Abstract
Purpose:
Receptor-interacting protein kinase RIPK3 phosphorylates effector molecule MLKL to trigger necroptosis. Although RIPK3 loss is seen in several human cancers, its role in malignant mesothelioma (MM) is unknown. This study aimed to determine if RIPK3 functions as a potential tumor suppressor to limit development of MM.
Experimental Design:
RIPK3 expression was examined in 66 MM tumors and cell lines. Promoter methylation and DNMT1 siRNA studies were performed to assess the mode of RIPK3 silencing in RIPK3-deficient MM cells. Restoration of RIPK3 expression in RIPK3-negative MM cells, either by treatment with 5-aza-2’-deoxycytidine or lentiviral expression of cDNA, was performed to assess effects on cell viability, necrosis, and chemosensitization.
Results:
Loss of RIPK3 expression was observed in 42/66 (63%) primary MMs and MM cell lines, and RT-PCR analysis demonstrated that downregulation occurs at the transcriptional level, consistent with epigenetic silencing. RIPK3-negative MM cells treated with 5-aza-2’-deoxycytidine resulted in re-expression of RIPK3 and chemosensitization. Ectopic expression of RIPK3 also resulted in chemosensitization and led to necroptosis, the latter demonstrated by phosphorylation of downstream target MLKL and confirmed by rescue experiments. Mining of RIPK3 expression and survival outcomes among MM patients available from The Cancer Genome Atlas repository revealed that promoter methylation of RIPK3 is associated with reduced RIPK3 expression and poor prognosis.
Conclusions:
These data suggest that RIPK3 acts as a tumor suppressor in MM by triggering necroptosis and that epigenetic silencing of RIPK3 by DNA methylation impairs necroptosis and contributes to chemoresistance and poor survival in this incurable disease.
Keywords: Mesothelioma, RIPK3, necroptosis, cell death, patient prognosis
Introduction
Human pleural malignant mesothelioma (MM) is an incurable cancer often associated with prior exposure to asbestos. How asbestos induces MM is still elusive, although the chronic inflammation caused by asbestos fibers is believed to be an important contributing factor (1). Recurring inactivation of a specific set of tumor suppressor genes, rather than activating mutations of protooncogenes, is considered a hallmark of MM pathogenesis. More than two decades ago, two key tumor suppressor genes, CDKN2A and NF2, were first shown to be frequently inactivated in human pleural MM cells and primary tumors (2,3). More recently, another tumor suppressor gene, BAP1, was also found to be frequently inactivated in MM (4), and germline mutations in BAP1 were shown to predispose carriers to MM (5). In addition to these three major tumorigenic drivers, more recent next generation sequencing studies have also identified other putative or known tumor suppressor genes to be significantly mutated in tumors from pleural MM patients, e.g., CUL1 (6) and TP53, SETD2, and DDX3X (7).
Tumor suppressors function by maintaining genome integrity, inhibiting malignant cell proliferation, or triggering cell death via various mechanisms, including apoptosis, necroptosis, and autophagy (8). Apoptosis is characterized by activation of a caspase cascade, which eventually results in DNA condensation and cellular disintegration into apoptotic bodies (9). Tumors can evolve anti-apoptotic strategies to evade this barrier, e.g., by overexpressing anti-apoptotic proteins such as Bcl-2, or by loss of expression of pro-apoptotic proteins such as Bax or Bad. In MM, one report showed that Bcl-2 is overexpressed in 40% of tumors, whereas 42% of samples exhibited loss of Bax expression (10). Moreover, decreased expression of BAP1 in BAP1-mutation carriers has been shown to cause a reduction in type 3 inositol-1,4,5-trisphosphate receptor levels and of Ca2+ flux, compromising the ability of BAP1+/− cells that accumulate DNA damage from effecting apoptosis, potentially leading to a higher rate of asbestos-induced malignant transformation (11).
Necrosis was initially thought to be an uncontrolled process that results in cell rupture under conditions such as infection, exposure to toxins, and trauma. Recently, however, it was recognized that necrosis can actually be programmed and carried out in an orchestrated manner. Such so-called ‘necroptosis’ is driven by a cellular signaling complex dubbed the necrosome, which is comprised, at a minimum, of RIPK1, RIPK3, and MLKL (12). RIPK3 can be activated by numerous upstream pathways, and once activated, phosphorylates MLKL, which then traffics to the plasma membrane and triggers cell rupture (13,14). The apoptosis and necroptosis pathways are intertwined: the apoptotic pathway components caspase-8 and FADD are also associated with the necrosome core machinery and have both activating (15,16) and repressive roles in RIPK activation in vivo (14,17–19). On the other hand, the kinase-independent scaffolding function of RIPK1 inhibits caspase 8-induced apoptosis as well as RIPK3-induced necroptosis (17,20–22). Furthermore, RIPK3 deficiency protects RIPK1 knockout-related triggering of necrosis and inflammation (17,20–22). Whether RIPK3-mediated necroptosis can serve as gatekeeper to protect against the development of certain malignancies is largely unknown, although loss of expression of necroptosis components have been reported in human cancers. In breast cancer, 85% of patients have reduced RIPK3 expression due to promoter hypermethylation (23). In esophageal squamous cell carcinoma, 78% of cases showed down regulation of RIPK3, which was linked to a poor clinical outcome and moderate amount of cisplatin resistance (24).
DNA methyltransferase I (DNMT1) can mediate hypermethylation of the RIPK3 promoter in glioma, and such impaired necrosis promotes oncogenesis (25). In colorectal cancer, RIPK3 expression has been reported to be downregulated in tumor tissue compared to adjacent normal tissue (26). Ripk3 knockout mice have been shown to be susceptible to colitis-induced colorectal cancer, which was proposed to occur via the presence of enhanced proinflammatory mediators (27). Moreover, apoptosis-resistant colon cancers are highly sensitive to SMAC mimetic-induced necroptosis (28). In the liver, loss of one of the necrosome components, RIPK1, activates caspase 8 and promotes hepatocellular carcinoma (29). Here, we report that RIPK3 is frequently down regulated in human pleural MM cells and that restoration of RIPK3 expression triggers necroptosis and chemosensitization, suggesting that RIPK3 acts as a tumor suppressor in mesothelial cells and that its silencing in MM may contribute to poor survival in this disease by inhibiting therapy-induced necroptosis.
Materials and Methods
Reagents
Wild type human RIPK3 GFP (RIPK3 WT) and kinase domain mutant D160N (kinase dead, KD) pEGFP-RIPK3-D160N (RIPK3-KD) in pEGFP-N1 were obtained from Addgene (Watertown, MA) as plasmids #41387 and #41386, respectively, and were originally deposited there by the Francis Chan laboratory (30). pEGFP-N1 encodes a red-shifted variant of GFP that has been optimized for brighter fluorescence and higher expression in mammalian cells. The vector backbone also contains an SV40 origin for replication in mammalian cells expressing the SV40 T antigen. A neomycin-resistance cassette (Neor), consisting of the SV40 early promoter, the neomycin/kanamycin resistance gene of Tn5, and polyadenylation signals from the Herpes simplex virus thymidine kinase (HSV TK) gene, allows stably transfected eukaryotic cells to be selected using G418. RIPK3 cDNAs were tagged with HA by PCR and subcloned into pWPI lentiviral vector (31). pWPI expresses GFP via an IRES sequence, and the RIPK3 lentivirus constructs express GFP at its C-terminus.
