Abstract
Background context:
Perioperative ischemic optic neuropathy (ION) is a devastating complication of spinal fusion surgery.
Purpose:
To develop predictive models of this blinding condition using longitudinal medical administrative claims databases, which provide temporal sequence of perioperative ischemic optic neuropathy and potential risk factors.
Design:
Nested case control study
Patient sample:
Participants in Cliniformatics® Data Mart medical claims database (2007–2017) with hospitalization involving lumbar or thoracic spinal fusion surgery and no history of ION.
Outcome measures:
Peri-operative ION (or not) during hospitalization for lumbar or thoracic spinal fusion surgery.
Methods:
65 ION cases and 106,871 controls were identified. Matched controls (n=211) were selected based on year of surgery and zip code. Chronic and peri-operative variables were assigned based on medical claims codes. Least absolute shrinkage and selection (LASSO) penalized conditional logistic regression with ten-fold cross validation was used to select variables for the optimal predictive model from the subset of variables with p < 0.15 between cases and matched controls (unadjusted conditional logistic regression). Receiver operating characteristic (ROC) curves were generated for the strata-independent matched and full sample.
Results:
The predictive model included age 57–65 years, male gender, diabetes with and without complications, chronic anemia, hypertension, heart failure, carotid stenosis, perioperative hemorrhage and perioperative organ damage in the predictive model. Area under ROC curve was 0.75 (95% CI: 0.68, 0.82) for the matched sample and 0.72 (95% CI: 0.66, 0.78) for the full sample.
Conclusions:
This predictive model for ION in spine fusion considering chronic conditions and perioperative conditions is unique to date in its use of longitudinal medical claims data, inclusion of ICD-10 codes and study of ophthalmic conditions as risk factors. Similar to other studies of this condition the multivariable model included age, male gender, peri-operative organ damage and peri-operative hemorrhage. Hypertension, chronic anemia and carotid artery stenosis were new predictive factors identified by this study.
Keywords: spinal fusion, complications, vision loss, ischemic optic neuropathy, medical claims, prediction, epidemiology
INTRODUCTION
Peri-operative vision loss in the setting of non-ophthalmic surgery is a devastating and unexpected complication.1 ION during spine fusion is particularly serious as the injury tends to be bilateral, leading to severe visual impairment or blindness, significantly impacting quality of life. Although perioperative ION after spinal fusion has decreased, concern for this complication continues due to the increasing volume of these procedures in the United States,2 and practice advisories have been issued by the American Society of Anesthesiologists with multidisciplinary support, in 2006,3 2012,4 and 2019.5
Determining the risk factors for perioperative ION is desirable both to allow for modification and for appropriate patient counselling.6 A retrospective case control study based on a surgical data registry, identified both patient (male sex, obesity) and procedural factors (blood loss, percent colloid replacement fluid, Wilson frame use, surgical duration) to be associated with ION in spinal fusion.7 Analysis of administrative data from a large population-based sample of US hospitalizations, the National Inpatient Sample (NIS), also found male sex and obesity to be associated with ION as well as age, transfusion (likely a surrogate for blood loss) and cerebral infarction.2 More recently, NIS data was used to develop risk prediction models for ION in spinal fusion that included sex, age and obstructive sleep apnea (pre-operative model) and sex, age, obesity and transfusion (perioperative model).8 External validation had fair receiving operator characteristic.9 Ophthalmic conditions such as glaucoma and age related macular degeneration, have been reported to be associated with ION in cardiac surgery, but have not been studied with respect to spinal fusion.10
NIS is a cross-sectional database comprised solely of inpatient discharge data based upon International Classification of Disease (ICD) diagnostic codes.11 This limits definitive determination of whether a diagnosis preceded the admission, and does not provide follow-up of new diagnoses following discharge. Accordingly, the objective of this study was to analyze a longitudinal medical administrative claims data base to provide more accurate temporal sequence of perioperative ION and the associated risk factors. Compared with cross sectional databases, a longitudinal database captures the health record over time. This enables looking back prior to the admission with the ION event to better capture chronic conditions (predictive factors) coded in prior outpatient and inpatient encounters. It also allows looking forward from the ION event to clarify uncertain vision loss diagnoses from the inpatient record.
MATERIALS & METHODS
This is a nested case-control study of patients undergoing lumbar or thoracic spine fusion surgery with and without perioperative ischemic optic neuropathy. Summary of methods is below (see Supplement 1 for detailed methods).
Data Source
Analysis was conducted using Clinformatics® Data Mart Database (OptumInsight, Eden Prairie, MN), which includes the medical administrative claims data for a large national health insurer in the United States. There are > 60 million unique members with > 15 million members in each year. The covered population is representative of the US population as a whole. This database has previously been used to study risk factors for spontaneous non-arteritic anterior ischemic optic neuropathy,12 and recently for external validation of a predictive model for ION in spinal fusion developed using cross sectional data.9 Analysis included data from 2007 −2017 using the ZIP5 data set. The data use agreement precludes reporting data with sample size < 10. Research adhered to the Declaration of Helsinki, and was deemed exempt by the Stanford IRB Committee.
Study Population
Subject selection – spinal fusion
Clinformatics® Data Mart Database members > 18 years of age who had eligible CPT and ICD-PCS for thoracic or lumbar spine fusion (Supplement 2 has screening codes to identify subjects) or either an eligible CPT or ICD-PCS code and forensic review of the claim supporting spinal fusion were included.13 ICD-9 and −10 codes were both used in order to enable use of data collected before and after 2015 (ICD-10 was introduced in the US in mid-2015). The admission and discharge dates for the inpatient confinement record corresponding to qualifying spine surgery were used to define the peri-operative period.
Case selection – ischemic optic neuropathy
Among subjects with qualifying spinal fusion surgery, cases with ischemic optic neuropathy were identified as those with first instance of ICD9 377.31 or ICD10 h47.01* (*=blank, 1, 2, 3, 9) between the admission and discharge dates (inclusive). Additional cases were identified if they had a non-specific vision loss ICD-CM code between the admission and discharge dates (see supplement 3, showing non-specific vision loss codes) and first instance of identifying ION codes in the 12 months following discharge from the hospitalization with spine fusion surgery. Each subject contributed only one case, which was associated with a specific hospitalization involving spinal fusion surgery.
