Abstract
After decades of notoriety for its adverse cardiovascular, proinflammatory and profibrotic actions, the renin-angiotensin system (RAS) began to be cast in a more favorable light with the discovery of angiotensin-converting enzyme-2 (ACE2) in 2000. This monocarboxypeptidase, best known for its ability to metabolize angiotensin (Ang) II to Ang 1–7, counteracts the adverse effects of Ang II mediated by the AT1 Ang II receptor. Ang peptides are classically considered to be metabolized by aminopeptidases, by which the nomenclature Ang III (des-Asp1Ang II, 2–8 heptapeptide) and Ang IV (des-Asp1des-Arg2Ang II, 3–8 hexapeptide) are derived. This report compares the ability of recombinant human ACE2 (rhACE2) to metabolize Ang III, Ang IV and Ang V, (4–8 pentapeptide) relative to Ang II to form corresponding des-omega-Phe metabolites. rhACE2 has highest affinity (lowest Km) for Ang III, followed by Ang II ~ Ang V, followed by Ang IV. However, rhACE2 has the highest Kcat for metabolising Ang IV followed by Ang V, Ang III and Ang II. The enzymatic efficiency (Kcat/Km) is highest for Ang V and Ang III followed by Ang IV and is lowest for Ang II. As a gluzincin metallopeptidase, ACE2 requires a zinc molecule at its active site for catalysis. This report also documents inhibition of ACE2 activity by concentrations of zinc exceeding 10 μM. These observations extend the functional significance of ACE2 to include the metabolic inactivation of Ang III, Ang IV and Ang V, reemphasizing the importance of monitoring zinc intake to maintain metabolic homeostasis.
Keywords: Angiotensin-converting enzyme-2 (ACE2), Angiotensin II, Angiotensin III, Angiotensin IV, Angiotensin V (4–8 pentapeptide), Zinc
Graphical abstract
1. Introduction
Known more for its pathophysiological actions, the renin-angiotensin system (RAS) turned over a new leaf with the recognition of the counterregulatory actions of its ACE2/Ang 1–7/Mas axis (see review [1] selected from 212 citations in PubMed as of 11/14/20). By not only metabolically inactivating angiotensin (Ang) II [2], ACE2 also forms Ang 1–7, the agonist component of the ACE2/Ang 1–7/Mas axis [3], making it doubly powerful as a counterregulator of the classical ACE/Ang II/AT1 receptor axis responsible for the pathophysiological actions of the RAS [4]. While the RAS performs the important physiological functions of short-term systemic blood pressure, maintaining fluid and electrolyte homeostasis, and in utero development, the widespread use of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) [5], shown in Figure 1, reveals that in many individuals, the RAS is pathologically overactive.
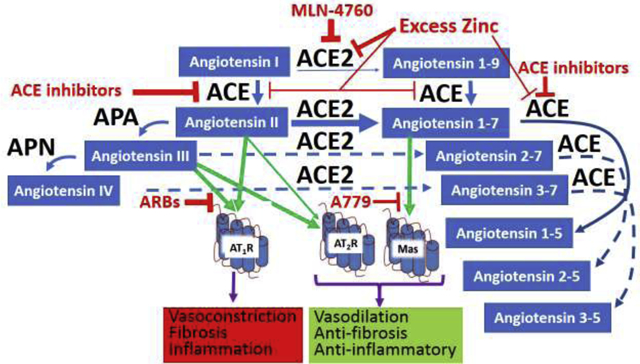
Figure 1.

Simplified diagram of renin-angiotensin system. Blue arrows denote metabolic pathways, green arrows denote receptor agonistic mechanisms, purple arrows denote physiological responses to receptor agonism, Red horizontal T’s represent enzyme inhibition or receptor antagonism. Width of the arrows represents relative significance of effects they represent. Dotted lines depict proposed metabolic pathways. IRAP is insulin-regulated aminopeptidase; HGF is hepatocyte growth factor; C-Met is a tyrosine kinase receptor, reported to also be a site of action of Ang IV [54]; ACE is angiotensin-converting enzyme; ACE2 is angiotensin-converting enzyme-2;A779 is D-Ala7 Ang 1–7 which blocks Mas receptor activation; ARBs are angiotensin receptor blockers which block activation of the AT1 receptor by both Ang II and III; PD123319 blocks both Ang II and III activation of the AT2 receptor.
Angiotensin-converting enzyme-2 (ACE2) was first discovered as a peptidase homolog of ACE (sometimes now referred to as ACE1), that converted Ang I to Ang 1–9 by hydrolysing the carboxy terminal leucine from Ang I [6]. Later it was discovered that ACE2 has a 400-fold higher catalytic efficiency to metabolize Ang II over Ang I [7] (Figure 1). Considering this preference to form Ang 1–7, that in general, has an opposite effect from Ang II through the activation of the Mas receptor [1, 3] ACE2 is now considered a major component of the RAS. The vasoprotective and antiproliferative effects of ACE2, which are likely the result of reduction of Ang II-mediated stimulation of AT1 receptors, has been demonstrated in animal studies in which it was shown that transgenic ACE2 overexpression reduces hypertension, [8], whereas suppression of ACE2 expression elevates blood pressure [9].
ACE2 is a type-I transmembrane monocarboxypeptidase and zinc metallopeptidase consisting of 805 amino-acids. It has an extracellular (ecto) domain (amino acids 18–739), a transmembrane region (amino acids 740–768), and an intracellular tail of 37 amino acids [10]. The extracellular part of ACE2 contains the catalytic domain (amino acids 147–555), which has a substrate binding region (amino acids 273–345) and a HEMGH metalloproteinase zinc-binding site motif (amino acids 374–378) as well as an additional glutamic acid at 402 which also contributes to zinc binding, categorizing it as a gluzincin type of zinc metallopeptidase [6, 11, 12]. The catalytic domain of ACE2 is a deep cleft, which consists of two subdomains linked by an α-helix hinge region [10]. The protein sequence in the catalytic domain of human ACE2 is 42% identical to its homolog angiotensin I-converting enzyme (ACE) [7].
The peptidase activity of ACE2 is dependent on the C-terminus sequence of the substrate [10]. ACE2 substrates generally have a hydrophobic or basic residue at the C-terminal end, preceded by a Pro-X-Pro motif, where either one of the two proline residues is sufficient to allow ACE2 to hydrolyze its peptide substrates [13]. In this circumstance, ACE2 can effectively cleave the carboxy terminal amino acids of Ang II (Pro-Phe) and Ang I (Pro- Phe-His-Leu), [10]. Based upon this model, it can be hypothesized that ACE2 should also be able to metabolize the pro-phe bonds of Ang III, Ang IV and Ang V (4–8 pentapeptide) to yield the des-omega-phe metabolites; Ang 2–7, Ang 3–7 and Ang 4–7, respectively. However, the kinetics of these metabolic pathways have not been characterized.
The renin-angiotensin system has been a developing story for more than 100 years with the discovery of novel angiotensin peptides arising from angiotensinogen. While Ang II (Ang 1–8) remains as the primary hormone of the RAS, other fragments of Ang II, in addition to Ang 1–7 possess biological activity; most notably Ang III [14] and Ang IV [15], (Figure 1). As with Ang II, the loss of the carboxy terminal phenylalanine may also remove or alter their biological activity.
Prior to the determination that ACE2 was a mediator of the counterregulatory arm of the RAS, a specific inhibitor of ACE2 activity, MLN-4760, was developed by Millenium Pharmaceuticals. It has a half maximum inhibitory concentration (IC50) of 0.4 nmol/L [16]. As noted above, the catalytic domain consists of two subdomains forming a deep cleft [10]. MLN-4760 binds to both subdomains with its two carboxylate groups preventing substrate binding. One carboxylate group binds to the zinc atom through displacement of the bound water molecule in the enzyme structure [12].
