Abstract
Background –
Venous thromboembolism (VTE) is a major cause of cardiovascular morbidity and mortality with a known genetic contribution. We tested the performance of a genetic risk score (GRS) for its ability to predict VTE in three cohorts of patients with cardiometabolic disease.
Methods –
We included patients from the FOURIER, PEGASUS-TIMI 54, and SAVOR-TIMI 53 trials (history of atherosclerosis, myocardial infarction, and diabetes, respectively) who consented for genetic testing and were not on baseline anticoagulation. We calculated a VTE GRS based on 297 SNPs with established genome-wide significance. Patients were divided into tertiles of genetic risk. Cox proportional hazards models were used to calculate hazard ratios for VTE across genetic risk groups. The polygenic risk score was compared to available clinical risk factors (age, obesity, smoking, history of heart failure, diabetes) and common monogenic mutations.
Results –
A total of 29,663 patients were included in the analysis with a median follow-up of 2.4 years, of whom 174 had a VTE event. There was a significantly increased gradient of risk across VTE genetic risk tertiles (p-trend <0.0001). After adjustment for clinical risk factors, patients in the intermediate and high genetic risk groups had a 1.88-fold (95% CI 1.23–2.89, p=0.004) and 2.70-fold (95% CI 1.81–4.06, p<0.0001) higher risk of VTE compared to patients with low genetic risk. In a continuous model adjusted for clinical risk factors, each standard deviation increase in the GRS was associated with a 47% (95% CI 29–68) increased risk of VTE (p<0.0001).
Conclusions –
In a broad spectrum of patients with cardiometabolic disease, a polygenic risk score is a strong, independent predictor of VTE after accounting for available clinical risk factors, identifying 1/3 of patients who have a risk of VTE comparable to that seen with established monogenic thrombophilia.
Keywords: genetics, genomics, venous thromboembolism, pulmonary embolism, deep vein thrombosis, cardiometabolic disease, genetic risk score
Journal Subject Terms: Genetics, Thrombosis, Embolism, Vascular Disease
Introduction
Venous thromboembolism (VTE) is a major cause of cardiovascular (CV) morbidity and mortality. In the United States, there are approximately 900,000 VTEs annually, resulting in up to 100,000 deaths.1, 2 While acute precipitants and clinical risk factors are often the focus of determining the etiology of VTE, a small minority of patients have a mutation in a limited number of genes leading to an inherited thrombophilia.3 To that end, hypercoagulability and/or genetic testing can identify some uncommon genetic mutations such as factor V Leiden, antithrombin deficiency, protein C or S deficiency, or a prothrombin gene mutation.3 However, standard testing is usually unrevealing, with mutations present in only about 5% of the general population.1, 2 Thus, for many patients with VTE, no clear precipitant or risk factor is ever identified.
In contrast to uncommon thrombophilias, recent work has used genome-wide association studies (GWAS) to identify 297 independent single nucleotide polymorphisms (SNPs) associated with VTE, from which a polygenic risk score was developed.4 Application of the genetic risk score in the general population led to the identification of many more individuals with a genetic predisposition to VTE than previously recognized. In this study, we tested the performance of this polygenic risk score in three TIMI (Thrombolysis in Myocardial Infarction) trials to evaluate whether a polygenic risk score predicts VTE in patients across the spectrum of cardiometabolic disease. In addition, we contrast the magnitude of risk compared to established clinical risk factors for VTE and classic monogenic thrombophilias.
Methods
This study was approved by the local institutional review committees at each study site. Complete methods outlining the study design, study population, genotyping, imputation, genetic risk score, clinical endpoints, and statistical analysis are included in the supplemental appendix. Although data and study material will not be made universally available, we encourage parties interested in collaboration to contact the corresponding author directly.
