Abstract
Background -
The Val122Ile mutation in Transthyretin (TTR) gene causes a rare, difficult to diagnose hereditary form of cardiac amyloidosis. This mutation is most common in the United States and mainly present in people of African descent. The carriers have an increased risk of congestive heart failure, peripheral edema, and several other non-cardiac phenotypes such as carpal tunnel syndrome, and arthroplasty which are top reasons for ambulatory/outpatient surgeries in the country.
Methods -
We conducted first-ever epigenome-wide association study using the Illumina’s EPIC array, in Val122Ile carriers of African descent for heart disease (HD) and multiple outpatient surgeries (OS) - an early disease indicator. Differential methylation across genome wide CpG sites was tested between carriers with and without HD and OS. Significant CpG sites were investigated for cis-mQTLs loci, followed by gene ontology and protein-protein interaction (PPI) network. We also investigated the significant CpG sites in a secondary cohort of carriers for replication.
Results -
Five differentially methylated sites (p≤2.1e-08) in genes – FAM129B, SKI, WDR27, GLS, and an intergenic site near RP11-550A5.2, and one differentially methylated region containing KCNA6 and GALNT3 (p=1.1e-12) were associated with HD. For OS, we observe four sites – two sites in UBE2E3 and SEC14L5, and other two in intergenic regions (p≤1.8e-07) and three regions overlapping SH3D21, EVA1B, LTB4R2 and CIDEB (p≤3.9e-07). Functional protein-interaction module analysis identified ABCA1 (p=0.001) for HD. Six cis-mQTLs were associated with one of the significant CpG sites (FAM129B; p=4.1e-24). We replicated two CpG sites (cg18546846 and cg06641417; p<0.05) in an external cohort of biopsy- confirmed cases of TTR amyloidosis. The genes identified are involved in transport and clearance of amyloid deposits (GLS, ABCA1, FAM129B); cardiac fibrosis (SKI); and muscle tissue regulation (SKI, FAM129B).
Conclusions -
These findings highlight the link between a complex amyloid circuit and diverse symptoms of Val122Ile.
Keywords: amyloid, race and ethnicity, epigenetics, transthyretin, methylation, cardiac amyloidosis, Cardiomyopathy
Introduction
Hereditary transthyretin amyloidosis, caused by specific disease-causing mutations, is due to a gradual extracellular deposition of amyloid in multiple tissues primarily leading to several clinical signs and symptoms1. There are 113 known mutations in the transthyretin (TTR) gene2 giving rise to hereditary form of TTR amyloidosis. The tetrameric structure of TTR protein dissociates into dimers and monomers resulting in formation of fibrils. The heterogeneous symptoms of hereditary TTR amyloidosis (hATTR) arises from amyloid deposition in different tissues and organs2,3. Val122Ile mutation (NP_000362.1:p.Val142Ile; rs76992529) is the most prevalent TTR amyloidogenic variant in the United States and is primarily observed in populations of African descent4. This point mutation results in the substitution of an isoleucine with a valine at 122 position of TTR mature protein. According to latest report by International Society of Amyloidosis, the recommended nomenclature is named based on the substitution or deletion of the TTR protein, hence here we use Val122ILe 5. Extensive amyloid deposition seems to resemble hypertrophic cardiomyopathy such as enlargement or wall thickening leading to heart failure and atrial fibrillation6. These symptoms often are attributed to other population-prevalent cardiovascular risk factors resulting in underestimation of the clinical penetrance of the Val122Ile7. The estimated age of onset for non-cardiac precursor phenotypes for hereditary transthyretin amyloidosis is between 30 to 40 years of age8. In two retrospective studies, carpal tunnel syndrome preceded hereditary transthyretin amyloidosis diagnosis by 9-10 years9–11. According to The Transthyretin Amyloid Outcome Survey (THAOS), several cardiac, gait, gastrointestinal, neurological and renal disorders are prevalent in Val122Ile carriers4. Parallel to these findings, another study reported atrial fibrillation, myopathy related to ventricular thickness, gastrointestinal and kidney dysfunction including nausea, vomiting, and neuromuscular dysfunction to be associated with TTR mutations12.
We previously investigated medical history phenotypes in the Yale-Penn cohort associated with Val122Ile carriers of African descent and found heart disease history and having had 10 or more outpatient (ambulatory) surgeries significantly associated with this amyloidogenic variant13. One of the top reasons for ambulatory surgery in the United States is arthroplasty14, which occurs in TTR-carriers years before the expected cardiac dystrophy at advanced ages15. These epidemiological findings indicate that atypical phenotypes occurring earlier in life could be connected to the risk of heart failure in Val122Ile carriers. Findings from several studies including ours raise the possibility of non-regulatory molecular factors contributing to the genotype-phenotype correlation12,16–19. Therefore, understanding the underlying biological changes in Val122Ile carriers is key to explaining the symptom heterogeneity and earlier onset of atypical phenotypes.
DNA methylation is a heritable non-coding regulatory mechanism causing phenotypic variation18. Epigenetic modifications arising from the addition of methyl groups on cytosine-phosphate-guanosine (CpG) sites20 could contribute to molecular mechanisms involved in TTR amyloidosis. So far, no study has investigated this hypothesis. Aberrant methylation profiles have been implicated in increasing accelerating the progression of common and rare diseases21 . The accumulation of amyloid-fibrils within or around cellular structures of the tissue result in damage invoking an immune response22. The inter-individual variation in response to site of damage invokes an acute phase response22. DNA methylation profiles have the potential to capture individual-level variability and highlight mechanisms involved in TTR amyloidosis23. Thus, we conducted the first epigenome-wide association study of TTR Val122Ile carriers to investigate the association of methylation changes with medical history of heart disease and outpatient surgeries.
Methods
The intensity files of methylation data have been uploaded to Gene Expression Omnibus (GSE154683). The authors declare that all supporting data are available within the article [and its online supplementary files]. For further inquiries, please contact the corresponding author. Author contributions
Yale-Penn study was approved by the institutional review boards at each participating site including receiving participants consent. The current study was approved under the protocol 2000023750 by the institutional review board (IRB) at Yale University School of Medicine. All intensity files of methylation data have been made publicly available at the GSE154683 and can be accessed at https://www.ncbi.nlm.nih.gov/geo/. The authors declare that all supporting data are available within the article [and its supplementary files]. Detailed methods are available in the Supplementary section.