Control selection – full set
All subjects ≥ 18 years of age with qualifying spine fusion surgery and without any vision loss or ION event were included as controls. Only one surgery, defined by hospital admission date was included in the study for each subject such that each subject served as a either a single case or a single control.
Control selection – matched set
A subset of all controls was selected using a matching strategy to avoid sparse data bias due to the relatively rare events of ION occurring after spine surgery.14 Among subjects with qualifying spinal fusion and without any instance of ION diagnosis code, controls were individually matched to cases by five digit zip code and year of surgery, the latter chosen based on prior evidence of association with ION in spine fusion.2 Geographic matching was performed to account for regional differences in practice.
Subject characteristics
Age at date of admission for spine fusion hospitalization and gender were obtained from the enrollment table.10 ICD-CM, ICD-PCS and CPT codes between date of admission and date of discharge were used to assign peri-operative diagnoses (Supplement 4 shows codes classifying chronic and acute conditions). Chronic subject characteristics were assigned based on ICD-CM and CPT codes for two date ranges (all information pre-discharge, pre-admission). Conditions previously shown to be associated with peri-operative ION in spine or cardiac surgery were included.2,10 Spinal fusion complexity, captured in other models using ICD-9 coding for number of levels, was not included because this is not available in ICD-10.
This classification improves upon prior analyses in NIS2,8 by providing differentiation of chronic and perioperative conditions, by the use of CPT and ICD-10 codes and consideration of ophthalmic conditions shown to be associated with ION in cardiac surgery. It is not possible to differentiate missing data due to lack of negative diagnosis coding in claims data.
Due to small numbers, diabetes mellitus with eye complications was combined with diabetes mellitus with complications other than eye. Similarly, although not necessarily pathophysiologically related, peri-operative acute myocardial infarction, acute kidney injury and acute stroke were combined as “peri-operative organ damage.” Due to concerns that acute stroke coding might have been used to represent ION, peri-operative organ damage was considered both inclusive of and exclusive of acute stroke. Hypertensive retinopathy and thrombocytopenia were excluded due to low numbers.
Statistical analysis
Factors and characteristics were compared between groups in unadjusted analyses using t-test for independent samples for age, Wilcoxon rank sum test for length of hospital stay and chi-square test for other variables. Pre-discharge (all information) and pre-admission coding of chronic conditions were considered separately. Variables with p <0.15 in unadjusted analysis were considered for inclusion in the multiple variable models, consistent with common practice of relaxed statistical criterion for initial model entry.15 Three multiple variable models were constructed, differing by included variables: All information including peri-operative acute stroke (model 1); all information excluding peri-operative stroke (model 2); and pre-admission information (model 3).
Given the rarity of ION and the sparse data, a nested case-control approach design was utilized for multiple variable model generation. Conditional logistic regression examined unadjusted associations of demographics, chronic conditions and peri-operative conditions with ION using cases and matched controls. Strata were defined to each contain one case and its matched controls based on zip code and year of surgery.
The goal of the multiple variable models was prediction over statistical inference. Therefore, penalized regression, a regularization method that avoids overfitting data, minimized total error including bias and variance, and useful for generation of predictive models, was applied to generate the multivariable model with the best predictive power.16 The cyclic coordinate descent algorithm developed by Reid and Tibshirani was used to fit each conditional logistic regression model with LASSO penalty (α=1).17, 18 Model predictive capabilities for different values of the penalty term (λ) were assessed using 10-fold cross validation with data split by leaving out entire strata. The penalized model that minimized the difference between predicted and actual conditional likelihoods was selected as the final model, defined by the β coefficients for variables included in that model. A nomogram was constructed to aid in application of results.
To generalize assessment of the three multiple variable models so they are not conditional on the strata (e.g., zip code and year of surgery), the receiver operating characteristic (ROC) curve was created based on the matched sample (ignoring strata),17 as well as the full sample including non-matched controls.19
Since this is a longitudinal study and chronic conditions may not be fully captured for the patients in the database less than half year before the admission of spine surgery, sensitivity analysis was performed by repeating the analysis details above for the all information including peri-operative stroke and pre-admission models, limited to patients in the database longer than a half year prior to the qualified spine surgery.
The multivariable analysis was performed with the package clogitL117 in R (Vienna, Austria), and all other analyses were conducted in SAS Enterprise Guide, version 7.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Study population characteristics
This study included 65 ION case subjects and 106,871 control subjects without ION after spinal fusion. Of the cases, 52 had ION codes during the period of hospitalization and 13 had a non-specific vision loss code during the hospitalization and a subsequent ION outpatient code. Surgical coding was based on ICD-9 for 46 (70.7%) cases and 79,930 (74.8%) controls, ICD-10 for 12 (18.4%) cases and 26,942 (25.2%) controls, and CPT only for 7 (10.8%) cases and no controls.
Compared to those who did not develop ION, those who did were on average older and more likely to be male (Table 1). Considering all available information, compared to those who did not develop ION, those who did were more likely to be diagnosed with diabetes, hyperlipidemia, hypertension, heart failure, ischemic heart disease and carotid artery stenosis. They were more likely to sustain peri-operative hemorrhage and peri-operative organ damage during the hospitalization for spine fusion (Table 1). Median length of stay was 6 days (IQ range 4–9) for cases vs. 4 days (IQ range 3–6) for controls (p<.001). When considering only pre-admission information, differences in diabetes, hyperlipidemia, hypertension, and carotid artery stenosis between those with and without ION were also seen (Table 2). However, there was no difference in ischemic heart disease and heart failure between groups for pre-admission coding.