Results
A total of 29,663 patients from the three trials were included in these analyses including 12,981 from FOURIER, 10,607 from PEGASUS-TIMI 54, and 6,075 from SAVOR-TIMI 53. The baseline characteristics by tertile of genetic risk are included in Table 1; there were no clinically significant differences across genetic risk groups. The median follow-up across the study cohort was 2.4 years. There were 174 VTE events (95 DVT and 79 PE), 1,232 MIs, and 387 ischemic strokes.
Table 1:
Baseline Characteristics by Tertile of Genetic Risk
| Participants (n) | 9,888 | 9,887 | 9,888 | |
|---|---|---|---|---|
| FOURIER (%) | 42 | 44 | 45 | |
| PEGASUS (%) | 37 | 36 | 35 | |
| SAVOR (%) | 21 | 20 | 20 | |
| Demographics | ||||
| Age (yr) (+/−SD) | 64.5 ± 8.7 | 64.2 ± 8.6 | 64.0 ± 8.5 | <0.001 |
| Male Sex (%) | 7,365 (74) | 7,442 (75) | 7,319 (74) | 0.12 |
| BMI (+/−SD) | 29.9 ± 5.1 | 30.0 ± 5.2 | 29.9 ± 5.1 | 0.33 |
| Medical History (%) | ||||
| Myocardial Infarction | 7,896 (80) | 7,954 (80) | 7,905 (80) | 0.53 |
| Stroke | 872 (9) | 906 (9) | 942 (10) | 0.23 |
| Peripheral Artery Disease | 1,037 (11) | 979 (10) | 1,093 (11) | 0.03 |
| Hypertension | 7,833 (79) | 7,913 (80) | 7,892 (80) | 0.33 |
| Heart Failure | 1,900 (19) | 1,982 (20) | 2,043 (21) | 0.04 |
| Diabetes | 4,487 (45) | 4,536 (46) | 4,463 (45) | 0.56 |
| Current Smoker | 2,164 (22) | 2,150 (22) | 2,302 (23) | 0.16 |
yr = years; SD = standard deviation; BMI = body mass index
Performance of GRS
There was a significantly increased gradient of risk across VTE genetic risk tertiles (p-trend <0.0001) (Figure 1). After adjustment for clinical risk factors, patients in the intermediate genetic risk group had a 1.88-fold increased risk of VTE (95% CI 1.23–2.89, p=0.004) and patients in the high-risk group had a 2.70-fold higher risk of VTE (95% CI 1.81–4.06, p<0.0001) (Figure 2) compared to the low genetic risk group. The risk of VTE increased linearly throughout the spectrum of genetic risk (Supplemental Figure 1), such that each standard deviation increase in the GRS carried a 47% increased risk of VTE (Adj. HR 1.47 [1.29–1.68], p<0.0001). The genetic risk score for VTE was not associated with an increase in arterial events such as MI or ischemic stroke (Supplemental Table 1). There was no heterogeneity in the performance of the VTE genetic risk score across multiple clinical subgroups (Figure 3).