Interest in ACE2 beyond its role in the RAS developed when it was identified as the receptor by which the SARS coronavirus gained entry into cells [17]. With the subsequent discovery that ACE2 plays the same role for SARS-CoV-2 infections [18, 19] ACE2 gained a notoriety akin to that of the RAS as a pathophysiological mediator. Early concerns were voiced that ACE inhibitors and ARBs could increase ACE2 expression, thereby worsening the infectivity of SARS-CoV-2 [20, 21]. However, beyond the fact that it has not been established that ACE inhibitors and ARBs increase ACE2 expression in the human lungs, treatment strategies to reduce lung ACE2 expression leading to the loss of the counterregulatory actions of ACE2 to reduce the proinflammatory actions of the ACE/Ang II/AT1 axis is ill-advised [22–26].
Materials and Methods
The metabolism of Ang II, Ang III, Ang IV and Ang V to Ang 1–7, Ang 2–7 (Phoenix Pharmaceuticals), Ang 3–7 and Ang 4–7 (Bachem), respectively, by rhACE2 (933-zn, R&D Systems) was determined by quantitative HPLC analysis using a Microsorb-MV 100–5 C18 column, 250×4.6mm×1/4”. For the HPLC analysis a PerkinElmer series 200lc pump, series 200 refrigerated autosampler and a 200 UV/VIS detector was used. These were all connected to a computer via a PerkinElmer 600 series LINK. The software used to record and quantify the peak elution data by integration of the area under the curves (AUC) was TotalChrom version 6.3.0. The peptides were eluted isocratically with 14.5% acetonitrile: 85.5% triethylamine phosphate, 83 mM PO4, pH 3.0, at a flow rate of 1.6 ml/min. Identification of peptide product elution times and their quantification was based upon comparison with the elution times of standards of known amounts of Ang 1–7, Ang 2–7 and Ang 3–7 (Supplementary Table S1, Figures S1, S2 and S3 of HPLC chromatogram elution profiles ).
To assess ACE2 specific metabolism of the peptides, 1 μM of MLN-4760, a specific inhibitor of ACE2 (provided by Craig Thomas, NCATS, NIH) was added to alternate samples to enable subtraction of non-ACE2-mediated metabolism of the peptides.
The peptides and rhACE2, supplied as a solution of 1:1aqueous Tris, NaCl, ZnCl2:Glycerol, were incubated in 50 mM Hepes, 100 mM NaCl, pH 7.0 or other buffer as noted, for 30 min at 37°C based upon previous studies [13, 27, 28]. Zinc was added in the form of zinc acetate (ZnAc) for near optimum rhACE2 activity at a concentration of 10 μM, or at different concentrations to determine its effects on metabolism of Ang II by rhACE2. HEPES, Tris HCl, MES, NaCl and zinc acetate were obtained from Fisher Scientific or Sigma-Aldrich.
The duration of the incubation was 30 min based upon a target criterion of ≤10% metabolism of the peptide substrate by rhACE2, so as to maintain pseudolinear rates of metabolism.
Zinc inhibition study
Angiotensin II was diluted in a mix of HPLC water and 50 mM Hepes, 100 mM NaCl, pH 7.0, buffer to a final concentration of 100 μM. The samples were spiked with different zinc concentrations varying from 10 μM to 10 mM in steps of half log intervals to yield final zinc concentrations of 1 μM to 1 mM. Immediately prior to incubation rhACE2 was added to give a final concentration of 48.4 ng/ml. For this a 1:100 diluted rhACE2 stock was diluted another 10 times, which resulted in an added concentration of 0.484 μg/mL of rhACE2 enzyme in each sample, which was diluted another 10 times in the final sample volume to an end concentration of 48.4 ng/mL rhACE2 per sample. Using the value 85000 Daltons (specified by R & D Systems) as the molecular weight of ACE2, there were 569 fmoles of rhACE2/mL (569 pM) in each sample. The final assay volume was 0.5 mL, so there was 284 fmoles of rhACE2 per sample. As soon as the enzyme was added, the samples were vortexed and put into a 37°C water bath for 30 min incubation. The reaction was stopped by placing the samples in an 80°C water bath for 2 minutes, to denature the ACE2, after which samples were placed on ice.
The ability of zinc to inhibit metabolism of a fluorescent substrate by rhACE-2 was also determined. rhACE2 was diluted to a concentration of 10 pg/μL in 50 mM HEPES, tris HCl or MES, 100 mM NaCl, pH 7.0; with or without the ACE-2 inhibitor MLN-4760 (1 μM) with varying concentrations 0, 1, 3, 10, 30 100, 300 or 1000 μM of added zinc acetate, and 50 μM Mca-APK(Dnp) (BML P163, Enzo Life Sciences). Metabolism of the fluorogenic substrate was measured at 393 nm with excitation at 328 nm at 37°C. Data shown is % of rate of substrate metabolism in the absence of added zinc acetate.
Angiotensins II, III, IV and V metabolism
The Ang II, III and IV peptides were each run in duplicate and diluted in the final sample volumes of 500 μL to different final concentrations of 100 μM, 30 μM, 10 μM and 3 μM. To a second duplicate of 100 μM samples 1 μM (final concentration) MLN-4760 was added to inhibit the metabolism of the peptides by rhACE2. The assays were run in 50 mM Hepes, 100 mM NaCl, pH 7.0. To every sample zinc acetate at a final concentration of 10 μM was added to be at the near optimal concentration for optimal rhACE2 activity (based upon the zinc inhibition study). The incubation was initiated with the addition of diluted rhACE2 to give a final concentration of 21.1 ng/mL. Using the molecular weight 85000 Daltons for ACE2 there was 124 fmoles of rhACE2 in the 0.5 ml sample volume. As soon as the enzyme was added the samples were vortexed and incubated at 37°C in a water bath for 30 minutes. The reaction was stopped by placing the samples in an 80°C water bath for 5 minutes, which denatured the enzyme, and placement on ice.
In a separate series of assays the ability of rhACE2 to metabolize Ang V (4–8 pentapeptide) was also determined in comparison with metabolism of Ang II using the same protocol.
Mass Spectrometric analysis of Ang II metabolite
An aliquot of the metabolite fraction eluting at 2.9 min, corresponding to the elution time of Ang 1–7 from the HPLC column was subjected to mass spectroscopic analysis to verify its identity. Samples were analyzed by direct injection into the Mass spectrometer for electrospray ionization (ESI). The Ang 1–7 in the sample was determined by comparison with a standard curve of known amounts of Ang 1–7.
Statistical analyses
Graphical representation and statistical analysis of the data was done with GraphPad PRISM versions 6.0 and 8.0 for Windows, (GraphPad Software, La Jolla California USA, www.graphpad.com). One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using GraphPad Prism.
The inhibition of rhACE2 activity is presented as mean IC50 value ± SEM (n=2) of a one site fit equation to determine log IC50: Y = 100/(1 + 10(x-log IC50)) where Y = % activity in the absence of added zinc, and X = log concentration of zinc.
Determination of Km and Vmax values for rhACE2 activity in the metabolism of Ang II, III and IV used a Michaelis-Menten equation to fit the data points: V = Vmax * S/(Km + S) where V = velocity of the reaction at a given concentration of the substrate S. The data is expressed as mean Km, Vmax, Kcat and Kcat/Km values ± SEM (n=5). Comparisons of the kinetic values of specific rhACE2 activity in the metabolism of the mentioned substrates were done by one-way repeated measures analysis of variance (ANOVA). Dunnett’s multiple comparison tests were used to determine any statistically significant effects between Ang II and Ang III or Ang IV.