Table 1:
Characteristics of spinal fusion subjects with and without ischemic optic neuropathy in the Clinformatics® Data Mart database using all available information
| Spinal fusion with ION (cases) | Spinal fusion without ION (controls) | Comparison | ||||
|---|---|---|---|---|---|---|
| Full set | Matched set | full set* | matched set** | |||
| n=65 | n=106,871 | n=211 | P value | Odds ratio | P value | |
| Demographics | ||||||
| Age, mean ± SD | 66.1 ± 9.5 | 58.5 ± 13.6 | 61.3 ± 13.3 | <.001 | ||
| Age category, n(%) | <.001 | < 0.15 | ||||
| 18-<57 | <10 | 44018 (41.2) | 55 (26.1) | |||
| 57–65 | 23 (35.4) | 26503 (24.8) | 43 (20.4) | |||
| 66–74 | 19 (29.2) | 23868 (22.3) | 71 (33.7) | |||
| >75 | 14 (21.5) | 12482 (11.7) | 42 (19.9) | |||
| Female, n(%) | 27 (41.5) | 60542 (56.7) | 137 (64.9) | 0.01 | 0.43 (0.23, 0.8) | 0.01 |
| Chronic conditions (all information), n(%) | ||||||
| Atherosclerosis | <10 | 9656 (9.04) | 23 (10.9) | ≥ 0.15 | ||
| Anemia | 26 (40) | 32658 (30.56) | 71 (33.65) | 0.1 | 1.67 (0.88, 3.18) | 0.12 |
| Carotid artery stenosis | <10 | 6164 (5.77) | 15 (7.11) | < 0.15 | ||
| Cerebrovascular disease | <10 | 6538 (6.12) | 15 (7.11) | ≥ 0.15 | ||
| Chronic kidney disease | <10 | 9350 (8.75) | 26 (12.32) | ≥ 0.15 | ||
| Diabetes mellitus | <.001 | 0.003 | ||||
| Complications | 11 (16.92) | 10601 (9.92) | 22 (10.43) | 3.21 (1.24, 8.33) | ||
| No complications | 20 (30.77) | 18362 (17.18) | 27 (12.8) | 2.99 (1.42, 6.31) | ||
| No diabetes | 34 (52.31) | 77908 (72.9) | 162 (76.78) | Reference | ||
| Heart failure | 10 (15.38) | 7621 (7.13) | 12 (5.69) | 0.01 | 3.86 (1.37, 10.83) | 0.01 |
| Hypercoagulability | <10 | 876 (0.82) | <10 | ≥ 0.15 | ||
| Hyperlipidemia | 53 (81.54) | 68786 (64.36) | 142 (67.3) | 0.00 4 | 2.07 (1.01, 4.22) | 0.05 |
| Hypertension | 57 (87.69) | 73593 (68.86) | 151 (71.56) | 0.001 | 2.61 (1.11, 6.13) | 0.03 |
| Ischemic heart disease | 20 (30.77) | 21939 (20.53) | 48 (22.75) | 0.04 | 1.65 (0.83, 3.26) | 0.15 |
| Obesity | 19 (29.23) | 31324 (29.31) | 69 (32.7) | 0.99 | 1.03 (0.55, 1.91) | 0.94 |
| Obstructive sleep apnea | 14 (21.54) | 15390 (14.4) | 29 (13.74) | 0.1 | 1.96 (0.88, 4.38) | 0.10 |
| Smoking | 20 (30.77) | 40907 (38.28) | 75 (35.55) | 0.21 | 1.04 (0.55, 1.96) | 0.90 |
| Thrombocytopenia | <10 | 3707 (3.47) | <10 | ≥ 0.15 | ||
| Chronic ophthalmic conditions (all information), n(%) | ||||||
| AMD | <10 | 3630 (3.4) | 35 (4.1) | ≥ 0.15 | ||
| Cataract category | ≥ 0.15 | |||||
| Surgery | <10 | 6547 (6.13) | 19 (9) | |||
| No surgery | 17 (26.15) | 17566 (16.44) | 53 (25.12) | 1.30 (0.65, 2.59) | ||
| No Cataract | 42 (64.62) | 82758 (77.44) | 139 (65.88) | Reference | ||
| Glaucoma | 11 (16.9) | 8820 (8.3) | 83 (9.7) | 1.39 (0.61, 3.17) | 0.43 | |
| HTN retinopathy | <10 | 1031 (1) | 10 (1.2) | ≥ 0.15 | ||
| Peri-operative conditions, n(%) | ||||||
| Organ damage | 11 (16.92) | 3609 (3.38) | <10 | <.001 | < 0.15 | |
| Acute kidney failure | <10 | 2798 (2.62) | <10 | |||
| Acute MI | <10 | 558 (0.52) | <10 | |||
| Acute stroke | <10 | 454 (0.42) | <10 | |||
| Hemorrhage | 20 (30.77) | 18505 (17.32) | 46 (21.8) | 0.00 4 | 1.94 (0.99, 3.79) | 0.05 |
| Transfusion | <10 | 12447 (11.65) | 24 (11.37) | ≥0.15 | ||
t-test for continuous variables or chi-square for categorical variables, p value not reported for if <10 subjects in either group
conditional logistic regression, unadjusted odds ratios, p value reported relative to threshold of 0.15 if < 10 subjects in either group
ION ischemic optic neuropathy, AMD age related macular degeneration, HTN hypertensive
Table 2:
Characteristics of spinal fusion subjects with and without ischemic optic neuropathy in the Clinformatics® Data Mart database using pre-admission information