Figure 1:
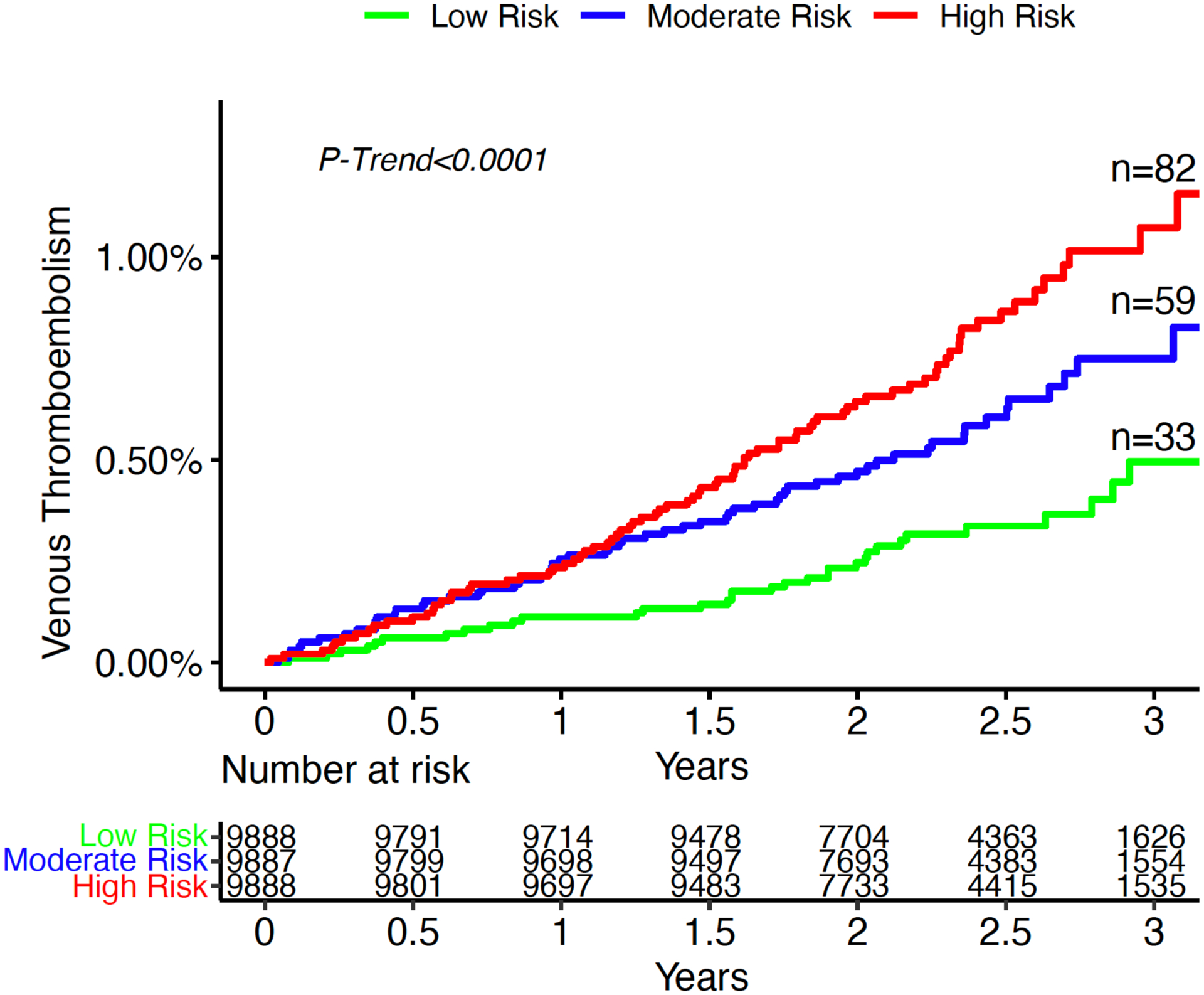
Three-year incidence of venous thromboembolism by tertile of genetic risk.
