Abstract
Targeted prevention of heart failure (HF) remains a critical need given the high prevalence of HF morbidity and mortality. Similar to risk-based prevention of atherosclerotic cardiovascular disease, optimal HF prevention strategies should include quantification of risk in the individual patient. In this review, we discuss incorporation of a quantitative risk-based approach into the existing HF staging landscape and the clinical opportunity that exists to translate available data on risk estimation to help guide personalized decision making. We first summarize the recent development of key HF risk prediction tools that can be applied broadly at a population level to estimate risk of incident HF. Next, we provide an in-depth description of the clinical utility of biomarkers to personalize risk estimation in select patients at the highest risk of developing HF. We also discuss integration of genomics-enhanced approaches (e.g. TTN) and other risk enhancing features to reclassify risk with a precision medicine approach to HF prevention. While sequential testing is very likely to identify low and high-risk individuals with excellent accuracy, whether or not interventions based on these risk models prevent HF in clinical practice requires prompt attention including randomized placebo-controlled trials of candidate therapies in risk-enriched populations. We conclude with a summary of unanswered questions and gaps in evidence that must be addressed to move the field of HF risk assessment forward.
Keywords: Heart Failure, Risk Prediction, Biomarkers, Genetics
Introduction
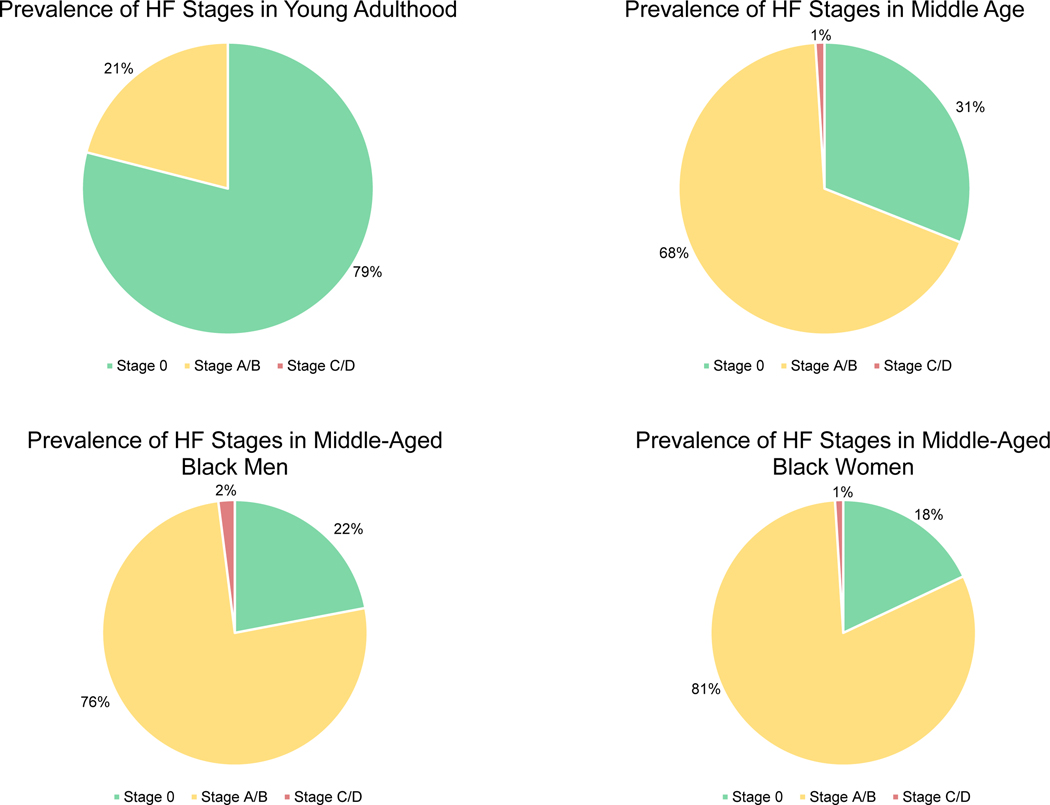
The high lifetime risk of heart failure (HF) in the US population is well established and estimates range from 20–46%.1,2 More than 8 million US adults are expected to have HF by 2030.3 Therefore, it is crucial to develop strategies focused on HF prevention that can be implemented broadly across populations and within health systems. The current construct of HF stages defined by the American Heart Association (AHA) and the American College of Cardiology (ACC) categorizes asymptomatic individuals at risk of developing HF as Stage A or B. An analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) cohort showed that the prevalence of Stage A/B increased from 21% between the ages of 22 and 37 to 68% between the ages of 47 and 62 (Figure 1).4 However, there is substantial heterogeneity of risk within HF Stage A/B, and there remain specific groups that are not included in the current HF staging schema but are nonetheless at increased risk for symptomatic HF.
Figure 1.
Prevalence of asymptomatic and symptomatic American Heart Association/American College of Cardiology Stages of Heart Failure (adapted from the Coronary Artery Risk Development in Young Adults study)4
To-date, much of the focus in HF has been on prevalent, symptomatic Stage C patients. Numerous risk prediction models using different methodologies including machine learning have been developed to estimate prognosis in symptomatic HF.5–7 In spite of significant advances in pharmacological, device, and surgical interventions for HF,8, 9 overall morbidity and mortality remain high and quality of life remains poor once symptomatic HF has developed.3, 10, 11 Thus, the focus needs to shift upstream to Stages 0 through B to prevent or delay the onset of symptomatic HF (Figure 2). The availability of management strategies (e.g. lifestyle modification,12–14 intensive blood pressure lowering15) and novel therapies (e.g. sodium glucose co-transporter 2 [SGLT2] inhibitors16) that can prevent or delay onset of symptomatic HF provides a compelling basis for the need to transform towards a risk-based paradigm in HF prevention. Specifically, earlier detection of high-risk individuals within Stage A or those with subclinical disease in Stage B who may derive the greatest benefit will inform a targeted approach to preventive interventions as has been demonstrated in biomarker-based trials for HF prevention.17, 18 Finally, identifying high-risk individuals may be utilized in clinical trial screening to study novel therapies in a risk-enriched population.
Figure 2.
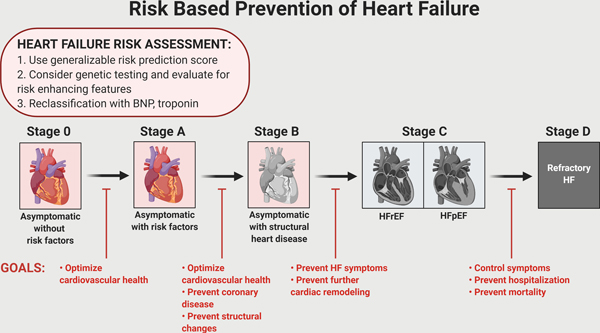
Overarching conceptual diagram of heart failure prevention (primordial, primary, and secondary) across the American Heart Association/ American College of Cardiology Stages
In order to match the intensity of prevention efforts with the absolute risk of the individual, a comprehensive understanding of HF risk prediction, reclassification, and personalization is needed. We aim to summarize the relevant data and create a framework for refining risk prediction within the heterogeneity of the current classification system to inform interventions focused on risk-based prevention of HF. In this review, we describe (1) available HF risk prediction models to calculate risk; (2) use of biomarkers to reclassify HF risk; (3) novel risk enhancers including genetics to personalize risk; (4) selective use of noninvasive imaging to identify subclinical dysfunction; and (5) unanswered questions and gaps in evidence that must be addressed in order to move the field of HF risk assessment forward.