| Spinal fusion with ION (cases) | Spinal fusion without ION (controls) | Comparison | ||||
|---|---|---|---|---|---|---|
| Full set | Matched set | full set* | matched set** | |||
| n=65 | n=106,871 | n=211 | P value | Odds ratio | P value | |
| Demographics | ||||||
| Age, mean ± SD | 66.1 ± 9.5 | 58.5 ± 13.6 | 61.3 ± 13.3 | <.001 | ||
| Age category, n(%) | <.001 | < 0.15 | ||||
| 18-<57 | <10 | 44018 (41.2) | 55 (26.1) | |||
| 57–65 | 23 (35.4) | 26503 (24.8) | 43 (20.4) | |||
| 66–74 | 19 (29.2) | 23868 (22.3) | 71 (33.7) | |||
| >75 | 14 (21.5) | 12482 (11.7) | 42 (19.9) | |||
| Female, n(%) | 27 (41.5) | 60542 (56.7) | 137 (64.9) | 0.01 | 0.43 (0.23, 0.8) | 0.01 |
| Chronic conditions (PA information), n(%) | ||||||
| Atherosclerosis | <10 | 9447 (8.84) | 23 (10.90) | ≥ 0.15 | ||
| Anemia | 19 (29.23) | 29655 (27.75) | 63 (29.86) | 0.79 | 1.23 (0.61, 2.46) | 0.56 |
| Carotid artery stenosis | <10 | 6063 (5.67) | 15 (7.11) | < 0.15 | ||
| Cerebrovascular disease | <10 | 1062 (0.99) | <10 | ≥ 0.15 | ||
| Chronic kidney disease | <10 | 9112 (8.53) | 24 (11.37) | ≥ 0.15 | ||
| Diabetes mellitus | <.001 | 0.003 | ||||
| Complications | 11 (16.9) | 10438 (9.77) | 22 (10.43) | 3.21 (1.24, 8.33) | ||
| No complications | 20 (30.77) | 18204 (17.03) | 27 (12.80) | 2.99 (1.42, 6.31) | ||
| No diabetes | 34 (52.31) | 78229 (73.2) | 162 (76.78) | Ref | ||
| Heart failure | <10 | 7184 (6.72) | 11 (5.21) | < 0.15 | ||
| Hypercoagulability | <10 | 823 (0.77) | <10 | 0.48 | ≥ 0.15 | |
| Hyperlipidemia | 53 (81.54) | 68514 (64.11) | 142 (67.30) | 0.00 3 | 2.07 (1.01, 4.23) | 0.05 |
| Hypertension | 57 (87.69) | 72973 (68.28) | 151 (71.56) | <.001 | 2.61 (1.11, 6.13) | 0.03 |
| Ischemic heart disease | <10 | 4917 (4.6) | <10 | ≥ 0.15 | ||
| Obesity | 19 (29.23) | 30799 (28.82) | 65 (30.81) | 0.94 | 1.16 (0.61, 2.19) | 0.65 |
| Obstructive sleep apnea | 14 (21.54) | 15151 (14.18) | 28 (13.27) | 0.09 | 2.1 (0.93, 4.77) | 0.08 |
| Smoking | 19 (29.23) | 40575 (37.97) | 75 (35.55) | 0.15 | 0.99 (0.53, 1.85) | 0.97 |
| Thrombocytopenia | <10 | 3380 (3.16) | <10 | ≥0.15 | ||
| Chronic ophthalmic conditions (PA information), n(%) | ||||||
| AMD | <10 | 3582 (3.35) | 12 (5.69) | 0.21 | 1.46 (0.41, 5.16) | ≥ 0.15 |
| Cataract category | 0.16 | ≥ 0.15 | ||||
| Surgery | <10 | 6547 (6.13) | 19 (9.00) | 1.47 (0.50, 4.30) | ||
| No surgery | 15 (23.08) | 17469 (1635) | 52 (24.64) | 1.12 (0.55, 2.30) | ||
| No Cataract | 44 (67.69) | 82855 (77.53) | 140 (66.35) | Ref | ||
| Glaucoma | 11 (16.92) | 8661 (8.10) | 22 (10.43) | 0.00 9 | 1.4 (0.61, 3.21) | 0.42 |
| HTN retinopathy | <10 | 1030 (0.96) | <10 | ≥ 0.15 | ||
t-test for continuous variables or chi-square for categorical variables, p value not reported for if <10 subjects in either group
conditional logistic regression, unadjusted odds ratios, p value reported relative to threshold of 0.15 if < 10 subjects in either group
ION ischemic optic neuropathy, AMD age related macular degeneration, HTN hypertensive
Unadjusted risk factors identified in the matched sample
211 matched controls were selected based on zip code and the year of surgery for the 65 ION cases. Compared to the matched controls and considering all information (Table 1), the cases were more likely to be older, male, diagnosed with diabetes with or without complications, heart failure, hyperlipidemia, hypertension, and to have perioperative organ damage. When considering only information available prior to hospitalization (Table 2), the ORs for diabetes, hyperlipidemia, and hypertension for subjects with and without ION remained significant. However, the ORs for carotid artery stenosis were no longer significant.