Results
Differentially methylated sites
We investigated differentially methylated sites with respect to two binary outcomes: – a) self-reported heart disease and b) a history of 10 or more outpatient surgeries. After performing the recommended quality control procedure, we investigated 737,385 sites in 96 individuals. In addition to performing standard association analysis, we permuted the phenotypes (pperm: p-value from permutation), which accounted for the case-control imbalance, yielding nine significant CpG sites (Figure 1). Five sites were hypomethylated in individuals with heart disease: cg06641417 (FAM129B; logFC=−1.822; pperm=1.6e-08), cg26033908 (SKI; logFC=−1.615; pperm=1.7e-08), cg14890866 (WDR27; logFC=−2.028; pperm=3.0e-08), cg15522719 (GLS; logFC=−1.731; pperm=4.7e-08) and cg18546846 (RP11-550A5.2; intergenic; logFC=−0.786; pperm=2.2e-08). The CpG sites mapped to FAM129B and SKI are located in gene bodies, cg14890866 is between the 5’UTR (Un-Translated Region) and TSS200 (− 200 nt upstream of Transcription start site24) of WDR27, while cg15522719 is in TSS150 at GLS. Four methylation sites were associated with 10 or more outpatient surgeries: cg13998023 (UBE2E3; logFC=−2.632; pperm=1.8e-07), cg05189127 (intergenic; logFC=1.885; pperm=1.4e-07), cg03718655 (SEC14L5; logFC=−2.673; pperm=1.5e-07) and cg25814327 (intergenic; logFC=−2.075; pperm=3e-08). Three sites were hypomethylated, while cg05189127 (intergenic) was hypermethylated. The two sites that were annotated to genes were in the 5’UTR (UBE2E3) and TSS200 (SEC14L5). Details of the association result and annotation are reported in Supplementary file (Table S1).
Figure 1:
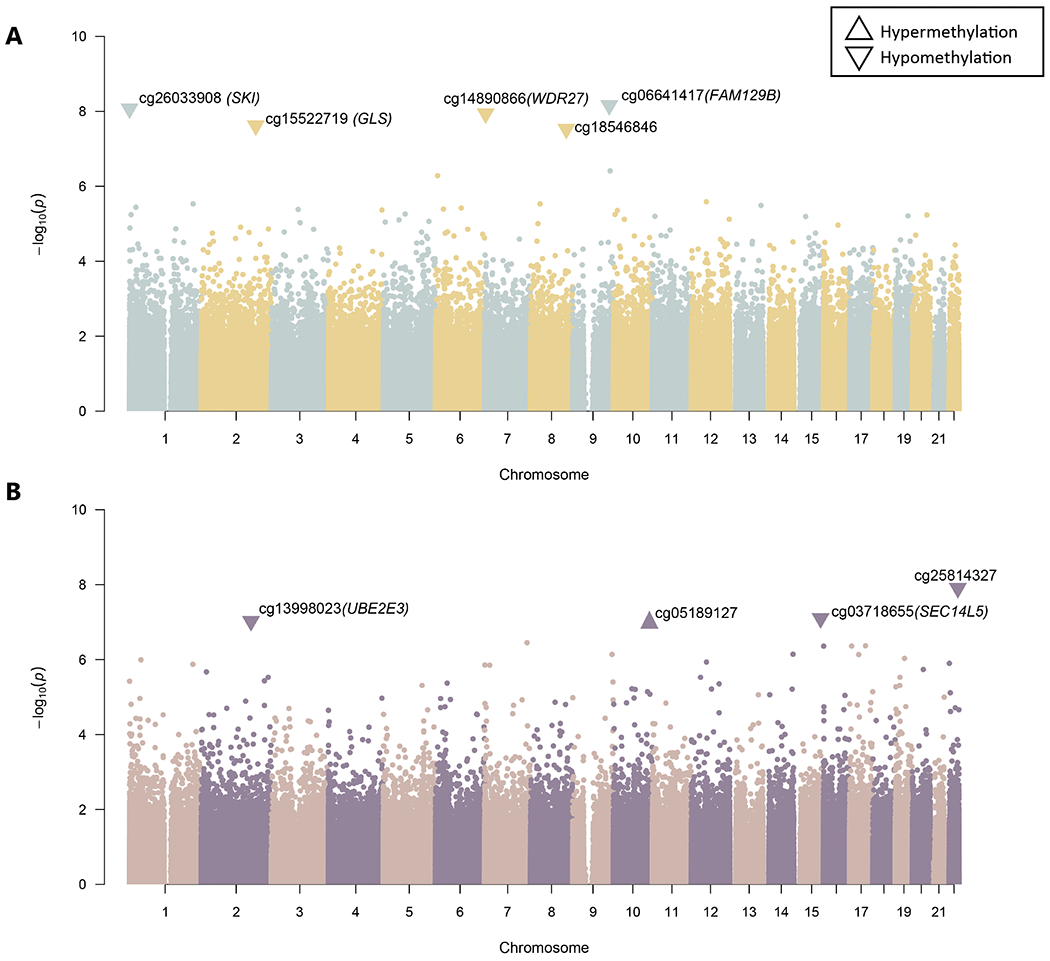
Differentially methylated sites in African American TTR-Val122Ile carriers. (A) Methylation sites that were significantly associated with medical history of heart disease. (B) Methylation sites that were significantly associated for having had 10 or more outpatient surgeries. Each CpG site is represented as a data point, with the x-axis being the genomic location, grouped by chromosome and wherein colors represent alternating chromosomes. The y-axis is the −log10 of the p-value of the CpG site. Significant sites are shown as triangles and labelled with CpG probe name and genic annotation in parentheses, triangles pointing upwards signify hypermethylation, whereas triangles pointing downwards signify hypomethylation.