Anti-RIPK3 antibody (E1Z1D, Cat# 13526, RRID:AB_2687467; 1:1000), anti-RIP (D94C12, Cat# 3493, RRID:AB_2305314; 1:2000), anti-MLKL (D2I6N, Cat# 14993, RRID:AB_2721822; 1:4000), anti-Caspase-8 (D35G2, Cat# 4790, RRID:AB_10545768; 1:1000), anti-FADD (Cat# 2782, RRID:AB_2100484; 1:1000), anti-HA-Tag (C29F4, Cat# 3724, RRID:AB_1549585; 1:5000), anti-NF2 (D3S3W, Cat# 12888, RRID:AB_2650551; 1:1000), anti-p16 INK4A (D7C1M, Cat# 80772, RRID:AB_2799960; 1:500), anti-p14 ARF (4C6/4, Cat# 2407, RRID:AB_490785; 1:500), anti-BAP1(D7W70, Cat#13271, RRID:AB_2798168; 1:1000) were purchased from Cell Signaling Technology (Danvers, MA). Anti-BAP1 (C-4, Cat# sc28383, RRID:AB_626723; 1:1000), anti-p15INK4B (D-12, Cat# sc271791, RRID:AB_10709436; 1:500), anti-GAPDH (6C5, Cat# sc-32233, RRID:AB_627679; 1:50000), anti-DNMT1 (H-12, Cat# sc-271729, RRID:AB_10710384; 1:1000) and anti-β-actin (C4, Cat# sc-47778, RRID:AB_2714189; 1:50000) were purchased from Santa Cruz Biotechnology. Anti-MLKL (phospho-S358, EPR9514, Cat# ab187091, RRID:AB_2619685; 1:2000) was purchased from Abcam. Anti-rabbit IgG, peroxidase-linked species-specific whole antibody (from donkey) secondary antibody (45-000-682; 1:5000), anti-mouse IgG, peroxidase-linked species-specific whole antibody (from sheep) secondary antibody (45-000-679; 1:5000-50000, depending on the primary antibody) were both from Fisher Scientific (Waltham, MA). Immunoblots were imaged using Immobilon Western Chemiluminescent HRP Substrate (ECL) (WBKLS0500, MilliporeSigma, Ontario Canada). MTS assays were performed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (G3582; Promega, Madison, WI). Z-VAD-FMK (50-101-2258), RIPK3 inhibitor GSK’872 (GSK2399872A, 50-194-8004), cisplatin (22-515-0) and doxorubicin hydrochloride (22-521-0) were obtained from Fisher Scientific. 5-Aza-2’-deoxycytidine (5-Aza-dC, A3656) and trichostatin A (TSA,T8552) were from Sigma.
Knockdown of DNMT1
Lipofectamine RNAiMAX, Silencer Pre-designed siRNAs against DNMT1 (ID: 110914 and 110915) and Negative Control #1 siRNA were obtained from ThermoFisher Scientific and used according to the manufacturer’s instructions. Thirty pmoles of siRNAs were transfected on 6-well plates, and cells were harvested for RNA and protein 72 hours after transfection.
Transient expression of RIPK3/RIPK3-KD
Nucleofections were performed using Amaxa Cell Line Kit R (Lonza AG), with programs A23 for cell lines MSTO-211H and M8, and T20 for M12. After 48 hours, MM cells were seeded for clonogenic assays and harvested for immunoblot analysis.
Clonogenic assay
At 48 hours post-nucleofection, MM cells were trypsinized, counted and seeded in 10-cm Petri dishes, in triplicate, at a density of 10,000 cells per dish. Cells were selected with 4 mg/mL of G418 for approximately 3 weeks. Colonies were stained using a Diff-Quik™ stain kit (Siemens). For each condition, colonies in triplicate dishes were counted by two separate individuals independently.
Immunoblot analysis
Protein lysates were prepared using cell lysis buffer (Cell Signaling) supplemented with 2 mM PMSF. PDX and tumor protein lysates were prepared by pulverizing frozen tumor pieces in liquid nitrogen, using a mortar and pestle, and then disrupting the cells in lysis buffer supplemented with 2 mM PMSF. All protein lysates were incubated for 30 min on ice with frequent vortexing before being cleared by centrifugation for 20 min at 4°C. Bradford reagent was used to measure protein concentrations. Then, 50 μg cell lysates were loaded into Bis-Tris gels (Invitrogen) and transferred onto PVDF membranes (Millipore). Membranes were blocked with 5% non-fat milk in TBST for 1 hour and then incubated with primary antibodies at 4°C overnight. After washing three times in TBST, membranes were incubated with secondary antibody at room temperature for 1 hour, and further washed three times.
Primary tumors/patient-derived xenografts (PDXs)
Tumor specimens were obtained from 27 primary pleural MM patients whose tumors were surgically resected at Icahn School of Medicine at Mount Sinai, New York, NY. Samples were obtained through an Institutional Review Board approved study (HSM#−00135) and MTA agreement AGR-12930. Patient background information for these samples is summarized in Supplementary Table 1. Samples were immediately frozen and stored for molecular studies. In some cases, a portion of the tumor was placed in cold, sterile transport medium (F12 media with no serum, but containing Pen/Strep, nystatin, fungizone, ciprofloxacin, and gentamycin) and transferred to Fox Chase Cancer Center to establish PDXs in NSG mice using standard techniques. Tissues were minced using disposable scapels and suspended in 200 μl of a 1:1 mix of Matrigel:RPMI medium per injection site. The suspensions were injected subcutaneously in both flanks of NSG mice. After the first passage in mice, a portion of each of three tumor specimens was frozen for molecular studies.
Cell lines and cell culture
Most of the pleural MM cell lines used in this study were generated as previously reported (32). Other MM lines included NCI2052 (RRID:CVCL_1518), NCI2452 (RRID:CVCL_1553) and MSTO-211H (CLS Cat# 300450/p759_MSTO-211H, RRID:CVCL_1430), which were obtained from ATCC (Manassas, VA). H-MESO-1 (H-Meso) cells (RRID:CVCL_5760) were a kind gift of Dr. Frank R. Reale (U. Massachusetts). NM311A primary mesothelial cells were obtained from Zenbio (Research Triangle Park, NC). All MM cell lines were periodically screened for mycoplasma by our Cell Culture Facility by transferring supernatant from cell cultures to indicator cultures of Vero monkey kidney cells and then staining for cytoplasmic DNA (mycoplasma) using Hoechst dye and fluorescence microscopy. For experiments conducted in this study, M8, M12, M17, M22, M29, M49, M217, and MSTO-211H were tested for mycoplasma infection using a LookOut Mycoplasma PCR Detection Kit following the manufacturer’s instructions (Sigma, MP0035). All cell lines tested were negative for mycoplasma contamination. Cells were maintained in RPMI1640 medium supplemented with 10% FBS containing 100 μg/mL penicillin and streptomycin and 2 mM L-glutamine.
HEK293T cells (ATCC Cat# CRL-3216, RRID:CVCL_0063) were maintained in DMEM with the same supplements. To prepare lentivirus, pWPI containing a GFP marker, psPAX2, and pMD2.G plasmids were co-transfected into HEK293T cells using lipofectamine2000 (ThermoFisher Scientific), and supernatant was collected after 24 hours. MM cells in 6-well plates were infected by lentivirus in medium containing polybrene (8 μg/mL), and then the plates were centrifuged at 2000 rpm for 2 hours. The infected cells were placed in a 37ºC incubator for an additional 4 hours, the supernatant was removed, and then fresh medium was added. After incubating for an additional 24–48 hours, various assays were performed. To determine the infection efficiency of MM cell lines M8, M12, M22 and MSTO-211H, we infected each of the lines with a pWPI lentivirus that expresses GFP alone or with a pWPI lentivirus that expresses both GFP and either RIPK3 WT or RIPK3-KD. Infected cells were examined by fluorescence and brightfield microscopy (Supplementary Figure 1A, B), and immunoblotting demonstrated abundant levels of RIPK3 WT or RIPK3-KD in infected cells (Supplementary Figure 1C). MM cell lines were treated with 30 μM 5-Aza-dC for 4 days, and then RNA was collected for semi-quantitative RT-PCR analysis of RIPK3 expression. Expression of RIPK3 was also determined by semi-quantitative RT-PCR, quantitative real-time PCR (qRT-PCR), and immunoblot analyses after treatment of MM cell lines with 2.5 μM, 10 μM or 30 μM 5-Aza-dC alone for 7 days or with 2.5 μM, 10 μM or 30 μM 5-Aza-dC for 6 days with TSA for the final 24 hours (7 days total).