Multivariable predictive model using the matched sample
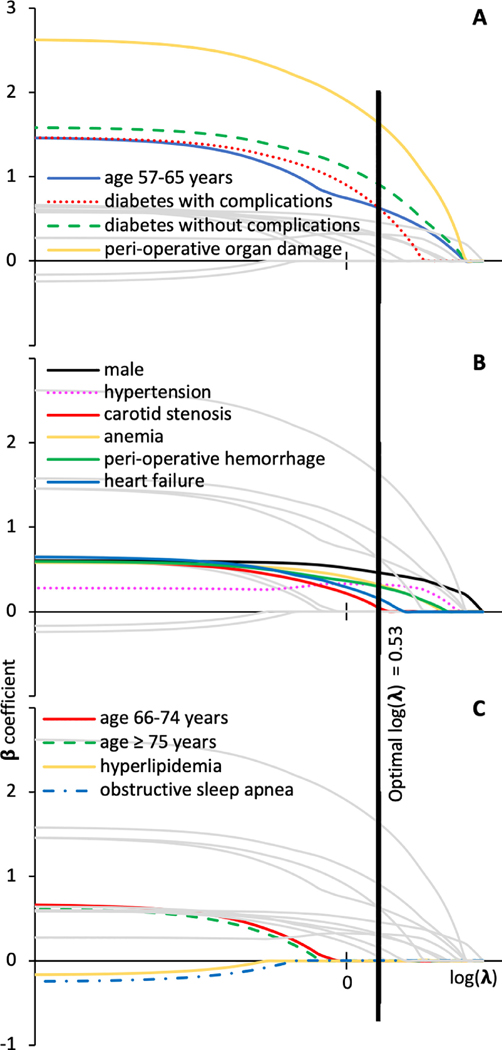
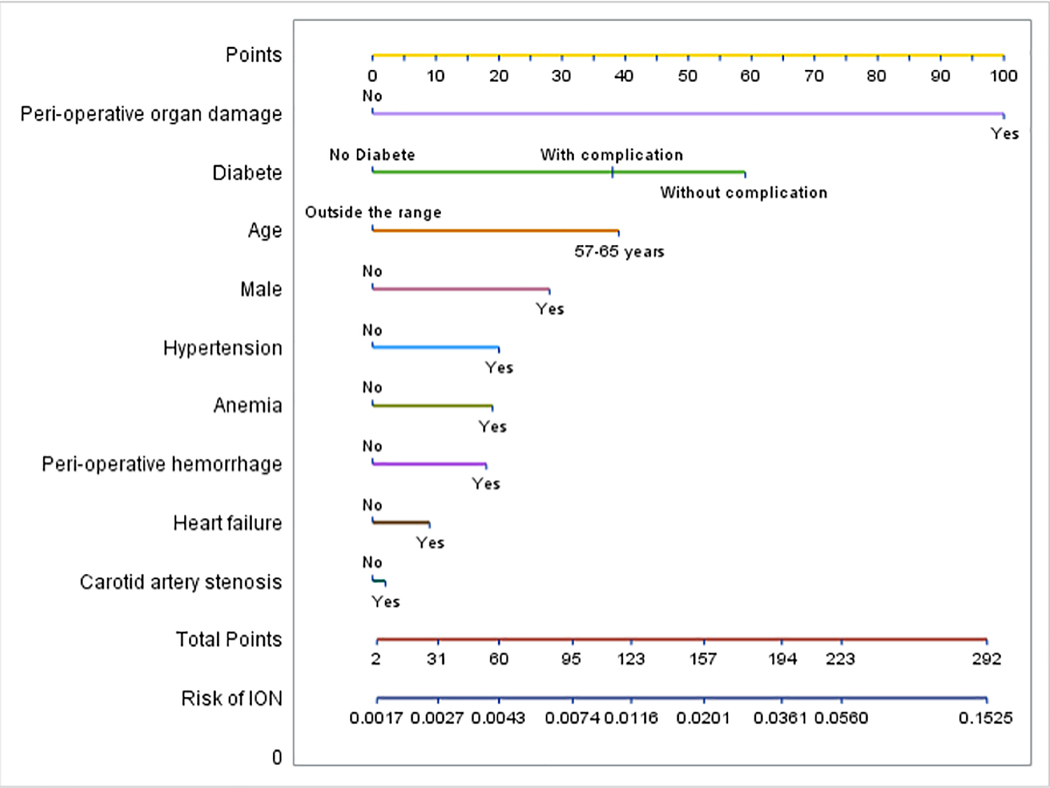
Model 1 considered all available information including peri-operative stroke. There were 11 potential risk factors with a total of 14 levels included in the initial model (Figure 1). Perioperative organ damage, age 57–65, and diabetes with and without complications were the three dominating risk factors in the matched sample as indicated by having the highest β coefficient estimates at small values of penalizing coefficient λ. Cross validation found conditional likelihood deviance for testing vs. training strata to be minimized at a log(λ) where there were 10 variables in the model. These were selected in order of “entering” the model as λ was decreased and include age, gender, anemia, carotid artery stenosis, hypertension, diabetes, heart failure, peri-operative hemorrhage and peri-operative organ damage. Table 3 summarizes β coefficients from the final model, corresponding matched odds ratios and receiver operating characteristic for matched and full samples. β coefficient > 0 indicates increased risk for ION and odds ratio >1 when comparing to reference group (e.g., with vs. without disease). Hyperlipidemia and obstructive sleep apnea were excluded from the multivariable model because β coefficients were zero at the optimal λ The area under the curve (AUC) resulting from the combination of these coefficients of the risk factors was 0.75 (95% CI: 0.68, 0.82) in the matched sample and 0.72 (95% CI: 0.66, 0.78) in the full sample (Supplement 5 shows the receiver operating characteristic). Figure 2 shows a nomogram for this model.
Figure 1: Parameter profile for conditional logistic regression with LASSO penalty used to select final predictive model for ischemic optic neuropathy in spinal fusion surgery (model 1 – all information).
Conditional regression derived β coefficients (y axis) for each model variable as a function of the log(λ), where λ is the coefficient for the penalization term in the regression equation displayed in three panels to improve readability (dominant variables (panel A), other variables included in final model (panel B) and variable excluded from final model (panel C). As λ approaches 0 (log λ becomes more negative) this is equivalent to a non-penalized model. The vertical line denotes the final model, corresponding to optimal λ, which is that minimizes the conditional likelihood deviance. Variables included in the final multivariable model are those with non-zero β coefficients for the model with optimal λ (panels A, B). Variables not included in the final multivariable model have β coefficients equal to zero for the model with optimal λ (panel C).