Differentially methylated regions
For heart disease, one region on chromosome 12 overlapping KCNA6 and GALNT3 (p=1.1e-12) was differentially methylated. Associations with more than 10 outpatient surgeries were identified on chromosome 1 (SH3D21; EVA1B; p=1.3e-09), chromosome 10 (intergenic region; p=1.7e-08) and chromosome 14 (LTB4R2; CIDEB; p=3.9e-07). Methylation levels among all sites were positively correlated within each region. (Figure 2; Supplementary file; Table S2)
Figure 2:
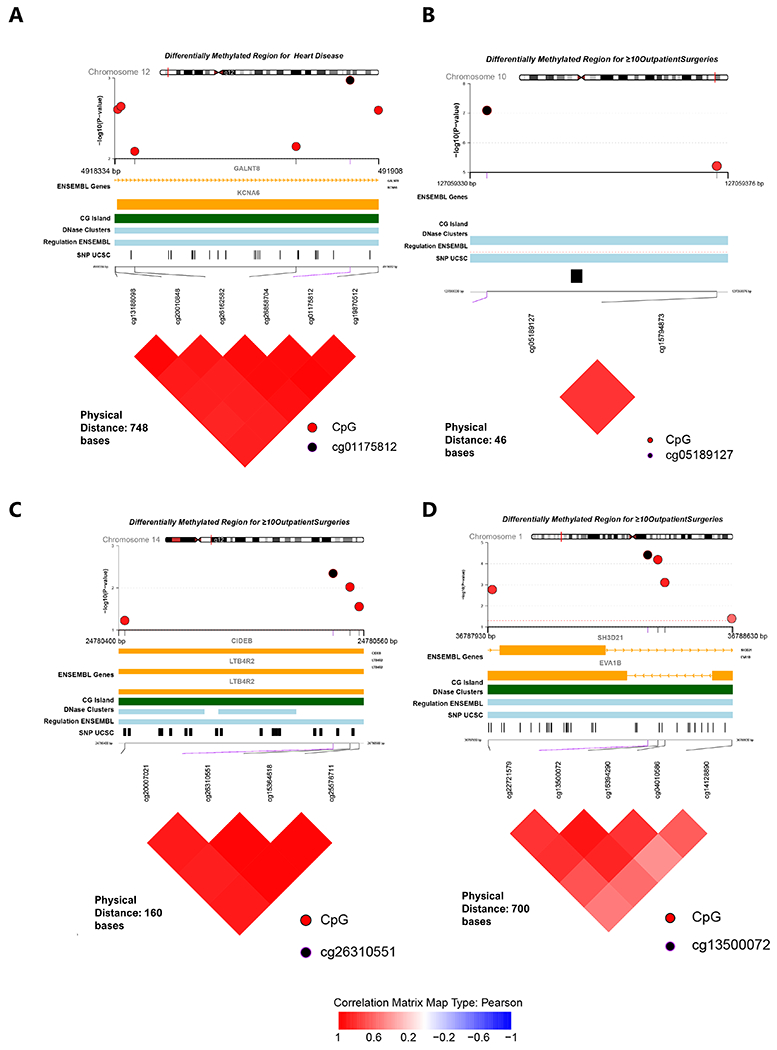
Differentially methylated regions in TTR-Val122Ile carriers. (A) Regional association with heart disease (B-D) Regional association with 10 or more outpatient surgeries. Each panel displays the association of sites within each region, followed by genomic location, ENSEMBL gene name, DNAse, regulation, SNP tracks (from UCSC browser) and correlation of CpG sites shown as a heatmap.
Overrepresented gene ontology and PPI networks
Differentially methylated sites and regions were annotated to their respective genes using UCSC RefGene: hg19 genome build. The gene ontology (GO) analysis identified 15 significantly enriched pathways in GO’s biological process. GLS, SKI, GALNT8, and KCNA6 are involved in protein oligomerization (FDRp-value=4.8e-03) and KCNA6 and GALNT8, which are located near one another, are involved in potassium ion transport (FDRp-value=4.9e-02). FAM12B and SKI (FDRp-value=4.8e-03 to 3.2e-02) participate in the development of various tissue types – myotubules, and skeletal and striated muscles (Figure 3; Supplementary file, Table S3).
Figure 3:

Enriched gene ontology (GO) biological processes. The dendrogram shows the FDRpvalue of the pathway associations and are grouped by similarity of function. The genes involved in each of the processes are highlighted in orange bars.
We also investigated the methylation sites for differentially methylated functional modules using the R package FEM 25. The CpG sites are weighted based on their location in the genes, which are then mapped to a protein-protein interaction (PPI) network. For each module (i.e. PPI network) identified, the seed gene is the primary gene to which other functionally related genes are connected. For heart disease, we found the ABCA1 module to be significant (p=0.001) and target genes identified within the module were: ABCA1, SNTB2, BLOC1S2 and LIN7B (p<0.05). The EXOSC4 gene module was associated with the phenotype of 10 or more outpatient surgeries, and it was the only gene that was a target (Figure 4; Supplementary file, Table S4).
Figure 4:
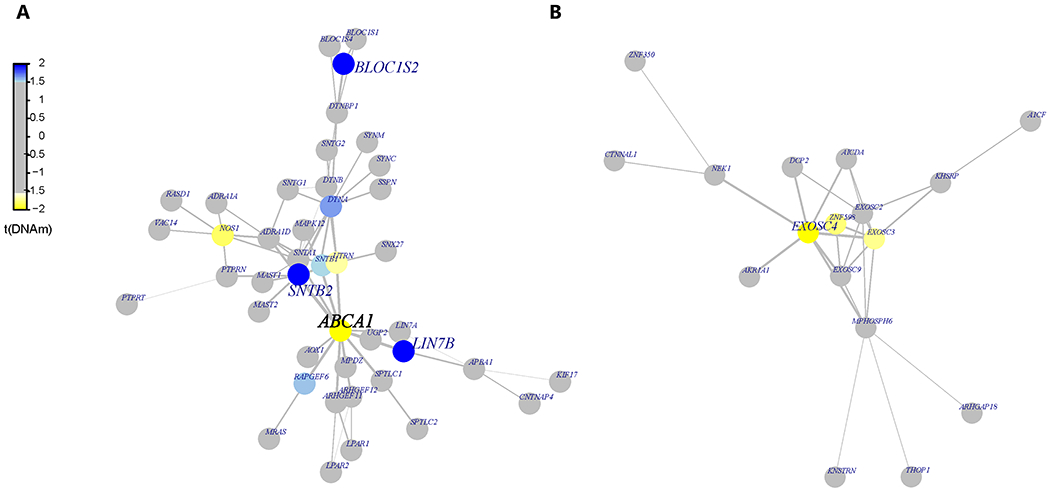
Functional Protein-Protein Interaction (PPI) Networks. The differentially methylated modules consist of a network of genes based on their functional connectivity using protein-protein interaction. Each module has primary gene which is connected to other target genes in the network. Each module was significant p<0.05 using the FEM method (see methods). The genes in blue show hypermethylation and yellow represents hypomethylation. A) ABCA1 module was associated with heart disease and the significant target genes in addition to ABCA1 were SNTB2, BLOC1S2 and LIN7B. B) EXOSC4 module was associated with outpatient surgeries and also was the only significant gene in the network.