To verify that the metabolism of the angiotensin peptides was mediated by rhACE2, separate tubes contained the specific ACE2 inhibitor MLN4760 at a final concentration of 1 μM and Ang II at 100 μM. The amount of inhibition by MLN4760 for each concentration was extrapolated by linear regression between 0 and 100 μM Ang II. A two-way ANOVA was performed to statistically analyse the MLN-4760 inhibition of the metabolism for each studied substrate. The kinetic values were calculated for MLN4760 displaceable rhACE2 metabolism.
Results
Zinc inhibition of rhACE2 enzymatic activity
While ACE2 is a zinc-dependent metallopeptidase, the stoichiometry of zinc to ACE2 is 1:1 [2, 29, 30]. As provided by the manufacturer, R & D systems, rhACE2 0.44 mg/ml is dissolved in a 50:50 mix of aqueous 12.5 mM Tris-HCl, 100 mM NaCl, 5 mM ZnCl:glycerol. Using the calculated molecular weight of 85 kDa the concentration of rhACE2 in the solution is 518 nM. Thus as provided there is a 4.8 fold excess of Zn to rhACE2. In view of the tight binding of Zn by ACE2, it can be assumed that nearly all of the ACE2 molecules in solution contain Zn. Thus additional zinc would not be expected to enhance ACE2 activity. The concentration of rhACE2 in this assay was 569 pM, suggesting that the commonly used zinc concentration of 10 μM would be ~17,500 times that of ACE2 in this study.
Over the range of 1 to 1000 μM, increasing concentration of zinc inhibited the metabolism of Ang II by rhACE2 with an IC50 = 69.1 ±1.1 μM (Figures 2 and 3)
Figure 2.
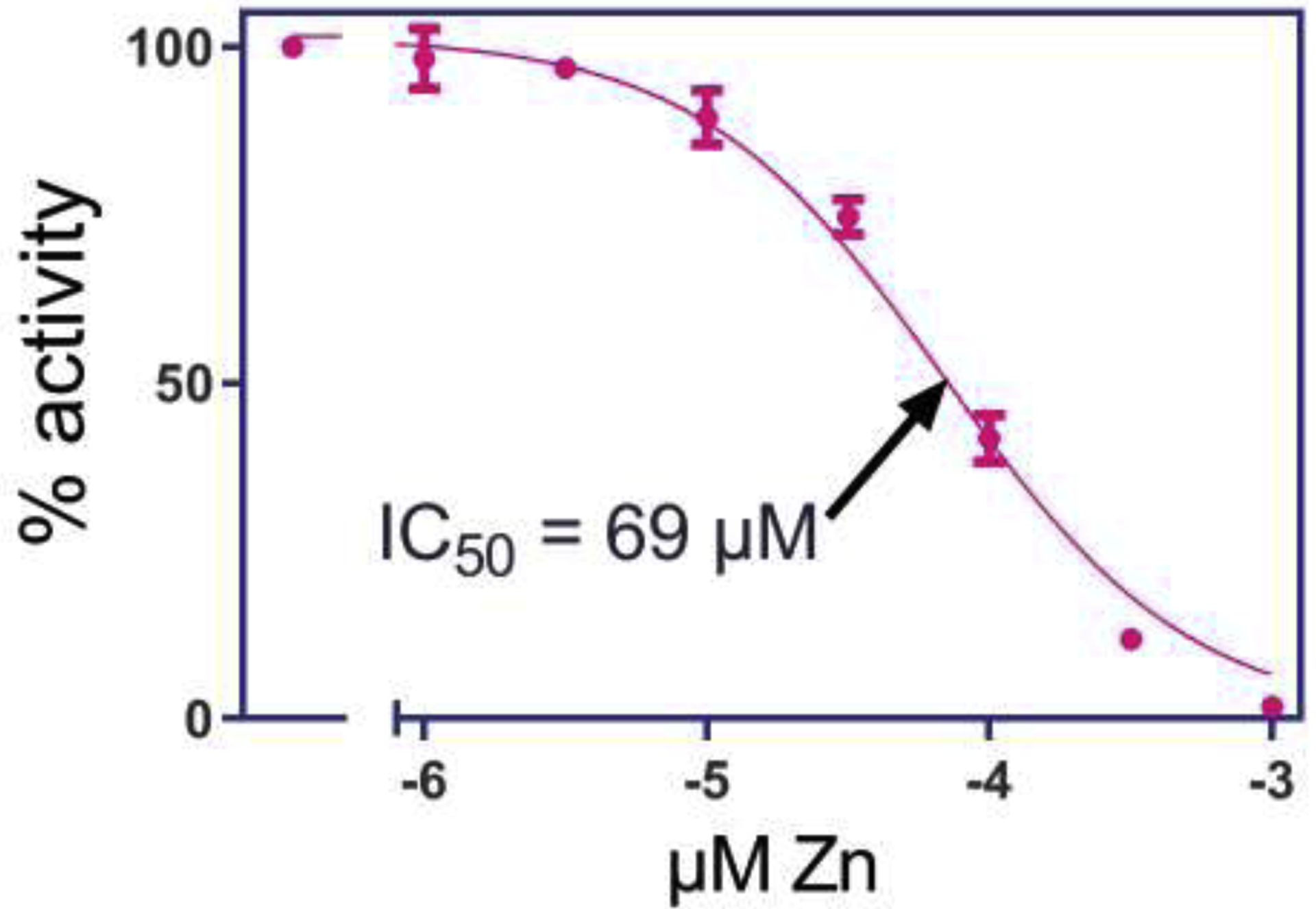
Inhibition of metabolism of Ang II by rhACE2 by increasing concentrations of zinc. The IC50.for inhibition by zinc of rhACE2 metabolism of Ang II to Ang 1–7 is 69 μM.
Figure 3.