Figure 2:
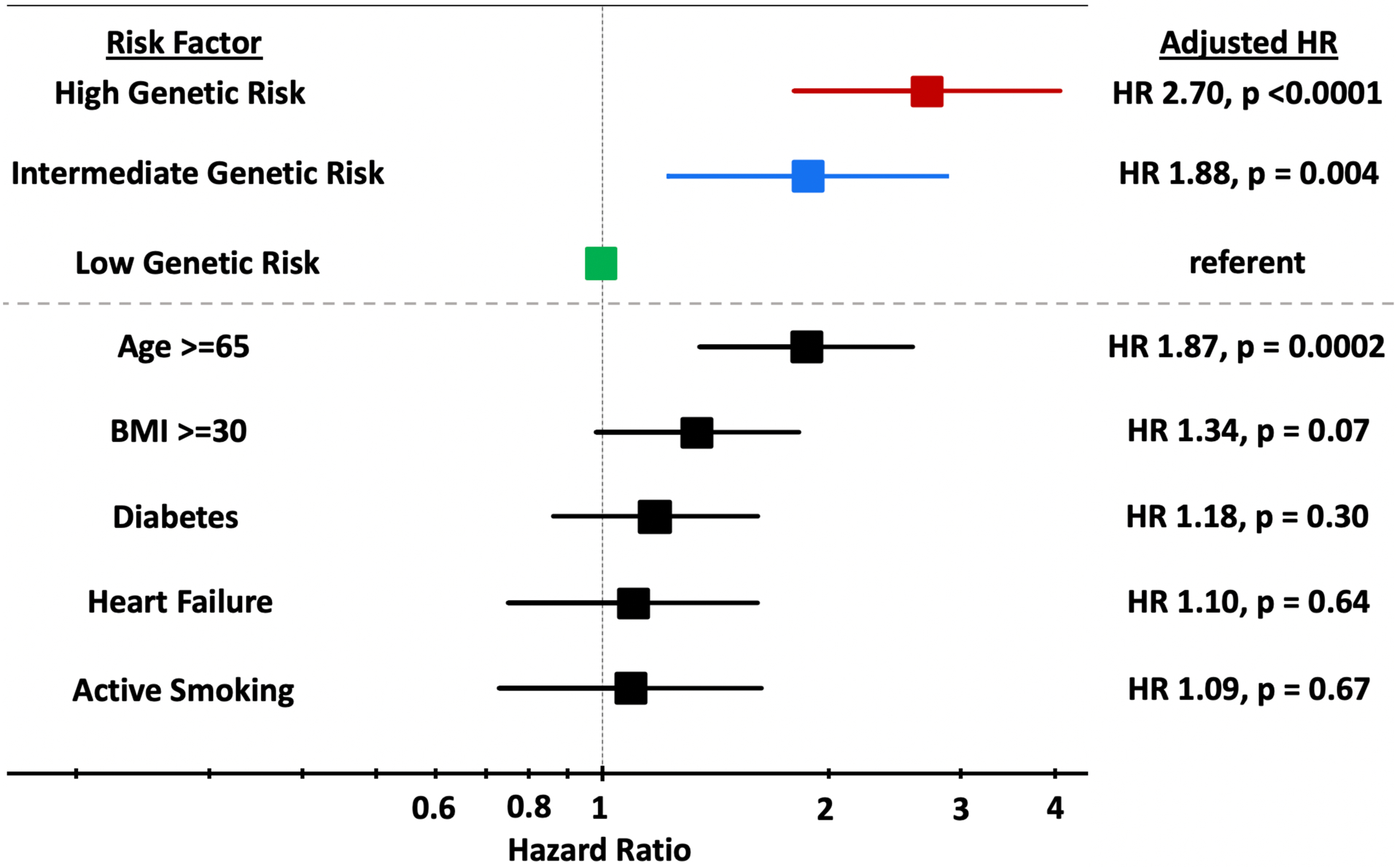
Forest Plot comparing High and Intermediate Genetic Risk to Clinical Risk Factors for VTE. Adjusted for age, sex, ancestry, obesity, active smoking, history of heart failure, and diabetes.
Figure 3.