Flow cytometry analysis
Flow cytometry (FACS, BD Biosciences) was used to analyze cell death markers. Annexin V-FITC and Propidium Iodide (PI) were purchased as a kit from BD Pharmingen (556420). Live cells were stained with these two dyes and analyzed by FACS using FlowJo, RRID:SCR_008520 software.
Quantitative real-time PCR (qRT-PCR)
RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Ambion) and a mixture of anchored oligo-dT and random decamers (IDT). Two reverse-transcription reactions were performed for each sample using either 100 or 25 ng of input RNA in a final volume of 50 μl. Taqman or SYBR Green assays were used in combination with Life Technologies Universal Master mixes and run on a 7900 HT sequence detection system (Life Technologies). Cycling conditions were 95°C for 15 min, followed by 40 (two-step) cycles (95°C, 15 s; 60°C, 60 s). Fold changes in gene expression were calculated using the delta‐delta Ct method and with IPO8 as the normalization control. MM cell line M49, which has high RIPK3 expression, was used as a control. The primer sequences for RIPK3 were as follows: forward primer: GAGCCAAATCCAGTAACAGGG and reverse primer: GTCTGTTGCATAGTCAAGTAGTTGTTGT.
Semi-quantitative reverse transcriptase PCR (RT-PCR) analysis
Total RNA was extracted with Trizol (ThermoFisher Scientific) following the manufacturer’s instructions, and RNA quality was determined using a Bioanalyzer (Agilent). For semi-quantitative RT-PCR analysis, 1 μg of total mRNA was retrotranscribed into cDNA from a subset of MM cell lines and NM311A normal mesothelial control cells (Zen-Bio, Inc.). Reverse-transcription was performed at 42°C with SuperScript™ II Reverse Transcriptase (ThermoFisher Scientific). To verify the RNA expression of RIPK3, RIPK1, p21 (CDKN1A), DNMT1 and GAPDH, we performed PCR using REDTaq (Sigma) and specific primer sets, with the primer sequences shown in Supplementary Table 2.
To clone full-length RIPK3, the following primers were used: RIPK3 forward (5’- ATGTCGTGCGTCAAGTTATGGC-3’) and RIPK3 reverse (5′- TTATTTCCCGCTATGATTATACCAACCC-3’). PCR was performed using Turbo PFU (Agilent), with annealing temperature at 58°C. For all semi-quantitative RT-PCR experiments, the PCR products were run out on 2% ethidium bromide gels.
RIPK3 Promoter Methylation Analysis
A MethylCollector Ultra kit (Active Motif, Carlsbad, CA) was used to enrich for methylated-CpG DNA. In brief, His-tagged MBD2b/MBD3L1 recombinant proteins were used to bind methylated CpGs of genomic DNA fragments. Magnetic beads were used to capture the protein-DNA complexes, and an NGS library (NGSadmix, RRID:SCR_003208) was made from the enriched DNA for sequencing of the RIPK3 promoter region. FASTQ files of the methyl-capture sequencing of the RIPK3 promoter were uploaded to GEO (accession GSE158768).
Bioinformatics analyses
For data from the TCGA data set, gene expression, methylation, mutation, DNA copy number and patient clinical data including overall survival (OS) were obtained from the Broad Institute Firehose pipeline (Data version 2016_01_28) (http://firebrowse.org). The normalized mRNA expression data in RSEM format was transformed (log) and categorized into 3 classes: a) downregulated (Low): expression data < first quartile; b) up-related (High): expression data > third quartile; and c) no change (NC): mRNA expression between first and third quartiles. In the case of methylation data, promoter annotations were obtained using the Illumina Human Methylation 450k Bioconductor package (Bioconductor, RRID:SCR_006442). To summarize the methylation data, we obtained median β-values by grouping probes mapped to promoter CpG shores and first exon. These values were categorized based on mRNA expression categories as described above. Spearman rank test was used to calculate the correlations between summarized β-values and mRNA expression. For survival analyses, the OS between Low and High expression categories was evaluated using Kaplan-Meier methods. OS was derived based on vital status and ‘days to death’ from initial pathologic diagnosis and days to last follow up. Survival curves were compared using the log-rank test. All calculations were done in the R programming environment using the ‘survival’ package.
Chemosensitization and cell viability assays
To assess the effect of RIPK3 expression on sensitivity to chemotherapeutic drugs, RIPK3-negative MM cell lines MSTO-211H and M12 and, as controls, RIPK3-positive lines M49 and M217 were treated or not with 5 or 10 μM 5-Aza-dC for 6 days, the final 2 days combined with either doxorubicin (0, 0.625 or 1.25 μM) or cisplatin (0, 6.25 or 12.5 μM). Cell viability was determined via MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega) per the manufacturer’s instruction. In a subsequent experiment, RIPK3 cDNA was ectopically re-expressed via lentiviral transduction (WPI-RIPK3 vs. WPI control virus) in RIPK3-negative MM cell lines MSTO-211H, M8, M12, and M17, with preparation and infection by lentivirus as described above. Twenty-four hours post-transduction, WPI control and RIPK3-expressing MM cells were seeded on 96-well plates (2000 cells/well), and 24 hours after seeding, the cells were treated with cisplatin (0, 12.5 or 25 μM) for 48 hours, and then cell viability was assessed by MTS assay. In a third experiment, MSTO-211H, M8, M12 and M17 were infected with control or RIPK3-expressing lentivirus, and 24 hours after infection, the cells were seeded on a 96-well plate (2000 cells/well). Cell viability was determined after 48 hours via MTS and LDH release assays per the manufacturer’s instructions (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega).
Cell viability assays
For each human MM cell line tested, cells were seeded onto 96-well plates at a density of 3,000 cells per well. 24 h after seeding, MM cell lines were treated with cisplatin (2.5 μM, 5 μM or 10 μM), doxorubicin (0.156 ηM, 0.321 ηM and 0.625 ηM) or DMSO vehicle control for 72 h. Cell viability was determined using MTS assay (G3582; Promega, Madison, WI). MTS reagent was added to each well and absorbance was determined at 490 nm as a read out of cell viability.
RIPK3 rescue experiments and imaging of necroptosis
RIPK3-negative MM cell lines MSTO-211H, M12 and M22 were trypsinized, and equivalent numbers of cells were seeded in multiple 6-well plates. 24h after seeding, the cells were pretreated for 2 h with the apoptosis inhibitor zVAD (50μM) and/or the RIPK3 inhibitor GSK’872 (5μM), and then transduced with WPI lentivirus expressing RIPK3 WT or empty vector control for 6 h at 37°C in a CO2 incubator. At that point, the media containing the virus and polybrene was aspirated and was replaced with media containing DMSO, the apoptosis inhibitor zVAD and/or the RIPK3 inhibitor GSK’872 for 24 h, 48 h, or 4 d. After drug treatment for 24 h, samples in one set of plates were collected for immunoblot analysis to assess RIPK3 re-expression and expression of total MLKL, p-MLKL, and GAPDH. At 48 h after beginning treatment with inhibitors, a second set of corresponding plates was trypsinized to assess cell viability, using a hemocytometer and trypan blue solution (T8154, Sigma) to calculate the percentage of viable versus dead cells in each well. This experiment was performed in triplicate. The third set of plates was used to performed a clonogenic growth experiment. After day 4 from the beginning of the experiment, a third set of corresponding plates was fixed and stained using a Diff-Quik™ stain kit (Siemens, Munich Germany) to access rescue of cell death caused by necroptosis.