Clinical Risk Prediction Models for Incident HF
The primary goal of a risk prediction model is to accurately quantify risk in the general population using readily available clinical variables. As HF can be caused by a wide array of etiologies, from familial dilated cardiomyopathy to ischemic heart disease, a single risk prediction model will not capture all of those at risk. This highlights the need to incorporate non-traditional risk enhancers in HF risk assessment to account for the heterogeneity within HF. However, traditional cardiovascular risk factors remain the predominant contributors to the population burden of HF.19, 20 Thus, the risk prediction model, which can ideally be used in the primary care setting for a large fraction of the general population, should be focused on the major cardiovascular risk factors. Herein, we restricted our discussion to risk prediction models that use factors readily available to primary care clinicians, specifically clinical history, routine lab values from lipid and metabolic panels, and/or electrocardiogram findings. In order to identify risk prediction scores that were generalizable, we focused on models derived from population-based cohorts free of baseline HF with external validation in a separate population-based cohort.
Criteria for evaluating risk prediction models have been previously described and most commonly include discrimination and calibration.21, 22 Discrimination is the ability of the model to distinguish those who will get the disease from those who will not and is assessed by determining the area under the receiver-operating-characteristic curve (AUC). The AUC incorporates both sensitivity and specificity of the prediction model with a value of 0.5 representing no discrimination and a value of 1.0 representing perfect discrimination. Calibration refers to the agreement between predicted and observed risks across the spectrum of baseline risk. Calibration is commonly measured using the Hosmer-Lemeshow χ2 or Greenwood-Nam-D’Agostino (GND) statistics, with a p-value >0.05 representing no significant difference. We report the AUC and calibration statistic, when available, for each of the models discussed here.
The utility of an easy to use risk calculator that clinicians can incorporate into their clinical visits has been well demonstrated with the Pooled Cohort Equations (PCE), a 10-year risk prediction model for atherosclerotic cardiovascular disease (ASCVD). The current AHA/ACC guidelines for primary prevention of ASCVD use the PCE to personalize and guide both cholesterol and blood pressure management.23, 24 Development of a validated HF risk calculator comprised of clinical variables that can be easily obtained during a clinic visit may similarly allow for targeted implementation of preventive therapies.
General Population-Based Cohort Risk Models of Incident HF
HF risk prediction models from population-based cohort studies with published external validation are summarized in Table 1. If available, we also included in Table 1 the risk prediction models derived in the cohorts that were used for external validation. One of the earliest HF risk prediction scores was developed in the Framingham Heart Study (FHS) cohort. This 10-year risk model included a mix of clinical risk factors such as age, systolic blood pressure (SBP), heart rate (HR), T2DM, body mass index (BMI), left ventricular hypertrophy (LVH) on electrocardiogram, coronary artery disease (CAD), and significant valvular disease on auscultation.25 In this cohort, CAD was the strongest predictor of incident HF. External validation of the FHS HF risk score was attempted in both the Atherosclerosis Risk in Communities (ARIC) and the Health Aging and Body Composition (ABC) study cohorts. It did not perform well in the ARIC cohort with an AUC of only 0.61.26 In the Health ABC study cohort, the FHS HF risk score was adapted to provide a 5-year HF risk prediction.27 The risk score discriminated better in men (AUC 0.74) than in women (AUC 0.68). The overall poor performance of the FHS risk score was likely due to differences in the age and racial composition of the cohorts. These findings underscore the need for external validation of risk predictions scores in multiple cohorts with diverse populations.
Table 1.
Incident heart failure risk prediction models from population-based cohorts
| Study Cohort | Demographics | HF Predictors | Internal Validation | External Validation |
|---|---|---|---|---|
| Free of CVD from Population-Based Cohorts | ||||
| Pooled Cohort Equations to Prevent Heart Failure (ARIC, CARDIA, CHS, FOF, MESA) | Ages: 30–79 White: 78%, Black: 22% Follow-up: 12 years |
Age, race, sex, smoking status, SBP, HTN medication, glucose, diabetes medication, TC, HDL-C, LDL-C, QRS duration |
Whites
Men – AUC: 0.79; Calibration: p = 0.06 Women – AUC: 0.85; Calibration: p = 0.14 Blacks Men – AUC: 0.71; Calibration: p = 0.78 Women – AUC: 0.78; Calibration: p = 0.33 |
PREVEND (Whites) Men – AUC: 0.80; Calibration: p = 0.03 Women – AUC: 0.87; Calibration: p = 0.30 JHS (Blacks) Men – AUC: 0.74; Calibration: p = 0.75 Women – AUC: 0.76; Calibration: p = 0.06 |
| MESA | Ages: 45–84 White: 39%, Black: 28%, Hispanic: 22%, Chinese: 12% Follow-up: 5 years |
Age, sex, smoking status, BMI, SBP, HR, DM, creatinine | AUC: 0.87 | -- |
| Inclusion of Participants with Prior CVD from Population-Based Cohorts | ||||
| Framingham | Ages: 45–94 White: 100%, Black: 0% Follow-up: 38 years |
Age, SBP, HR, LVH, CAD, DM, valvular disease, BMI | AUC: NR Calibration: NR |
ARIC – AUC: 0.614; Calibration: NR Health ABC – AUC: 0.735 (men), AUC 0.684 (women); Calibration: NR |
| ARIC | Ages: 45–64 White: 73%, Black: 27% Follow-up: 15 years |
Age, race, sex, CAD, SBP, HTN medication, DM, smoking status, HR, BMI | AUC: 0.797 Calibration: NR |
-- |
| Health ABC | Ages: 70–79 White: 59%, Black: 41% Follow-up: 6 years |
Age, SBP, HR, smoking status, LVH, CAD, creatinine, glucose, albumin | AUC: 0.72 Calibration: p=0.62 |
ARIC – AUC: 0.785; Calibration: NR CHS – AUC: 0.74; Calibration: p = 0.14 |
| International Collaboration of HF subtypes (FHS, CHS, PREVEND) |
Ages: 30–79 22048 White: 95%, Black: 5% Follow-up: 13 years |
HFpEF: age, SBP, BMI, HTN medication, prior MI HFrEF: age, sex, SBP, BMI, HTN medication, prior MI, LVH, LBBB, DM |
HFpEF – AUC: 0.79; Calibration: p = 0.34 HFrEF – AUC: 0.80; Calibration: p = 0.08 |
MESA
HFpEF – AUC: 0.76; Calibration: p = 0.81 HFrEF – AUC: 0.76; Calibration: p = 0.48 |