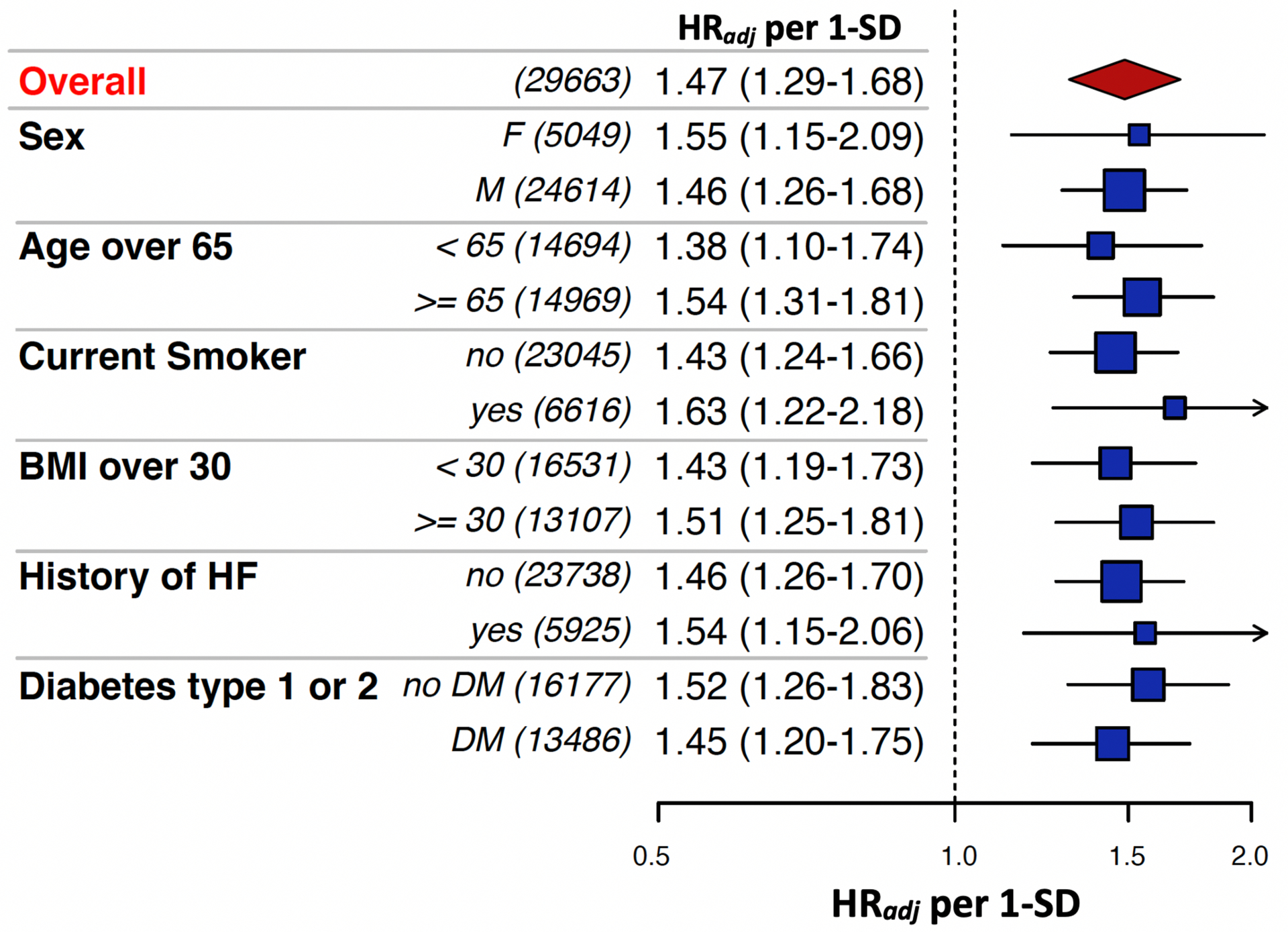
Subgroup Analysis of VTE Risk per 1-SD increase in Genetic Risk Score. There were no significant interactions across subgroups
Comparison to Clinical Risk Factors
Of the available clinical risk factors, only age ≥65 (HR 1.87 [1.35–2.59], p=0.0002) was a significant predictor of VTE, with risk similar to that conferred by an intermediate genetic risk score. Obesity (HR 1.34 [0.98–1.83], p=0.07) was a weaker predictor of VTE and there was no appreciable risk associated with diabetes (HR 1.18 [0.86–1.61], p=0.30), heart failure (HR 1.1 [0.75–1.61], p=0.64) or active smoking (HR 1.09 [0.73–1.63], p=0.67) (Figure 2). The c-index for VTE for all the clinical factors was 0.63 (0.59–0.67), whereas for the genetic risk score alone, it was 0.67 (0.63–0.71). The addition of the genetic risk score to clinical risk factors increased the c-index from 0.63 (0.59–0.67) to 0.67 (0.63–0.71) (p<0.0001).
