Abstract
Background:
Hyperinsulinemia and inflammation are inter-related pathways that link diet with the risk of several chronic diseases. Evidence suggests that these pathways may also increase prostate cancer risk.
Objective:
To determine whether hyperinsulinemic diet and inflammatory diet are associated with prostate cancer incidence and mortality.
Design, setting, and participants:
We prospectively followed 41 209 men in the Health Professionals Follow-up Study (1986–2014). Scores for two validated dietary patterns were calculated from food frequency questionnaires at baseline and updated every 4 yr.
Outcome measurements and statistical analysis:
Total, advanced, and lethal prostate cancer outcomes were assessed. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were determined for associations between two empirical hypothesis-oriented dietary patterns—empirical dietary index for hyperinsulinemia and empirical dietary inflammatory pattern—and prostate cancer risk estimated using Cox proportional hazard regression.
Results and limitations:
During 28 yr of follow-up, 5929 incident cases of total prostate cancer, including 1019 advanced and 667 fatal, were documented. In multivariable-adjusted models, there was a 7% higher risk of advanced prostate cancer (HR: 1.07; 95% CI: 1.01–1.15) and a 9% higher risk of fatal prostate cancer (HR: 1.09; 95% CI: 1.00–1.18) per standard deviation (SD) increase in the hyperinsulinemic diet. When stratified by age, the hyperinsulinemic diet was associated with only earlier-onset aggressive prostate cancer (men under 65 yr), with per SD HRs of 1.20 (95% CI: 1.06–1.35) for advanced, 1.22 (1.04–1.42) for fatal, and 1.20 (1.04–1.38) for lethal. The inflammatory diet was not associated with prostate cancer risk in the overall study population, but was associated with earlier-onset lethal prostate cancer (per SD increase HR: 1.16; 95% CI: 1.00–1.35).
Conclusions:
Hyperinsulinemia and inflammation may be potential mechanisms linking dietary patterns with the risk of aggressive prostate cancer, particularly earlier-onset disease.
Patient summary:
Avoiding inflammatory and hyperinsulinemic dietary patterns may be beneficial for the prevention of clinically relevant prostate cancer, especially among younger men.
Keywords: Cohort study, Diet, Hyperinsulinemia, Inflammation, Prostate cancer
1. Introduction
The considerably higher burden of prostate cancer in developed countries [1], along with higher incidence rates for men who move from low- to high-risk countries than for those in their native countries [2,3], suggests that dietary and lifestyle factors may play a role in the disease’s etiology. However, established risk factors are limited to older age, African ancestry, family history, and certain genetic factors. There remains a critical need to identify modifiable risk factors to help guide prevention strategies.
To date, most studies of diet and prostate cancer incidence have focused on individual dietary components [4]. Although there is suggestive evidence that certain food and nutrients are associated with prostate cancer, such as dairy increasing risk [5] and lycopene reducing risk [6], many other individual dietary factors have shown null or mixed findings. Assessment of dietary patterns is an alternative approach, providing the advantage of better accounting for additive effects and complex interactions among dietary components [7]. Dietary patterns also allow for multiple approaches to achieve a healthy diet and have been increasingly used to develop dietary recommendations [8]. Another factor complicating studies of diet and prostate cancer is the biological heterogeneity of the disease, with evidence suggesting differences in the etiology of indolent and aggressive cancer.
Hyperinsulinemia and inflammation are two inter-related biological pathways that link diet with risk of several cancers [9], and studies have suggested that these may also increase prostate cancer risk [10,11]. Therefore, we investigated the association of the two empirical hypothesis-oriented dietary patterns based on these pathways with the risk of prostate cancer overall, as well as more aggressive forms of disease by clinical stage, Gleason score, and lethal outcomes, in a prospective cohort study of US men.
2. Patients and methods
2.1. Study population
The Health Professionals Follow-up Study (HPFS) is an ongoing cohort of 51,529 male health professionals aged 40–75 yr at baseline in 1986. Questionnaires were administered at baseline and every 2 yr thereafter to collect information on demographics, lifestyle, medical history, and disease outcomes. Dietary intake was assessed every 4 yr using a validated semiquantitative food frequency questionnaire (FFQ) [12]. Follow-up rates at each 2-yr cycle have exceeded 90%. The study protocol was approved by the institutional review board of Harvard T.H. Chan School of Public Health and those of participating registries as required. Each participant provided written informed consent.
