in November 2019.
Eligible participants were randomized 1:1 in a double-blinded fashion by an Interactive Web Response System (Sharp Clinical Services; Allentown, PA) using block-stratified randomization, with menopausal status (pre-menopausal; post-menopausal), chest radiation dose (1200-2599cGy; ≥2600cGy) and age at radiation (<18y; 18-40y) as stratification factors and a block size of 4. Sharp Clinical Services provided 5mg tamoxifen and identical placebo tablets.
Study Intervention
Women received low-dose tamoxifen or placebo daily for 2y. After a screening visit, research staff enrolled participants and provided them the study drug every 90d. Mammograms and collection of a morning fasting blood and urine sample occurred at baseline (t0), 1y (t1) and 2y (t2). Participants, treating clinicians, and research staff were masked to treatment assignments.
Study End Points
Mammographic dense area was the primary endpoint. Participating sites submitted de-identified mammograms for study participants. Three study radiologists evaluated every mammogram independently on Mammography Quality Standards Act-certified monitors, and determined breast density by visually estimating the proportion of dense breast tissue (fibroglandular tissue) to nondense tissue (fatty tissue) to the nearest 5%. The percentage given was based on the 4th edition of the ACR BI-RADS Atlas. The radiologists were masked to study arm assignment and study timepoint. Concordance among the three radiologists was 0.89 (95%CI, 0.87-0.92).
Serum IGF-1 and IGFBP-3 levels were measured using chemiluminescent immunoassay (ARUP laboratories, Salt Lake City, UT).
Safety and tolerability
Safety endpoints included serum bone-specific alkaline phosphatase (BSAP: marker of bone formation), urine N-telopeptide crosslinks (NTX: marker of bone resorption), serum anti-thrombin III levels [AT-III: for thrombophilic propensity), and fasting serum lipid panel. Participating sites reported AEs (graded using Common Terminology Criteria for Adverse Events), and likely attribution to study drug. Study participants returned their pill kits every 90d for calculation of adherence rates using pill count. Patient-reported symptoms were recorded every 90d. Voluntary withdrawals were tabulated at study end.
Statistical analysis
We provide details regarding sample size and power calculations in Appendix Table A2. Assuming a Type I error=0.05, 2-sided test, 10% attrition between annual visits, and correlation=0.8 between measurements, we projected that a sample size of 115/arm would provide 80% power to detect an effect size of 0.25 at t2.(8, 9) Slower than expected accrual, and financial constraints of supporting study drug costs necessitated an interim analysis for futility; results showed a separation of the two arms. Sample size was adjusted using the between-measurement correlation (r=0.95; lower 95%CI=0.9) obtained from the interim data. Assuming an annual attrition rate of 10%, r=.95 (r=0.9), n=31/arm (n=59/arm) would be needed to detect an effect size of 0.25 at t2 with 80% power and a type I error=0.05 (Table A2). We performed all analyses using SAS version 9.4.
Efficacy
Mammographic dense area:
Using an intention-to-treat analysis, we examined the efficacy of low-dose tamoxifen in reducing mammographic dense area by applying the linear mixed effects (LME) model for normally distributed data. All patients with a minimum of baseline (t0) mammographic data were included. Mammographic dense area data from each breast was square root transformed to normality. We used an average of the left and right mammographic dense area as the dependent variable (given between breast r=0.99). Random effects were assumed for the intercept (to account for within-person correlations) and for the three radiologists. We treated time as a categorical variable using two indicator variables, and examined the treatment arm*time interaction to determine the efficacy of low-dose tamoxifen. We adjusted the analysis for baseline mammographic dense area. We considered low-dose tamoxifen to be efficacious if the 2-df test was significant, or if mammographic dense area was lower at t2 alone for low-dose tamoxifen group when compared to the placebo group. The two-sided significance level was set at .05.
Serum IGF-1 and IGFBP-3 levels:
By applying LME models, we examined the treatment arm*time interaction to determine the efficacy of low-dose tamoxifen in reducing IGF-1 levels, increasing IGFBP-3 levels, adjusting for baseline levels. We considered low-dose tamoxifen to be efficacious if the 2-df test was significant, or if the levels differed at t2 alone, when compared to placebo. Two-sided significance level was set at .05.
Safety and tolerability of low-dose tamoxifen
Adverse events:
AEs were graded as not present (grade 0), mild (grade 1), moderate (grade 2), serious (grade 3), life-threatening (grade 4) or fatal (grade 5). We dichotomized AEs (grade ≤2 vs. ≥3) and examined the difference in proportion of AEs by treatment group. Cholesterol (total, HDL, LDL), triglycerides, AT-III, BSAP and NTX were treated as continuous variables. We used generalized LME model with random intercepts, and used treatment arm*time interaction to assess the effect of low-dose tamoxifen on these measurements.
Patient-reported symptoms:
We scored patient-reported symptoms on a 5-point Likert-type scale (0 to 4), and compared the proportion of patients with moderate-to-severe symptoms between treatment groups.