The HF risk score developed in the Health ABC study cohort consisted of age, SBP, HR, smoking status, LVH, CAD as well as routine laboratory values such as serum creatinine, glucose, and albumin.27 Since the baseline age was older (70–79 years) for this cohort, the HF risk score was based on a 5-year risk prediction model. Prevalent CAD was again the strongest predictor of incident HF. The Health ABC HF model had an AUC of 0.72 by internal validation and good calibration (χ2 6.24, p = 0.62). The Health ABC HF risk score was externally validated in the ARIC cohort and performed well with respect to discrimination (AUC 0.79).26 It was also externally validated in the Cardiovascular Health Study (CHS) cohort and performed relatively well with good discrimination (AUC 0.74) and calibration (χ2 14.72, p = 0.14).28
Both the FHS and Health ABC HF risk scores are inherently limited in generalizability as they are derived from single cohorts focused on specific population subgroups. A pooled study of participants from the FHS, PREVEND (Prevention of Renal and Vascular End-stage Disease), and CHS cohorts developed risk prediction models specific for HF subtypes (heart failure with persevered ejection fraction, HFpEF and heart failure with reduced ejection fraction, HFrEF).29 The HFpEF specific model included age, sex, SBP, BMI, antihypertensive treatment, and previous myocardial infarction (MI). The model was applied to a validation sample and had an AUC of 0.79 with good calibration (χ2 9.02, p = 0.34) The HFrEF specific model additionally included smoking, LVH, left bundle branch block (LBBB), and T2DM. In the validation sample, the model had an AUC of 0.80 with reasonable calibration (χ2 14.19, p = 0.08). Both models were then externally validated in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort. Both models performed well with good discrimination (HFpEF AUC 0.76, HFrEF AUC 0.76) and good calibration (HFpEF χ2 4.54, p = 0.81, HFrEF χ2 7.56, p = 0.48). However, over 95% of the discovery cohort was white, limiting generalizability. Furthermore, one global HF risk score, may make clinical implementation easier and is more clinically relevant given shared risk factors between HFrEF and HFpEF.
The prediction models discussed so far were developed in cohorts that included individuals with prevalent CAD. Their applicability is limited in the context of identifying high risk individuals for targeted primary prevention as individuals with CAD or MI should already be considered at high risk for HF and be on preventive therapies. An optimal derivation cohort for development of incident HF prediction model should exclude individuals with baseline ASCVD. The Pooled Cohort equations to Prevent HF (PCP-HF) were developed using pooled individual participant-level data free of ASCVD from 5 diverse cohorts, including ARIC, CARDIA (Coronary Artery Risk Development in Young Adults), CHS, FOF (Framingham Offspring Study), and MESA.30 Pooling across multiple contemporary cohorts allowed for a large enough sample size to generate race- and sex-specific models. The variables in the risk score included age, sex, race, SBP, hypertension treatment, fasting plasma glucose, T2DM treatment, BMI, smoking status, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and QRS duration (optional). Among white men and women, and Black men and women in the internal validation sample, the AUC in each race-sex group was 0.79, 0.71, 0.85, and 0.78, respectively. The equations had good calibration in the internal validation sample as assessed by GND statistic (p > 0.05 for all). The models were then externally validated in white participants from the PREVEND cohort and in Black participants from the Jackson Heart Study (JHS) with good discrimination (AUC ranging from 0.71 to 0.88) and strong calibration. The models have been further validated in a diverse cohort from a single integrated health system leveraging electronic health record data.31 Limitations of this risk score include the use of cohorts from earlier time periods and unclear applicability to other racial/ethnic ancestry groups such as Latinx or Asian.
Similar to the PCE for ASCVD, a tool such as the PCP-HF score can provide a quick and easy method for initial risk stratification to identify individuals at high risk for developing symptomatic HF. For example, a threshold of >5% predicted 10-year risk, which represents the top 10th percentile of the US population, could be proposed to categorize individuals at high risk who may benefit from enhanced surveillance with sequential risk stratification and application of preventive therapies and behavioral interventions aimed at preventing HF. Further studies are needed to examine different risk thresholds for interventions.
An important limitation in the studies discussed here is the difference in the definition of HF between cohorts. For example, most cohorts only reviewed hospitalizations while certain cohorts such as FHS, CHS, and MESA reviewed study and clinic examinations in addition to hospitalizations to determine HF events. HF events in the ARIC cohort were identified using administrative diagnosis codes from HF hospitalizations and death certificates, while the other cohorts had independent adjudication of using a combination of symptoms, physical exam findings, and imaging.
Clinical Risk Prediction Models for Incident HF in T2DM
A HF risk prediction model specifically for individuals with T2DM is of considerable interest given the strong association of T2DM with HF and the emergence of SGLT2 inhibitors, which can reduce HF in patients with T2DM by 23%.16, 32 Incident HF is also the most common initial cardiovascular presentation in patients with T2DM and given the rising prevalence of T2DM, stratifying HF risk in this population is of importance.33, 34
The predictors and performance characteristics of HF risk prediction models specific to T2DM that have been externally validated are described in Table 2. A 10-year HF risk prediction score (QDiabetes) was developed in individuals free from HF from the QResearch cohort, a patient-level database of over 1000 general practices covering a population >20 million patients in England.35 The risk score performed well with good discrimination and calibration in the internal validation dataset as well as in an external validation cohort of 357 separate general practices in England. T2DM-specific HF risk scores have also been developed using clinical trial populations. The WATCH-DM score was created using participants free from HF at baseline from the ACCORD (Action to Control Cardiovascular Risk in T2DM) trial and validated in individuals with T2DM from ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial).36 Similarly, a clinical risk score was developed using participants randomized to placebo in SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with T2DM Mellitus-Thrombolysis in Myocardial Infarction 53) and externally validated in the placebo arm of DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58).37
Table 2.