For the current study, we excluded participants who had a history of cancer (other than nonmelanoma skin cancer), cardiovascular disease, or diabetes at baseline, as well as those who left more than 70 items blank on the FFQ or reported implausible values for total energy intake (<800 or >4200 kcal/d). The remaining 41 209 men were included in the analysis.
2.2. Assessment of dietary scores
Development and validation of the empirical dietary index for hyperinsulinemia and empirical dietary inflammatory pattern have previously been described in detail [13,14]. Briefly, 39 predefined food groups were entered into reduced rank regression models followed by stepwise linear regression to identify a dietary pattern that was most predictive of plasma measures of interleukin-6, C-reactive protein, and tumor necrosis factor-α receptor 2 for the inflammatory diet. A similar approach was used to create the hyperinsulinemic diet score, with the exception of food groups being regressed onto plasma C-peptide. The two types of diet were significantly predictive of the biomarkers in validation analyses [13,14]. For example, the adjusted relative concentration of C-peptide in the highest versus lowest quintile of the hyperinsulinemic diet was 1.29 (95% confidence interval [CI]: 1.22, 1.39) for men. Both dietary scores are the weighted sum of 18 food groups (nine overlapping) and are moderately correlated [15]. Higher scores indicate more inflammatory and insulinemic diet. Food groups that comprise the two scores, including serving sizes, are listed in Supplementary Table 1. Dietary scores were calculated for each participant at every 4-yr cycle from 1986 to 2010.
2.3. Ascertainment of prostate cancer
Participants reported incident diagnoses of prostate cancer on biennial questionnaires. Permission was obtained to acquire medical records and pathology reports for cases, which were reviewed by trained personnel blinded to exposure status to confirm the diagnosis, diagnosis date, and clinical features. When records were unavailable, diagnoses were confirmed by linkage to state tumor registries. Deaths were identified by reports from the next of kin, postal service, and National Death Index. Information on treatment, disease progression, and development of metastases was obtained through biennial disease-specific questionnaires.
Stage T1a cases were excluded from analyses. Given the disease’s considerable heterogeneity in metastatic potential, we included indolent and aggressive prostate cancers, classified into the following clinical subgroups: localized (stage T1/T2 and N0, M0 at diagnosis), advanced (stage T3b/T4/N1/M1 at diagnosis, or prostate cancer death or lymph node metastasis or distant metastasis over follow-up), fatal (prostate cancer death), lethal (prostate cancer death or distant metastasis), low grade (Gleason 2–6 or 3 + 4), and high grade (Gleason 4 + 3 or 8–10).
2.4. Statistical analysis
Person time was calculated starting from the date of return of the baseline questionnaire to the date of incident prostate cancer diagnosis, date of death, or end of follow-up (January 31, 2014), whichever came first. Hyperinsulinemic and inflammatory diet scores were calculated as cumulative averages through follow-up to best represent long-term diet and minimize within-person variation. Dietary scores were adjusted for total energy intake using the residual method.
We used Cox proportional hazard regression models stratified by age and time period to estimate hazard ratios (HRs) and 95% CIs for the association of the two dietary scores with prostate cancer overall and by clinical subgroup. Dietary scores were modeled continuously, as per standard deviation (SD) increase. To address potential bias from reverse causation, we used a 2-yr lag between dietary assessment and prostate cancer incidence in the main analysis. For example, we used cumulative average dietary scores from 1986 to 1990 as the exposure for follow-up from 1992 to 1994, and cumulative average scores from 1986 to 1994 for follow-up from 1996 to 1998. Multivariable-adjusted models were adjusted for race, height, body mass index (BMI) at age 21 yr, smoking status, family history of prostate cancer, prostate-specific antigen (PSA) test in previous cycle, PSA testing in >50% of previous cycles, multivitamin use, vitamin E supplement use, alcohol intake, physical activity, and aspirin use. In sensitivity analyses, we additionally adjusted for current BMI, a potential mediator in the association of the dietary patterns with prostate cancer.
We also conducted several other secondary analyses. First, we conducted analyses stratified by current age (<65 vs ≥65 yr) and current BMI (<25 vs ≥25 kg/m2). We hypothesized that the dietary patterns would be more strongly associated with earlier-onset disease and among leaner men, as shown for other dietary factors such as energy balance [16]. The likelihood ratio test was used to assess the statistical significance of any interaction between each dietary score and age or BMI by comparing models with and without these terms. Second, we used modified versions of the two dietary scores, which excluded tomatoes. Third, we analyzed the effect of latency time on the association between the dietary patterns and prostate cancer by modeling the scores with 4-, 8-, and 12-yr latencies, using a similar lag approach to the primary analysis. Fourth, we addressed potential residual confounding from PSA testing by starting follow-up in 1994 and restricting analyses to a subcohort of men who reported having a PSA test in 1994, the year the US Food and Drug Administration approved the use of PSA testing for prostate cancer screening.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical tests were two sided, with p < 0.05 considered statistically significant.