Table 3:
Final multivariable conditional logistic regression models for ischemic optic neuropathy in spinal fusion patients in the Clinformatics® Data Mart database using the matched control sample and considering all available information
| All available data | Pre-admission data only | |||||
|---|---|---|---|---|---|---|
| Model 1 Peri-operative stroke included | Model 2 Peri-operative stroke excluded | Model 3 No peri-operative data | ||||
| Variable | β coefficient | odds ratio (adjusted) | β coefficient | odds ratio (adjusted) | β coefficient | odds ratio (adjusted) |
| Age 57–65 years | 0.63 | 1.88 | 0.69 | 1.99 | 0.72 | 2.05 |
| Male | 0.46 | 1.58 | 0.53 | 1.70 | 0.68 | 1.97 |
| Diabetes with complications | 0.62 0.90 |
0.77 0.99 |
0.55 0.73 |
1.73 2.08 |
||
| Diabetes without complications | 1.86 2.46 |
2.16 2.69 |
||||
| Anemia | 0.31 | 1.36 | 0.48 | 1.62 | NS | |
| Hypertension | 0.32 | 1.38 | 0.24 | 1.27 | 0.4 | 1.49 |
| Hyperlipidemia | 0.06 | 1.06 | 0.1 | 1.11 | ||
| Heart failure | 0.15 | 1.16 | 0.17 | 1.19 | 0.39 | 1.48 |
| Carotid artery stenosis | 0.04 | 1.04 | 0.37 | 1.45 | 0.37 | 1.45 |
| Obstructive sleep apnea | NS | NS | 0.19 | 1.21 | ||
| Peri-operative hemorrhage | 0.29 | 1.34 | 0.36 | 1.43 | NI | |
| Peri-operative organ damage* | 1.62 | 5.05 | 1.91 | 6.75 | NI | |
| AUC matched sample | 0.75 (0.68, 0.82) | 0.74 (0.67–0.81) | 0.72 (0.65–0.80) | |||
| AUC full sample | 0.72 (0.66, 0.78) | 0.71(0.65–0.77) | 0.69 (0.62–0.75) | |||
β coefficients from the final model with > 0 indicate increased risk for ION and odds ratio >1 when comparing to reference group (e.g., with vs. without disease). Adjusted odds ratio for each variable indicates odds for ION vs. without ION in spinal fusion for subjects with that variable vs without that variable, who are the same status for all other variables in the model.
NS did not reach significance threshold in unadjusted analysis and was therefore not considered for inclusion in the final model
NI not included in unadjusted analysis because variables were defined on a time frame distinct from that considered by the model.
Figure 2: Nomogram for the final multivariable model for ischemic optic neuropathy in spinal fusion surgery (model 1 – all information).
To utilize the nomogram, use a vertical line between the patient’s value for each variable and the upper number line to assign points for each variable. Sum the points for each variable and use a vertical line between the bottom two number lines to translate total points to ION risk.
Model 2 considered all available information excluding peri-operative stroke (Table 3). Compared to model 1, the dominating risk factors remained the same. Obstructive sleep apnea was excluded from the final model as well. The only difference was that hyperlipidemia made a very small contribution (β coefficients 0.06 at optimal λ).
Model 3 considered information available prior to hospitalization and therefore excluded peri-operative variables (Table 3). There were 9 potential risk factors with 11 levels. There were still 9 variables with 9 levels in the prediction model. Similar to models 1 and 2, at optimal λ these were age 57–65, male, hypertension, carotid artery stenosis, diabetes with complications, diabetes without complications, and heart failure. Similar to model 2, model 3 included hyperlipidemia. Different from models 1 & 2, model 3 included obstructive sleep apnea and excluded anemia.
The sensitivity analyses restricted to subjects in the database longer than a half year prior to spine surgery (54 cases, 189 controls). These generated comparable results. In the sensitivity analysis for model 1 (all information), the three dominating factors were also perioperative organ damage, age of 57–65, and diabetes with complications. Cross validation results indicated that there were also 10 variables in the final prediction model. Similar to the full matched analysis, the resulting prediction model excluded obstructive sleep apnea. A difference in the sensitivity sample model was the inclusion of hyperlipidemia instead carotid artery stenosis. In the sensitivity analysis for model 3 (pre-admission information), cross validation results indicated that there were 8 variables with 9 levels in the final prediction model. Similar to the full matched analysis the resulting model excluded age 66–74. A difference in the sensitivity sample model was the inclusion of age > 75 and exclusion of heart failure.
DISCUSSION
Through application of conditional logistic regression analysis using a LASSO penalty to a longitudinal medical claims database, we contribute a predictive model for ION in spinal fusion surgery based on chronic medical conditions and perioperative factors. Considering information available prior to discharge, we identified age, male gender, diabetes, hypertension, heart failure, anemia, carotid artery stenosis, peri-operative blood loss, and peri-operative organ damage to predict ION in spine fusion surgery. This model is most applicable to understanding the contribution of peri-operative factors to risk of ION in spine fusion. Considering information available prior to admission, the model differed in exclusion of peri-operative hemorrhage, peri-operative organ damage and anemia and inclusion of obstructive sleep apnea. This model is most applicable to understanding the contribution of patient factors to risk of ION in spine fusion. This analysis differs from prior efforts in that we were able to use longitudinal data to improve case selection and enhance classification of chronic conditions. It is the first analysis on this topic to include data after 2015 when ICD-10 coding was instituted. Furthermore, we addressed issues of sparse data and determined the optimal model using advanced statistical methods.
Prior knowledge regarding associations with ION in spinal fusion has come from two major sources: a case control study using a post-operative vision loss registry (POVLR) and cross-sectional analyses using the National Inpatient Sample (NIS).2,7,8,20 Though the number of cases in the current study was similar to that used in the POVLR, the current study has less selection bias since it did not rely on voluntary reporting of cases, and controls selected from a group of institutions.21 Although the number of cases is fewer than that in the NIS, the current cohort study with nested case-control analysis leveraged inpatient and outpatient data to distinguish chronic and peri-operative conditions. In rare, but devastating complications such as ION in spine fusion, it is important to utilize different data sources and approaches to inform the overall picture of the condition.
The statistical approach used for final variable selection was based on prediction capability of the model rather than statistical significance levels (e.g. p=0.05). This is an appropriate strategy given our goal of developing a predictive model as well as concerns that P value based variable selection approaches are prone to instability, bias, and overfitting or underfitting when there is sparse data.22 However, a penalized regression approach does not inherently generate confidence intervals for model coefficients which limits statistical inference regarding the selected variables. While bootstrapping approaches have been applied to estimate confidence intervals for some penalized regression models, these have not yet been developed for penalized conditional logistic regression.23
Similar to both POVLR and NIS analyses, we identified male gender as a predictive factor, supporting its role as a likely risk factor for ION in spine surgery. As in the NIS and POVLR samples, the majority of spinal fusions were performed in women, while the majority of ION events were in men. Older age was also a predictive factor in our models, with higher odds of ION for patients between 56 and 65 years old. The NIS analyses found an association between ION and older age while the POVLR did not. Interestingly both case and control samples in the current study were slightly older than the POVLR and NIS samples.