RESULTS
Patient Characteristics
We enrolled 116 women and randomized 84; 23 were ineligible at screening and nine declined to participate after providing informed consent. A central review identified mammographic dense area to be <25% for 11 patients; one patient withdrew after randomization (before study start), yielding 72 participants at t0 (low-dose tamoxifen: n=34; placebo: n=38) (Figure 1). Most patients (86%) carried a history of Hodgkin lymphoma. Median age at primary cancer diagnosis was 21.5y (IQR, 16-29), and at enrollment was 43.8y (35-49). Median dose of chest radiation was 30.3Gy (21-37.3). Forty-four participants (61%) were pre-menopausal at trial enrollment. Baseline patient characteristics were comparable between treatment groups (Table 1).
Figure 1:
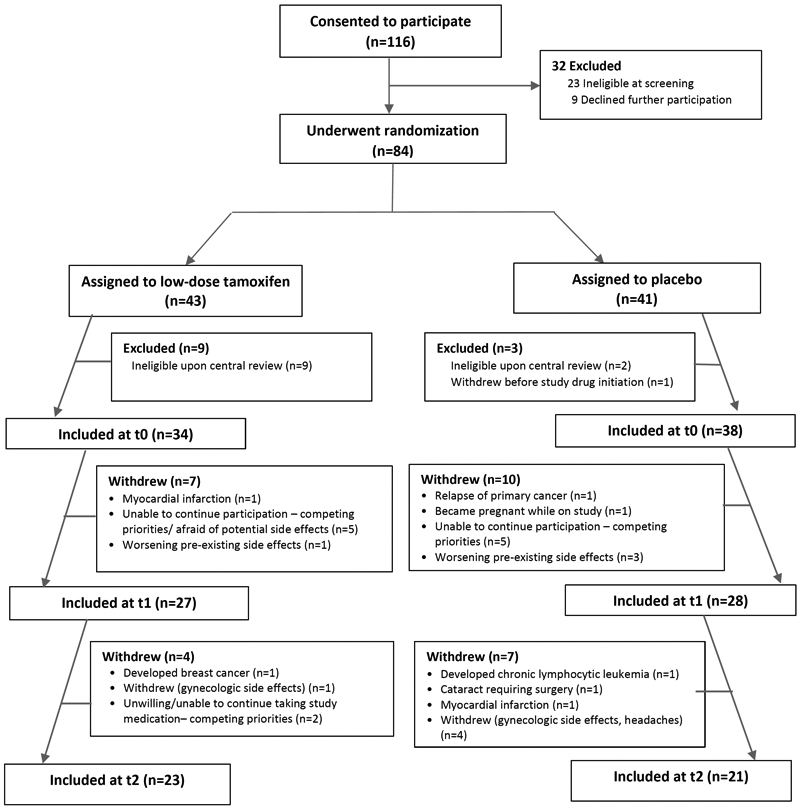
CONSORT diagram for enrollment of patients on the trial
Table 1.
Participant Characteristics at Baseline
| Low-dose tamoxifen (n=34) | Placebo (n=38) | |
|---|---|---|
| Age at study in years | ||
| Median (IQR) | 43.7 (34.3 to 48.8) | 44.1 (34.6 to 47.0) |
| Race/ ethnicity (n, %) | ||
| Non-Hispanic whites | 27 (79%) | 31 (82%) |
| Body Mass Indexin Kg/m2 | ||
| Median (IQR) | 25.0 (21.9 to 29.0) | 25.4 (22.1 to 30.8) |
| Primary Cancer diagnosis (n, %) | ||
| Hodgkin lymphoma | 29 (85%) | 33 (87%) |
| Non-Hodgkin lymphoma | 5 (15%) | 2 (5%) |
| Other | 0 (0%) | 3 (8%) |
| Time between cancer diagnosis and study in years | ||
| Median (IQR) | 16.6 (12.5 to 24.8) | 17.1 (10.7 to 26.1) |
| Age at diagnosis of primary cancer in years | ||
| Median (IQR) | 23.4 (17.5 to 29.0) | 19.9 (15.9 to 28.4) |
| Dose of radiation to chest for primary cancer in Gy | ||
| Median (IQR) | 30.3 (21.0 to 36.6) | 29.8 (21.0 to 38.0) |
| 1200-2599 cGy | 15 (44.1%) | 16 (42.1%) |
| ≥2600 cGy | 19 (55.9%) | 22 (57.9%) |
| Radiation field | ||
| Mantle | 21 (61.8%) | 26 (68.4%) |