3. Results
During 28 yr of follow-up, we documented 5929 incident cases of total prostate cancer and 667 cases of fatal prostate cancer. Men consuming a more hyperinsulinemic diet were less likely to use multivitamins and undergo PSA screening, and had lower physical activity levels (Table 1). Age and race were comparable across quintiles. A similar pattern with lifestyle and demographic factors was observed for those consuming a more inflammatory diet. Men in the higher quintiles for both dietary patterns also tended to have lower intake of fiber, calcium, and magnesium (Supplementary Table 2).
Table 1 –
Participant characteristics weighted by person-years in quintiles of hyperinsulinemic and inflammatory diet scores in the Health Professionals Follow-up Study (1986–2014)
| Quintile 1 | Quintile 3 | Quintile 5 | |
|---|---|---|---|
| Empirical dietary index for hyperinsulinemia | |||
| Median score | −1.18 | −0.01 | 1.19 |
| Age (yr) | 64.9 (10.9) | 64.7 (10.9) | 61.2 (10.5) |
| White (%) | 92 | 91 | 91 |
| Height (inches) | 70.1 (3.4) | 70.2 (3.2) | 70.3 (3.4) |
| Body mass index at age 21 (kg/m2) | 22.1 (5.6) | 22.1 (5.3) | 22.2 (5.6) |
| Ever smoker (%) | 56 | 52 | 50 |
| Family history of prostate cancer (%) | 12 | 12 | 12 |
| PSA screening in 1994 (%) | 39 | 37 | 31 |
| Multivitamin use (%) | 56 | 54 | 49 |
| Alcohol consumption (g/d) | 17.2 (18.6) | 10.5 (13.9) | 8.1 (13.0) |
| Physical activity (METs-h/wk) | 36.1 (30.1) | 31.8 (27.2) | 29.7 (25.9) |
| Aspirin use (%) | 44 | 44 | 41 |
| Total energy intake (kcal/d) | 2129 (631) | 1903 (605) | 2054 (662) |
| Body mass index (kg/m2) | 24.7 (3.6) | 25.2 (3.8) | 25.8 (4.6) |
| Empirical dietary inflammatory pattern | |||
| Median score | −1.20 | 0.05 | 1.14 |
| Age (yr) | 63.0 (10.4) | 65.0 (11.0) | 62.7 (11.0) |
| White (%) | 93 | 91 | 88 |
| Height (inches) | 70.3 (3.2) | 70.2 (3.5) | 70.0 (3.4) |
| Body mass index at age 21 (kg/m2) | 22.2 (5.4) | 22.0 (5.3) | 22.1 (5.7) |
| Ever smoker (%) | 62 | 51 | 46 |
| Family history of prostate cancer (%) | 12 | 12 | 12 |
| PSA screening in 1994 (%) | 37 | 37 | 32 |
| Multivitamin use (%) | 57 | 54 | 48 |
| Alcohol consumption (g/d) | 21.3 (20.2) | 9.7 (12.4) | 6.1 (11.3) |
| Physical activity (METs-h/wk) | 34.6 (29.1) | 32.2 (27.3) | 30.5 (26.4) |
| Aspirin use (%) | 47 | 43 | 40 |
| Total energy intake (kcal/d) | 2095 (627) | 1916 (608) | 2055 (663) |
| Body mass index (kg/m2) | 24.9 (3.8) | 25.0 (3.8) | 25.8 (4.4) |
MET = metabolic equivalent of task; PSA = prostate-specific antigen; SD= standard deviation.
All variables other than age are standardized to the age distribution of all participants. Values are presented as mean (SD) for continuous variables.
The hyperinsulinemic diet was not associated with total prostate cancer, but was associated with advanced and fatal prostate cancer in multivariable-adjusted analyses (Table 2). For each SD increase in the hyperinsulinemic diet, there was a 7% higher risk of advanced (HR: 1.07; 95% CI: 1.01–1.15) and a 9% higher risk of fatal (HR: 1.09; 95% CI: 1.00–1.18) prostate cancer. Although the inflammatory diet was associated with a lower risk of total prostate cancer in the age-adjusted model, there were no significant associations for total prostate cancer or any clinical subgroup after adjusting for potential confounders, including PSA screening, in the overall study population. These results for both dietary scores did not change materially after additionally adjusting for current BMI. Associations for the two types of diet were similar when excluding tomatoes from the score (Supplementary Table 3).