Multiple chronic vascular risk factors were identified as predictive factors for ION in spinal fusion, including diabetes with complications, diabetes without complications, and hypertension. Hyperlipidemia was included in 2 of 3 models. Vascular risk factors for ischemic events such as ION are scientifically plausible. Interestingly, ischemic heart disease, a chronic end organ vascular disease, was associated with ION in the full sample but did not meet the threshold for inclusion in the matched model. This is in contrast to previously published multivariable models derived from POVLR and NIS, which did not include vascular risk factors. This might be attributed to sample as well as differences in variable definition underlying higher prevalence of vascular risk factors in the controls for the Clinformatics® Data Mart sample compared to prior samples (e.g. diabetes 27% vs. 8% in POVLR vs. 13% in NIS for controls; hypertension 69% vs. 36% POVLR vs. 42% in NIS for controls). This may also reflect differences in variable definition using longitudinal medical claims data in the current study compared to using only cross-sectional inpatient data captured in NIS and POVLR.
Carotid artery stenosis was identified as a predictive factor, which mechanistically suggests local hypoperfusion as a contributing factor. This was also studied in NIS where it was not found to be associated. Similar to vascular risk factors, there was also a substantial difference in carotid stenosis rates in controls between the two studies (6% Clinformatics® Data Mart vs. 0.2% NIS for controls), which may reflect a difference between inpatient and outpatient coding of this diagnosis.
NIS analysis had shown anemia and cerebral infarct to be associated with ION in spine fusion. However, chronic and peri-operative conditions could not be distinguished using the cross-sectional inpatient sample. In this analysis using a prospective cohort, we were able to make this distinction. Anemia was selected for inclusion in the models that included hospitalization data, but not in the model restricted to pre-admission data. Chronic cerebrovascular disease did not meet the threshold for inclusion in multivariable models in the Clinformatics® sample. These differences from NIS suggest that anemia and stroke might be considered peri-operative risk factors. Inclusion of both anemia and peri-operative hemorrhage in the models considering all information suggests possible mechanisms for local hypoxia causing ION. Peri-operative stroke, together with the other variables including in peri-operative end organ damage suggest that hypoperfusion/hypoxic complications of surgery are associated with ION. The persistence of peri-operative organ damage as a dominant variable with exclusion of peri-operative stroke re-enforces this possibility.
Prior analyses (POVLR, NIS) have identified obstructive sleep apnea and obesity to be associated with ION in spine fusion. Interestingly, OSA was identified as a predictive factor when considering preadmission information, but not when considering all information in the current study. The difference between models 1/ 2 and model 3, reflect differences in predictive contribution of OSA is less when peri-operative factors are included in the model. The difference between models 1/ 2 and NIS/POVLR results may reflect varying disease prevalence in the reference group: our sample had slightly lower obesity rates in controls than POVLR and higher obesity rates than the NIS (29% Clinformatics® Data Mart vs. 35% POVL vs. 9.8% NIS in controls). While obesity has been shown to be under coded in medical claims data a recent comparison using Optum Integrated Claims-Clinical Database showed > 90% sensitivity, specificity and positive predictive value for obesity codes in a variety of surgeries.24 This, and a likely less healthy population than the third party insured individuals captured in Cliniformatics® Data Mart, likely account for the slightly higher prevalence in the POVLR. The three-fold difference with NIS suggests a difference in outpatient and inpatient obesity coding.
Ophthalmic conditions, including glaucoma, were not associated with ION in spine surgery in this analysis. These have not been previously studied in spine surgery but were found to be associated with NIS analysis of ION in cardiac surgery.10 This may be due to differences in pathophysiology between ION in cardiac surgery, which is more likely to be anterior ION, possibly influenced by the vascular status of the eye versus spine surgery, which tends to be posterior ION. It may also be attributable to differential diagnostic bias in cross sectional inpatient data due to those with a vision loss event having an eye exam and therefore being more likely to have inpatient codes for ophthalmic conditions.
As in NIS and POVLR, peri-operative conditions were identified as predictive factors in our model. Peri-operative hemorrhage and transfusion were separately associated with ION in POVLR and NIS analyses respectively. Our model included peri-operative hemorrhage. Peri-operative organ damage was also in our model which likely relates to the association between ION in spinal fusion and stroke found in NIS analyses. Unfortunately, the sample size for damage to specific organs in Clinformatics® Data Mart precluded individual consideration in our multivariable model. We cannot determine if there is a causal relationship between peri-operative organ damage and ION, or rather, if perioperative organ damage and peri-operative ION share common causes.
The main limitation of this analysis is similar to that for NIS in that variable definition was based on medical claims data with the possibility of misclassification bias. While this has been studied and is likely minimal for spine surgery definition, it has not been studied for ION or all considered risk factors.13 Furthermore, available information precluded quantifying factor severity and inclusion of of modifiable surgical/anesthesia factors. Though Clinformatics® Data Mart compares favorably with the US population it is not a random sample and this also introduces bias. For example, patients insured by traditional Medicare, Medicaid and other private insurers are not included. Use of ICD-9 and 10 coding precluded adjusting models for intra-operative variables and complexity of surgery. Our statistical methodology to generate the best predictive model does not generate confidence intervals for coefficients and this limits statistical inference based on the multivariable model.