Monogenic vs. Polygenic Risk
Of the entire genetic cohort, 2,474 patients (8.3%) had at least 1 of the 2 monogenic mutations (Factor V Leiden or Prothrombin) with frequency and overlap displayed in Table 2. Monogenic mutations were enriched among patients with VTE (14.9%, 26/174). Polygenic risk for VTE was balanced across patients with and without monogenic risk. In the 8.3% of patients with monogenic variants, polygenic risk did not improve risk prediction of VTE. However, the performance of the polygenic risk score strengthened when restricting the analysis to the 91.7% patients without a monogenic predisposition to VTE (HRadj per 1 SD: 1.53 (1.30–1.82); HRadj for Q3 vs. Q1: 2.88 (1.85–4.49) (Supplemental Table 2).
Table 2:
Prevalence of Monogenic Mutations
| Factor V Leiden | |||||
|---|---|---|---|---|---|
| Wild Type | Heterozygote | Homozygote | Total | ||
| Prothrombin | Wild Type | 27,189 | 1,583 | 37 | 28,809 |
| Heterozygote | 802 | 42 | 1 | 845 | |
| Homozygote | 9 | 0 | 0 | 9 | |
| Total | 28,000 | 1,625 | 38 | 29,663 | |
Discussion
When a patient experiences a VTE event without an acute precipitant such as recent surgery, immobilization, or trauma, one often considers clinical risk factors5 and contemplates testing for a handful of known, monogenic thrombophilia disorders. However, use of thrombophilia testing has fallen out of favor in part due to the low number of patients identified.3 These data demonstrate that consideration of broader polygenic risk can identify a much larger proportion of patients at risk for VTE and is a stronger predictor than many chronic clinical risk factors.
These findings are consistent with, and build upon, recent work done by Klarin, et al., who derived and validated this polygenic risk score for VTE in a general population. We now test the same score in a population with higher baseline risk and found the top one-third of patients had more than a 2-fold increased risk of VTE, suggesting that polygenic risk offers important insight into VTE risk among those with cardiometabolic disease. Also unique to this analysis is the comparison of the genetic risk score to both established clinical risk factors and monogenic thrombophilias.
Importantly, this genetic risk score was specific to venous thrombotic events and did not predict arterial thrombotic events such as MI or ischemic stroke. This is not unexpected as the score is distinct from a previously published 27-SNP score for coronary artery disease that we and others have studied.6, 7 Although there is some overlap, there is growing appreciation that risk factors and mechanism differ between arterial and venous thrombosis. The ability of VTE GRS to strongly predict venous thromboembolic events but not arterial, such as MI, supports this premise.
Physicians sometimes pursue hypercoagulability testing to identify uncommon but impactful etiologies for their patients with VTE. For example, Factor V Leiden (p.R506Q) is a monogenic mutation that is present in <5%3 of the population but carries a 2.3-fold increased risk of incident VTE4. Similarly, prothrombin mutation carries an approximately 2.8-fold increased risk.8 This degree of VTE risk is similar to that observed in one third of cardiometabolic patients with high polygenic risk. These data suggest the VTE polygenic risk score would identify far more patients with genetic risk compared to standard hypercoagulability testing. Whether this increased identification of genetic risk would improve the clinical utility of hypercoagulability testing is an area requiring further investigation.
Limitations
The study was made up of patients from three clinical trial populations that spanned the spectrum of cardiometabolic disease, however the results may not be generalizable to other disease domains. In particular, malignancies and pregnancies were excluded, which are major predisposing factors for VTE, and acute precipitants such as surgery and prolonged immobility were not captured. Thus, this analysis focuses on chronic risk factors for VTE. Moreover, not all trials collected use of hormonal therapies. Additionally, this analysis only included patients of European ancestry, as this is the population for which the genetic risk score was developed, and it is unclear how well it translates to other ethnicities. Finally, VTE events were collected as investigator reported adverse events, rather than predefined CEC adjudicated events. A total of 174 VTE events led to wide confidence intervals, which limited statistical power and precision. Nonetheless, in a model with clinical risk factors and GRS, the latter remained statistically significantly associated with VTE.