Local quantitative trait loci for methylated sites (mQTL)
We tested SNP associations with nine methylation sites that were epigenome-wide significant with the two phenotypes investigated. The cis-mQTL loci were defined as SNPs within ±1 Mb of the significant CpG site. The sites were considered significant based on an FDRp-value < 0.05 and genomic corrected p-value (pgc<0.05). We found six SNPs, rs192528579, rs182192023, rs114553373, rs187644239, rs114896522, and rs139996037 significantly associated (Figure 5; p=4.1e-24; Table S5) with site cg06641417. The SNPs are in high linkage disequilibrium (LD>0.8), rs192528579 is in the intronic region of neighboring gene –- GARNL3; rs182192023, rs114553373, rs187644239 and rs114896522 map to LRSAM1. Rs139996037 is a non-coding transcript variant of the FAM129B.
Figure 5:
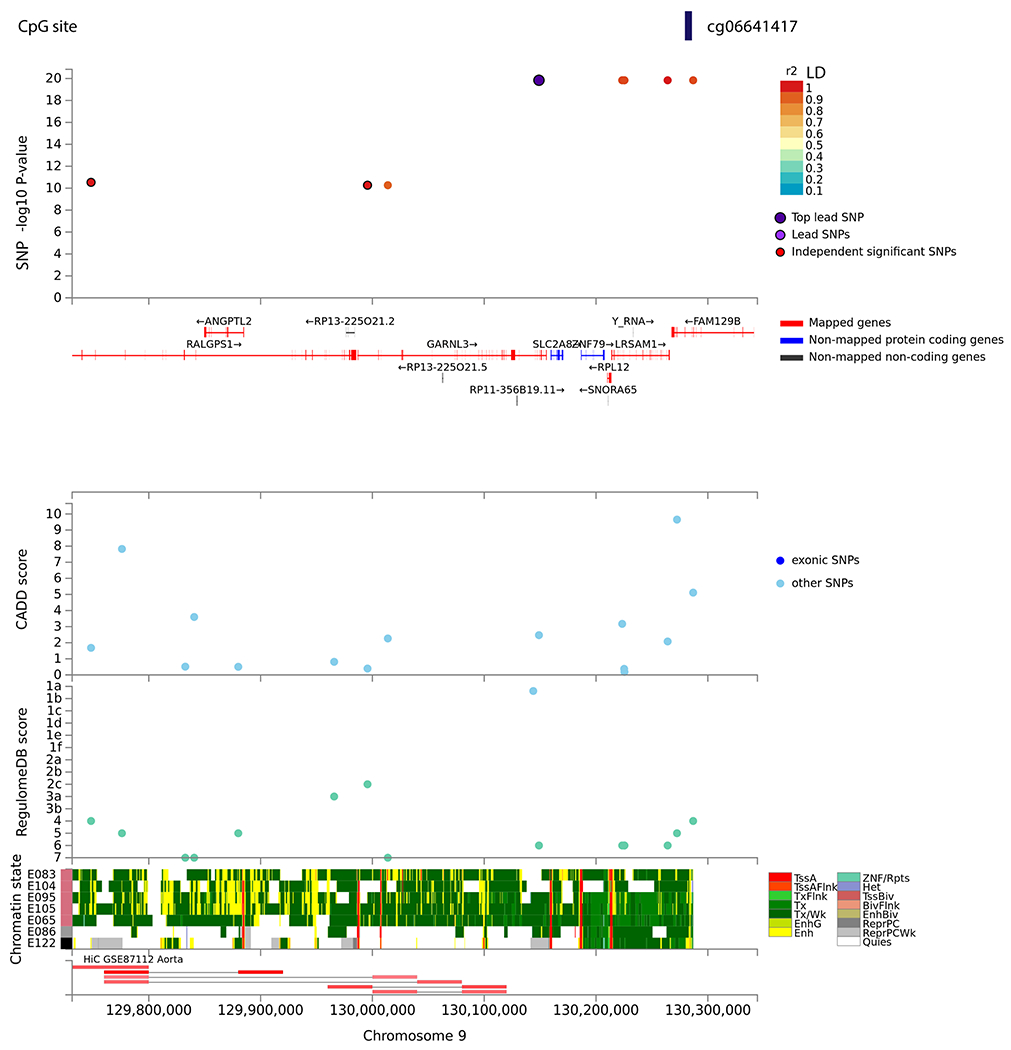
Local-mQTL associated with site – cg06641417 mapped to the FAM129B gene. The top panel displays single nucleotide polymorphisms (SNPs) associated with CpG site – cg06641417 as a data point and color coded based on linkage disequilibrium with the top lead SNP in purple. The x-axis shows gene annotation (hg19) of the region and the y-axis displays the −log10 of p-value. The following panels present various annotations of the reported SNPs i.e. CADD - Combined Annotation Dependent Depletion, and RegulomeDB – score to identify regulatory variants. The bottom panel highlights the chromatin states of various regulatory features being putatively affected from chromatin markers observed in aorta tissue cell line. Visualization made in FUMA.