Incident heart failure risk prediction models in clinical trial populations with Type 2 diabetes mellitus
| Study Cohort | Demographics | HF Predictors | Internal Validation | External Validation |
|---|---|---|---|---|
| ACCORD Trial (WATCH-DM) |
Ages: 55–79 White: 63%, Black: 19%, Hispanic: 7%, Others: 11% Follow-up: 5 years |
Age, BMI, SBP, DBP, glucose, creatinine, HDL-C, QRS duration, prior MI, prior CABG | AUC: 0.72 Calibration: p = 0.23 |
ALLHAT Trial AUC: 0.74 Calibration: p = 0.20 |
| SAVOR-TIMI 53 | Ages: 55–75 White: 75%, Hispanic: 22% Other: 3% Follow-up: 2 years |
Atrial fibrillation, CAD, GFR, UACR | AUC: 0.81 Calibration: NR |
DECLARE-TIMI 58 AUC: 0.78 Calibration: p = 0.20 |
| QResearch | Ages: 25–84 White: 83% South Asian: 9% Black: 4% Other: 4% |
Age, BMI, SBP, TC/HDL-C ratio, HbA1c, material deprivation, ethnicity, smoking, duration of diabetes, atrial fibrillation, CVD, chronic kidney disease | Men – AUC: 0.764; Calibration: NR Women – AUC: 0.770; Calibration: NR |
CPRD Men – AUC: 0.769; Calibration: NR Women – AUC: 0.783; Calibration: NR |
However, these diabetes-specific risk scores have limitations. The QDiabetes risk score was developed and derived in databases that used diagnostic codes, which may lead to ascertainment bias. The clinical trial-based scores clearly cannot be generalized at a population level since trial inclusion criteria creates significant selection bias. In addition, the risk models included participants with established ASCVD or very high baseline cardiovascular risk. Therefore, it is unclear whether these risk scores would be useful in discriminating HF risk in the broader population of those with diabetes without underlying ASCVD. It is unknown whether they improve risk classification compared with the more generalizable HF risk scores discussed in the prior section.
HF Risk Reclassification
While clinical risk scores can be broadly applied to a general primary prevention population and are a key first step in risk assessment, validated biomarkers can further personalize risk estimates and minimize misclassification in Stage A/B HF patients. Net reclassification index (NRI) is an important, clinically relevant measure that helps quantify how well a new marker reclassifies patients when added to the existing the model.38 It is a sum of the proportion of patients that are correctly up-classified and down-classified with the introduction of a new marker. We limit our discussion primarily to widely available biomarkers such as B-type natriuretic peptide (BNP) and troponin, which can be readily adopted in clinical practice. We also briefly discuss the role of a multi-biomarker approach.
Natriuretic Peptide System
In response to ventricular myocardial wall stretch, pre-proBNP is synthesized and processed to pro-BNP, which is further processed into the biologically inactive N terminal-proBNP (NT-proBNP) and the biologically active BNP. The BNP pathway plays a fundamental role in cardiovascular remodeling and volume homeostasis and has been extensively studied in diagnosis of clinical HF, HF risk stratification, and as a pharmaceutical target for HF treatment.39, 40
A few studies have evaluated the improvement in HF risk prediction, as measured by categorical NRI, when BNP or NT-proBNP is added to the model. In the MESA cohort, addition of NT-proBNP significantly improved the HF risk prediction model (categorical NRI = 0.37). The improvement was primarily due to upward reclassification of individuals who subsequently developed HF.41 In the ARIC cohort, NT-proBNP was added to three separate HF risk prediction models.26 The addition of NT-proBNP significantly improved the FHS, Health ABC, and ARIC HF risk prediction models, as measured using categorial NRI, by 0.18, 0.12, and 0.13, respectively. The addition of NT-proBNP to the Health ABC HF risk score was also assessed in the CHS cohort.42 NRI was observed in 11% of the individuals with participants classified as intermediate risk by the clinical model deriving the greatest benefit. Similarly, in the Malmo Diet and Cancer Study, a community-based cohort in southern Sweden, NRI was observed in 16% of individuals with the addition of NT-proBNP to conventional risk factors.43 Again, this was mostly due to substantial upward reclassification to a higher risk category.
High-Sensitivity Troponin
High sensitivity (hs)-troponin is another commonly used biomarker that has been shown to be associated with incident HF and represents subclinical myocardial damage from underlying nonischemic etiology.44, 45 A prospective cohort of elderly individuals from CHS illustrated a 2.5-fold higher risk of HF in participants with the highest level of hs-troponin T (>12.94 pg/mL) compared to those with an undetectable level of hs-troponin T (<3 pg/mL).46 The higher risk did not attenuate even after further adjustment for BNP. In those who had repeat measures during follow-up, a rising trajectory was associated with a greater risk of HF while a declining trajectory was associated with a lower risk of HF. However, the addition of troponin T to a clinical risk model led to a modest NRI of 0.04. In the ARIC cohort, addition of hs-troponin I to the PCE led to a NRI of 0.09 for incident HF.47
Multi-biomarker Approach
A multi-biomarker approach is an appealing way to incorporate information from different pathways implicated in HF development. In the FOF cohort, the strength of association of soluble ST2, growth differentiation factor-15, and hs-troponin I with HF was similar to that of BNP.48 When these biomarkers were added to C-reactive protein and BNP to create a multi-biomarker score, individuals with scores in the highest quartile had a 6-fold higher risk of HF. Addition of the multi-biomarker score to the best-fit clinical model for HF led to a categorical NRI of 0.13. Another study in the FOF cohort demonstrated urinary albumin-to-creatinine ratio (UACR) to be a key predictor of HF.49 Addition of UACR and BNP to a clinical risk model led to NRI of 0.13. Higher UACR has been associated with impaired endothelial dysfunction in different patient populations50, 51, which is increasingly recognized as an important pathway in HF.52 Findings from these two studies suggest a modest additive value of incorporating multiple biomarkers beyond BNP in HF risk prediction models. Additional research is needed to determine which individuals may benefit from sequential multi-biomarker screening.
Risk Enhancers for Incident HF
HF risk prediction models based on traditional cardiovascular risk factors can perform well, but they likely underestimate risk in individuals with non-traditional risk factors. Specifically, genetic susceptibility for HF is identified as a key risk enhancer (Stage A) and will be the focus of this section to personalize risk stratification. Additional risk enhancing features also need to be considered to better identify individuals at high risk for HF. These include a myriad of comorbidities outlined in Table 3 and include chronic kidney disease, chronic liver disease, adverse pregnancy outcomes, chronic inflammatory diseases, radiation therapy, and history of cardiotoxic chemotherapy exposure.
Table 3.