| Mediastinal | 4 (11.8%) | 3 (7.9%) |
| Mini-mantle | 4 (11.8%) | 2 (5.3%) |
| Other | 5 (14.7%) | 7 (18.4%) |
| Pelvic radiation for primary cancer | ||
| Yes (n, %) | 4 (12%) | 4 (11%) |
| Alkylating agent exposure (n, %) | ||
| Yes | 6 (18%) | 5 (14%) |
| Postmenopausal at study (n, %) | ||
| Yes | 13 (38%) | 15 (40%) |
| Age at menarche in years | ||
| Median (IQR) | 12 (11 to 14) | 13 (12 to 14) |
| Age at first childbirth in years in years | ||
| Median (IQR) y | 28 (27 to 31) | 26 (21 to 31) |
| Family history of breast cancer in first-degree relatives (n, %) | ||
| Yes | 12 (35%) | 15 (40%) |
| Baseline estradiol in pg/mL | ||
| Pre-menopausal | 85 (0 to 389) | 87 (0-242) |
| Post-menopausal | 14.7 (1.3 to 226) | 9.8 (4.6-145) |
| Baseline Percent Mammographic Breast Dense area | ||
| Median (range) | 51.0% (25% to 85%) | 50.0% (25% to 95%) |
| Mean (±SD) | 52.6%±18.5% | 50.4±20.7% |
| Baseline Insulin-like growth factor-1 levels (ng/mL) | ||
| Median (range) | 166 (82 to 258) | 148 (78 to 289) |
| Mean (±SD) | 160.1 (41.0) | 156.8 (48.8) |
| Baseline Insulin-like growth factor binding protein levels (ng/mL) | ||
| Median (range) | 4730 (2430 to 6090) | 4260 (1990 to 8880) |
| Mean (±SD) | 4601.8 (782.0) | 4382.2 (1147.8) |
Biologic endpoints
Mammographic dense area:
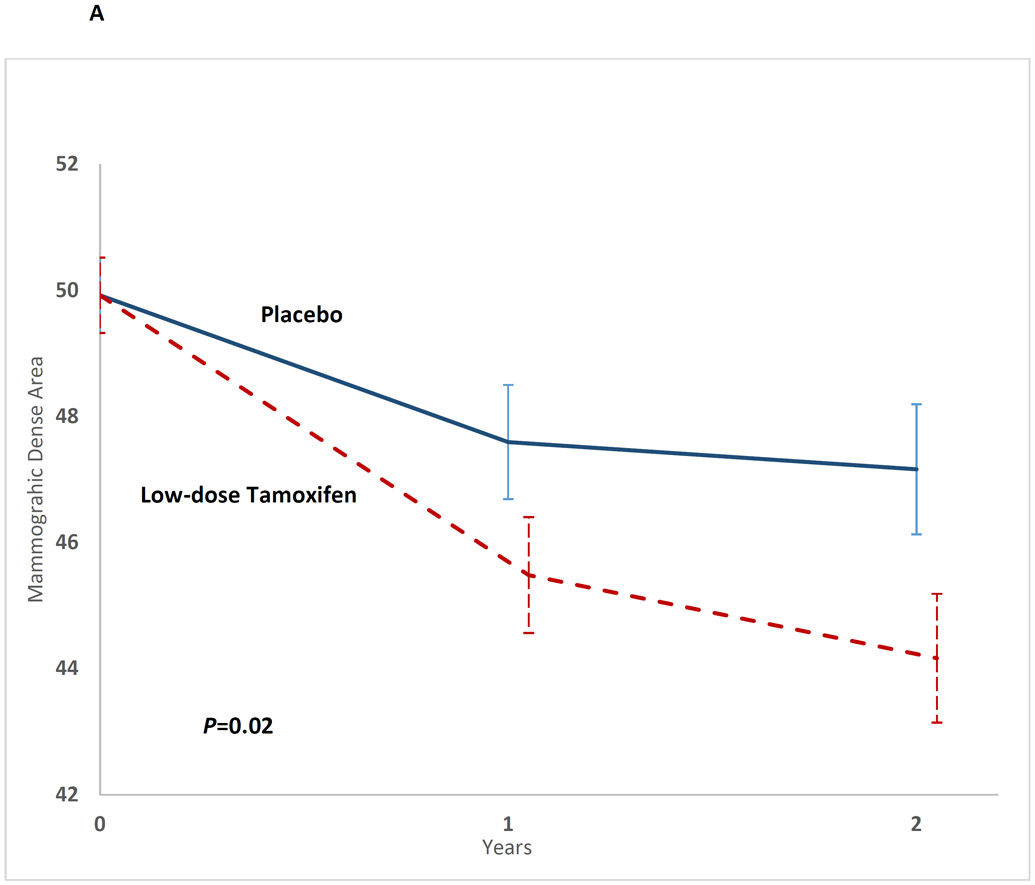
The 2-df test was significant (P=0.02). The mean mammographic dense area was lower among participants on the low-dose tamoxifen arm at t1 (low-dose tamoxifen: 44.9 vs. placebo: 47.8; mammographic dense areatamoxifen-placebo: −2.9, 95%CI, −3.35 to −2.47, P=0.02) and at t2 (low-dose tamoxifen: 43.7 vs. placebo: 46.8; mammographic dense areatamoxifen-placebo: −3.13, 95%CI, −3.57 to −2.68, P=0.03) (Table 2, Figure 2A). This represented a 10.2% relative reduction from t0 to t2 in the low-dose tamoxifen arm and 4.4% in the placebo arm.
Table 2.