Table 2 –
Hazard ratios and 95% confidence intervals for the associations of hyperinsulinemic and inflammatory diet with the risk of prostate cancer in the Health Professionals Follow-up Study (1986–2014)
| Per SD increase | |||
|---|---|---|---|
| Cases | Age-adjusted | Multivariable | |
| Empirical dietary index for hyperinsulinemia | |||
| Total prostate cancer | 5929 | 0.99 (0.96–1.01) | 1.00 (0.97–1.03) |
| Localized prostate cancer | 4115 | 0.96 (0.93–0.99) | 0.98 (0.95–1.02) |
| Advanced prostate cancer | 1019 | 1.07 (1.01–1.14) | 1.07 (1.01–1.15) |
| Fatal prostate cancer | 667 | 1.10 (1.02–1.19) | 1.09 (1.00–1.18) |
| Lethal prostate cancer | 811 | 1.07 (1.00–1.15) | 1.07 (0.99–1.15) |
| Low-grade prostate cancer | 3705 | 0.98 (0.94–1.01) | 1.00 (0.96–1.04) |
| High-grade prostate cancer | 1337 | 0.97 (0.92–1.03) | 0.99 (0.93–1.05) |
| Empirical dietary inflammatory pattern | |||
| Total prostate cancer | 5929 | 0.96 (0.94–0.99) | 0.98 (0.95–1.01) |
| Localized prostate cancer | 4115 | 0.94 (0.91–0.97) | 0.97 (0.93–1.00) |
| Advanced prostate cancer | 1019 | 1.02 (0.95–1.08) | 1.03 (0.96–1.10) |
| Fatal prostate cancer | 667 | 1.04 (0.96–1.12) | 1.04 (0.95–1.13) |
| Lethal prostate cancer | 811 | 1.03 (0.96–1.11) | 1.04 (0.96–1.12) |
| Low-grade prostate cancer | 3705 | 0.95 (0.92–0.98) | 0.99 (0.95–1.02) |
| High-grade prostate cancer | 1337 | 0.92 (0.87–0.97) | 0.94 (0.88–1.00) |
BMI = body mass index; MET = metabolic equivalent of task; PSA = prostate-specific antigen; SD = standard deviation.
Dietary scores were adjusted for total energy intake using the residual method, with higher scores indicating hyperinsulinemic and inflammatory diets.
Localized prostate cancer: stage T1/T2 and N0, M0 at diagnosis; advanced prostate cancer: stage T3b/T4/N1/M1 at diagnosis, or prostate cancer death or lymph node metastasis or distant metastasis over follow-up; fatal prostate cancer: prostate cancer death; lethal prostate cancer: prostate cancer death or distant metastasis; low-grade prostate cancer: Gleason 2–6 or 3 + 4; and high-grade prostate cancer: Gleason 4 + 3 or 8–10.
Age-adjusted models were stratified by age and time period. Multivariable-adjusted models were stratified by age and time period, and adjusted for race (white and nonwhite), height (≤68, >68–70, >70–72, and >72 inches), BMI at age 21 (≤20, 21–<23, 23–<25, and ≥25 kg/m2), smoking status (never, former, and current), family history of prostate cancer (yes or no), PSA test in previous cycle (yes or no), PSA testing in >50% of previous cycles (yes or no), multivitamin use (yes or no), vitamin E supplement use (yes or no), alcohol intake (g/d; quintiles), physical activity (METs-h/wk; quintiles), and aspirin use (yes or no).
We next examined the associations of the dietary patterns by age. In line with our hypothesis, both dietary patterns tended to show stronger positive associations for aggressive cancers among men under 65 yr of age (Fig. 1A and 1B). Each SD increase in the hyperinsulinemic diet was associated with a 20% higher risk of advanced prostate cancer (HR: 1.20; 95% CI: 1.06–1.35), 22% higher risk of fatal prostate cancer (HR: 1.22; 95% CI: 1.04–1.42), and 20% higher risk of lethal prostate cancer (HR: 1.20; 95% CI: 1.04–1.38) among younger men. For the inflammatory diet, there was a statistically significant association for lethal prostate cancer (per SD increase HR: 1.16; 95% CI: 1.00–1.35) and a suggestive association for advanced prostate cancer (per SD increase HR: 1.13; 95% CI: 0.99–1.28). These HRs were similar when additionally adjusted for current BMI and incident diabetes. There were no statistically significant differences for associations between either of the dietary patterns and prostate cancer risk when stratifying by BMI (Supplementary Table 4).