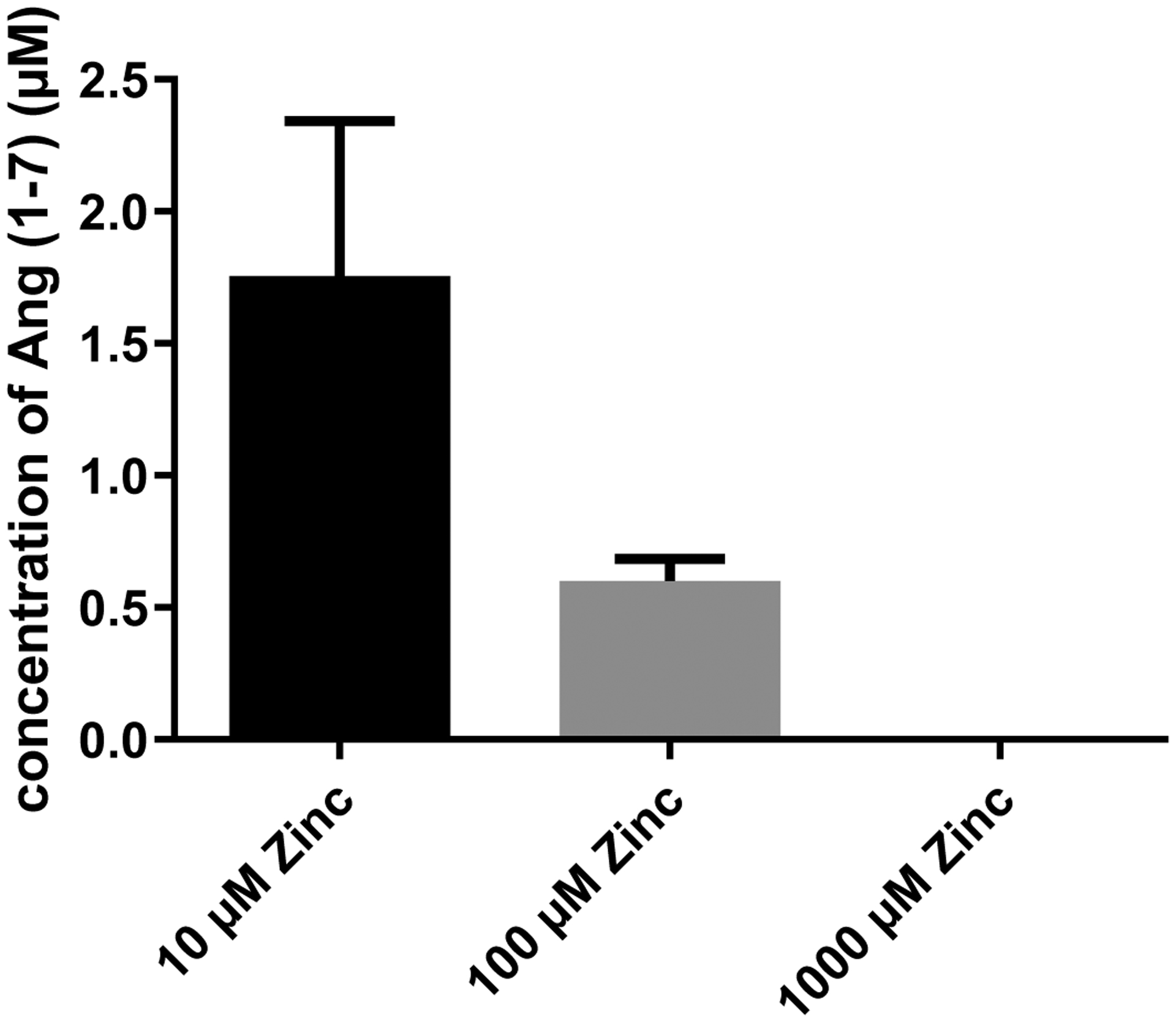
Retrieved Ang 1–7 concentrations (μM) as a result of Ang II metabolism by rhACE2 in presence of 10, 100 or 1000 μM Zinc.
Zinc was slightly less potent at inhibiting metabolism of the MCA-APK-dnp substrate by rhACE2. Figure 4 shows the inhibition curve of zinc for rhACE2 activity in 3 different buffers: HEPES, Tris HCl and MES all at pH 7.0. IC50 values were 170, 150 and 142 μM, respectively.
Figure 4.
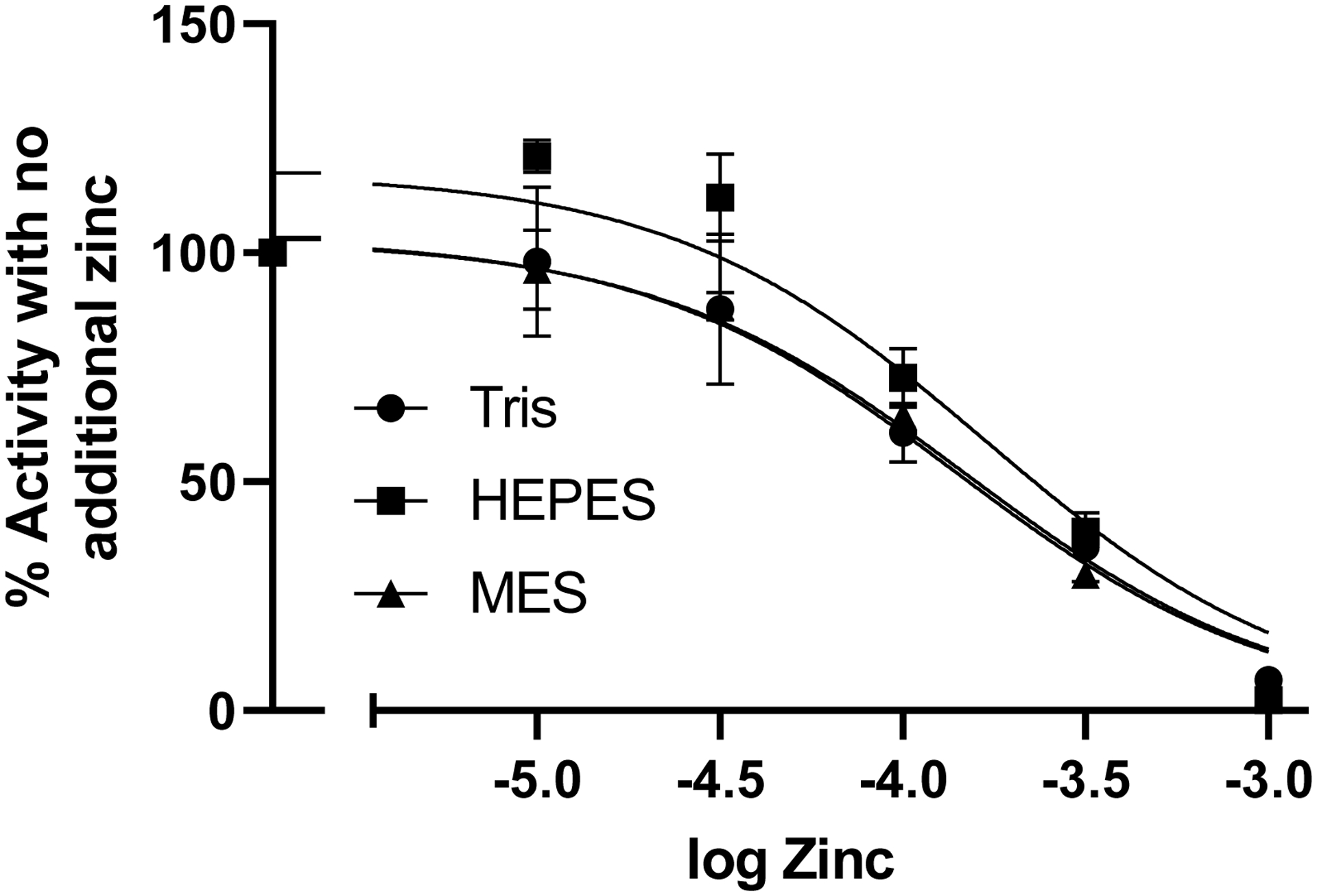
Inhibition of metabolism of fluorogenic substrate Mca-APK(dnp) by rhACE2 by increasing concentrations of zinc in 3 different buffers. Log IC50 values for Tris, HEPES and MES buffers were −3.82 (151 μM), −3.77 (170 μM), −3.85 (141 μM), respectively.
Ang II, III, IV and V metabolism by rhACE2
Table 2 describes the kinetics of the metabolism of Ang II, III and IV by rhACE2. Ang III was the preferred substrate with a Km of 2.87 μM, followed by Ang II and Ang IV. However, the Kcat and Vmax of rhACE2 for Ang IV was the highest followed by Ang III and Ang II. This gave the highest Kcat/Km value to Ang III and the lowest value to Ang II. Figure 5 shows a representative Michaelis-Menten plot of rhACE2 metabolism of Ang II, III and IV.
Table 2.
rhACE2 Km, Vmax, Kcat and Kcat/Km values for the metabolism of Ang II vs. Ang III vs. Ang IV.
| Substrate | Km (μM) | Vmax (nmol /30 min | Kcat (sec−1) | Kcat/Km (μm−1 sec−1) |
|---|---|---|---|---|
| Ang II | 8.53 ±1.43 | 1.83 ± 0.23 | 7.82 ± 0.97 | 0.90 ± 0.08 |
| Ang III | 2.87 ± 0.45*** | 3.65 ± 0.56* | 15.6 ± 2.4* | 6.52 ± 1.05** |
| Ang IV | 19.6 ± 0.97† | 14.3 ± 0.44† | 61.3 ± 1.3† | 3.07 ± 0.10 |
p < 0.05 greater than Ang II;
p < 0.0001 less than Ang II;
p < 0.001 greater than Ang II and p < 0.01 greater than ang IV;
p < 0.0001 greater than Ang II and Ang III. Values are mean ± SEM, n=5.