Efficacy and Safety of Low dose tamoxifen
| Efficacy of low-dose tamoxifen | ||||
|---|---|---|---|---|
| Efficacy Endpoints | Difference between low-dose tamoxifen and placebo |
95% CI | p-value | p-value 2df at t1 and t2 |
| Percent Mammographic Dense area (square root transformed)* | ||||
| At t1 | −0.21 | −0.40 to −0.03 | 0.02 | 0.02 |
| At t2 | −0.23 | −0.44 to −0.03 | 0.03 | |
| Percent Mammographic Dense area (mean)* | ||||
| At t1 | −2.9% | −3.35 to −2.47 | 0.02 | 0.02 |
| At t2 | −3.13% | −3.57 to −2.68 | 0.03 | |
| Insulin-like Growth Factor-1 (IGF-1)**† | ||||
| At t1 | −34.53 | −45.75 to −23.30 | <.0001 | <0.0001 |
| At t2 | −16.99 | −29.41 to −4.56 | 0.008 | |
| Insulin-like Growth Factor Binding Protein-3***‡ | ||||
| At t1 | 295.53 | −23.52 to 614.59 | 0.07 | 0.02 |
| At t2 | 433.44 | 79.81 to 787.06 | 0.02 | |
| Free IGF-1 (IGF-1/IGF-BP3)*100 | ||||
| At t1 | −0.89 | −1.15 to −0.64 | <.0001 | <.0001 |
| At t2 | −0.66 | −0.95 to −0.38 | <.0001 | |
| Safety of low-dose tamoxifen | ||||
| Safety Biomarkers | Difference between low-dose tamoxifen and placebo at t2 |
95% Confidence Interval |
p-value | |
| Lipids | ||||
| Total cholesterol | −2.59 | −21.30 to 16.10 | 0.80 | |
| Low-density lipoprotein | −3.23 | −18.30 to 11.80 | 0.70 | |
| High-density lipoprotein | 1.13 | −6.60 to 8.90 | 0.80 | |
| Triglycerides | 21.51 | −14.50 to 57.10 | 0.20 | |
| Pro-coagulation markers | ||||
| Anti-thrombin-III levels | −8.9 | −22.90 to 5.20 | 0.20 | |
| Bone markers | ||||
| Bone-specific Alkaline Phosphatase | −0.27 | −1.80 to 1.30 | 0.70 | |
| Urinary N-telopeptide, cross-linked | 1.1 | −9.00 to 11.20 | 0.80 | |
Adjusted for baseline mammographic dense area
Adjusted for baseline IGF-1 levels
Adjusted for baseline IGF-BP3
Figure 2A:
Impact of low-dose tamoxifen on mammographic dense area at t1 and t2, shown as mean mammographic dense area and the corresponding standard error
IGF1 levels:
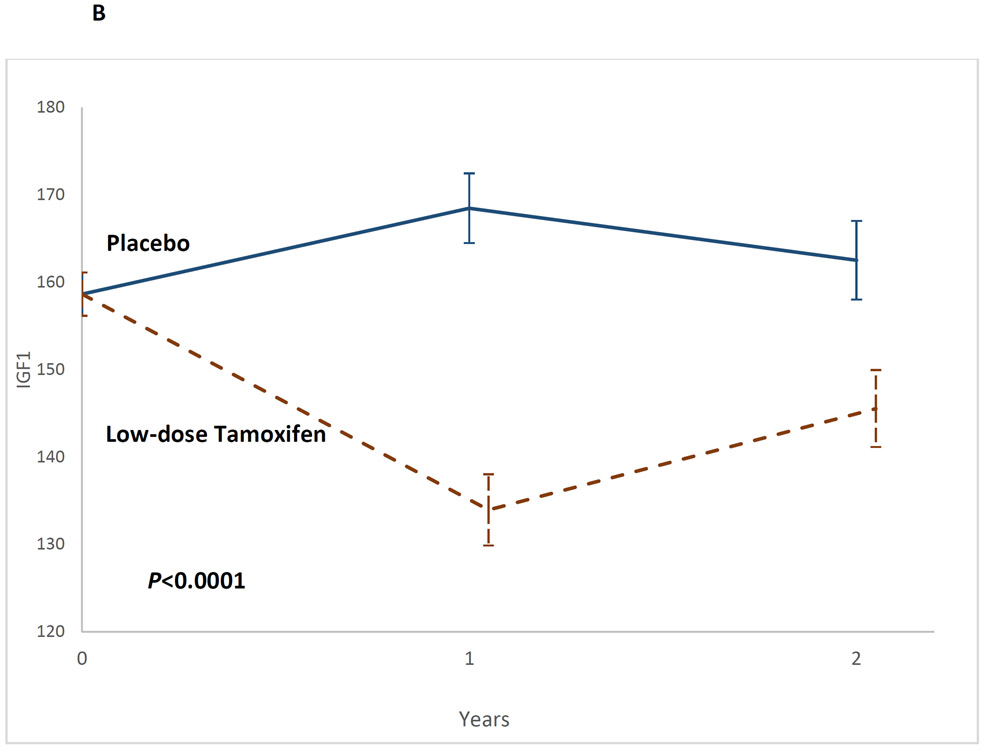
The 2-df test was significant (P<0.0001), as were study arm differences at t1 (Least Square Means [LSMeans]: low-dose tamoxifen: 133.95 vs. placebo: 168.48; IGF1tamoxifen-placebo: −34.53, 95%CI, −45.8 to −23.3, P<0.0001) and at t2 (LSMeans: 144.55 vs. 162.53; IGF1tamoxifen-placebo: −16.99 95%CI, −29.41 to −4.56, P=0.008) (Table 2, Figure 2B). The decline in IGF-1 levels on the low-dose tamoxifen arm were steeper among post-menopausal women (Table A3).