Fig. 1 –
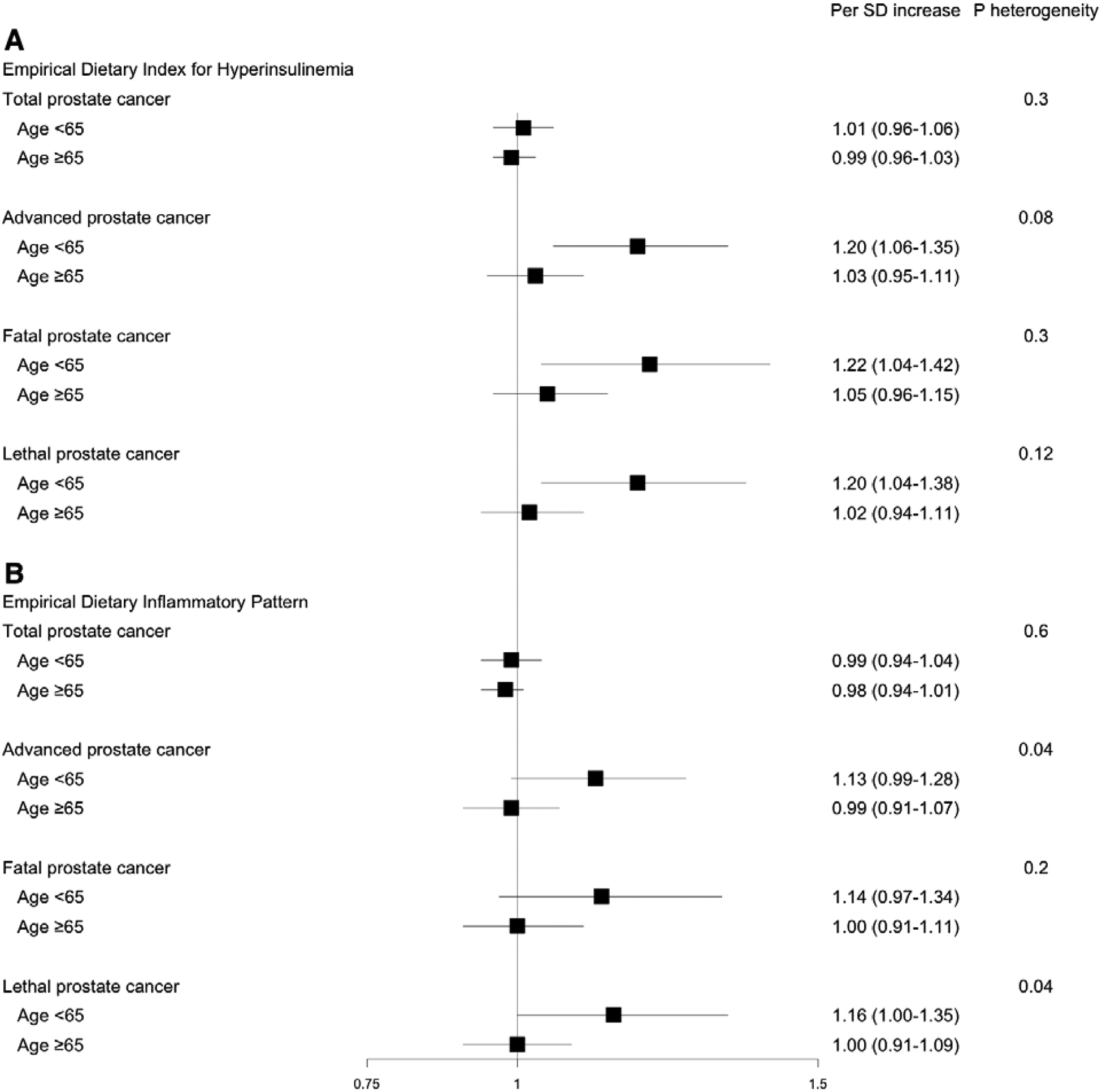
Multivariable-adjusted hazard ratios and 95% confidence intervals for the associations of (A) hyperinsulinemic and (B) inflammatory diet with prostate cancer risk by age subgroup. Dietary scores were adjusted for total energy intake using the residual method. Localized prostate cancer: stage T1/T2 and N0, M0 at diagnosis; advanced prostate cancer: stage T3b/T4/N1/M1 at diagnosis, or prostate cancer death or lymph node metastasis or distant metastasis over follow-up; fatal prostate cancer: prostate cancer death; lethal prostate cancer: prostate cancer death or distant metastasis; low-grade prostate cancer: Gleason 2–6 or 3 + 4; and high-grade prostate cancer: Gleason 4 + 3 or 8–10. Multivariable-adjusted models were stratified by age and time period, and adjusted for race, height, BMI at age 21, smoking, family history of prostate cancer, PSA test in previous cycle, PSA testing in >50% of previous cycles, multivitamin use, vitamin E supplement use, alcohol intake, physical activity, and aspirin use. The p value for heterogeneity was calculated using the likelihood ratio test comparing the models with and without the product term between each dietary pattern and age. BMI = body mass index; MET = metabolic equivalent of task; PSA = prostate-specific antigen; SD = standard deviation.