Summary of cardiac dysfunction and heart failure risk secondary to risk-enhancing features
| Risk Enhancing Feature | HF and Cardiac Dysfunction Risk | Disease-specific Risk Factors |
|---|---|---|
| Chronic Kidney Disease53–55 | 1. 2-fold increase with eGFR <60 ml/min/1.73m2
2. 1.5 to 5-fold increase with increasing levels of albuminuria |
Anemia, insulin resistance, inflammation |
| Chronic Liver Disease56 | 1. Prevalence of cirrhotic cardiomyopathy as high as 40% | Hyperdynamic circulation, inflammation |
| Chronic Inflammatory Diseases57 | 1. RA: 1.3 to 2-fold increase58, 59 2. SLE: 3-fold increase60 3. SSc: 3 to 7-fold increase61 4. Psoriasis: 1.2 to 1.5-fold increase62 5. HIV: 1.5 to 2-fold increase63, 64 |
1. RA: +RF, elevated ESR, severe extraarticular manifestations 2. SLE: disease severity especially nephritis 3. SSc: diffuse cutaneous subtype, positive SSc specific serology, peripheral myositis65 4. Psoriasis: disease severity 5. HIV: low nadir CD4, low current CD4, high viral load |
| Cardiotoxic Chemotherapy66 | 1. Anthracyclines: 2–20% incidence rate of cardiac dysfunction 2. HER2 inhibitors: 3% incidence rate of cardiac dysfunction 3. Alkylating agents: 22% incidence rate of cardiac dysfunction 4. Taxanes: 0.7% incidence rate of cardiac dysfunction 5. VEGF inhibition: 0.2–20% incidence rate of cardiac dysfunction 6. Immune checkpoint inhibitors: 0.06 to 1% incidence rate of myocarditis |
Advanced age, female sex, CAD, cardiovascular risk factors, prior history of chest radiation, concurrent or sequential use of multiple cardiotoxic chemotherapies |
| Radiation Therapy67 | 1. Decreased LVEF in 7–15% with anterior radiation 2. Diastolic dysfunction in 22% of survivors of childhood cancer |
CVD risk factors, concomitant anthracycline use, anterior or left chest irradiation |
| Adverse Pregnancy Outcomes68–70 | 1. Pre-term birth: 1.6-fold increase 2. Pre-eclampsia: 2.2-fold increase 3. Maternal placental syndromes: 1.5-fold increase |
Hypertension, older age at pregnancy |
Genetic or Inherited Cardiomyopathies
Nearly one-fifth of the community burden of HF can be attributed to heritable factors.71 Both Mendelian (single gene) and non-Mendelian (common variants) genetic underpinnings of HF have been well-described. For the single gene mutations, there is a well described complexity of variable penetrance and expressivity of the genetic mutations which typically follow autosomal dominant inheritance patterns. In some gene mutations like TTN (titin) and certain arrhythmogenic right ventricular cardiomyopathy gene mutations, earlier or more severe manifestation of the phenotype may occur with concomitant exposure to environmental insults (e.g. hypertension), providing an opportunity for more intensive prevention strategies.72 The range of genetic contribution to HF is starting to be better understood, from rare pathogenic variants involved in inherited cardiomyopathies (development of dilated [DCM] and hypertrophic [HCM] cardiomyopathies) to more prevalent genetic variants that are increasingly being recognized as potential risk enhancers for HF.
Conservative estimates place the prevalence of DCM and HCM at 1 in 250 and 1 in 500, respectively. Currently, nearly 30% of DCM cases and over 50% of HCM cases have an identified genetic cause.73 The genes in which DCM-associated pathogenic variants most commonly occur include TTN, lamin A/C (LMNA), and β-myosin heavy chain (MYH7).73 In HCM, mutations in MYH7 and cardiac myosin binding protein-c (MYBPC3) account for 80% of inherited cases. The age of onset of DCM and HCM associated with specific mutations varies from adolescence to early middle-age or even later for DCM mutations. Therefore, it is crucial to obtain a targeted three-generation family history to identify potential asymptomatic individuals who are at significantly higher risk than their clinical HF risk score would otherwise suggest. An underlying genetic cardiomyopathy should be considered when two or more family members have been reported to have HF or a first-degree relative has had a premature sudden cardiac death without a well-defined cause.74 A positive family history should lead to cascade clinical assessment of the patient with ECG, echocardiography, and possibly heart rhythm monitoring.
Broadening the Role of Genetic Testing in HF
While cascade testing is essential for asymptomatic individuals with family history, family history is often an insensitive tool in identifying individuals with a genetic component to their HF.75 Even in individuals with isolated LV dysfunction without HCM or DCM, nearly 1 in 6 individuals without family history had a pathogenic mutation in DCM-related genes.76 Similarly, there is evidence that certain pathogenic mutations, primarily in TTN, create a genetic predisposition to HF due to other causes such as alcohol, chemotherapy and peripartum cardiomyopathy.77–79 Given the emerging understanding of this interaction between genetics and other etiologies of HF, a more broad and systematic approach to genetic testing should be considered in individuals with HF.80 Determining a potential genetic component is important for subsequent family screening, which would lead to identification of asymptomatic individuals at risk, as well as to inform life-saving changes to management (e.g. implantable cardioverter defibrillator) and prognostication.81
Furthermore, a genomics-informed (or genomics-first) approach may be used for certain genetic variants that have a high enough prevalence in certain population subgroups and may serve to guide risk reduction strategies. As genetic risks can be identified at birth, earlier diagnosis and initiation of preventive therapies may be a possibility. In this setting, such gene variants are considered risk alleles for HF and do not necessarily represent monogenic causes of HF. Examples of such genetic variants include titin truncating variants (TTNtv) in individuals with European ancestry, a transthyretin (TTR) variant in African Americans, and a MYBPC3 variant in south Asians. While nearly 15–20% of patients with DCM have a TTNtv, we have only recently started to understand the significance of TTNtv in the general population. The prevalence of TTNtv was approximately 0.5% in individuals with European ancestry and was associated with a 4.1-fold higher risk of incident HF.82 Similarly, the variant leading to valine to isoleucine amino acid substitution at position 122 (V122I) of transthyretin is present in nearly 4% of the African American population.83 Multiple studies have shown that the presence of the V122I TTR variant increases the risk of incident HF by approximately 1.5-fold.84, 85 The frequency of a 25 base-pair deletion in the MYBPC3 gene is 4% in south Asians. This deletion is associated with a nearly 7-fold higher risk of HF.86 Therefore, current strategies incorporating genetics into HF prevention should focus on screening for common variants with clear underlying pathophysiology in race-specific subgroups.