Figure 2B:
Impact of low-dose tamoxifen on serum insulin-like growth factor-1 at t1 and t2, shown as mean IGF-1 levels and the corresponding standard error
IGFBP-3 levels:
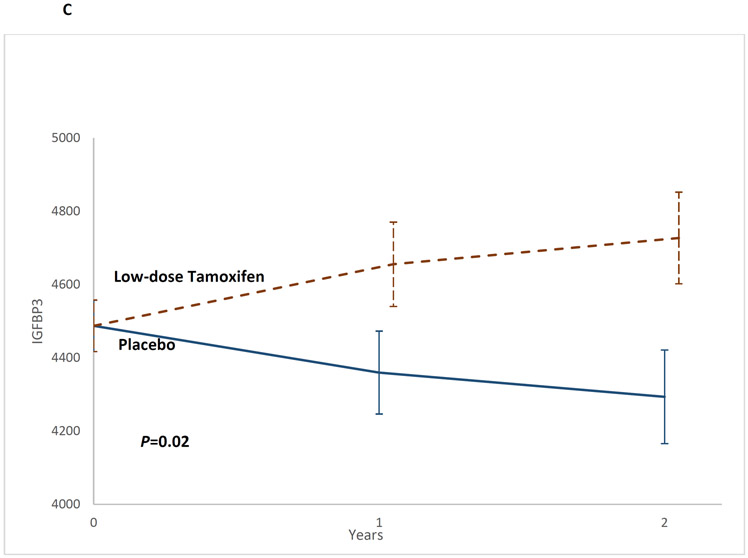
The 2-df test was significant (P=0.02) as were study arm differences at t2 (LSMeans: low-dose tamoxifen: 4727.0 vs. placebo: 4293.6; IGF-BP3tamoxifen-placebo: −433.4, 95%CI, 79.8 to 787.1, P=0.02) (Table 2, Figure 2C).
Figure 2C:
Impact of low-dose tamoxifen on serum insulin-like growth factor binding protein-3 at t1 and t2, shown as mean IGF-BP3 levels and the corresponding standard error
Safety and Tolerability
Biomarkers of toxicity:
Lipids, AT-III, BSAP and NTX levels were comparable between the arms at baseline (Appendix Table A4). Treatment arm*time interaction was not significant indicating no difference in these markers between the low-dose tamoxifen and placebo arms (Table 2).
Adverse events:
We did not find a statistically significant difference in AEs between study arms (grades 1-2: low-dose tamoxifen: 18% vs. placebo: 18%, P=1.0; grades 3-4: 12% vs. 18%, P=0.5) (Table 3). Breast cancer developed in one participant on the low-dose tamoxifen arm and in three participants on the placebo arm. None of the AEs was attributable to low-dose tamoxifen.
Table 3:
Adverse events and Patient-reported Symptoms by Study Arm
| Low-dose tamoxifen (n=34) | Placebo (n=38) | P-value* | |
|---|---|---|---|
| N (%)* | N (%)* | ||
| Patient-reported symptoms | |||
| Arthralgia/arthritis | 6 (18%) | 5 (13%) | 0.7458 |
| Back pain | 7 (21%) | 4 (11%) | 0.3288 |
| Myalgias | 7 (21%) | 1 (3%) | 0.0227 |
| Malaise | 6 (18%) | 5 (13%) | 0.7458 |
| Hot flashes | 9 (27%) | 11 (29%) | 1.0000 |
| Night sweats | 7 (21%) | 6 (16%) | 0.7606 |
| Irregular menstruation | 9 (27%) | 6 (16%) | 0.3843 |
| Vaginal discharge | 8 (24%) | 5 (13%) | 0.3593 |
| Vaginal spotting/bleeding | 5 (15%) | 8 (21%) | 0.5512 |
| Breast pain | 5 (15%) | 3 (8%) | 0.4630 |
| Urinary incontinence/frequency/urgency | 7 (21%) | 10 (26%) | 0.5926 |
| Weight gain/ loss | 17 (50%) | 19 (50%) | 1.0000 |
| Insomnia | 4 (12%) | 7 (18%) | 0.5225 |
| Somnolence | 9 (27%) | 4 (11%) | 0.1241 |
| Fatigue | 10 (29%) | 3 (8%) | 0.0296 |
| Irritability | 6 (18%) | 6 (16%) | 1.0000 |
| Memory impairment | 6 (18%) | 2 (5%) | 0.1375 |
| Inability to concentrate | 7 (21%) | 3 (8%) | 0.1748 |
| Restlessness | 7 (21%) | 4 (11%) | 0.3288 |
| Paresthesia | 7 (21%) | 2 (5%) | 0.0746 |
| Mood swings | 5 (15%) | 4 (11%) | 0.7265 |
| Feelings of depression | 7 (21%) | 8 (21%) | 1.0000 |
| Diarrhea | 6 (18%) | 5 (13%) | 0.7458 |
| Constipation | 7 (21%) | 6 (16%) | 0.7606 |
| Heartburn | 9 (27%) | 7 (18%) | 0.5713 |
| Headaches | 8 (24%) | 6 (16%) | 0.5527 |
| Adverse Events** | |||
| Grades 3-4 Adverse Events | |||
| Any grade 3-4 adverse events | 4 (12%) | 7 (18%) | 0.5225 |
| Breast cancer | 1 (3%) | 3 (8%) | 0.6167 |
| Myocardial infarction | 2 (6%) | 2 (5%) | 1.0000 |
| Carotid artery occlusion | 1 (3%) | 0 (0%) | 0.4722 |
| Relapse of primary disease | 0 | 2 (5%) | 0.4945 |
| Grades 1-2 Adverse Events | |||
| Any grade 1-2 adverse events | 6 (18%) | 7 (18%) | 1.0000 |
| Pregnancy | 0 | 1 (3%) | 1.0000 |
| Cataract | 2 (6%) | 2 (5%) | 1.0000 |
| Basal cell carcinoma | 0 | 3 (8%) | 0.2418 |
| Endometriosis | 0 | 1 (3%) | 1.0000 |
| Thyroid nodules | 4 (12%) | 0 (0%) | 0.0451 |
P-value calculated using Fisher’s exact test.