Epigenetic age
The epigenetic age (DNAm) was measured using the biological clock developed by Horvath and colleagues, which uses 353 CpG sites26 and also with a second clock based on 71 CpG sites from Hannum and colleagues27. The ‘Horvath’ clock is considered to be a pan-tissue epigenetic clock, while the ‘Hannum’ clock is considered to be accurate for whole-blood tissue28. Both clocks estimated that carriers with heart disease are of older epigenetic age than carriers without heart disease (pHorvath=0.007 and pHannum=0.0009) (Supplementary file; Table S6). However, the (delta) ΔDNAm age (difference in chronological and biological age) was not significant between the two groups (pΔHorvath=0.31 and pΔHannum=0.57; Supplementary file; Table S7).
Replication of methylation sites in the Italian cohort
We tested the nine CpG sites identified in Val122Ile carriers in an independent cohort of biopsy-confirmed TTR amyloidosis cases and healthy controls. We replicated cg18546846 (intergenic; near to RP11-550A5.2; p= 0.021) and cg06641417 (FAM129B; p=0.016) at nominal significance (p<0.05).
Discussion
The clinical consequences of the TTR Val122Ile mutation remain underappreciated and the syndrome that accompanies this risk mutation, under-diagnosed. Individuals exhibiting early TTR-amyloidosis symptoms are more likely to be diagnosed with another condition prior to receiving the diagnosis of TTR-amyloidosis29. There is nonetheless a greater burden over time towards developing ventricular hypertrophy, reduced left ventricular ejection fraction, and atrial dilation, at a later age3,6. We previously showed that African-American carriers of the Val122Ile mutation had a higher prevalence of heart disease and having multiple outpatient surgeries than individuals without the mutation13. In the present study, we identified methylation changes associated with these same phenotypes in Val122Ile carriers. We also replicated two CpG sites (RP11-550A5.2; cg18546846 and FAM129B; cg06641417) at nominal significance in an external cohort including biopsy confirmed cases of TTR amyloidosis30. Thus, we hypothesize that the epigenetic changes associated with the pathogenesis heart disease differs from the methylation profile of carriers who are not affected by the disease. Lastly, we used GeneMANIA31 to interpret the interaction among the significant genes (Supplementary file). We observed that major genes identified in the present study physically interact and share pathways with TTR (Figure 6).
Figure 6:
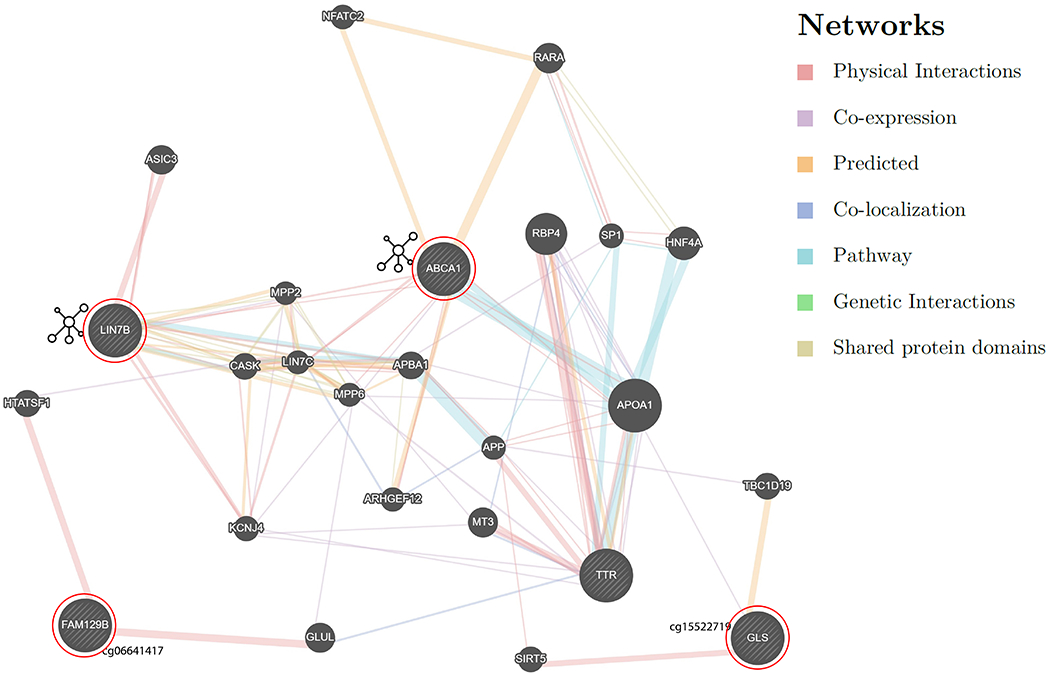
Gene network of significant genic hits and their relationship with TTR. The genes circled in red are the query genes that were identified from the reported analysis. The network shows intermediate genes that connect the query genes based on different interactions as shown in the legend. The lines are colored based on the type of network domain shown in the figure.
ABCA1 (ATP binding cassette transporter A1) identified via the functional network analysis encodes a transporter of cholesterol from apolipoproteins32. ABCA1 regulates Apolipoprotein E (ApoE) levels, with lower expression of ABCA1 reducing ApoE levels. However, ApoE with ApoA1 (Apolipoprotein A) reduces amyloid deposition twice as fast as inhibition of the expression of ApoE. Additionally, amyloid-beta levels were the lowest for the dual-knockout of APP (which encodes amyloid precursor protein) and ABCA133.
GLS (glutaminase) is a key contributor to the metabolizing of glutamine to glutamate34. Amyloid-beta-treated neurons show elevated glutaminase expression, which increases glutamate levels and disrupts calcium neural regulation35. Additionally, neurofibrillary tangles have been shown to coexist with higher glutaminase activity36. The hypomethylated site in the transcription start site of the GLS gene may indicate its potential involvement in the central nervous system, which supports the recent finding of cerebral amyloid angiopathy in individuals with mutated TTR cardiac amyloidosis37. FAM129B (aliases; MEG-3 and NIBAN2) is downregulated in tissues with amyloid deposition and animal studies have shown that enhancing the expression of FAM129B reduces oxidative damage by reducing amyloid-beta production via PI3K/Akt signaling38. Cardiac hypertrophy increases the risk of heart failure. FAM129B is overexpressed in heart failure samples, and rodent experiments indicate a potential role of the gene in the apoptosis of cardiac myocytes after myocardial infarction39. Rescuing the expression levels of FAM129B reverses hypertrophic responses, thus the hypomethylation of the CpG site in FAM129B observed in our finding supports the overexpression of the gene in heart failure40. The African-American population has a high prevalence of diabetes41. FAM129B is also overexpressed in cardiomyocytes under high glucose concentration reflecting its role in diabetic cardiomyopathy42. Although SKI is an inhibitor of TGF-beta-induced fibrosis and is under expressed in cardiac fibrosis, other epigenetic modulators such as miRNAs-34a and 93-c affect both SKI and TGF-beta, targeting the inhibitory factors of SKI, which could rescue cardiac fibrosis43. The gene enrichment analysis identified a role for FAM129B and SKI in the development of myotube cells and skeletal muscle fiber and organ, and striated muscle cell development.