Refining Risk with Detailed Phenotyping of Cardiac Mechanics
Identification of high-risk Stage 0/A HF patients with quantitative risk assessment and sequential testing with biomarkers (including possible genetic risk and other risk enhancers) can help create an enriched pool that would achieve the greatest benefit from direct myocardial imaging to aid in early detection of Stage B HF. Classically, Stage B HF refers to structural heart disease such as prior MI, LVH, reduced left ventricular ejection fraction (LVEF), and valvular disease. Data from the Olmsted County, Minnesota cohort found the prevalence of systolic dysfunction, as defined by LVEF ≤ 50%, to be 6% and the prevalence of moderate or severe diastolic dysfunction with normal LVEF to be 5.6%.87 The prevalence of Stage B HF, encompassing a wide range of structural abnormalities, was estimated to be 34% in a cross-sectional study of Olmsted County residents.88 In the more recent analysis from the CARDIA cohort, 26% of middle-aged adults had Stage B HF.4 Individuals with Stage B HF and especially a decreased EF are at a significantly greater risk of developing HF, estimated to be near 10% risk over 10 years.89
As demonstrated in the SOLVD prevention trial, early identification of asymptomatic LV dysfunction in patients can lead to implementation of beneficial preventive therapies, such as beta-blockers and angiotensin converting enzyme inhibitors, that help reduce progression to Stage C HF.90, 91 In addition to medical therapy, multiple studies have demonstrated the importance of lifestyle in HF prevention. Specifically, physical activity, defined as ≥150 min/week of moderate intensity or ≥75 min/week of high intensity activity, has been associated with a lower risk of HF.12–14 While direct evidence of a specific diet reducing HF risk is lacking, different types of diet have been effective in preventing HF risk factors such as T2DM, hypertension, and ASCVD.92–94 Since these lifestyle changes are directly tied to social determinants of health, they need to be addressed by health systems and public health institutions via broader policy changes. Whether other therapies effective for treatment of Stage C HFrEF, such as mineralocorticoid receptor antagonists (MRA), angiotensin receptor-neprilysin inhibitor, and SGLT2 inhibitors, are also of benefit for the prevention of HF in patients with asymptomatic left ventricular dysfunction warrants further investigation. Furthermore, the use of more sensitive imaging markers such as LV global longitudinal strain (LV-GLS) may also better identify individuals at risk that can be intervened upon earlier to prevent HF.95, 96
Risk Based HF Prevention Trials
Our discussion on risk assessment, reclassification, and personalization identifies a pathway for selecting high-risk individuals for intervention trials focused on HF prevention. There have been two landmark studies that have evaluated the effect of BNP-based screening on HF prevention. The STOP-HF trial included adults with at least one HF risk factor and no evidence of systolic dysfunction or symptomatic HF with a mean age of 65 years.17 The participants randomized to the BNP-guided group had 45% lower development of LV dysfunction with or without HF over a 4-year period compared with those randomized to routine primary care. Along with more diagnostic interventions, there was a greater use of RAAS modifying therapies in the BNP-guided group. The PONTIAC trial was a similar study in patients with T2DM and no history of HF, who had an NT-proBNP level greater than 125 pg/mL. The participants randomized to the intensive therapy group were treated with up-titration of RAAS antagonists and beta-blockers, which resulted in a 5-fold lower incidence of hospitalization for HF compared with the routine care group.18
These two trials led to the class IIB recommendation of BNP screening in patients at high risk for HF in the 2017 AHA/ACC HF guidelines.97 However, it remains unclear how to identify these high-risk individuals, thus highlighting the need for a readily available risk score to appropriately select an enriched population that would benefit from biomarker testing, as opposed to shotgun screening. While 40% of the BNP-guided arm in STOP-HF had a BNP value above the threshold, nearly 25% of the treatment group had known ASCVD including prior MI. In a broader population without underlying ASCVD or prior MI, indiscriminate BNP screening is likely to be of low yield. Therefore, measuring BNP as a secondary testing strategy to reclassify individuals with an intermediate clinical risk score is likely to be of more value, while keeping in mind BNP-deficient states associated with higher BMI and African ancestry where the clinical utility of high sensitivity troponin and other biomarkers may be greater.98–100
Future Directions
The next phase in HF prevention research must be focused on examining efficacy of novel therapies in reducing incident HF in populations at greatest risk (Table 4). Risk-based trials utilizing risk scores, such as the race- and sex-specific PCP-HF tool, are needed to investigate the benefits of a management strategy guided by the PCP-HF risk score followed by sequential biomarker testing and cardiac imaging in high-risk individuals compared with usual care. The risk-based management strategy should evaluate a variety of interventions, including disease modifying therapies (e.g. RAAS agents), targeted uptake of SGLT2 inhibitors in those with or without DM, intensive risk factor modification, and lifestyle education. In addition, longitudinal studies are needed to determine how a genomics-enhanced approach with highly prevalent risk alleles for HF can best be translated into actionable clinical interventions. Specifically, it is not clear whether these risk alleles have synergy with HF risk factors and thus carriers may benefit from aggressive risk factor modification earlier in life. Novel therapies targeted at certain culprit genes such as TTR need to be studied in the context of HF prevention in carriers of risk alleles who have Stage B HF.
Table 4.
Future directions and unmet needs
| Question | Significance | Future Directions |
|---|---|---|
| Does using a HF risk score to identify individuals for preventive interventions reduce incident HF? | Important to identify patients that will gain the greatest benefit from early use of emerging preventive therapies | Trials comparing a clinical risk score-based strategy of management including subsequent use of biomarkers, imaging, and interventions focused on HF prevention compared with usual care |
| Does intensive lifestyle intervention in high-risk adults reduce incident HF? | Programs like the diabetes prevention program have shown significant benefits for risk factor management, and a similar framework needs to be explored with HF prevention | Creation and implementation of an intensive lifestyle program focused on adults at high-risk for HF with longitudinal follow-up to determine efficacy |
| When should biomarker or imaging-based screening be started in individuals with highly prevalent risk alleles to identify subclinical cardiac dysfunction? | The V122I TTR variant and the MYBPC3 deletion variant are present in nearly 4% of the African American and South Asian populations, respectively | Longitudinal cohort studies of young to middle-aged African American and South Asian adults with genetic data and contemporary cardiac imaging |
| In adults with TTR risk alleles, does initiation of TTR-specific therapies or aggressive risk factor modification during the subclinical phase prevent onset of HF? | Interaction of TTR risk alleles with clinical risk factors is not known; rationale for early use of TTR-specific therapies is based on the underlying pathophysiology and may significantly reduce morbidity and mortality | Large longitudinal cohorts studies to evaluate the interaction between TTR risk alleles and clinical risk factors; clinical trials of TTR-specific therapies in patients with TTR risk alleles and Stage B HF |
| How should HF risk be assessed in individuals with different risk enhancing features? | Clinical risk scores validated in the general population have not been specifically evaluated in these populations and disease-specific characteristics may need to be added to the score to improve its performance | Use of multicenter registries, specialized cohort studies, and electronic health record data to identify large enough cohorts for these more rare risk enhancing diseases |
Conclusions
Given the growing burden of HF on the healthcare system, a systematic approach to risk assessment of HF is necessary to inform personalized clinical approaches for precision prevention in those at highest risk for developing HF. We advocate for an easy-to-use clinical risk tool that can be applied broadly in the US population to estimate risk of HF. Risk for HF can be further reclassified using biomarkers such as BNP and UACR. Risk enhancing features including genetic risk also must be considered when determining an individual’s risk of HF. Those at high risk should undergo echocardiography to evaluate for structural heart disease and adverse cardiac mechanics, which may help refine risk and identify those who would benefit most from preventive strategies. In order to create this paradigm shift in HF prevention towards a risk-based approach, randomized clinical trials in risk-enriched populations are needed to generate the evidence base to support that this structured approach can decrease incident HF by focusing preventive strategies on those with the highest risk.
Acknowledgements:
The funding sponsors did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding:
Dr. Arjun Sinha is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL069771. Dr. Sadiya Khan is supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (KL2TR001424) and the American Heart Association (#19TPA34890060). Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number KL2TR001424. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:
Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GSK, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Shifamed, Tenax, and United Therapeutics.