In the latency analysis, associations between hyperinsulinemic diet and aggressive prostate cancers tended to show stronger trends for 4-yr lag than for 8- and 12-yr lag (Supplementary Table 5). There was no significant trend for total prostate cancer at any latency period. Associations between inflammatory diet and prostate cancer risk were largely similar across different latency periods (Supplementary Table 6). There were no significant associations between either dietary score and prostate cancer risk among the subcohort with PSA screening in 1994, possibly due in part to the reduced sample size (Supplementary Table 7).
4. Discussion
Men and their families often seek advice on the optimal diet and lifestyle patterns to engage in, to reduce the risk of prostate cancer. Results from this large prospective cohort study support the hypothesis that consuming a diet with higher insulinemic potential is associated with a greater risk of developing aggressive prostate cancer. Of note, these associations remained even after additional adjustment for BMI, and tended to be stronger for more recent diet and among younger men. Proinflammatory diet consumption was not associated with prostate cancer risk in the overall study population, but was associated with lethal prostate cancer among men under 65 yr.
Most studies on diet and prostate cancer have focused on individual food or nutrients, and have typically shown suggestive evidence or conflicting results [4]. Among the more extensively studied food are dairy and tomatoes/lycopene, with meta-analyses showing modest effects for both [5,17]. While individual food and nutrients may have relatively smaller influence on disease risk, overall dietary patterns can better capture the added effects and complex interactions between the included dietary components. Results to date from prospective studies of dietary patterns and prostate cancer have been mixed. The Mediterranean diet [18–21] and dietary patterns derived from data-driven approaches [22–24], such as factor analysis or principal component analysis, have not been associated with prostate cancer incidence, while the Alternate Healthy Eating Index-2010 has been shown to be beneficial in some but not all studies [18,21]. However, there is evidence that postdiagnostic diet may influence prostate cancer–specific and overall mortality [19,25]. In line with these findings, the 2018 World Cancer Research Fund International/American Institute for Cancer Research (WCRF/AICR) Continuous Update Project lists evidence for all individual dietary factors and dietary patterns as limited [26].
The two types of diet used in our study include a number of food groups that are not typically captured in other dietary patterns, including specific vegetable categories (eg, green leafy vegetables), coffee, and animal-based food besides red and processed meat, such as eggs. Several of these have been shown to be potentially important individually in the context of prostate cancer, particularly for more aggressive cancers [4]. Thus, dietary patterns that do not account for these food items may be missing critical aspects of diet that influence prostate cancer risk and could partially explain previous null findings.
To our knowledge, this is the first prospective cohort study examining dietary insulinemic potential and prostate cancer development and progression. Although case-control studies of circulating insulin or C-peptide and prostate cancer risk have had somewhat mixed results [27–31], a number of other lines of evidence from both epidemiologic and experimental studies suggest a role for hyperinsulinemia in prostate cancer. Serum insulin has been associated with prostate cancer recurrence [32], while the Physicians’ Health Study found prediagnostic C-peptide to be predictive of prostate cancer mortality [33]. Additionally, metabolic syndrome has been associated with a higher prostate cancer risk, especially for more aggressive tumor features and biochemical recurrence [34], and metformin use has been reported to improve survival among prostate cancer patients [35]. Diet-induced hyperinsulinemia has also been shown to increase tumor growth in mice injected with human prostate cancer cells [36,37].