One of the clinical findings associated with cardiac amyloidosis is increased left ventricular wall thickness, which can lead to heart failure44. Electrical perturbations resulting from lower potassium repolarizing current leads to a prolonged action potential in heart failure45. The role of KCNA6 and GALNT8 is associated with potassium ion transport and the transmembrane transport complexes. One of the cardiovascular symptoms of the TTR amyloidosis is pronounced diastolic hypertension7, and diastolic dysfunction is one of the symptoms associated with transthyretin amyloidosis46 . WDR27 was reported to be differentially methylated in individuals with significant differences in diastolic blood pressure26.
Aging is a common denominator to the symptomology of Val122Ile and DNA methylation47. Age-related methylation changes measured via “epigenetic clocks” help to identify molecular aging and its disconnect with chronological age. The Horvath clock based on 353 CpG sites and the Hannum clock based on 71 CpG sites have been extensively replicated in various tissues48. While these clocks were developed using blood tissues26,27, Horvath’s clock is validated across multiple tissues, while Hannum’s clock is more consistent in samples originating from blood tissues. Higher epigenetically derived age has been associated with several cardiovascular disease traits. Hypermethylation of genes that are protective against heart disease, lead to cardiovascular aging and increased risk for coronary disease49. The dysregulation of the ABCA1 gene, the product of which is involved in the transport of cholesterol from the periphery to liver tissue50 has been associated with different cardiovascular pathologies. The hypermethylation of the ABCA1 promoter region silences its expression and is associated with coronary artery disease51. In contrast, the increased expression of ABCA1 regulated by ApoA1 leads to reverse cholesterol efflux in hepatic tissue 52. Elevated high density lipoprotein (HDL) in the liver is a target site for serum amyloid A, an acute phase response protein that is expressed during amyloidosis53. The observed hypomethylation of ABCA1 and putative increase in gene expression underscores its likely involvement in shifting the methylation milieu and could perhaps explain the cardiac symptomology in a comparatively younger group of Val122Ile carriers. These findings reflect the observational symptomology of carpal tunnel, a common denominator to arthroplasty and hATTR54.
These findings provide unique insights into epigenomic contrasts related to symptomology in Val122Ile carriers. However, our study has limitations. First, we investigated the Val122Ile polymorphism only for heart disease, though it is possible that we could identify additional differences with individuals who are non-Val122Ile carriers or who present with wild-type transthyretin amyloidosis. Additionally, due to the low frequency of the disease-causing mutation investigated, our study suffers from an imbalance in the ratio of cases to controls. Although the permutation analysis accounting for this imbalance confirmed our results and we replicated two associations in an independent cohort (with mostly different risk variants). Our findings would benefit from replication in a larger, more balanced study to further dissect the underlying disease mechanisms. While the DNAm age was significantly different in the two groups, the ΔDNAm age was not different. It is possible that our study is underpowered to detect delta-DNAm age. Additionally, there may be some biases in applying DNAm age measures developed largely on individuals of European descent to individuals of other ancestries, and such ancestry-stratified DNAm differences have been reported by other studies as well 55–57.
Conclusions
Our study is the first to explore the epigenetic changes in TTR Val122Ile carriers. Certain Val122Ile carriers in our study presented with heart disease earlier than usually reported by individuals affected by cardiac amyloidosis. This could be due to modifier effects accelerating the pathogenicity of Val122Ile mutation. Due to the underestimated clinical penetrance of the mutation in the African American population, we leveraged an external secondary dataset with confirmed clinical phenotype as a random population sample. The purpose of this study was to understand possible non-coding mechanisms that may explain the heterogeneous phenotypes observed in Val122Ile carriers with history of heart disease. The epigenetic changes identified affect the regulation of genes involved in the transport of amyloid and regulating striated and smooth muscle, which form key components of amyloidosis and cardiac tissue susceptibility. These findings provide higher resolution on mechanisms underlying the TTR-Val122Ile mutation.
Supplementary Material
Acknowledgments
Sources of Funding: The study was supported by ‘Global ASPIRE TTR Amyloidosis Competitive Grant’ from Pfizer Inc. We are grateful to the participants of the Yale-Penn cohort, which was funded under grants RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01AA11330, and R01 AA017535. The investigation conducted in the Italian cohort was supported by an Investigator-Initiated Research from Pfizer Inc. to the University of Rome “Tor Vergata”. The content reported in the manuscript is solely the responsibility of the authors and does not represent the official views of the NIH or Pfizer. The funding agencies had no role in the study design, data analysis, and results interpretation of the present study.
Disclosures: H.R.K. is a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which over the last three years was sponsored by Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Amygdala Neurosciences, Inc. H.R.K. and J.G. are named as inventors on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed on 24 January 2018. The other authors report no conflict of interest.