Immunofluorescence was also performed 24h after beginning treatment of MSTO-211H cells with zVAD and/or GSK’872. These cells were then seeded and transduced with WPI lentivirus expressing RIPK3 WT or WPI alone for 6 h at 37°C in a CO2 incubator on sterile 8-well glass slides (Millicell EZ Slides, PEZGS0896, Sigma). After 24h, the cells were washed 3 times in HBSS buffer and stained with Sytox Green at a final concentration of 167 ηM in HBBS for 20 min while protected from light (SYTOX Green Nucleic Acid Stain, 5 mM solution in DMSO, S7020, ThermoFisher, 1:30000). After the incubation, the staining solution was removed and the cells were washed 3 times in HBSS buffer and then fixed with freshly-prepared 4% (w/v) paraformaldehyde for 10 min, permeabilized in 0.1% (v/v) Triton X-100 for 15 min, blocked with MAXblock Blocking Medium (Active Motif) for 1 h at 37°C, and incubated overnight with primary antibodies at 4°C. After three washes in PBS, slides were incubated with fluorophore-conjugated secondary antibodies for 1 h at room temperature. Following an additional three washes in PBS, slides were mounted in ProLong Gold antifade reagent (ThermoFisher) and imaged by confocal microscopy on a Leica SP8 (Wetzlar, Germany). Primary antibodies were used at the following dilutions for immunofluorescence studies: phosphorylated human MLKL (phospho-S358, EPR9514, Cat# ab187091, RRID:AB_2619685,1:500), anti-alpha 1 Sodium Potassium ATPase (Cat# ab7671, RRID:AB_306023; 1:500), each from Abcam.
Knockdown of MLKL
Lentivirus plasmids expressing shMLKL were purchased from SIGMA, and the sequences are shown in Supplementary Table 3. To prepare lentivirus expressing shMLKL and shGFP control plasmids, psPAX2 and pMD2.G plasmids were co-transfected into HEK293T cells using lipofectamine 2000 (ThermoFisher Scientific), and supernatant was collected after 24 h. MM cell lines MSTO-211H, M12, and M22 were each seeded in 6-well plates and infected with lentivirus in medium containing polybrene (8 μg/mL), and then the plates were centrifuged at 2000 rpm for 2 h. The infected cells were placed in a 37ºC incubator for an additional 4 h, the supernatant was removed, and then fresh medium was added. After 48 h, the transduced MM cell lines were selected with puromycin (2 μg/ml) for 72h. Stable cells expressing shMLKL or shGFP were then infected with WPI or WPI expressing RIPK3. Immunoblotting and cell viability were assessed at 48 h as described above.
Results
RIPK3 loss of expression is a frequent finding in tumor cells from MM patients
Necroptosis, one of the major forms of cell death, is executed by the necrosome. To investigate the role of necroptosis in MM, we initially examined the expression of the key components of the necrosome complex: RIPK1, RIPK3, caspase 8, FADD, and MLKL. Cell lysates from 36 human pleural MM cell lines were subjected to immunoblotting. Compared with normal primary human mesothelial cells, NM311A, only 9 (25%) MM cell lines retained expression of RIPK3, 4 of which had relatively low expression levels (Fig. 1A; Supplementary Fig. 2). In contrast, only two MM cell lines expressed little or no MLKL, and all cell lines showed expression of RIPK1, caspase 8, and FADD. These data suggest a specific selective pressure favoring loss of RIPK3 expression in MM cells. We further found that the MM cells without RIPK3 protein expression also had very low or undetectable RIPK3 transcript levels as shown by semi-quantitative RT-PCR analysis (Fig. 1B). Note that hereafter we refer to these cells as RIPK3-negative MM cell lines. Many of the same MM cell lines reported here were previously evaluated for DNA copy number imbalances, using Affymetrix SNP mapping arrays, and no homozygous losses in the vicinity of the RIPK3 locus at chromosome band 14q12 were observed, although ~30% of the cell lines showed heterozygous deletions encompassing this gene (32). Western blotting did not show any specific band for RIPK3 in any MM cell lines, even upon long film exposure. We used multiple sets of primer pairs that covered the full length RIPK3 mRNA transcript, and we could not amplify any region of the mRNA in several MM cell lines tested that did not express RIPK3, indicating that RIPK3 was completely silenced in these cell lines. Moreover, RNA-seq analysis detected no or extremely low levels of RIPK3 transcript in these cells compared with the RIPK3-positive cell lines M49, M29, M217, and M34 or with primary human normal mesothelial cells. Moreover, no RIPK3 fusion transcripts were detected using the RNA-fusion pipelines Arriba and JAFFA. Moreover, an interrogation of the TCGA database revealed no pathogenic mutations and only 1 missense mutation of unknown significance in RIPK3 out of 87 MMs studied.
Figure 1.
RIPK3 expression is frequently down regulated or undetectable in human MM cells, primary tumors and PDXs from pleural MM patients. A, Immunoblot analysis of RIPK1, RIPK3, MLKL, β-actin, FADD, caspase 8, NF2/Merlin, BAP1 and p16INK4A protein expression in a panel of human pleural MM cell lines. B, Semi-quantitative RT-PCR analysis (ethidium bromide gel) demonstrating transcriptional down regulation of RIPK3 mRNA in human MM cell lines. In both immunoblot and semi-quantitative RT-PCR analyses, the human mesothelial cell line NM311A was used as a normal control. C, Immunoblot analysis of primary human pleural MM specimens showing down regulated or absent expression of RIPK3 in 13 of 27 (48%) samples and of RIPK1 in 10 of 27 (37%) samples. Separate blots shown below depict expression of BAP1 and NF2 tumor suppressors in the same set of tumors for comparison.
We next examined the expression of RIPK3 in 27 primary pleural MM samples. Altogether, 13 of 27 (48%) primary tumors showed very low or undetectable levels of RIPK3 protein expression (Fig. 1C). We also examined expression of various MM-related tumor suppressors, including BAP1, NF2, p16INK4A, p14ARF, and p15INK4B in the same sets of MM tumors and MM cell lines (Fig. 1A, C; Supplementary Figs. 2 and 3A). No obvious correlation was observed between loss of expression of RIPK3 or RIPK1 and loss of expression of any of the tumor suppressors tested in our primary MM tumors, PDXs, or MM cell lines.
One possible reason for the lower percentage of RIPK3 loss in primary tumors compared to tumor-derived cell lines may be the presence of RIPK3-expressing stromal cells in the tissue specimens. Given that the antibodies we tested did not work for immunohistochemistry, we could not test this possibility. We also examined the expression of RIPK3 and RIPK1 in four patient-derived xenografts (PDXs) that we established from human pleural MMs, and we found that all four PDXs had extremely low or undetectable levels of RIPK3 protein expression (Supplementary Fig. 3B). Two of the PDX samples also had undetectable levels of RIPK1 protein expression and two others showed reduced levels of expression (Supplementary Fig. 3B). Thus, all four PDXs would be expected to have impaired necroptosis signaling.