Of note, no episodes of venous thromboembolism, or uterine cancer were observed
Patient-reported symptoms:
There was no difference in the prevalence of patient-reported symptoms between the treatment groups, with the exception of myalgias (low-dose tamoxifen: 21% vs. placebo: 3%, p=0.02) and fatigue (low-dose tamoxifen: 29% vs. placebo: 8%, p=0.03) (Table 3).
Voluntary withdrawals and adherence to low-dose tamoxifen:
Voluntary withdrawals did not differ between the two arms (low-dose tamoxifen: 26.5% vs. placebo: 31.6%, P=0.60). Median adherence rates over 2y were comparable (low-dose tamoxifen: 97.5% vs. placebo: 96.7%, P=0.9).
DISCUSSION
In this phase IIb randomized, double-blinded, placebo-controlled trial of chest-irradiated cancer survivors, low-dose tamoxifen (5mg/d for 2y) resulted in a 10.2% reduction in mammographic dense area compared with 4.4% reduction in the placebo arm. We observed statistically significant changes in serum IGF-1 and IGF-BP3 levels. Importantly, low-dose tamoxifen was well tolerated and without any serious AEs.
Breast cancer risk-reduction strategies in other high risk populations include surgical and pharmacologic interventions.(10) Prophylactic bilateral mastectomy is associated with a 90-95% reduction in risk of familial breast cancer(11); however, not all women are comfortable with this option(12). Although bilateral oophorectomy confers a 50-70% reduction in breast cancer risk if performed under age 45, the associated osteoporosis, dyslipidemia, and cardiovascular disease are deterrents, unless the patient is also at increased risk of ovarian cancer.(13) A synergistic effect between radiation and estrogen exposure in mammary carcinoma models(14) and the partial protection afforded to women from radiation-related breast cancer after ovarian radiation(3), suggests that estrogen plays a role in the etiology of radiation-related breast cancer, supporting investigation of an estrogen-blocking intervention as a prevention strategy. While the US Preventive Services Task Force(4) and the American Society of Clinical Oncology(15) recommend SERMs or AIs for women at high risk for breast cancer, they do not include chest-irradiated women in this recommendation, because of insufficient evidence.
The only FDA-approved option for breast cancer chemoprevention in premenopausal women is tamoxifen. Tamoxifen results in decreased incidence of radiation-induced rodent mammary carcinoma.(16) In clinical trials enrolling women at elevated risk of breast cancer based on the Gail Model, 20mg/d of tamoxifen given for 5y decreased the incidence of breast cancer by ~50%.(17) However, tamoxifen at 20mg/d is associated with uterine malignancies, stroke, venous thromboembolism, and vasomotor/gynecological symptoms, contributing to its limited use for breast cancer prevention.(4) These concerns prompted studies exploring tamoxifen at 5mg/d, which demonstrated breast cancer risk-reduction without AEs.(5, 18) These studies provided us with the rationale for selecting tamoxifen at 5mg/d for a mixed population of pre- and post-menopausal chest-irradiated women. Indeed, we found that biomarkers of toxicity (BSAP, NTX, ATIII and lipid profile) were not different between the low-dose tamoxifen and placebo arms, nor was the prevalence of patient-reported symptoms with the exception of a higher prevalence of myalgias and fatigue in the low-dose tamoxifen arm. Four patients developed breast cancer, one on the low-dose tamoxifen arm and three on the placebo arm. Given the younger age of the study population (median age ~44y), one would expect a lower prevalence of AEs; however, this population is uniquely vulnerable to radiation-related severe/life-threatening adverse events such as second cancers, stroke, cardiomyopathy, and cardiovascular disease.(19) Nonetheless, the burden of treatment-related morbidity should have been comparable between the placebo and low-dose tamoxifen arms, allowing us to evaluate the additional impact of low-dose tamoxifen on AEs.