REFERENCES
- 1.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML and Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. Journal of the American College of Cardiology. 2013;61:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP and Rana JS. Association Between Aging of the US Population and Heart Disease Mortality From 2011 to 2017. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.Gidding SS, Lloyd-Jones D, Lima J, Ambale-Venkatesh B, Shah SJ, Shah R, Lewis CE, Jacobs DR Jr., and Allen NB. Prevalence of American Heart Association Heart Failure Stages in Black and White Young and Middle-Aged Adults: The CARDIA Study. Circ Heart Fail. 2019;12:e005730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 6.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. [DOI] [PubMed] [Google Scholar]
- 7.Adler ED, Voors AA, Klein L, Macheret F, Braun OO, Urey MA, Zhu W, Sama I, Tadel M, Campagnari C, et al. Improving risk prediction in heart failure using machine learning. Eur J Heart Fail. 2020;22:139–147. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Hernandez AF, Solomon SD and Yancy CW. Potential Mortality Reduction With Optimal Implementation of Angiotensin Receptor Neprilysin Inhibitor Therapy in Heart Failure. JAMA Cardiol. 2016;1:714–7. [DOI] [PubMed] [Google Scholar]
- 9.Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG and Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–61. [DOI] [PubMed] [Google Scholar]
- 10.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND and Roger VL. Hospitalizations after heart failure diagnosis a community perspective. Journal of the American College of Cardiology. 2009;54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S and Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Failure Reviews. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Uijl A, Koudstaal S, Vaartjes I, Boer JMA, Verschuren WMM, van der Schouw YT, Asselbergs FW, Hoes AW and Sluijs I. Risk for Heart Failure: The Opportunity for Prevention With the American Heart Association’s Life’s Simple 7. JACC Heart Fail. 2019;7:637–647. [DOI] [PubMed] [Google Scholar]
- 13.Spahillari A, Talegawkar S, Correa A, Carr JJ, Terry JG, Lima J, Freedman JE, Das S, Kociol R, de Ferranti S, et al. Ideal Cardiovascular Health, Cardiovascular Remodeling, and Heart Failure in Blacks: The Jackson Heart Study. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, et al. Life’s Simple 7 and Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 17.Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 18.Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, Adlbrecht C, Prager R, Luger A, Pacher R, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62:1365–72. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Yamagishi K, Hozawa A and Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubicki DM, Xu M, Akwo EA, Dixon D, Muñoz D, Blot WJ, Wang TJ, Lipworth L and Gupta DK. Race and Sex Differences in Modifiable Risk Factors and Incident Heart Failure. JACC Heart Fail. 2020;8:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr., Hong Y, Howard BV, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PWF and Levy D. Profile for Estimating Risk of Heart Failure. Archives of Internal Medicine. 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PW, Newman AB, Harris TB, et al. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010;3:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, et al. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, Mentz RJ, O’Brien E, Correa A, Suthahar N, et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. Journal of the American College of Cardiology. 2019;73:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bavishi A, Bruce M, Ning H, Freaney PM, Glynn P, Ahmad FS, Yancy CW, Shah SJ, Allen NB, Vupputuri SX, et al. Predictive Accuracy of Heart Failure-Specific Risk Equations in an Electronic Health Record-Based Cohort. Circ Heart Fail. 2020:Circheartfailure120007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Negishi K, Otahal P and Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta-analysis. Open Heart. 2015;2:e000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Standl E, Schnell O and McGuire DK. Heart Failure Considerations of Antihyperglycemic Medications for Type 2 Diabetes. Circ Res. 2016;118:1830–1843. [DOI] [PubMed] [Google Scholar]
- 34.Selvin E and Ali MK. Declines in the Incidence of Diabetes in the U.S.-Real Progress or Artifact? Diabetes Care. 2017;40:1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hippisley-Cox J and Coupland C. Development and validation of risk prediction equations to estimate future risk of heart failure in patients with diabetes: a prospective cohort study. BMJ Open. 2015;5:e008503-e008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segar MW, Vaduganathan M, Patel KV, McGuire DK, Butler J, Fonarow GC, Basit M, Kannan V, Grodin JL, Everett B, et al. Machine Learning to Predict the Risk of Incident Heart Failure Hospitalization Among Patients With Diabetes: The WATCH-DM Risk Score. Diabetes Care. 2019;42:2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation. 2019;140:1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr. and Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 39.Lee CY and Burnett JC Jr. Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–42. [DOI] [PubMed] [Google Scholar]
- 40.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA and Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. The New England journal of medicine. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 41.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart (British Cardiac Society). 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalogeropoulos AP, Georgiopoulou VV, deFilippi CR, Gottdiener JS and Butler J. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the Cardiovascular Health Study. JACC Cardiovasc Imaging. 2012;5:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engstrom G, Wang TJ, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggers KM, Lind L, Ahlström H, Bjerner T, Ebeling Barbier C, Larsson A, Venge P and Lindahl B. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. European heart journal. 2008;29:2252–2258. [DOI] [PubMed] [Google Scholar]
- 45.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. The New England journal of medicine. 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M and Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia X, Sun W, Hoogeveen RC, Nambi V, Matsushita K, Folsom AR, Heiss G, Couper DJ, Solomon SD, Boerwinkle E, et al. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation. 2019;139:2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velagaleti RS, Gona P, Larson MG, Wang TJ, Levy D, Benjamin EJ, Selhub J, Jacques PF, Meigs JB, Tofler GH, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malik AR, Sultan S, Turner ST and Kullo IJ. Urinary albumin excretion is associated with impaired flow- and nitroglycerin-mediated brachial artery dilatation in hypertensive adults. J Hum Hypertens. 2007;21:231–238. [DOI] [PubMed] [Google Scholar]
- 51.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ and den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet (London, England). 1992;340:319–323. [DOI] [PubMed] [Google Scholar]
- 52.Alem MM. Endothelial Dysfunction in Chronic Heart Failure: Assessment, Findings, Significance, and Potential Therapeutic Targets. Int J Mol Sci. 2019;20:3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE and Coresh J. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. [DOI] [PubMed] [Google Scholar]
- 54.Bailey LN, Levitan EB, Judd SE, Sterling MR, Goyal P, Cushman M, Safford MM and Gutiérrez OM. Association of Urine Albumin Excretion With Incident Heart Failure Hospitalization in Community-Dwelling Adults. JACC Heart failure. 2019;7:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He J, Shlipak M, Anderson A, Roy JA, Feldman HI, Kallem RR, Kanthety R, Kusek JW, Ojo A, Rahman M, et al. Risk Factors for Heart Failure in Patients With Chronic Kidney Disease: The CRIC (Chronic Renal Insufficiency Cohort) Study. Journal of the American Heart Association. 2017;6:e005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xanthopoulos A, Starling RC, Kitai T and Triposkiadis F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart failure. 2019;7:87–97. [DOI] [PubMed] [Google Scholar]