Hyperinsulinemia may promote tumor progression directly through insulin receptors or regulation of insulin-like growth factors (IGFs) and their binding proteins (IGFBPs), which are involved in cell proliferation and survival. The IGF signaling pathway, especially IGF-1, has been associated with prostate cancer risk in a number of studies, with a pooled analysis of prospective studies reporting an odds ratio (OR) of 1.29 (95% CI: 1.16–1.43) for men in the highest versus lowest quintile [38]. Similarly, low levels of IGFBP-1 have been associated with an elevated risk of prostate cancer [39]. Our findings of significant associations between hyperinsulinemic diet and aggressive prostate cancer among men under 65 yr of age further support the potential role of IGF-1, as evidence suggests the hormone to be a stronger risk factor for prostate cancer among younger men, with a meta-analysis reporting ORs of 1.80 (95% CI: 1.34–2.42) and 1.20 (95% CI: 1.03–1.38) per 80 percentile IGF-1 increase for men under 60 and ≥70 yr at diagnosis, respectively [38]. IGF-1 could be a more important factor in younger men due to higher circulating concentrations earlier in life.
Aspects of inflammation are closely related to hyperinsulinemia, and the potential role of inflammation on the IGF axis through hyperinsulinemia and insulin resistance [9] could contribute to the associations between inflammatory diet and aggressive prostate cancer among younger men in our study. A number of studies have reported associations between circulating inflammatory biomarkers and prostate cancer risk [40–44]. An inflammatory diet could also contribute to the etiology of prostate cancer through tissue-level inflammation, as prostatitis is particularly common among younger and middle-aged men and inflammation is frequently observed in prostate tumor biopsies. This is supported by epidemiologic studies of sexually transmitted infections and infectious agents in prostate tissue [45].
Our study has several strengths, including a high follow-up rate in a large prospective cohort that allowed us to utilize repeated dietary assessments and analyze clinical subtypes of prostate cancer. We also had detailed information on a wide range of prostate cancer risk variables that allowed for rigorous adjustment of important confounding factors.
However, our study is not without limitations. First, dietary assessment by an FFQ may be subject to measurement error. However, previous validation studies in HPFS have shown reasonably good correlations between FFQs and diet records, suggesting that dietary intake is generally well measured. Moreover, the empirical scores are robustly associated with C-peptide and inflammatory biomarkers in independent validation datasets. We additionally used multiple FFQs over follow-up to reduce within-person variation and better approximate habitual long-term diet. Second, although we adjusted for many potential confounding variables, residual confounding cannot be ruled out given the observational nature of this study. Third, our cohort was predominantly white male health professionals, which could limit generalizability of our findings. However, this may also potentially reduce residual confounding by socioeconomic status. Studies of these dietary patterns and prostate cancer risk in other populations, including other racial/ethnic groups, are warranted.
5. Conclusions
In summary, our results suggest hyperinsulinemia and inflammation to be possible mechanisms linking dietary patterns and risk of aggressive, but not indolent, prostate cancer. Avoiding dietary patterns with insulinemic or inflammatory potential may be beneficial for the prevention of clinically relevant prostate cancer, especially among younger men.
Supplementary Material
Acknowledgments:
We would like to thank the participants and staff of the Health Professionals Follow-up Study for their valuable contributions, as well as the cancer registries of the following US states for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY.
Financial disclosures: Benjamin C. Fu certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by grants from the National Institutes of Health (U01 CA167552, R00 CA215314, T32 CA009001, R00 CA207736) and intercancer center collaborations (P30 CA016058 and P30 CA006516).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 1991;63:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu H, Harris RE, Gao YT, Gao R, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol 1991;20:76–81. [DOI] [PubMed] [Google Scholar]
- [4].Lin PH, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med 2015;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aune D, Navarro Rosenblatt DA, Chan DS, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87–117. [DOI] [PubMed] [Google Scholar]
- [6].Rowles JL 3rd, Ranard KM, Smith JW, An R, Erdman JW Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2017;20:361–77. [DOI] [PubMed] [Google Scholar]
- [7].Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- [8].DeSalvo KB, Olson R, Casavale KO. Dietary guidelines for Americans. JAMA 2016;315:457–8. [DOI] [PubMed] [Google Scholar]
- [9].Giovannucci E A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2018;29:1–6. [DOI] [PubMed] [Google Scholar]