Figure 5.
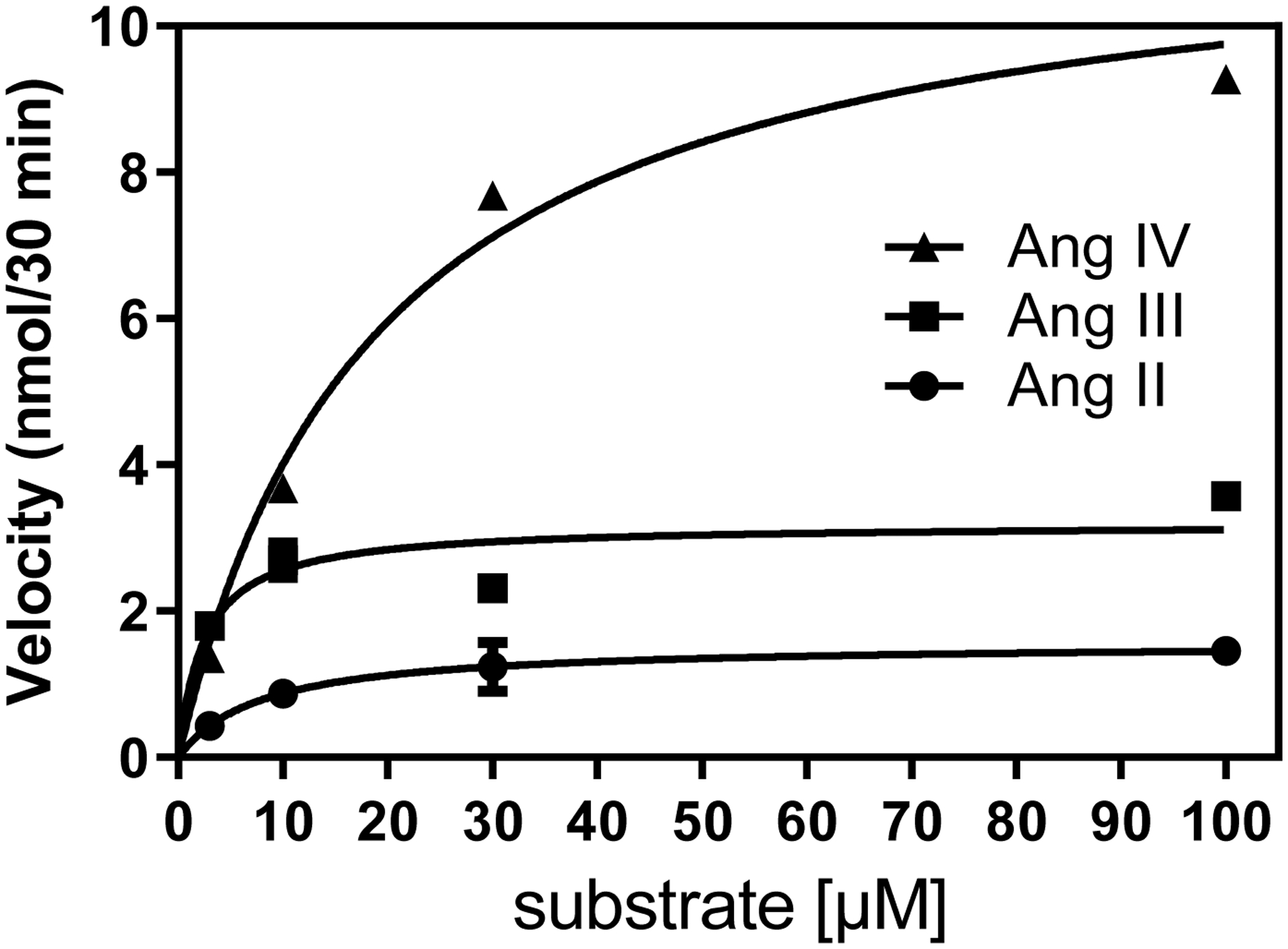
Representative Michaelis Menten plots with non-linear regression curves for specific (MLN-4760 inhibitable) rhACE2 activity in the metabolism of Ang II (●), Ang III (■) and Ang IV (▲), reaction time is 30 minutes, n=5.
Table 3 describes the kinetics of the metabolism of Ang V (4–8 pentapeptide) relative to Ang II in a separate series of assays. The Km of rhACE2 for Ang V was similar to that of Ang II, but its Kcat was significantly higher. Figure 6 shows a representative MLN-4760 displaceable and non-MLN4760 displaceable Michaelis-Menten plot of rhACE2 metabolism of Ang V relative to that of Ang II.
Table 3.
rhACE2 Km, Vmax, Kcat and Kcat/Km values for the metabolism of Ang II and Ang V.
| Substrate | Km (μM) | Vmax (nmol /30 min | Kcat (sec−1) | Kcat/Km (μm−1 sec−1) |
|---|---|---|---|---|
| Ang II | 8.41 ± 2.69 | 2.21 ± 0.18 | 8.42 ± 1.61 | 1.13 ± 0.25 |
| Ang V | 8.23 ± 3.23 | 7.13 ± 0.49* | 31.7 ± 2.6* | 6.22 ± 3.24 |
p < 0.05 greater than Ang II; Values are mean ± SEM, n= 3
Figure 6.
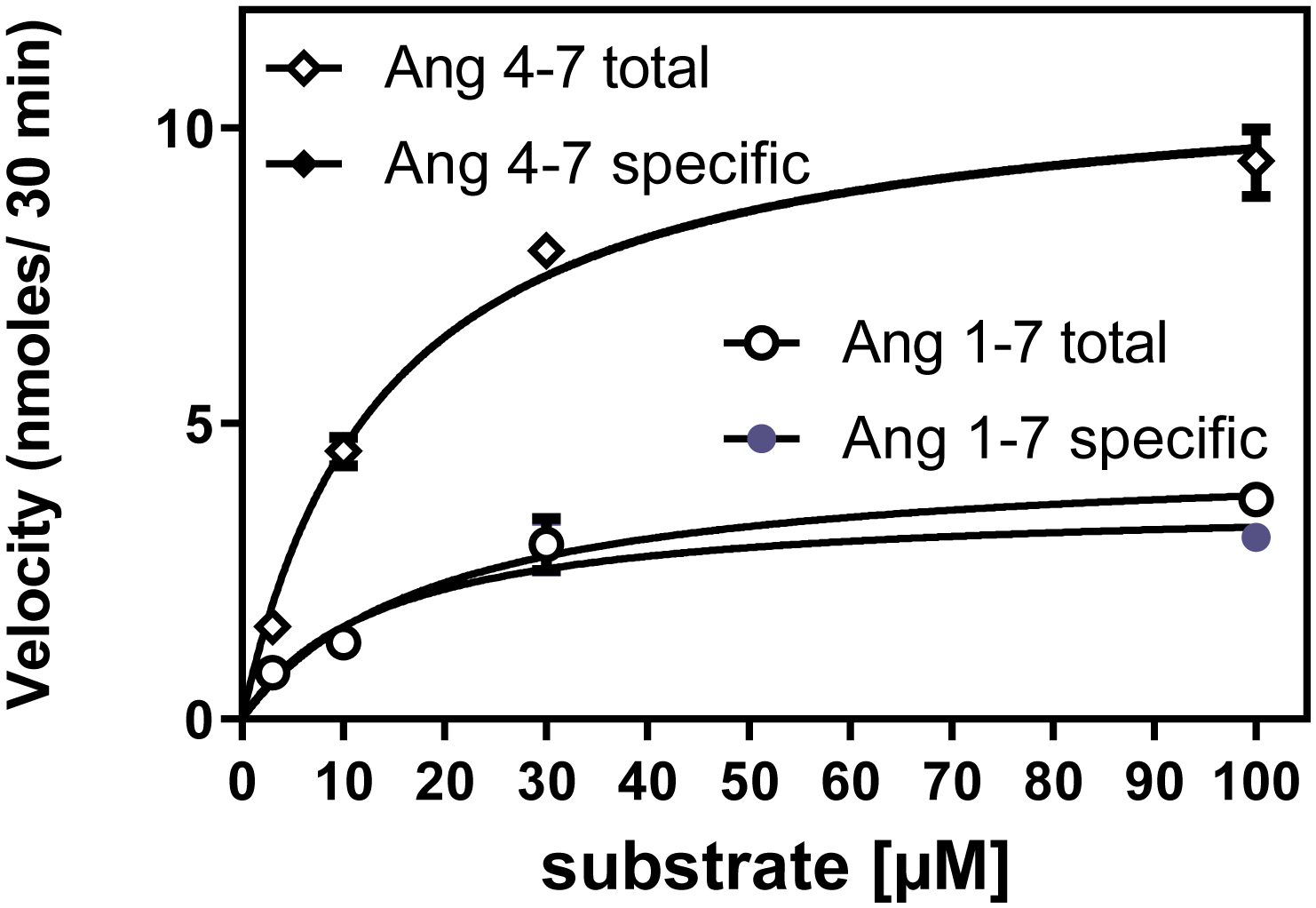
Representative Michaelis Menten plots with non-linear regression curves for total and specific (MLN-4760 inhibitable) rhACE2 activity in the metabolism of Ang II (○●) and Ang V (◊♦), reaction time is 30 minutes, n=3. The near perfect overlap of total and MLN displaceable Ang V metabolism hides the ♦ symbols.