- 57.Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, Chadha SA, Ahmad FS, Khan SS, Achenbach C, et al. Differential Associations of Chronic Inflammatory Diseases With Incident Heart Failure. JACC Heart Fail. 2020;8:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantel Ä, Holmqvist M, Andersson DC, Lund LH and Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69:1275–1285. [DOI] [PubMed] [Google Scholar]
- 59.Khalid U, Egeberg A, Ahlehoff O, Lane D, Gislason GH, Lip GYH and Hansen PR. Incident Heart Failure in Patients With Rheumatoid Arthritis: A Nationwide Cohort Study. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M and Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart. 2017;103:227–233. [DOI] [PubMed] [Google Scholar]
- 61.Butt SA, Jeppesen JL, Torp-Pedersen C, Sam F, Gislason GH, Jacobsen S and Andersson C. Cardiovascular Manifestations of Systemic Sclerosis: A Danish Nationwide Cohort Study. J Am Heart Assoc. 2019;8:e013405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khalid U, Ahlehoff O, Gislason GH, Kristensen SL, Skov L, Torp-Pedersen C and Hansen PR. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail. 2014;16:743–8. [DOI] [PubMed] [Google Scholar]
- 63.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez-Barradas MC, Lim J, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bissell L-A, Md Yusof MY and Buch MH. Primary myocardial disease in scleroderma—a comprehensive review of the literature to inform the UK Systemic Sclerosis Study Group cardiac working group. Rheumatology. 2016;56:882–895. [DOI] [PubMed] [Google Scholar]
- 66.Perez IE, Taveras Alam S, Hernandez GA and Sancassani R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin Med Insights Cardiol. 2019;13:1179546819866445–1179546819866445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mrotzek SM, Rassaf T and Totzeck M. Cardiovascular Damage Associated With Chest Irradiation. Front Cardiovasc Med. 2020;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silverberg O, Park AL, Cohen E, Fell DB and Ray JG. Premature Cardiac Disease and Death in Women Whose Infant Was Preterm and Small for Gestational Age: A Retrospective Cohort Study. JAMA cardiology. 2018;3:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellamy L, Casas J-P, Hingorani AD and Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ray JG, Schull MJ, Kingdom JC and Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98:1136. [DOI] [PubMed] [Google Scholar]
- 71.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O’Donnell CJ, Nam B-H, Larson MG, D’Agostino RB and Vasan RS. Association of parental heart failure with risk of heart failure in offspring. The New England journal of medicine. 2006;355:138–147. [DOI] [PubMed] [Google Scholar]
- 72.Liew C-C and Dzau VJ. Molecular genetics and genomics of heart failure. Nature Reviews Genetics. 2004;5:811–825. [DOI] [PubMed] [Google Scholar]
- 73.Rosenbaum AN, Agre KE and Pereira NL. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat Rev Cardiol. 2019: 10.1038/s41569-019-0284-0. [DOI] [PubMed] [Google Scholar]
- 74.Sweet M, Taylor MRG and Mestroni L. Diagnosis, prevalence, and screening of familial dilated cardiomyopathy. Expert Opin Orphan Drugs. 2015;3:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bailly C, Henriques S, Tsabedze N and Krause A. Role of family history and clinical screening in the identification of families with idiopathic dilated cardiomyopathy in Johannesburg, South Africa. S Afr Med J. 2019;109:673–678. [DOI] [PubMed] [Google Scholar]
- 76.Hazebroek MR, Krapels I, Verdonschot J, van den Wijngaard A, Vanhoutte E, Hoos M, Snijders L, van Montfort L, Witjens M, Dennert R, et al. Prevalence of Pathogenic Gene Mutations and Prognosis Do Not Differ in Isolated Left Ventricular Dysfunction Compared With Dilated Cardiomyopathy. Circ Heart Fail. 2018;11:e004682. [DOI] [PubMed] [Google Scholar]
- 77.Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchía J, García-Pinilla JM, Pascual-Figal DA, Nuñez J, Guzzo-Merello G, et al. Genetic Etiology for Alcohol-Induced Cardiac Toxicity. Journal of the American College of Cardiology. 2018;71:2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, et al. Genetic Variants Associated With Cancer Therapy-Induced Cardiomyopathy. Circulation. 2019;140:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, et al. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N Engl J Med. 2016;374:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musunuru K, Hershberger RE, Day SM, Klinedinst NJ, Landstrom AP, Parikh VN, Prakash S, Semsarian C and Sturm AC. Genetic Testing for Inherited Cardiovascular Diseases: A Scientific Statement From the American Heart Association. Circ Genom Precis Med. 2020;13:e000067. [DOI] [PubMed] [Google Scholar]
- 81.McNally EM and Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res. 2017;121:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pirruccello JP, Bick A, Friedman S, Chaffin M, Aragam KG, Choi SH, Lubitz SA, Ho C, Ng K, Philippakis A, et al. Prevalence and clinical importance of titin truncating variants in adults without known congestive heart failure. medRxiv. 2019:19005058. [Google Scholar]
- 83.Jacobson DR, Alexander AA, Tagoe C and Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22:171–174. [DOI] [PubMed] [Google Scholar]
- 84.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E and Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. The New England journal of medicine. 2015;372:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Damrauer SM, Chaudhary K, Cho JH, Liang LW, Argulian E, Chan L, Dobbyn A, Guerraty MA, Judy R, Kay J, et al. Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. JAMA. 2019;322:2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dhandapany PS, Sadayappan S, Xue Y, Powell GT, Rani DS, Nallari P, Rai TS, Khullar M, Soares P, Bahl A, et al. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR and Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 88.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Jr. and Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. [DOI] [PubMed] [Google Scholar]
- 89.Jong P, Yusuf S, Rousseau MF, Ahn SA and Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet (London, England). 2003;361:1843–1848. [DOI] [PubMed] [Google Scholar]
- 90.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Jr. and Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The New England journal of medicine. 1992;327:685–691. [DOI] [PubMed] [Google Scholar]
- 91.Exner DV, Dries DL, Waclawiw MA, Shelton B and Domanski MJ. Beta-adrenergic blocking agent use and mortality in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a post hoc analysis of the Studies of Left Ventricular Dysfunction. Journal of the American College of Cardiology. 1999;33:916–923. [DOI] [PubMed] [Google Scholar]
- 92.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA and Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. Jama. 2003;289:2083–93. [DOI] [PubMed] [Google Scholar]
- 94.Martínez-González MA, Gea A and Ruiz-Canela M. The Mediterranean Diet and Cardiovascular Health. Circ Res. 2019;124:779–798. [DOI] [PubMed] [Google Scholar]
- 95.Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, Shah AM and Jensen JS. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL and Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 98.Gupta DK, de Lemos JA, Ayers CR, Berry JD and Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart failure. 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, Selvin E, Coresh J, Konety S, Butler KR, et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. Journal of the American Heart Association. 2015;4:e001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clerico A, Giannoni A, Vittorini S and Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2012;17:81–96. [DOI] [PubMed] [Google Scholar]