Nonstandard Abbreviations and Acronyms
- TTR
Transthyretin
- OS
Outpatient surgery
- HD
Heart Disease
- CpG
Cytosine-phosphate Guanine
- hATTR
hereditary TTR amyloidosis
- SNP
Single Nucleotide Polymorphisms
- TSS
Transcription Start Site
References:
- 1.Kristen AV, Maurer MS, Rapezzi C, Mundayat R, Suhr OB, Damy T, THAOS investigators. Impact of genotype and phenotype on cardiac biomarkers in patients with transthyretin amyloidosis - Report from the Transthyretin Amyloidosis Outcome Survey (THAOS). Plos One. 2017;12:e0173086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson DR, Alexander AA, Tagoe C, Garvey WT, Williams SM, Tishkoff S, Modiano D, Sirima SB, Kalidi I, Toure A, Buxbaum JN. The prevalence and distribution of the amyloidogenic transthyretin (TTR) V122I allele in Africa. Mol Genet Genomic Med. 2016;4:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (transthyretin amyloid outcome survey). J Am Coll Cardiol. 2016;68:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25:215–219. [DOI] [PubMed] [Google Scholar]
- 6.Mankad AK, Shah KB. Transthyretin Cardiac Amyloidosis. Curr Cardiol Rep. 2017;19:97. [DOI] [PubMed] [Google Scholar]
- 7.Shah KB, Mankad AK, Castano A, Akinboboye OO, Duncan PB, Fergus IV, Maurer MS. Transthyretin cardiac amyloidosis in black americans. Circ Heart Fail. 2016;9:e002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jercan A, Ene A, Jurcut R, Draghici M, Badelita S, Dragomir M, Dobrea C, Popescu M, Jardan D, Stoica E, Iacob S, Codita I, Stan C, Coriu D. Clinical characteristics in patients with hereditary amyloidosis with Glu54Gln transthyretin identified in the Romanian population. Orphanet J Rare Dis. 2020;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karam C, Dimitrova D, Christ M, Heitner SB. Carpal tunnel syndrome and associated symptoms as first manifestation of hATTR amyloidosis. Neurol Clin Pract. 2019;9:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milandri A, Farioli A, Gagliardi C, Longhi S, Salvi F, Curti S, Foffi S, Caponetti AG, Lorenzini M, Ferlini A, Rimessi P, Mattioli S, Violante FS, Rapezzi C. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. 2020;22:507–515. [DOI] [PubMed] [Google Scholar]
- 11.Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, Hoshii Y, Kato H, Ikeda S. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42:1785–1791. [DOI] [PubMed] [Google Scholar]
- 12.De Lillo A, De Angelis F, Di Girolamo M, Luigetti M, Frusconi S, Manfellotto D, Fuciarelli M, Polimanti R. Phenome-wide association study of TTR and RBP4 genes in 361,194 individuals reveals novel insights in the genetics of hereditary and wildtype transthyretin amyloidoses. Hum Genet. 2019;138:1331–1340. [DOI] [PubMed] [Google Scholar]
- 13.Polimanti R, Nuñez YZ, Gelernter J. Increased Risk of Multiple Outpatient Surgeries in African-American Carriers of Transthyretin Val122Ile Mutation Is Modulated by Non-Coding Variants. J Clin Med. 2019;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in Hospital-Based Ambulatory Surgery and Hospital Inpatient Settings, 2014: Statistical Brief #223 - In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. February 2017 May [updated 2020 Jul 20]. [PubMed] [Google Scholar]
- 15.Rubin J, Alvarez J, Teruya S, Castano A, Lehman RA, Weidenbaum M, Geller JA, Helmke S, Maurer MS. Hip and knee arthroplasty are common among patients with transthyretin cardiac amyloidosis, occurring years before cardiac amyloid diagnosis: can we identify affected patients earlier? Amyloid. 2017;24:226–230. [DOI] [PubMed] [Google Scholar]
- 16.Iorio A, De Lillo A, De Angelis F, Di Girolamo M, Luigetti M, Sabatelli M, Pradotto L, Mauro A, Mazzeo A, Stancanelli C, et al. Non-coding variants contribute to the clinical heterogeneity of TTR amyloidosis. Eur J Hum Genet. 2017;25:1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iorio A, De Angelis F, Di Girolamo M, Luigetti M, Pradotto LG, Mazzeo A, Frusconi S, My F, Manfellotto D, Fuciarelli M, Polimanti R. Population diversity of the genetically determined TTR expression in human tissues and its implications in TTR amyloidosis. BMC Genomics. 2017;18:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polimanti R, Di Girolamo M, Manfellotto D, Fuciarelli M. In silico analysis of TTR gene (coding and non-coding regions, and interactive network) and its implications in transthyretin-related amyloidosis. Amyloid. 2014;21:154–162. [DOI] [PubMed] [Google Scholar]
- 19.Polimanti R, Di Girolamo M, Manfellotto D, Fuciarelli M. Functional variation of the transthyretin gene among human populations and its correlation with amyloidosis phenotypes. Amyloid. 2013;20:256–262. [DOI] [PubMed] [Google Scholar]
- 20.Pathak GA, Silzer TK, Sun J, Zhou Z, Daniel AA, Johnson L, O’Bryant S, Phillips NR, Barber RC. Genome-Wide Methylation of Mild Cognitive Impairment in Mexican Americans Highlights Genes Involved in Synaptic Transport, Alzheimer’s Disease-Precursor Phenotypes, and Metabolic Morbidities. J Alzheimers Dis. 2019;72:733–749. [DOI] [PubMed] [Google Scholar]
- 21.Zoghbi HY, Beaudet AL. Epigenetics and human disease. Cold Spring Harb Perspect Biol. 2016;8:a019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10:1086–1097. [DOI] [PubMed] [Google Scholar]
- 23.Hachiya T, Furukawa R, Shiwa Y, Ohmomo H, Ono K, Katsuoka F, Nagasaki M, Yasuda J, Fuse N, Kinoshita K, et al. Genome-wide identification of inter-individually variable DNA methylation sites improves the efficacy of epigenetic association studies. NPJ Genom Med. 2017;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, Delano D, Zhang L, Schroth GP, Gunderson KL, Fan J-B, Shen R. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. [DOI] [PubMed] [Google Scholar]
- 25.Jiao Y, Widschwendter M, Teschendorff AE. A systems-level integrative framework for genome-wide DNA methylation and gene expression data identifies differential gene expression modules under epigenetic control. Bioinformatics. 2014;30:2360–2366. [DOI] [PubMed] [Google Scholar]
- 26.Horvath S DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan J-B, Gao Y, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Vallerga CL, Walker RM, Lin T, Henders AK, Montgomery GW, He J, Fan D, Fowdar J, Kennedy M, et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 2019;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bishop E, Brown EE, Fajardo J, Barouch LA, Judge DP, Halushka MK. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018;25:174–179. [DOI] [PubMed] [Google Scholar]