Mammographic dense area is a biologically plausible surrogate endpoint with a strong association with breast cancer.(6, 20, 21) Tamoxifen is associated with decreases in mammographic dense area after 1-2y of treatment.(22) These observations informed a 2y timepoint for the mammographic dense area as an efficacy endpoint in our trial. The absolute reduction in mammographic dense area of 5.1% after 2y of low-dose tamoxifen in our trial is comparable to the reduction (7.9%) with 20mg/d of tamoxifen for 18 months in the IBIS trial.(22)
IGF-1 (potent mitogen) binds to IGF-1 receptor, triggering a signaling cascade leading to proliferative and anti-apoptotic events in the mammary gland, playing an important role in breast carcinogenesis.(7) Similar to a previous low-dose tamoxifen trial in women with intra-epithelial neoplasia or a 5y Gail risk ≥1.3%,(23), our trial found a decline in serum IGF-1 and increase in the IGF-BP3 levels, providing further support for the potential efficacy of low-dose tamoxifen in chest-irradiated women at risk for breast cancer. We noted that the decline in IGF-1 levels on the low-dose tamoxifen arm was steeper among the post-menopausal women, as previously observed in breast cancer patients receiving adjuvant tamoxifen therapy.(24)
While the initial desired sample size was 230 participants, financial constraints and a slow accrual necessitated an interim analysis with a recalculation of the required sample size ranging between 62 and 118. Main reasons for the slow accrual included inability to travel to the site every 6mo as mandated by the trial (55%), lack of desire to participate in a placebo-controlled trial (39%). If low-dose tamoxifen were to become an accepted risk-reduction strategy for chest-irradiated female cancer survivors, the major deterrents (frequent travel to study site; participation in a placebo-controlled trial) would be obviated. A larger sample would have allowed analyses of subgroups that benefited most from low-dose tamoxifen. Nonetheless, in this first chemoprevention trial for young chest-irradiated cancer survivors, we provide evidence that 2y of low-dose tamoxifen significantly reduces mammographic dense area, and that the drug dosing of 5mg/d is safe and well tolerated. Radiation-related breast cancer is the most prevalent second cancer in survivors of AYA cancer;(25) low-dose tamoxifen could serve as an attractive option because of ease of administration, low cost, and a favorable safety profile, good tolerability and high adherence.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Young females treated with chest radiation for their primary cancer are at increased risk for breast cancer; this risk is as high as that observed in BRCA mutation carriers. Estrogen plays a role in the etiology of radiation-related breast cancer, supporting investigation of an estrogen-blocking intervention as a prevention strategy. Low-dose tamoxifen reduces breast cancer risk in high-risk populations with minimal side effects, but has not been tested in chest-irradiated cancer survivors. In an investigator-initiated randomized, phase IIb, double-blinded, placebo-controlled trial, we show that low-dose tamoxifen taken daily for 2y was efficacious in reducing mammographic dense area and IGF-1 levels, and increasing IGFBP-3 levels, when compared with placebo. There were no grade 3-4 toxicities attributable to low-dose tamoxifen. Low-dose tamoxifen can serve as an attractive chemoprevention option in chest irradiated cancer survivors because of its ease of dissemination and favorable safety profile.
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (R01CA140245 to SB, P30CA046592 to Benjamin Allen); and the Breast Cancer Research Foundation; and Tempting Tables. The funding sponsors had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or preparation of the manuscript and the decision to submit for publication.
Footnotes
Potential Conflicts of Interest: Dr. Palomares reports receiving financial support for medical monitoring services from Covance; Dr. Henderson reports research funding from Seattle Genetics. All other authors report no potential conflicts of interest.
Presented, in part, at the American Society of Hematology annual meeting, Orlando, FL, December 8, 2019.
REFERENCES
- 1.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, Mubdi NZ, Leisenring WM, Stovall M, Hammond S, Smith SA, Henderson TO, Boice JD, Hudson MM, Diller LR, Bhatia S, Kenney LB, Neglia JP, Begg CB, Robison LL, Oeffinger KC. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–23. Epub 2014/04/23. doi: 10.1200/JCO.2013.54.4601. PubMed PMID: 24752044; PMCID: PMC4100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskowitz CS, Chou JF, Neglia JP, Partridge AH, Howell RM, Diller LR, Novetsky Friedman D, Barnea D, Morton LM, Turcotte LM, Arnold MA, Leisenring WM, Armstrong GT, Robison LL, Oeffinger KC, Henderson TO. Mortality After Breast Cancer Among Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(24):2120–30. Epub 2019/07/02. doi: 10.1200/JCO.18.02219. PubMed PMID: 31260644; PMCID: PMC6698921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inskip PD, Robison LL, Stovall M, Smith SA, Hammond S, Mertens AC, Whitton JA, Diller L, Kenney L, Donaldson SS, Meadows AT, Neglia JP. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27(24):3901–7. Epub 2009/07/22. doi: 10.1200/JCO.2008.20.7738. PubMed PMID: 19620485; PMCID: PMC2734395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Doubeni CA, Epling JW Jr., Kubik M, Landefeld CS, Mangione CM, Pbert L, Silverstein M, Tseng CW, Wong JB. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(9):857–67. Epub 2019/09/04. doi: 10.1001/jama.2019.11885. PubMed PMID: 31479144. [DOI] [PubMed] [Google Scholar]