RIPK3 is silenced in MM cells via DNA methylation
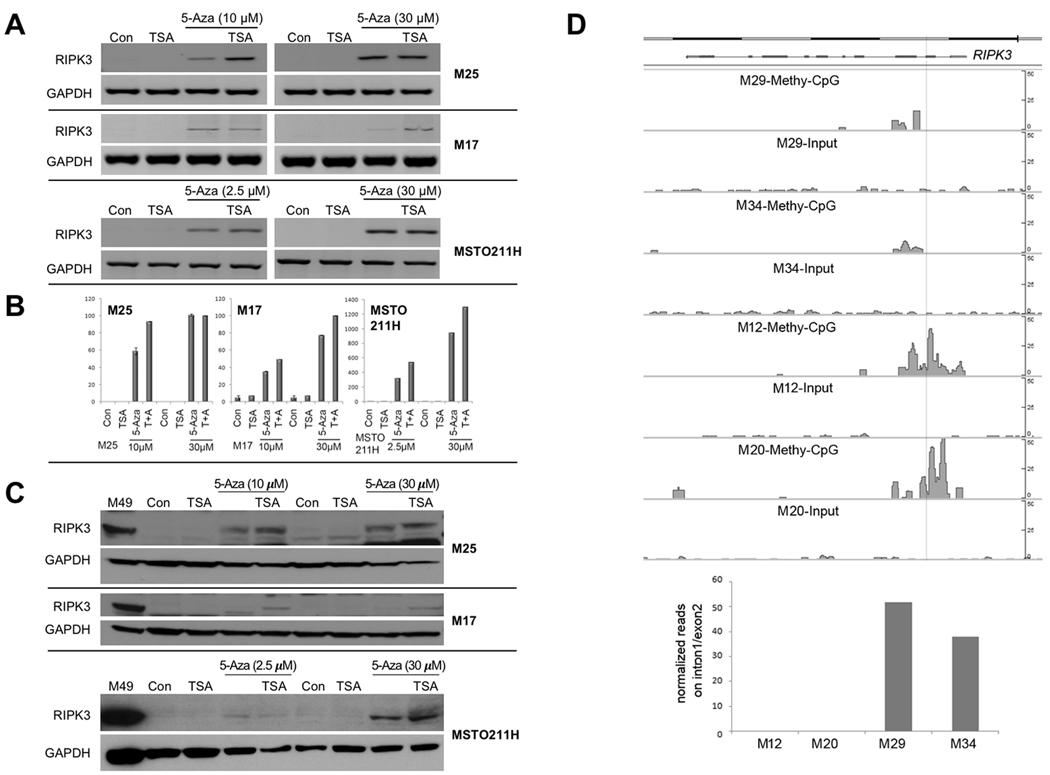
To test the possibility that downregulation of RIPK3 in MM cells occurs by epigenetic silencing, RIPK3-negative MM cell lines were treated with 30 μM of the DNA methylation inhibitor 5-Aza-dC for 4 days, followed by semi-quantitative RT-PCR analysis. The DNA methylation inhibitor strongly restored expression of RIPK3 in 13 of 13 (100%) RIPK3-negative MM cell lines tested, 5 of which are shown in Supplementary Fig. 4. Since the tumor suppressor p21WAF1 (CDKN1A) is frequently silenced in tumor cells (33), we used it here as a positive control for the reversion of promoter methylation by 5-Aza-dC. The histone deacetylase (HDAC) inhibitor trichostatin A (TSA) was able to potentiate the effect of 5-Aza-dC as shown by semi-quantitative RT-PCR (Fig. 2A) and Real-Time PCR (Fig. 2B) analyses (6 days with 5-Aza-dC followed by 1 day of TSA treatment). Restored expression of RIPK3 protein was also detected via immunoblotting by treating RIPK3-negative MM cells with 5-Aza-dC in combination with TSA (6 days with 5-Aza-dC followed by 1 day of TSA treatment) (Fig. 2C), the latter having an additive effect with 5-Aza-dC in reactivating expression of RIPK3.
Figure 2.
Treatment of MM cells with DNA methylation and/or histone deacetylase inhibitors results in restoration of RIPK3 expression. A-C, The expression of RIPK3 was also determined after treatment of MM cell lines with 2.5 μM, 10 μM or 30 μM 5-aza-2’-deoxycyditine alone for 7 days or 2.5 μM, 10 μM or 30 μM 5-aza-2’-deoxycyditine for 6 days with TSA for the final 24 hours (7 days total), followed by semi-quantitative RT-PCR (A), Real-Time PCR (B) and immunoblot analysis (C). In panel B, the Y-axis refers to relative RIPK3 mRNA expression in treated samples compared to control. Note that the Y-axis in the two graphs on the left and the one on the right differ, because the RIPK3-negative MM cell lines we tested showed variable levels of re-expression of RIPK3 following treatment with 5-aza-2’-deoxycyditine, possibly related to differences in degree of global methylation and/or chromatin compaction. D, RIPK3-positive cell lines (M29 and M34) and RIPK3-negative cell lines (M12 and M20) were used to study DNA methylation. Genomic DNA fragments with methylated-CpG were enriched with MBD2b/MBD3L1 and subjected to NGS and analyzed using MACS2 peak calling pipeline. Both RIPK3-negative cell lines show strong methylated-CpG peaks in RIPK3 intron1 and exon2, whereas both RIPK3-positive cell lines do not. The pre-captured DNAs serve as negative controls (Input). Histograph depicts fold changes of the methylated-CpG peaks among the cell lines studied.
To provide direct evidence that the promoter region of the RIPK3 gene is highly methylated in the RIPK3-low expressing MMs, we assessed the methylation status of the promoter region of the RIPK3 gene in two RIPK3-positive and two RIPK3-negative cell lines. RIPK3-negative cell lines showed highly methylated-CpG peaks in RIPK3 intron1 and exon2, whereas RIPK3-positive cell lines did not (Fig. 2D).
DNA methyltransferase DNMT1 suppresses RIPK3 transcription
RIPK3-negative cell lines M12, M22 and MSTO-211H (Fig. 1A; Supplementary Fig. 5) were transfected with two separate siRNAs against DNMT1 or an siControl, and DNMT1 protein levels were determined by immunoblot analysis 72 hours after transfection. Both siDNMT1_1 and siDNMT1_2 resulted in no detectable DNMT1 protein expression (Supplementary Fig. 5A). Semi-quantitative RT-PCR analysis revealed that knockdown of DNMT1 with either siRNA tested led to modest re-expression of RIPK3 mRNA (Supplementary Fig. 5B), suggesting that DNMT1 at least partially contributes to RIPK3 silencing.
Restoration of RIPK3 expression inhibits clonogenicity and triggers cell death in RIPK3-negative MM cells
To test the hypothesis that RIPK3-mediated cell death has a tumor suppressor role, we re-expressed RIPK3 in MM cells lacking endogenous expression of this gene. Human MM cells were nucleofected with pEGFP-N1, wild-type human pEGFP-RIPK3 and pEGFP-RIPK3-D160N (RIPK3-KD) plasmids. At 48 hours post-nucleofection, the human MM cells were seeded for clonogenic assays and selected with G418 for 3 weeks. MM cells nucleofected with wild type RIPK3 failed to form colonies compared to control EGFP-nucleofected cells (Fig. 3A, B). However, mutant RIPK3-KD was unable to greatly inhibit colony formation, suggesting that RIPK3 kinase activity is required to block colony formation (Fig. 3A, B). To determine if the programmed necrosis is initiated directly by RIPK3, we then tested whether there was activation of the direct RIPK3 target, MLKL. Western blotting demonstrated phosphorylation of MLKL in the wild type RIPK3-nucleofected MM cells but not in the control or the RIPK3-KD-nucleofected cells (Fig. 3C).
Figure 3.
RIPK3 suppresses clonogenic growth of MM cells. A, RIPK3-negative cells were nucleofected with pEGFP, pEGFP-RIPK3-WT or pEGFP-RIPK3-KD, and clonogenic assays were then performed. B, Quantification of colonies was performed in triplicate with error bars indicated. C, Immunoblot analysis of RIPK3, p-MLKL, MLKL (54 kDa) and GAPDH in nucleofected MM cells. The cells used for immunoblotting in panel C were harvested 48 h after nucleofection; and the remaining cells were seeded on dishes and selected in medium containing G418 and allowed to form the colonies shown in panel B.