- [10].Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology 2012;60:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Albanes D, Weinstein SJ, Wright ME, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst 2009;101:1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- [13].Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr 2016;146:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tabung FK, Wang W, Fung TT, et al. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr 2016;116:1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee DH, Fung TT, Tabung FK, et al. Dietary pattern and risk of multiple myeloma in two large prospective US cohort studies. JNCI Cancer Spectr 2019;3:pkz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Platz EA, Leitzmann MF, Michaud DS, Willett WC, Giovannucci E. Interrelation of energy intake, body size, and physical activity with prostate cancer in a large prospective cohort study. Cancer Res 2003;63:8542–8. [PubMed] [Google Scholar]
- [17].Rowles JL 3rd, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis 2018;21:319–36. [DOI] [PubMed] [Google Scholar]
- [18].Bosire C, Stampfer MJ, Subar AF, et al. Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am J Epidemiol 2013;177:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kenfield SA, DuPre N, Richman EL, Stampfer MJ, Chan JM, Giovannucci EL. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur Urol 2014;65:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ax E, Garmo H, Grundmark B, et al. Dietary patterns and prostate cancer risk: report from the population based ULSAM cohort study of Swedish men. Nutr Cancer 2014;66:77–87. [DOI] [PubMed] [Google Scholar]
- [21].Lavalette C, Adjibade M, Srour B, et al. Cancer-specific and general nutritional scores and cancer risk: results from the Prospective NutriNet-Sante Cohort. Cancer Res 2018;78:4427–35. [DOI] [PubMed] [Google Scholar]
- [22].Muller DC, Severi G, Baglietto L, et al. Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2009;18:3126–9. [DOI] [PubMed] [Google Scholar]
- [23].Wu K, Hu FB, Willett WC, Giovannucci E. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev 2006;15:167–71. [DOI] [PubMed] [Google Scholar]
- [24].Tseng M, Breslow RA, DeVellis RF, Ziegler RG. Dietary patterns and prostate cancer risk in the National Health and Nutrition Examination Survey Epidemiological Follow-up Study cohort. Cancer Epidemiol Biomarkers Prev 2004;13:71–7. [DOI] [PubMed] [Google Scholar]
- [25].Yang M, Kenfield SA, Van Blarigan EL, et al. Dietary patterns after prostate cancer diagnosis in relation to disease-specific and total mortality. Cancer Prev Res (Phila) 2015;8:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and prostate cancer. Continuous Update Project Expert Report; 2018. [Google Scholar]
- [27].Lai GY, Giovannucci EL, Pollak MN, et al. Association of C-peptide and leptin with prostate cancer incidence in the Health Professionals Follow-up Study. Cancer Causes Control 2014;25:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lai GY, Helzlsouer KJ, Clipp SL, Rifai N, Platz EA. Association between C-peptide concentration and prostate cancer incidence in the CLUE II cohort study. Cancer Prev Res (Phila) 2010;3:1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stevens VL, Jacobs EJ, Sun J, Gapstur SM. No association of plasma levels of adiponectin and c-peptide with risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2014;23:890–2. [DOI] [PubMed] [Google Scholar]
- [30].Stocks T, Lukanova A, Rinaldi S, et al. Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer 2007;120:2678–86. [DOI] [PubMed] [Google Scholar]
- [31].Hsing AW, Chua S Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst 2001;93:783–9. [DOI] [PubMed] [Google Scholar]
- [32].Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Increased serum insulin associated with increased risk of prostate cancer recurrence. Prostate 2002;50:1–3. [DOI] [PubMed] [Google Scholar]
- [33].Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008;9:1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gacci M, Russo GI, De Nunzio C, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis 2017;20:146–55. [DOI] [PubMed] [Google Scholar]
- [35].He K, Hu H, Ye S, Wang H, Cui R, Yi L. The effect of metformin therapy on incidence and prognosis in prostate cancer: a systematic review and meta-analysis. Sci Rep 2019;9:2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst 2007;99:1793–800. [DOI] [PubMed] [Google Scholar]
- [37].Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008;68:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Travis RC, Appleby PN, Martin RM, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res 2016;76:2288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cao Y, Nimptsch K, Shui IM, et al. Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int J Cancer 2015;136:2418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Commun Health 2007;61:824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stark JR, Li H, Kraft P, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer 2009;124:2683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Toriola AT, Laukkanen JA, Kurl S, Nyyssonen K, Ronkainen K, Kauhanen J. Prediagnostic circulating markers of inflammation and risk of prostate cancer. Int J Cancer 2013;133:2961–7. [DOI] [PubMed] [Google Scholar]
- [43].Arthur R, Williams R, Garmo H, et al. Serum inflammatory markers in relation to prostate cancer severity and death in the Swedish AMORIS study. Int J Cancer 2018;142:2254–62. [DOI] [PubMed] [Google Scholar]
- [44].Stikbakke E, Richardsen E, Knutsen T, et al. Inflammatory serum markers and risk and severity of prostate cancer: The PROCA-life study. Int J Cancer 2020;147:84–92. [DOI] [PubMed] [Google Scholar]
- [45].Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol 2004;171(2 Pt 2):S36–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