Conclusions
In a broad spectrum of patients with cardiometabolic disease, a polygenic risk score is a strong predictor of VTE, identifying one-third of patients with a risk of VTE similar to patients with monogenic inherited thrombophilia.
Supplementary Material
Acknowledgments:
NAM contributed to study design, literature search, statistical analysis, data interpretation, figures, and drafting of the manuscript. GEMM, YG, MPB, FKK, CR, and CL contributed to data preparation, study design, and statistical analysis. IC, RPG, BMS, DLB, PGS, MC, RFS, ACK, IR, OM, and EB contributed to data interpretation and critical review of the manuscript. SAL, PTE, MSS, and CTR contributed to study design, statistical analysis, data interpretation, figures, and critical review of the manuscript. CTR and MSS are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding: Amgen and AstraZeneca
Disclosures: Dr. Marston is supported by NIH grant funding. Drs. Melloni and Kamanu are members of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. Yared Gurmu is a member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. Dr. Bonaca discloses grant support from Amgen, AstraZeneca, Bayer, Sanofi and consulting fees from Amgen, AstraZeneca, Bayer, Sanofi. Ms. Roselli is supported by a grant from Bayer AG to the Broad Institute focused on the development of therapeutics for cardiovascular disease. Dr. Lee has nothing to disclose. Dr. Cavallari reports speaking fees from BMS-Pfizer, AstraZeneca, and Boehringer Ingelheim. Dr. Giugliano reports grants from Merck, during the conduct of the study; personal fees from Akcea, grants and personal fees from Amarin, personal fees from American College of Cardiology, grants and personal fees from Amgen, personal fees from Angel Med, personal fees from Beckman-Coulter, personal fees from Boeringer-Ingelheim, personal fees from Bristol Myers Squibb, personal fees from CVS Caremark, grants and personal fees from Daiichi Sankyo, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Lexicon, grants and personal fees from Merck, personal fees from Portola, personal fees from Pfizer, personal fees from St Jude, personal fees from Stealth Peptide, outside the submitted work; and Institutional research grant to the TIMI Study Group at Brigham and Women’s Hospital for research he is not directly involved in from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. Dr. Scirica has received research grants from AstraZeneca, Eisai, Novartis, and Merck and consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr Reddy’s Laboratories, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Merck, Novo Nordisk, Sanofi, and St Jude’s Medical; and has equity in Health [at] Scale. Dr. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr. Steg receives research grants from Amarin, Bayer, Sanofi, and Servier. Speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers-Squibb, Idorsia, Novartis, Novo-Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Dr. Cohen discloses honoraria for Speakers Bureau and advisory Boards (moderate) from AstraZeneca. Dr. Storey reports research grants, consultancy fees and honoraria from AstraZeneca; consulting fees and honoraria from Bayer and Bristol Myers Squibb/Pfizer; research grants and consultancy fees from GlyCardial Diagnostics and Thromboserin; consultancy fees from Amgen, Haemonetics, and Portola; honoraria from Medscape. Dr. Keech reports grants and personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Mylan, personal fees from Pfizer, grants from Sanofi, grants from Novartis, personal fees from Bayer, outside the submitted work. Dr Raz has received personal fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Con- center BioPharma and Silkim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Or- genesis, Pfizer, Sanofi, SmartZyme Innovation, Panaxia, FutuRx, Insuline Medical, Medial EarlySign, CameraEyes, Exscopia, Dermal Biomics, Johnson & Johnson, No- vartis, Teva, GlucoMe, and DarioHealth. Dr. Mosenzon reports serving on Advisory Boards for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, AstraZeneca, BOL