Re-expression of RIPK3 induces necrosis in RIPK3-negative MM cells and RIPK3 inhibition rescues cell death induced by necroptosis
To further investigate if cell death caused by restoration of RIPK3 was indeed due to necroptosis, we next carried out an apoptosis/necrosis assay. Lentivirus harboring RIPK3 WT, RIPK3-KD or empty virus was used to transduce MM cells at MOI=5 via a spin-infection method. 48 h after lentiviral infection, MM cells were photographed (Fig. 4A) and stained with the apoptotic marker Annexin V and the necrosis marker PI for flow cytometry analysis. RIPK3-WT-infected MM cells had markedly less cells and more floating, dead, cells per dish than RIPK3-KD-infected or control-infected dishes (Fig. 4A). MM cells transduced with wild type RIPK3 also exhibited necrotic cell death as demonstrated by the presence of a PI-only cell population, whereas MM cells transduced with RIPK3-KD or control MM cell lines did not (Supplementary Fig. 6A, B). To investigate if the cell death caused by the restoration of RIPK3 is indeed due to necroptosis, we performed multiple experiments. The RIPK3-negative MM cell lines MSTO-211H, M12, and M22 were each transduced with WPI lentivirus expressing RIPK3 WT or empty vector and treated with the apoptosis inhibitor zVAD and/or the RIPK3 inhibitor GSK’872 for 24 h, 48 h, and 4 d. As shown in Figure 4B, immunoblot analysis performed at 24 h revealed that re-expression of RIPK3 induced activation of MLKL, the effector of the necrosis, which was rescued by treatment with GSK’872. Re-expression of RIPK3 causes cell death and phosphorylation of MLKL in MM cell lines transduced and treated with DMSO or zVAD alone. The cell death caused by the re-expression of RIPK3 was rescued only when the cells were treated GSK’872 alone or in combination with zVAD and no p-MLKL was observed, consistent with cell death occurring via necroptosis. At 48h, a trypan exclusion assay revealed that when MSTO-211H cells were transduced with WPI lentivirus expressing wild type RIPK3, greater than half of all infected cells died. However, when MSTO-211H cells transduced with RIPK3 were treated with GSK’872 alone or in combination with zVAD, no dead cells were detected. Also, a 4-d clonogenic growth experiment demonstrated that only the cells transduced and treated with GSK’872 alone or combined with zVAD were able to rescue necrosis and form colonies (Fig. 4B). Moreover, fluorescence microscopy demonstrated the spatial activation of MLKL, which was rescued by the RIPK3 inhibitor GSK’872, but not by the apoptosis inhibitor zVAD (Fig. 4C). Similar results were obtained with the other RIPK3-negative MM cell lines tested, M12 and M22, as shown in Supplementary Fig. 7A, B.
Figure 4.
Restoration of RIPK3 expression triggers necroptosis in RIPK3-negative MM cells, and RIPK3 inhibition rescues the cell death caused by necroptosis. A, Lentivirus expressing RIPK3 WT, RIPK3-KD, or empty vector only was used to transduce RIPK3-negative MM cells at MOI=5, and light microscopy photographs depict representative transduced MM cells 48 h after transduction. B, RIPK3-negative MM cell line MSTO-211H was transduced with WPI lentivirus expressing RIPK3 WT or empty vector and treated with the apoptosis inhibitor zVAD and/or the RIPK3 inhibitor GSK’872 for 24 h, 48 h, and 4 d. Results of immunoblot analysis (24 h) and cell viability assays (48 h), and clonogenic growth (4 d) are shown. A hemocytometer and trypan blue dye exclusion test was used to determine cell viability. Expression of RIPK3 induced activation of MLKL loss of cell viability, which was rescued by treatment with GSK’872. C, Fluorescence microscopy of MSTO-211H cells demonstrating spatial activation of MLKL, which was rescued by the RIPK3 inhibitor (R3i) GSK’872. D, Transduction of MSTO-211H cells with lentivirus expressing three different shMLKL or shGFP and then selected for 72 h with puromycin. Stable cells expressing shMLKL or shGFP were then infected with WPI or WPI expressing RIPK3. Cell viability assays and immunoblotting were performed after 48 h to detect the activation of MLKL and proportions of alive vs. dead cells. Expression of RIPK3 induced necroptosis, which was rescued by knockdown of MLKL.
We also performed separate experiments, performed in triplicate, in which stable MSTO-211H cells expressing shMLKL or shGFP were infected for 48 h with WPI or WPI expressing RIPK3. Cells transduced with RIPK3 and shGFP expressed abundant p-MLKL, and ~40% of the cells were dead (Fig. 4D). Cell death was markedly decreased in cells expressing both shMLKL and RIPK3 (consistently ≤ 15% dead cells observed with any one of the three different shRNA against MLKL, ~5% in the experiment shown in Fig. 4D). Similar results were obtained with other RIPK3-negative MM cell lines tested (Supplementary Fig. 7A–D).
Treatment with 5-Aza-dC or re-expression of RIPK3 sensitizes RIPK3-negative MM cells to therapeutic drugs
To assess whether RIPK3 status correlates with chemosensitivity in MM cells, we treated 2 RIPK3-positive and 2 RIPK3-negative MM cell lines with either 5 or 10 μM 5-Aza-dC for 4 days followed by treatment for an additional 2 days with 5-Aza-dC plus either doxorubicin or cisplatin, and then MTS assays were performed to assess cell viability. Semi-quantitative RT-PCR was performed to verify re-expression of RIPK3. Treatment with 5-Aza-dC sensitized RIPK3-negative MM cell lines to cisplatin and doxorubicin, whereas treatment with 5-Aza-dC showed little or no sensitizing effect on RIPK3-positive MM cell lines (Fig. 5A–C).
Figure 5.
Re-expression of RIPK3 induces necrosis and sensitizes RIPK3-negative, RIPK3 (–), MM cells to chemotherapeutic drugs. A, B, RIPK3 (–) MSTO-211H and M12 and RIPK3-positive, RIPK3 (+), cell lines M49 and M217 were treated with 10 μM of 5-Aza-dC for 4 days followed by treatment for 48 hours with 5-Aza-dC plus two different doses each of doxorubicin (0 μM, 0.625 μM, 1.25 μM) (A) or cisplatin (0 μM, 6.25 μM, 12.5 μM) (B), and MTS assays were performed to assess cell viability. Treatment with 5-Aza-dC sensitized RIPK3 (–) MM cell lines to both cisplatin and doxorubicin, whereas treatment with 5-Aza-dC showed little or no effect on RIPK3 (+) cells. Similar differences between RIPK3 (–) and RIPK3 (+) MM cell lines were observed when a lower dose (5 μM) of 5-Aza-dC was used (not shown). C, Semi-quantitative RT-PCR analysis of RIPK3 RNA expression in MM cell lines treated with or without 10 μM 5-Aza-dC for 6 days (end date of the chemosensitization assays). Expression of GAPDH was used as a loading control. D, Immunoblot analysis of RIPK3 expression 24 post-infection with control WPI or WPI-RIP3K expressing lentivirus. M8, M12, M17 and MSTO-211H cells were transduced with control WPI or WPI-RIPK3 lentivirus for 24 hours before being harvested for immunoblot analysis as well as seeding for chemosensitization and cell viability assays. RIPK3, P-MLKL, and total MLKL levels were determined to demonstrate re-expression of RIP3K and induction of necrosis. GAPDH levels are shown as a loading control. E, Re-expression of RIP3K sensitizes MM cells to cisplatin. RIPK3-negative MM cell lines were transduced as above for 24 hour and then seeded on a 96-well plate; 24 hours later the cells were treated with 0, 12.5 or 25 μM cisplatin for 48 hours. Cell viability was determined using MTS assay.
To confirm that re-expression of RIPK3 induces necroptosis and potentially sensitizes RIPK3-negative MM cells to cytotoxic drugs, RIPK3-expressing lentivirus was used to infect RIPK3-negative MM cells. In multiple RIPK3-negative MM cell lines tested, ectopic expression of RIPK3 cDNA resulted in reduced cell viability based on MTS assay, as well as increased lactose dehydrogenase (LDH) release, the latter indicative of necrosis (Supplementary Fig. 8). Upregulation of phospho-MLKL, indicative of necroptosis, was also documented upon RIPK3 re-expression (Fig. 5D). In additional to causing necroptosis, expression of RIPK3 cDNA also caused sensitization of RIPK3-negative MM cells to cisplatin (Fig. 5E).
To determine if these differences in drug sensitivity are indicative of a general correlation between RIPK3 levels and chemosensitivity, we treated 15 MM cell lines (12 RIPK3-negative and 3 RIPK3-positive) with several different doses of either cisplatin or doxorubicin, and indeed there was an overall trend toward greater chemoresistance among the MM cell lines with loss of RIPK3 expression (Supplementary Fig. 9A, B). However, our data set was underpowered with regard to the number of cell lines that retained expression of this kinase, as only 3 of 5 RIPK3-positive MM cell lines grew well in culture.