- 5.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, Taverniti C, Pacquola MG, Falcini F, Gulisano M, Digennaro M, Cariello A, Cagossi K, Pinotti G, Lazzeroni M, Serrano D, Branchi D, Campora S, Petrera M, Buttiron Webber T, Boni L, Bonanni B. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol. 2019;37(19):1629–37. Epub 2019/04/12. doi: 10.1200/JCO.18.01779. PubMed PMID: 30973790; PMCID: PMC6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer. 2018;25(3):259–67. Epub 2018/04/14. doi: 10.1007/s12282-018-0857-5. PubMed PMID: 29651637; PMCID: PMC5906528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. Epub 2015/03/10. doi: 10.1186/s12943-015-0291-7. PubMed PMID: 25743390; PMCID: PMC4335664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. Journal of Educational and Behavioral Statistics. 1999;24:70–93. [Google Scholar]
- 9.Palomares MR, Machia JR, Lehman CD, Daling JR, McTiernan A. Mammographic density correlation with Gail model breast cancer risk estimates and component risk factors. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1324–30. Epub 2006/07/13. doi: 10.1158/1055-9965.EPI-05-0689. PubMed PMID: 16835331. [DOI] [PubMed] [Google Scholar]
- 10.Narod SA, Offit K. Prevention and management of hereditary breast cancer. J Clin Oncol. 2005;23(8):1656–63. Epub 2005/03/10. doi: 10.1200/JCO.2005.10.035. PubMed PMID: 15755973. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, Frost MH, Grant CS, Donohue JH, Woods JE, McDonnell SK, Vockley CW, Deffenbaugh A, Couch FJ, Jenkins RB. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93(21):1633–7. Epub 2001/11/08. doi: 10.1093/jnci/93.21.1633. PubMed PMID: 11698567. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MD, Lerman C, Brogan B, Peshkin BN, Halbert CH, DeMarco T, Lawrence W, Main D, Finch C, Magnant C, Pennanen M, Tsangaris T, Willey S, Isaacs C. Impact of BRCA1/BRCA2 counseling and testing on newly diagnosed breast cancer patients. J Clin Oncol. 2004;22(10):1823–9. Epub 2004/04/07. doi: 10.1200/JCO.2004.04.086. PubMed PMID: 15067026. [DOI] [PubMed] [Google Scholar]
- 13.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, Evans G, Isaacs C, Daly MB, Matloff E, Olopade OI, Weber BL, Prevention, Observation of Surgical End Points Study G. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346(21):1616–22. Epub 2002/05/25. doi: 10.1056/NEJMoa012158. PubMed PMID: 12023993. [DOI] [PubMed] [Google Scholar]
- 14.Segaloff A, Pettigrew HM. Effect of radiation dosage on the synergism between radiation and estrogen in the production of mammary cancer in the rat. Cancer Res. 1978;38(10):3445–52. Epub 1978/10/01. PubMed PMID: 688229. [PubMed] [Google Scholar]
- 15.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, Floyd JD, Garber JE, Hofstatter EW, Khan SA, Katapodi MC, Pruthi S, Raab R, Runowicz CD, Somerfield MR. Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37(33):3152–65. Epub 2019/09/04. doi: 10.1200/JCO.19.01472. PubMed PMID: 31479306. [DOI] [PubMed] [Google Scholar]
- 16.Peterson NC, Servinsky MD, Christian A, Peng Z, Qiu W, Mann J, Dicello J, Huso DL. Tamoxifen resistance and Her2/neu expression in an aged, irradiated rat breast carcinoma model. Carcinogenesis. 2005;26(9):1542–52. Epub 2005/04/30. doi: 10.1093/carcin/bgi103. PubMed PMID: 15860508; PMCID: PMC1224736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–52. Epub 2011/04/13. doi: 10.1093/jnci/djr079. PubMed PMID: 21483019. [DOI] [PubMed] [Google Scholar]
- 18.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, Veronesi P, Torrisi R, Cazzaniga M, Mora S, Sandri MT, Pelosi G, Luini A, Goldhirsch A, Lien EA, Veronesi U. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95(11):779–90. Epub 2003/06/05. doi: 10.1093/jnci/95.11.779. PubMed PMID: 12783932. [DOI] [PubMed] [Google Scholar]
- 19.Bhakta N, Liu Q, Yeo F, Baassiri M, Ehrhardt MJ, Srivastava DK, Metzger ML, Krasin MJ, Ness KK, Hudson MM, Yasui Y, Robison LL. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–34. Epub 2016/07/30. doi: 10.1016/S1470-2045(16)30215-7. PubMed PMID: 27470081; PMCID: PMC5029267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. Epub 2007/01/19. doi: 10.1056/NEJMoa062790. PubMed PMID: 17229950. [DOI] [PubMed] [Google Scholar]
- 21.Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3(4):314–21. Epub 2001/06/08. doi: 10.1007/s11912-001-0083-7. PubMed PMID: 11389815. [DOI] [PubMed] [Google Scholar]
- 22.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–8. Epub 2004/04/22. doi: 10.1093/jnci/djh106. PubMed PMID: 15100340. [DOI] [PubMed] [Google Scholar]
- 23.Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S, Gulisano M, Johansson H, Galimberti V, Cassano E, Moroni SM, Formelli F, Lien EA, Pelosi G, Johnson KA, Bonanni B. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27(23):3749–56. Epub 2009/07/15. doi: 10.1200/JCO.2008.19.3797. PubMed PMID: 19597031; PMCID: PMC2799048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedl A, Jordan VC, Pollak M. Suppression of serum insulin-like growth factor-1 levels in breast cancer patients during adjuvant tamoxifen therapy. Eur J Cancer. 1993;29A(10):1368–72. Epub 1993/01/01. doi: 10.1016/0959-8049(93)90003-x. PubMed PMID: 8398260. [DOI] [PubMed] [Google Scholar]
- 25.Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, Smith SA, Weathers RE, Henderson TO, Gibson TM, Leisenring W, Armstrong GT, Robison LL, Neglia JP. Temporal Trends in Treatment and Subsequent Neoplasm Risk Among 5-Year Survivors of Childhood Cancer, 1970-2015. JAMA. 2017;317(8):814–24. Epub 2017/03/01. doi: 10.1001/jama.2017.0693. PubMed PMID: 28245323; PMCID: PMC5473951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.