Pharma; Research grant support through Hadassah Hebrew University Hospital: Novo Nordisk, AstraZeneca and Bristol-Myers Squibb; Speaker’s Bureau: AstraZeneca and Bristol-Myers Squibb, Novo Nordisk, Eli Lilly, Sanofi, Novartis, Merck Sharp & Dohme, Boehringer Ingelheim. Dr. Braunwald reports research grants through the Brigham and Women’s Hospital from Astra Zeneca, Merck, and Novartis. Consultancies with Amgen, Cardurion, MyoKardia, NovoNordisk, and Verve. Uncompensated consultancies and lectures with The Medicines Company. Dr. Lubitz is supported by NIH grant 1R01HL139731 and American Heart Association 18SFRN34250007. Dr. Lubitz receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, and Boehringer Ingelheim, and has consulted for Bristol Myers Squibb / Pfizer and Bayer AG. Dr. Ellinor reports grants and personal fees from Bayer AG, personal fees from Novartis, personal fees from Quest Diagnostics, outside the submitted work. Dr. Sabatine reports research grant support from Significant Abbott Laboratories, Amgen, AstraZeneca, Bayer, Critical Diagnostics, Daiichi-Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Quark pharmaceuticals, Roche Diagnostics, and Takeda and has received consulting fees; Modest from Alnylam, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, Dyrnamix, Esperion, IFM Pharmaceuticals, Intarcia, Ionis, Janssen Research and Development, Medicines Company, MedImmune, Merck, MyoKardia, and Novartis. In addition, they report significant consulting fees from Amgen. Dr. Ruff reports grants from Boehringer Ingelheim, grants from Daiichi Sankyo, grants from MedImmune, grants from National Institute of Health, personal fees from Bayer, personal fees from Bristol Myers Squibb, personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from Janssen, personal fees from MedImmune, personal fees from Pfizer, personal fees from Portola, personal fees from Anthos, outside the submitted work; Dr. Ruff is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. Dr. Marston has nothing to declare.
Nonstandard Abbreviations and Acronyms:
- VTE
venous thromboembolism
- GRS
genetic risk score
- SNP
single nucleotide polymorphism
- CV
cardiovascular
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- HR
hazard ratio
- CI
confidence interval
References:
- 1.Data and Statistics on Venous Thromboembolism. CDC.gov. 2020. https://www.cdc.gov/ncbddd/dvt/data.html. [Google Scholar]
- 2.Beckman MG, Hooper WC, Critchley SE and Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495–501. [DOI] [PubMed] [Google Scholar]
- 3.Connors JM. Thrombophilia Testing and Venous Thrombosis. N Engl J Med. 2017;377:1177–1187. [DOI] [PubMed] [Google Scholar]
- 4.Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, Aragam K, Chaffin M, Haas M, Lindstrom S, et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet. 2019;51:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavallari I, Morrow DA, Creager MA, Olin J, Bhatt DL, Steg PG, Storey RF, Cohen M, Scirica BS, Piazza G, et al. Frequency, Predictors, and Impact of Combined Antiplatelet Therapy on Venous Thromboembolism in Patients With Symptomatic Atherosclerosis. Circulation. 2018;137:684–692. [DOI] [PubMed] [Google Scholar]
- 6.Marston NA, Kamanu FK, Nordio F, Gurmu Y, Roselli C, Sever PS, Pedersen TR, Keech AC, Wang H, Lira Pineda A, et al. Predicting Benefit From Evolocumab Therapy in Patients With Atherosclerotic Disease Using a Genetic Risk Score: Results From the FOURIER Trial. Circulation. 2020;141:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mega JL, Stitziel NO, Smith JG, Chasman DI, Caulfield M, Devlin JJ, Nordio F, Hyde C, Cannon CP, Sacks F, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simone B, De Stefano V, Leoncini E, Zacho J, Martinelli I, Emmerich J, Rossi E, Folsom AR, Almawi WY, Scarabin PY, et al. Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate reductase C677T: a meta-analysis involving over 11,000 cases and 21,000 controls. Eur J Epidemiol. 2013;28:621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