Loss of RIPK3 is associated with a poor prognosis in MM patients
In order to investigate the prevalence of RIPK3 promoter methylation and its association with expression and OS among MM patients, the genomic and survival outcomes data from 87 MM patients available from The Cancer Genome Atlas (TCGA) repository was analyzed. While there are no predicted deleterious mutations or copy-number alterations identified in this cohort of patients, there is frequent promoter CpG methylation (β-value ≥ 0.2) of RIPK3 (22%) (Fig. 6A). Further investigation to evaluate the relationship between methylation of CpG islands in the promoter region of RIPK3 and its corresponding mRNA expression revealed a negative correlation (Spearman rank correlation = −0.5; p < 0.001), validating the observation in our MM cell lines (Fig. 6B). While profiling of the MM tumor specimens was done prior to any treatment, most of the TCGA patients were treated with chemotherapeutic agents, so we compared RIPK3 mRNA expression with overall survival (OS). Interestingly, low RIPK3 mRNA expression was found to be associated with poor OS (Fig. 6C, E). MM patients with higher expression (Up) of RIPK3 mRNA showed a significantly favorable overall survival (2-year survival 72.4%; 95% CI 0.54–0.96) compared to cases with low (Down) expression (2 year survival 32.7%; 95% CI 0.17–0.6) (p= 0.008). The expression of RIPK3 did not differ significantly between histologic subtypes of MM (Kruskal-Wallis test p-value = 0.169) (Fig. 6D). Low RIPK1 mRNA expression was also observed in many of the MM tumors in the TCGA data set, and MM patients with higher expression of RIPK1 had a significantly better OS than patients with lower RIPK1 levels (Fig. 6F). Such an association (significance p < 0.05) was not observed with the expression levels of RIPK3’s downstream effector MLKL (Supplementary Fig. 10A, B) or those of caspase 8 (CASP8) (Supplementary Fig. 10C, D) and FADD (Supplementary Fig. 10E, F).
Figure 6.
Loss of RIPK3 mRNA is associated with promoter methylation and poor prognosis in pleural MM patients. Data mining was performed using TCGA pleural MM dataset. A, Box plot showing RIPK3 promoter methylation for various degrees of RIPK3 expression. X-axis shows RIPK3 expression categories partitioned into three groups: Down (lower quartile), No-change (inter quartile range, designated as No Change in expression), and Up (upper quartile); Y-axis shows methylation β-values of promoter probes. White bars in the box plot indicate median β-value, while each color dot indicates an MM patient in the respective category. B, Dot plot showing negative correlation between RIPK3 promoter methylation and RIPK3 mRNA expression (Pearson correlation coefficient r = −0.59, p-value < 0.001). Red line indicates regression, and blue dots represent individual cases. X- and Y-axes indicate log-transformed RSEM expression values and median of CpG probe β-values in promoter regions, respectively. C, Box plot of mRNA expression for RIPK3 where Y-axis indicates the log-transformed RSEM mRNA expression values for different expression categories partitioned into three groups: Down (lower quartile), No-change (inter quartile range), and Up (upper quartile). D, Box plot showing RIPK3 expression for different histologic subtypes of MM. E,. Kaplan-Meier curves indicating OS based on expression categories as shown in panel C for RIPK3 (logrank test, p = 0.01) of MM patients based on RIPK3 mRNA expression, with poor survival of MM patients having low expression of RIPK3 (blue line) compared to those with high levels (orange) or No-change levels (gray) of RIPK3 expression. F, Kaplan-Meier curves indicating OS of expression categories of MM patients based on RIPK1 mRNA expression (p = 0.001), with poor survival of MM patients having low expression of RIPK1 (blue line) compared to those with high levels (red) or No-change levels (gray) of RIPK1 expression.
Discussion
The role of RIPK3 in malignancy appears to be tissue type-dependent. In colorectal cancer, the induction of necrosis via activation of RIPK3 can eliminate apoptosis-resistant cancers (28). Similar results have been reported in acute myeloid leukemia (AML) patients treated with the second mitochondria-derived activator of caspases (Smac) mimetic BV (34). Interestingly, the AML subtypes M4 and M5 are resistant to RIPK3-mediated necrosis, and inhibition of RIPK3 augments IFNγ-induced differentiation (35). In pancreatic ductal carcinoma, RIPK1 and RIPK3 are highly expressed, and the necrosome is required to create the immune-suppressive microenvironment (36). Moreover, RIPK3 plays a role in tumor metastasis and T cell death (37). Interestingly, in primary tumors and in the few PDX samples that were tested, RIPK1 loss often accompanied RIPK3 loss, whereas in cell lines RIPK1 is mostly retained and only RIPK3 is silenced. These apparently-paradoxical findings can be explained by the observations that RIPK3 primarily has a pro-death role, whereas RIPK1 functions as both a pro-death and pro-survival molecule. Clearly, there would be some selective advantage to losing RIPK1 in vivo that is related to its pro-death functions, such as activation of apoptosis signaling downstream of cytokine receptors and TLR pathways. In cell lines, however, RIPK1 is known to supply a strong pro-survival signal via NF-kB, which we propose offsets any benefits there might be for its loss in cell culture. This strong pro-survival signal protects cultured cells from autocrine apoptotic stimuli, such as TNF-α (38). Although RIPK1 kinase activity has an essential role in classic TNF-driven necroptosis, the fact that human pleural MM cells frequently exhibit loss of expression of RIPK3 both in vitro and in primary tumors suggests a central role for RIPK3 in necrosis-related tumor suppression that is circumvented by epigenetic silencing in MM pathogenesis.
The inactivation of tumor suppressors is a hallmark of MM development and progression. Tumor suppressor inactivation in MM involves various mechanisms, including hetero- and homozygous deletions (CDKN2A) (2), biallelic inactivation via a combination of loss-of-function point mutations and loss of heterozygosity (NF2) (39) connected with monosomy 22 (40), and a combination of an inactivating point mutation and loss of one or more exons of the second allele (BAP1) (41). Thus, the main driver tumor suppressor genes identified to date in MM have been inactivated almost entirely via mutations and deletions. RIPK3 is therefore noteworthy, because it represents the first reported tumor suppressor gene in pleural MM that is inactivated via epigenetic silencing in a high percentage of cases and is associated with a potential marker of poor clinical outcome. Moreover, the fact that RIPK3 plays a role in a necroptotic pathway that is involved in asbestos-related inflammation is likely to be of particular relevance to MM pathogenesis. In the case of RIPK3, like with GPC3 (42), inactivation in MM occurs primarily via epigenetic silencing, and we found that the treatment of RIPK3-negative MM cells with 5-Aza-dC and TSA restored transcription of RIPK3 mRNA. Our data indicate that, as reported in breast cancer and glioma (25), DNMT1 can at least partially mediate hypermethylation of the RIPK3 promoter, thereby suppressing RIPK3.
Re-expression of RIPK3 in RIPK3-negative MM cells rapidly led to phosphorylation and activation of downstream MLKL, accompanying by widespread MM cell necroptosis. RIPK3-negative MM cells appear to be inherently very sensitive to the re-expression of RIPK3, which strongly activates the necroptosis pathway. We speculate that simple ectopic expression of RIPK3 activates necroptosis in RIPK3-negative cells, because these cells have become addicted to the loss of RIPK3 expression, perhaps because they are obligatorily reliant on autocrine TNF signaling (or some similar pathway) that supplies a necessary pro-survival signal, but will also activate necroptosis unless RIPK3 signaling is inactivated. As numerous pathways, including those initiated by TLRs, other innate-immune sensors, and the cytokines TNFα and interferons, can activate RIPK3 (43), the mechanism by which RIPK3 activation occurs in neoplasia remains an intriguing question. Specifically, knowing what kind of carcinogenic stimulus serves to activate RIPK3 would provide fundamental insights about MM biology. In this regard, asbestos, a known carcinogen causally linked to MM (44), is a potent activator of the NLRP3 inflammasome (45). Recent findings indicate that activation of NLRP3, in certain settings, is also accompanied by activation of RIPK3 (46,47). Speculatively, silencing of RIPK3 in MM cells may represent a prerequisite for tumor pathogenesis. It is also possible that exposure of macrophages to asbestos triggers production of pro-inflammatory cytokines, most notably TNFα and IL-1β, which could drive necroptosis in the neighboring mesothelium and thereby provide the selection pressure necessary for RIPK3 inactivation.