MLN-4760 displacement
The results presented in table 4 shows that 1 μM MLN-4760 inhibits ~ 90% of rhACE2 activity. A two-way ANOVA comparing total and specific (MLN-4760 inhibitable) for Ang II, Ang III and Ang IV peptide did not show a significant difference in Vmax (F1,24= 0.39) indicating that MLN-4760 inhibited nearly all of the enzymatic activity for all three of these peptides. There was no significant interaction between MLN-4760 displacement and substrate for Vmax for these four peptides.
Table 4.
Percentage of the mean of specific rhACE2 metabolic activity inhibited by MLN-4760 at a concentration of 1 μM.
| Substrate | Vmax % MLN 4760 displaceable |
|---|---|
| Ang II | 91.5 |
| Ang III | 85.4 |
| Ang IV | 93.6 |
Discussion
ACE2 is a zinc metallopeptidase, requiring zinc at the catalytic site to bind and metabolize substrates. However, the zinc inhibition study has shown that ACE2 does not need very much zinc to ensure optimum ACE2 activity. ACE2 like other gluzincins with a single catalytic sites binds zinc in 1:1 stoichiometry with high affinity at physiological pH, suggesting that low concentrations of zinc are sufficient for full activity of these enzymes. Indeed, the stock solution of rhACE2 available from R & D Systems contains a concentration of zinc sufficient to saturate the the enzyme. At a 10 μM concentration of zinc, there was ~ 17,500 times the concentration of zinc to rhACE2 in our zinc inhibition study. Indeed, there appeared to be a slight inhibition of ACE2 activity even at this routinely employed concentration of zinc, relative to 0 or 1 μM added zinc. In conditions where more than 10 μM zinc is present ACE2 shows less than optimal activity. In fact, higher (1 mM) zinc concentrations inhibited ACE2 activity completely.
It is noteworthy that the potency of zinc to inhibit metabolism of the fluorescent substrate was lower than for the endogenous peptide substrates. The fluorescent substrate contains a basic amino acid coupled to Dnp, which may fit into the binding pocket of the active site differently from the pro-phe dipeptide sequence, being less susceptible to steric hindrance from excess zinc molecules that may be interacting in the vicinity of the active site of the enzyme. Additional studies indicate an even lower potency of zinc to inhibit membrane bound ACE2 metabolism of Mca-APK(dnp) in rat lung (IC50 = 448 μM) [31], suggesting that differences in the amino acid sequences of ACE2 between humans and rats, as well as other vertebrate species [32] may affect excess zinc binding to ACE2. Of note, preliminary studies indicate that zinc is also a less potent inhibitor of rat testis ACE metabolism of the fluorescent substrate, Abz-FRK (DNP) at pH 7.0; IC50 = 584 μM [31]. Zinc inhibited rabbit lung ACE metabolism of furanacryloyl (FA) phe-gly-gly substrate activity with an IC50 ~ 1.4 mM [33].
It is reported that excess zinc inhibits the bacterial enzyme lysostaphin due to a second (lower affinity) zinc binding site to catalytic histidines that competes for substrate binding [34]. A similar inhibition of carboxypeptidase A occurs in which zinc binding to a second zinc binding site at millimolar concentrations distorts the catalytic site of the enzyme [35]. Excess zinc binding to a second zinc binding site in thermolysin displaces water bound to an active site histidine which sterically hinders substrate binding and may also interfere with hinge bending associated with catalysis of substrate, leading to inhibition of its enzymatic activity [36]. Human neutrophil collagenase (matrix metalloproteinase 8, MMP8) shows a U-shaped response to zinc, with activity increasing up to 100 μM, after which activity decreases rapidly to ~ 20% at 10 mM zinc, again suggesting a second, lower affinity zinc binding site which interferes with substrate binding [37].
Since there are many zinc metallopeptidases, zinc homeostasis is critical to survival [38]. Zinc is considered to be an essential nutrient with a recommended daily allowance [39]. It is often recommended to be taken as supplement [40]. However, it can cause toxicity if consumed in excess [41]. A recent study demonstrated a correlation between high zinc intake and increased risk of cardiovascular disease in women [42]. Levels of zinc in the brain are reported to approach 300 μM during a convulsive state suggesting that zinc contributes to the neurotoxicity associated with intense glutamatergic stimulation of neurons [43].
Additionally, zinc has been recommended as a possible therapy to block replication of SARS-CoV-2 and promote immune function [44, 45]. Clinicaltrials.gov (accessed October 11, 2020) lists 39 ongoing trials of zinc therapy for COVID-19. It has also been shown to reduce the duration of colds caused by coronaviruses [46] and is widely promoted on the internet, e.g. a major online retailer lists 100 different brands of zinc supplements with amounts well in excess of the Recommended Daily Allowance (RDA). As a result there is a potential danger that people may consume excess zinc and reduce the ability of their ACE2 to metabolize Ang II with resulting increases in AT1R activation. Dysregulation of zinc homeostasis increases vascular senescence caused by Ang II [47]. While this has been attributed to additive effects of 50 and 100 μM zinc with Ang II to promote reactive oxygen species formation, it could also reflect a preferential reduction in ACE2 activity (compared to ACE activity) leading to increased levels of Ang II. Ang II was shown to potentiate neuronal cell death caused by 300 μM zinc [48]. It is thus important to take into consideration that the daily intake of zinc via zinc supplements should be limited.
ACE2 metabolism of angiotensin peptides
ACE2, while best known for its ability to metabolize Ang II, appears to have the highest affinity for Ang III as a substrate. Ang III is also metabolized at a higher rate than Ang II, such that the catalytic efficiency of ACE2 for Ang III is > 7 times greater than that for Ang II (Table 3). This suggests that the N terminal Arg provides a better fit into the substrate binding pocket of ACE2 than does Asp or Val.