- 30.De Lillo A, Pathak G, De Angelis F, Di Girolamo M, Luigetti M, Sabatelli M, Perfetto F, Frusconi S, Manfellotto D, Fuciarelli M, Polimanti R. Epigenetic profiling of Italian patients identified methylation sites associated with hereditary Transthyretin amyloidosis. Clin Epigenetics. 2020;12:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montojo J, Zuberi K, Rodriguez H, Bader GD, Morris Q. GeneMANIA: Fast gene network construction and function prediction for Cytoscape. F1000Res. 2014;3:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki M, Komatsu T, Ikewaki K. Impact of Hepatic ABCA1 (ATP-Binding Cassette Transporter A1) Deletion on Reverse Cholesterol Transport A New Clue in Solving Complex HDL (High-Density Lipoprotein) Metabolism. Arterioscler Thromb Vasc Biol. 2019;39:1699–1701. [DOI] [PubMed] [Google Scholar]
- 33.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280:43224–43235. [DOI] [PubMed] [Google Scholar]
- 34.Cooper AJL, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules. 2016;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revett TJ, Baker GB, Jhamandas J, Kar S. Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci. 2013;38:6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchsberger T, Martínez-Bellver S, Giraldo E, Teruel-Martí V, Lloret A, Viña J. Aβ Induces Excitotoxicity Mediated by APC/C-Cdh1 Depletion That Can Be Prevented by Glutaminase Inhibition Promoting Neuronal Survival. Sci Rep. 2016;6:31158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaich CL, Maurer MS, Nadkarni NK. Amyloidosis of the brain and heart: two sides of the same coin? JACC Heart Fail. 2019;7:129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi J, Chen B, Yao X, Lei Y, Ou F, Huang F. Upregulation of the lncRNA MEG3 improves cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer’s disease through inactivating the PI3K/Akt signaling pathway. J Cell Biochem. 2019;120:18053–18065. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Zhao Z-A, Liu J, Hao K, Yu Y, Han X, Li J, Wang Y, Lei W, Dong N et al. Long noncoding RNA Meg3 regulates cardiomyocyte apoptosis in myocardial infarction. Gene Ther. 2018;25:511–523. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Liang Y, Huang X, Guo X, Liu Y, Zhong J, Yuan J. STAT3-induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR-361-5p/HDAC9 axis. Sci Rep. 2019;9:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow EA, Foster H, Gonzalez V, McIver L. The disparate impact of diabetes on racial/ethnic minority populations. Clin Diabetes. 2012;30:130–133. [Google Scholar]
- 42.Chen Y, Zhang Z, Zhu D, Zhao W, Li F. Long non-coding RNA MEG3 serves as a ceRNA for microRNA-145 to induce apoptosis of AC16 cardiomyocytes under high glucose condition. Biosci Rep. 2019;39:BSR20190444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Zhang Y, Zhu H, Hu J, Xie Z. MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro. Cell Signal. 2018;46:145–153. [DOI] [PubMed] [Google Scholar]
- 44.Suresh R, Grogan M, Maleszewski JJ, Pellikka PA, Hanna M, Dispenzieri A, Pereira NL. Advanced cardiac amyloidosis associated with normal interventricular septal thickness: an uncommon presentation of infiltrative cardiomyopathy. J Am Soc Echocardiogr. 2014;27:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang K-C, Nerbonne JM. Mechanisms contributing to myocardial potassium channel diversity, regulation and remodeling. Trends Cardiovasc Med. 2016;26:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González-Duarte A, Barroso F, Mundayat R, Shapiro B. Blood pressure and orthostatic hypotension as measures of autonomic dysfunction in patients from the transthyretin amyloidosis outcomes survey (THAOS). Auton Neurosci. 2019;222:102590. [DOI] [PubMed] [Google Scholar]
- 47.Maurer MS, Mundayat R, Rapezzi C. Reply: Val122Ile mt-ATTR Has a Worse Survival Than wt-ATTR Cardiac Amyloidosis. J Am Coll Cardiol. 2017;69:758–759. [DOI] [PubMed] [Google Scholar]
- 48.Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. 2019;195:172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Song M, Qu J, Liu G-H. Epigenetic modifications in cardiovascular aging and diseases. Circ Res. 2018;123:773–786. [DOI] [PubMed] [Google Scholar]
- 50.Ghaznavi H, Mahmoodi K, Soltanpour MS. A preliminary study of the association between the ABCA1 gene promoter DNA methylation and coronary artery disease risk. Mol Biol Res Commun. 2018;7:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guay S-P, Légaré C, Houde A-A, Mathieu P, Bossé Y, Bouchard L. Acetylsalicylic acid, aging and coronary artery disease are associated with ABCA1 DNA methylation in men. Clin Epigenetics. 2014;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, Cain WJ, Fruchart-Najib J, Neufeld ED, Remaley AT, et al. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M, Liu Y, Dai J, Li L, Ding X, Xu Z, Mori M, Miyahara H, Sawashita J, Higuchi K. Apolipoprotein A-II induces acute-phase response associated AA amyloidosis in mice through conformational changes of plasma lipoprotein structure. Sci Rep. 2018;8:5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aus dem Siepen F, Hein S, Prestel S, Baumgärtner C, Schönland S, Hegenbart U, Röcken C, Katus HA, Kristen AV. Carpal tunnel syndrome and spinal canal stenosis: harbingers of transthyretin amyloid cardiomyopathy? Clin Res Cardiol. 2019;108:1324–1330. [DOI] [PubMed] [Google Scholar]
- 55.Tajuddin SM, Hernandez DG, Chen BH, Noren Hooten N, Mode NA, Nalls MA, Singleton AB, Ejiogu N, Chitrala KN, Zonderman AB, et al. Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites. Clin Epigenetics. 2019;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Wang Z, Hardy T, Huang Y, Hui Q, Crusto CA, Wright ML, Taylor JY, Sun YV. Association of Obesity with DNA Methylation Age Acceleration in African American Mothers from the InterGEN Study. Int J Mol Sci. 2019;20:4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, Ritz BR, Chen B, Lu AT, Rickabaugh TM, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