Our findings support a role for RIPK3 as a tumor suppressor in MM cells and suggest that loss of expression of RIPK3 may contribute to the generally very poor OS characteristic of this disease, potentially by impairing therapy-induced necroptosis. Indeed, the fact that the MM patients in the TCGA cohort with higher mRNA expression of RIPK3 (and RIPK1) had a better OS than the patients with lower expression levels is consistent with our experimental in vitro findings that epigenetic silencing of RIPK3 impairs necroptosis and contributes to chemoresistance. Moreover, our data indicate that treatment of RIPK3-deficient MM cells with 5-Aza-dC or ectopic expression of RIPK3 sensitizes RIPK3-negative MM cells to chemotherapy. Furthermore, it will be important to determine whether other DNA and/or histone methyl transferases also regulate RIPK3 expression in MM—a line of inquiry we will focus on in future investigations.
Supplementary Material
Translational Relevance.
Although loss of expression of necroptosis signaling components has been reported in several human cancers, whether RIPK3-mediated necroptosis can serve as a gatekeeper to protect against tumor development is largely unknown. We report that reduced RIPK3 expression due to promoter methylation positively correlates with worse clinical outcome in MM patients. Mechanistically, we demonstrate that restoration of RIPK3 expression in MM cells triggers necroptosis and sensitization to chemotherapeutic agents, suggesting that RIPK3 acts as a tumor suppressor in mesothelial cells and that its silencing in MM may contribute to poor survival in this disease by inhibiting therapy-induced necroptosis. Thus, RIPK3 expression may limit MM development and progression, and represents a novel prognostic marker for this disease.
Acknowledgments
This work was supported by NCI grant CA190542 to J.R. Testa and S. Balachandran. Also supported by NCI grant CA06927 and an appropriation from the Commonwealth of Pennsylvania to Fox Chase Cancer Center. Other support was provided by a gift of the Local #14 Mesothelioma Fund of the International Association of Heat and Frost Insulators and Allied Workers (to J.R. Testa).
The authors thank the Flow Cytometry, Genomics, Tissue Culture and Biostatistics and Bioinformatics Facilities of Fox Chase Cancer Center for assistance. We thank Dr. Emmanuelle Nicolas for assistance with the Real-Time PCR analysis.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest with regard to this work.
References
- 1.Kadariya Y, Menges CW, Talarchek J, Cai KQ, Klein-Szanto AJ, Pietrofesa RA, et al. Inflammation-related IL1beta/IL1R signaling promotes the development of asbestos-induced malignant mesothelioma. Cancer Prev Res 2016;9:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng JQ, Jhanwar SC, Klein WM, Bell DW, Lee W-C, Altomare DA, et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res 1994;54:5547–51. [PubMed] [Google Scholar]
- 3.Bianchi AB, Mitsunaga S-I, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A 1995;92:10854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264–9. [DOI] [PubMed] [Google Scholar]
- 7.Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407–16. [DOI] [PubMed] [Google Scholar]
- 8.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nature Rev Cancer 2016;16:20–33. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Ann Rev Biochem 2004;73:87–106. [DOI] [PubMed] [Google Scholar]
- 10.O’Kane SL, Pound RJ, Campbell A, Chaudhuri N, Lind MJ, Cawkwell L. Expression of bcl-2 family members in malignant pleural mesothelioma. Acta Oncol 2006;45:449–53. [DOI] [PubMed] [Google Scholar]
- 11.Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 2017;546:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015;517:311–20. [DOI] [PubMed] [Google Scholar]
- 13.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012;148:213–27. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Yang Y, He W, L. S. Necrosome core machinery: MLKL. Cell Mol Life Sci 2016;73:2153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, et al. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ 2014;21:1600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, Ingram JP, et al. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe 2016;20:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A 2014;111:7753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep 2012;1:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011;471:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014;513:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 2014;157:1189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014;157:1175–88. [DOI] [PubMed] [Google Scholar]
- 23.Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 2015;25:707–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Zhai L, Ma S, Zhang C, Zhao L, Li N, et al. Down-regulation of RIP3 potentiates cisplatin chemoresistance by triggering HSP90-ERK pathway mediated DNA repair in esophageal squamous cell carcinoma. Cancer Lett 2018;418:97–108. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Jiang B, Wang Y, Ni H, Zhang J, Xia J, et al. 2-HG Inhibits Necroptosis by Stimulating DNMT1-Dependent Hypermethylation of the RIP3 Promoter. Cell Rep 2017;19:1846–57. [DOI] [PubMed] [Google Scholar]
- 26.Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 2015;62:592–601. [DOI] [PubMed] [Google Scholar]
- 27.Bozec D, Iuga AC, Roda G, Dahan S, Yeretssian G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget 2016;7:46384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He GW, Gunther C, Thonn V, Yu YQ, Martini E, Buchen B, et al. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J Exp Med 2017;214:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider AT, Gautheron J, Feoktistova M, Roderburg C, Loosen SH, Roy S, et al. RIPK1 suppresses a TRAF2-dependent pathway to liver cancer. Cancer Cell 2017;31:94–109. [DOI] [PubMed] [Google Scholar]
- 30.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009;137:1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997;15:871–5. [DOI] [PubMed] [Google Scholar]
- 32.Cheung M, Pei J, Pei Y, Jhanwar SC, Pass HI, Testa JR. The promyelocytic leukemia zinc-finger gene, PLZF, is frequently downregulated in malignant mesothelioma cells and contributes to cell survival. Oncogene 2010;29:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roman-Gomez J, Castillejo JA, Jimenez A, Gonzalez MG, Moreno F, Rodriguez MdC, et al. 5’ CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood 2002;99:2291–6. [DOI] [PubMed] [Google Scholar]
- 34.Safferthal C, Rohde K, Fulda S. Therapeutic targeting of necroptosis by Smac mimetic bypasses apoptosis resistance in acute myeloid leukemia cells. Oncogene 2017;36:1487–502. [DOI] [PubMed] [Google Scholar]
- 35.Xin J, You D, Breslin P, Li J, Zhang J, Wei W, et al. Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia 2017;31:1154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016;532:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Najafov A, Chen H, Yuan J. Necroptosis and cancer. Trends Cancer 2017;3::294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 2007;12:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng JQ, Lee WC, Klein MA, Cheng GZ, Jhanwar SC, Testa JR. Frequent mutations of NF2 and allelic loss from chromosome band 22q12 in malignant mesothelioma: evidence for a two-hit mechanism of NF2 inactivation. Genes Chromosomes Cancer 1999;24:238–42. [PubMed] [Google Scholar]
- 40.Flejter WL, Li FP, Antman KH, Testa JR. Recurring loss involving chromosomes 1, 3, and 22 in malignant mesothelioma: possible sites of tumor suppressor genes. Genes Chromosomes Cancer 1989;1:148–54. [DOI] [PubMed] [Google Scholar]
- 41.Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murthy SS, Shen T, De Rienzo A, Lee WC, Ferriola PC, Jhanwar SC, et al. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene 2000;19:410–6. [DOI] [PubMed] [Google Scholar]
- 43.Dondelinger Y, Vandenabeele P, Bertrand MJ. Regulation of RIPK1’s cell death function by phosphorylation. Cell Cycle 2016;15:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesotheliomas and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;12:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 2012;36:215–27. [DOI] [PubMed] [Google Scholar]
- 47.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013;38:27–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.