Of special interest is the purported hypertensinogenic potency of Ang III by activating CNS AT1 receptors which has led to the development of the aminopeptidase A inhibitor firibastat as a potential antihypertensive drug by virtue of its ability to inhibit formation of Ang III in the brain [49]. The greater ability of ACE2 to metabolize Ang III relative to Ang II, adds to its counterregulatory importance of ACE2 in balancing the activity of the RAS.
Ang IV is also a substrate for ACE2. While it is a lower affinity substrate for ACE2 compared to Ang II, it is more rapidly metabolized than either Ang II or Ang III. As a result, the catalytic efficiency of ACE2 for Ang IV is 3.2 times greater than that for Ang II (Table 3). The Ang V (4–8 pentapeptide) and its 4–7 tetrapeptide metabolite have not yet been demonstrated to have specific receptors or physiological significance.
No further metabolism of the des Phe metabolites of the Ang peptides was observed, which was expected since the critical Pro-X motif, critical for ACE2 metabolism of peptides [13] is absent in the des Phe metabolites. The average values for Km and Kcat 8.47 μM and 8.12 μM−1,sec−1 respectively, are moderately different from those previously reported 2.0 μM and 3.5 μM−1,sec−1 [50]. As a result the average Kcat/Km value for Ang II metabolism to Ang 1–7 by rhACE2 in this study: 1.02 μM−1,sec−1 is slightly lower than the value reported previously; 1.8 μM−1,sec−1 [50]. Differences in methodology: MES buffer, 2 hour incubation at room temperature slight differences in the ACE2 structures may explain the different results.
At a concentration of 1 μM MLN-4760 inhibited nearly all of the metabolism of Ang II to Ang 1–7 reaffirming its high potency as an ACE2 inhibitor [51]. The total metabolism of Ang II, Ang III and Ang IV peptides was statistically indistinguishable from the MLN-4760 inhibitable activity. While it has yet to be developed into a drug due to the adverse consequences of increased stimulation of AT1Rs by Ang II, it was registered for a clinical trial NCT01039597 as ORE-1001 for treatment of ulcerative colitis in 2009. However, no results of the trial were posted to the clinicaltrial.gov website.
The current interest in ACE2 as the receptor for SARS-CoV-2 has clearly revealed a schism in our approach to its existence. Emerging thought regarding its functional significance in the COVID-19 pandemic now suggests that ACE2 can mitigate the harmful cytokine storm associated with COVID-19. It may be that the loss of ACE2 through its internalization into the cells on which it resides by SARS-CoV-2 exacerbates the pathogenicity of this coronavirus . Early fears that ACE inhibitors or ARBs might increase ACE2 expression in human lungs [20], even if they could do so, thereby worsening COVID-19 infectivity; are now replaced by speculation that if ACE inhibitors or ARBs or any other therapeutic approach can increase ACE2 expression, it might reduce the pathogenicity of SARS-CoV-2 [22–25, 52, 53] by reducing Ang II, and now, Ang III,-mediated AT1R activation leading to an increased inflammatory response [4].
Conclusion
In conclusion, the present study demonstrated that even though ACE2 is a zinc metallopeptidase, more zinc does not necessarily mean higher enzyme activity. 10 μM zinc was found to be the near-optimal concentration for optimal rhACE2 activity. In conditions where more than 10 μM zinc is present, ACE2 activity is inhibited.
rhACE2 shows the highest catalytic efficiency for metabolism of Ang III followed by Ang IV and Ang V, all of which are greater than that for Ang II. This study demonstrates that in general, the smaller the Ang peptide, the more efficiently it is metabolized by ACE2.
Supplementary Material
Table 1.
Angiotensin peptides with corresponding amino acid sequence.
| Peptide | Amino acid sequence |
|---|---|
| Ang II | Asp-Arg-Val-Tyr-Ile-His-Pro-Phe |
| Ang 1–7 | Asp-Arg-Val-Tyr-Ile-His-Pro |
| Ang III | Arg-Val-Tyr-Ile-His-Pro-Phe |
| Ang 2–7 | Arg-Val-Tyr-Ile-His-Pro |
| Ang IV | Val-Tyr-Ile-His-Pro-Phe |
| Ang 3–7 | Val-Tyr-Ile-His-Pro |
| Ang V | Tyr-Ile-His-Pro-Phe |
| Ang 4–7 | Tyr-Ile-His-Pro |
Highlights.
At the 20th anniversary of the discovery of Angiotensin-Converting Enzyme-2 (ACE2) this protein has taken on an entirely new persona of being the receptor for SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic.
Initially thought to be another pathway for formation of angiotensin (Ang) II, the primary active hormone of the renin-angiotensin system (RAS), it is now recognized as part of the counter-regulatory arm of the RAS which exerts effects antagonizing the pathophysiological effects of the RAS.
While ACE2 is a zinc-dependent metallopeptidase, excess zinc, > 10 μM can inhibit ACE2 activity. Because zinc is reported to have anti-coronaviral effects it is important to guard against excessive prophylactic zinc intake, which could worsen COVID-19 by removing the Ang II to Ang 1–7 pathway which reduces inflammation, a major contributor to COVID-19 morbidity.
ACE2 inactivates Ang II, forming Ang 1–7, which by acting upon its receptor Mas exerts anti-inflammatory and vasodilator effects.
ACE2 inactivates Ang III more efficiently than Ang II which complements its ability to counter-regulate the pathophysiological arm of the RAS
Acknowledgments
The authors thank Craig Thomas, NCATS, NIH for providing the MLN-4760 used in this study, Eduardo “Eddy” Carrera, Andrea Linares, Arlene Joachim, Malaika Jean-Baptiste, Austin Price and Carmen De Jesus for assistance with the research techniques; Junho Jeon and Francisco Fernandez-Lima for the Mass Spectrometric analysis of the angiotensin 1-7 peaks. Funding for this work was provided by the Wijck-Stam-Caspersfonds foundation, the (KNMP) to YP, NIDDK “STEP-UP” fellowship to Carmen De Jesus through the American Physiological Society, and NIH HL-113905 to RCS with minority supplements to AJ and MJ-B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ, The ACE2/Angiotensin-(1–7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1–7), Physiological reviews 98(1) (2018) 505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ, A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase, J.Biol.Chem 275(43) (2000) 33238–33243. [DOI] [PubMed] [Google Scholar]
- [3].Santos RAS, Silva ACSE, Maric C, Silva DMR, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SVB, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T, Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas, Proceedings of the National Academy of Sciences of the United States of America 100(14) (2003) 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S, Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology, Physiological reviews 98(3) (2018) 1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perez A, Speth R, Saavedra J, Trends in Angiotensin Receptor Blocker Use Among those at Risk for COVID-19 Morbidity and Mortality in the United States, medRxiv (2020) 2020.07.24.20161851. [Google Scholar]
- [6].Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S, A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9, Circ Res 87(5) (2000) E1–9. [DOI] [PubMed] [Google Scholar]
- [7].Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P, Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase, J Biol Chem 277(17) (2002) 14838–43. [DOI] [PubMed] [Google Scholar]
- [8].Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E, Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension, Circ.Res 106(2) (2010) 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uri K, Fagyas M, Manyine Siket I, Kertesz A, Csanadi Z, Sandorfi G, Clemens M, Fedor R, Papp Z, Edes I, Toth A, Lizanecz E, New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure, PloS one 9(4) (2014) e87845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C, Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets, Nat Rev Cardiol 11(7) (2014) 413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guy JL, Jackson RM, Jensen HA, Hooper NM, Turner AJ, Identification of critical active-site residues in angiotensin-converting enzyme-2 (ACE2) by site-directed mutagenesis, Febs j 272(14) (2005) 3512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW, ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis, J Biol Chem 279(17) (2004) 17996–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guy JL, Jackson RM, Acharya KR, Sturrock ED, Hooper NM, Turner AJ, Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence, Biochemistry 42(45) (2003) 13185–13192. [DOI] [PubMed] [Google Scholar]