These predictive models for ION in spine fusion considering chronic conditions and perioperative conditions are unique to date in their use of longitudinal medical claims data, inclusion of ICD-10 codes and study of ophthalmic conditions as risk factors. Similar to other studies we found age, male gender, peri-operative organ damage and peri-operative hemorrhage to be predictive factors. Different from prior studies we also found diabetes, hypertension, chronic anemia and carotid artery stenosis to be predictive factors to ION. Neither obesity nor sleep apnea were predictive factors in our models that considered all information. Surgeons and anesthesiologists should continue to consider demographic and chronic medical conditions when providing pre-operative counselling to people scheduled to undergo spine fusion and should continue to implement practices to prevent perioperative hemorrhage and organ damage. Further study is needed to identify modifiable risk factors. Natural language processing of the medical record may offer opportunities in this regard.
Supplementary Material
Acknowledgments
Funding disclosure:
R21 EY027447 to Dr. Roth
K23 EY 024345 to Dr. Moss
P30 026877 to Dr. Moss
UL1TR002003 to the University of Illinois at Chicago
Research to Prevent Blindness to Stanford Department of Ophthalmology
Footnotes
Conflict of interest statement: Dr. Roth has been compensated for expert witness services on behalf of hospitals, physicians, and patients in cases of perioperative visual loss. Dr. Roth is Chair of the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. The statements and opinions in this manuscript are exclusively those of the authors and do not reflect the opinions of the American Society of Anesthesiologists or the United States Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Biousse V, Newman NJ: Ischemic Optic Neuropathies. N Engl J Med 2015; 372: 2428–36 [DOI] [PubMed] [Google Scholar]
- 2.Rubin DS, Parakati I, Lee LA, Moss HE, Joslin CE, Roth S: Perioperative Visual Loss in Spine Fusion Surgery: Ischemic Optic Neuropathy in the United States from 1998 to 2012 in the Nationwide Inpatient Sample. Anesthesiology 2016; 125: 457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology 2006; 104: 1319–28 [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaum JL, Roth S, Connis RT, Domino KB, Lee LA, Nickinovich DG, Warner MA: Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology 2012; 116: 274–285 [DOI] [PubMed] [Google Scholar]
- 5.Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery 2019: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss, the North American Neuro-Ophthalmology Society, and the Society for Neuroscience in Anesthesiology and Critical Care. Anesthesiology 2019; 130: 12–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corda DM, Dexter F, Pasternak JJ, Trentman TL, Nottmeier EW, Brull SJ: Patients’ perspective on full disclosure and informed consent regarding postoperative visual loss associated with spinal surgery in the prone position. Mayo Clin Proc 2011; 86: 865–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins DJ: Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog retin eye res 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S: Predicting Risk of Perioperative Ischemic Optic Neuropathy in Spine Fusion Surgery: A Cohort Study Using the National Inpatient Sample. Anesth Analg 2020; 130: 967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss HE, Xiao L, Shah SH, Chen YF, Joslin CE, Roth S: Peri-operative ischemic optic neuropathy in spinal fusion surgery: Validating a predictive scoring system. The Spine Journal 2020; on-line ahead of print June 6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin DS, Matsumoto MM, Moss HE, Joslin CE, Tung A, Roth S: Ischemic Optic Neuropathy in Cardiac Surgery: Incidence and Risk Factors in the United States from the National Inpatient Sample 1998 to 2013. Anesthesiology 2017; 126: 810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(HCUP) AfHRaQHCaUP: Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2011. Rockville, MD, 2013 [Google Scholar]
- 12.Cestari DM, Gaier ED, Bouzika P, Blachley TS, De Lott LB, Rizzo JF, Wiggs JL, Kang JH, Pasquale LR, Stein JD: Demographic, Systemic, and Ocular Factors Associated with Nonarteritic Anterior Ischemic Optic Neuropathy. Ophthalmology 2016; 123: 2446–2455 [DOI] [PubMed] [Google Scholar]
- 13.Faciszewski T, Jensen R, Berg RL: Procedural coding of spinal surgeries (CPT-4 versus ICD-9-CM) and decisions regarding standards: a multicenter study. Spine (Phila Pa 1976) 2003; 28: 502–7 [DOI] [PubMed] [Google Scholar]
- 14.Greenland S, Mansournia MA, Altman DG: Sparse data bias: a problem hiding in plain sight. Bmj 2016; 352: i1981. [DOI] [PubMed] [Google Scholar]
- 15.DW H, S L: Applied Logistic Regression. New York, John Wiley & Sons, Inc, 1989 [Google Scholar]
- 16.Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ : Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med 2016; 35: 1159–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid S, Tibshirani R: Regularization Paths for Conditional Logistic Regression: The clogitL1 Package. J Stat Softw 2014; 58 [PMC free article] [PubMed] [Google Scholar]
- 18.LDD D: A Review on Variable Selection in Regression Analysis. Econometrics 2018; 6: 1–27 [Google Scholar]
- 19.Lee HS, Krischer JP: A new framework for prediction and variable selection for uncommon events in a large prospective cohort study. Model Assist Stat Appl 2017; 12: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LA, Roth S, Posner KL, Cheney FW, Caplan RA, Newman NJ, Domino KB: The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology 2006; 105: 652–9 [DOI] [PubMed] [Google Scholar]
- 21.Lee LA, Roth S, Todd MM, Posner KL, Polissar NL, Neradilek MB, Torner J, Newman NJ, Domino KB: The Postoperative Visual Loss Study Group. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology 2012; 116: 15–24 [DOI] [PubMed] [Google Scholar]
- 22.van Smeden M, Moons KG, de Groot JA, Collins GS, Altman DG, Eijkemans MJ, Reitsma JB: Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat Methods Med Res 2019; 28: 2455–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor J, Tibshirani R: Post-Selection Inference for l1-Penalized Likelihood Models. Can J Stat 2018; 46: 41–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammann EM, Kalsekar I, Yoo A, Scamuffa R, Hsiao CW, Stokes AC, Morton JM, Johnston SS: Assessment of obesity prevalence and validity of obesity diagnoses coded in claims data for selected surgical populations: A retrospective, observational study. Medicine (Baltimore) 2019; 98: e16438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.