- [14].Goodfriend TL, Peach MJ, Angiotensin III: (des-aspartic acid1)-angiotensin II Evidence and speculation for its role as an important agonist in the renin-angiotensin system, Circ.Res 36 and 37, Supp.1 (1975) 38–48. [DOI] [PubMed] [Google Scholar]
- [15].Swanson GN, Hanesworth JM, Sardinia MF, Coleman JK, Wright JW, Hall KL, Miller-Wing AV, Stobb JW, Cook VI, Harding EC, et al. , Discovery of a distinct binding site for angiotensin II (3–8), a putative angiotensin IV receptor, Regulatory peptides 40(3) (1992) 409–19. [DOI] [PubMed] [Google Scholar]
- [16].Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, Gavin JM, Hales P, Kaushik VK, Stewart M, Tummino PJ, Vickers CS, Ocain TD, Patane MA, Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors, J Am Chem Soc 124(40) (2002) 11852–3. [DOI] [PubMed] [Google Scholar]
- [17].Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature 426(6965) (2003) 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell 181 (2020) 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wan Y, Shang J, Graham R, Baric RS, Li F, Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS, J Virol 94 (2020) e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Diaz JH, Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19, J Travel Med 27 (2020) taaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fang L, Karakiulakis G, Roth M, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir Med 8 (2020) e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Speth RC, Response to recent commentaries regarding the involvement of angiotensin-converting enzyme 2 (ACE2) and renin-angiotensin system blockers in SARS-CoV-2 infections, Drug Dev Res 81 (2020) 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Speth RC, Keep Taking Your ACE Inhibitors and ARBs During the COVID 19 Pandemic, J Travel Med 27 (2020) taaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD, Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19, New England Journal of Medicine (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].South AM, Diz DI, Chappell MC, COVID-19, ACE2, and the cardiovascular consequences, American journal of physiology. Heart and circulatory physiology 318(5) (2020) H1084–h1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saavedra JM, Angiotensin receptor blockers and COVID-19, Pharmacological research 156 (2020) 104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rushworth CA, Guy JL, Turner AJ, Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis, Febs j 275(23) (2008) 6033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM, Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism, Biochem J 383(Pt 1) (2004) 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW, ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis, J.Biol.Chem 279(17) (2004) 17996–18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM, ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors, Can.J.Physiol Pharmacol 80(4) (2002) 346–353. [DOI] [PubMed] [Google Scholar]
- [31].Speth RC, Carrera EJ, Jean-Baptiste M, Joachim A, Linares A , Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity, Faseb J. 28(S1) (2014) 1067.4. [Google Scholar]
- [32].Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, Koepfli K-P, Pfenning AR, Zhao H, Genereux DP, Swofford R, Pollard KS, Ryder OA, Nweeia MT, Lindblad-Toh K, Teeling EC, Karlsson EK, Lewin HA, Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates, Proceedings of the National Academy of Sciences (2020) 202010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bünning P, Holmquist B, Riordan JF, Substrate specificity and kinetic characteristics of angiotensin converting enzyme, Biochemistry 22(1) (1983) 103–10. [DOI] [PubMed] [Google Scholar]
- [34].Raulinaitis V, Tossavainen H, Aitio O, Juuti JT, Hiramatsu K, Kontinen V, Permi P, Identification and structural characterization of LytU, a unique peptidoglycan endopeptidase from the lysostaphin family, Scientific reports 7(1) (2017) 6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gomez-Ortiz M, Gomis-Rüth FX, Huber R, Avilés FX, Inhibition of carboxypeptidase A by excess zinc: analysis of the structural determinants by X-ray crystallography, FEBS letters 400(3) (1997) 336–40. [DOI] [PubMed] [Google Scholar]
- [36].Holland DR, Hausrath AC, Juers D, Matthews BW, Structural analysis of zinc substitutions in the active site of thermolysin, Protein Sci 4(10) (1995) 1955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mallya SK, Van Wart HE, Mechanism of inhibition of human neutrophil collagenase by Gold(I) chrysotherapeutic compounds. Interaction at a heavy metal binding site, The Journal of biological chemistry 264(3) (1989) 1594–601. [PubMed] [Google Scholar]
- [38].Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME, Zinc and human health: an update, Arch Toxicol 86(4) (2012) 521–34. [DOI] [PubMed] [Google Scholar]
- [39].Trumbo P, Yates AA, Schlicker S, Poos M, Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, J Am Diet Assoc 101(3) (2001) 294–301. [DOI] [PubMed] [Google Scholar]
- [40].Mayo-Wilson E, Imdad A, Junior J, Dean S, Bhutta ZA, Preventive zinc supplementation for children, and the effect of additional iron: a systematic review and meta-analysis, BMJ Open 4(6) (2014) e004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fosmire GJ, Zinc toxicity, The American journal of clinical nutrition 51(2) (1990) 225–7. [DOI] [PubMed] [Google Scholar]
- [42].Milton AH, Vashum KP, McEvoy M, Hussain S, McElduff P, Byles J, Attia J, Prospective Study of Dietary Zinc Intake and Risk of Cardiovascular Disease in Women, Nutrients 10(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Assaf SY, Chung SH, Release of endogenous Zn2+ from brain tissue during activity, Nature 308(5961) (1984) 734–6. [DOI] [PubMed] [Google Scholar]
- [44].Bauer SR, Kapoor A, Rath M, Thomas SA, What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19?, Cleve Clin J Med (2020). [DOI] [PubMed] [Google Scholar]
- [45].Rahman MT, Idid SZ, Can Zn Be a Critical Element in COVID-19 Treatment?, Biol Trace Elem Res (2020) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mossad SB, Effect of zincum gluconicum nasal gel on the duration and symptom severity of the common cold in otherwise healthy adults, Qjm 96(1) (2003) 35–43. [DOI] [PubMed] [Google Scholar]
- [47].Patrushev N, Seidel-Rogol B, Salazar G, Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells, PloS one 7(3) (2012) e33211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Park MH, Kim HN, Lim JS, Ahn JS, Koh JY, Angiotensin II potentiates zinc-induced cortical neuronal death by acting on angiotensin II type 2 receptor, Mol Brain 6 (2013) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Arendse LB, Danser AHJ, Poglitsch M, Touyz RM, Burnett JC Jr., Llorens-Cortes C, Ehlers MR, Sturrock ED, Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure, Pharmacological reviews 71(4) (2019) 539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P, Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase, J.Biol.Chem 277(17) (2002) 14838–14843. [DOI] [PubMed] [Google Scholar]
- [51].Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, Gavin JM, Hales P, Kaushik VK, Stewart M, Tummino PJ, Vickers CS, Ocain TD, Patane MA, Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors, J Am.Chem Soc 124(40) (2002) 11852–11853. [DOI] [PubMed] [Google Scholar]
- [52].Sriram K, Insel PA, Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence, Clin Pharmacol Ther 108 (2020) 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gurwitz D, Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics, Drug Dev Res 81 (2020) 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yamamoto BJ, Elias PD, Masino JA, Hudson BD, McCoy AT, Anderson ZJ, Varnum MD, Sardinia MF, Wright JW, Harding JW, The angiotensin IV analog Nle-Tyr-Leu-psi-(CH2-NH2)3–4-His-Pro-Phe (norleual) can act as a hepatocyte growth factor/c-Met inhibitor, Journal of Pharmacology and Experimental Therapeutics 333(1) (2010) 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


