Abstract
Multisystem inflammatory syndrome in children (MIS-C) during the COVID-19 pandemic raised a global alert from the Centers for Disease Control and Prevention’s Health Alert Network. The main manifestations of MIS-C (also known as pediatric MIS (PMIS)) in the setting of a severe inflammatory state include fever, diarrhea, shock, and variable presence of rash, conjunctivitis, extremity edema, and mucous membrane changes. In some cases, these symptoms progressed to multi-organ failure. The low percentage of children with asymptomatic cases compared with mild illness and moderate illness could be correlated with the rare cases of MIS-C. One potential explanation for the progression to severe MIS-C disease despite the presence of readily detectable anti-SARS-CoV-2 antibodies could be due to the potential role of antibody-dependent enhancement (ADE). We reason that the incidence of the ADE phenomenon whereby the pathogen-specific antibodies can promote pathology should be considered in vaccine development against SARS-CoV-2.
Keywords: antibody-dependent enhancement, COVID-19, Kawasaki syndrome, multisystem inflammatory syndrome, SARS-CoV-2
1 |. INTRODUCTION
In April 2020, clinical reports documented the occurrence of a multisystem inflammatory syndrome in children (MIS-C) during the COVID-19 pandemic in the United States, United Kingdom, Italy, and France. In the United States, during the period from April 16, 2020, to May 4, 2020, 15 children aged 2–15 years with MIS-C syndromes were admitted to the intensive care unit. On May 12, 2020, 102 children with similar presentations were reported in a study from New York, many of whom tested positive for SARS-CoV-2 infection by either a serologic assay or by RT-PCR.1 On July 12, 2020, two children are the first in the South Carolina with a confirmed diagnosis of MIS-C. On May 14, 2020, the Centers for Disease Control (CDC) and Prevention’s Health Alert Network issued a global alert for MIS-C after identifying a possible link between this critical illness in children and infection from the novel coronavirus SARS-CoV-2, the virus that causes the COVID-19 disease.1 A CDC-supported surveillance study for MIS-C conducted, and as of August 6, 2020, in pediatric health centers across the United States documented 570 confirmed cases of MIS-C and 10 deaths in 40 states with a median age of 8.3 years. The study also showed that about 55% of MIS-C patients were male and 99% of the patients were positive for SARS-CoV-2 by RT-PCR or antibody testing, and 88% of them were hospitalized.2,3 In general, more than 1,000 cases of MIS-C were documented worldwide and most patients have antibodies against SARS-CoV-2, and the virus is detected in a smaller proportion.4
The main manifestations of MIS-C in the setting of a severe inflammatory state include fever, diarrhea, shock, and variable presence of rash, conjunctivitis, extremity edema, and mucous membrane changes, and in some cases, this can lead to the development of multi-organ failure.5 The multi-organ failure in MIS-C is manifested by neurologic involvement, hyperferritinemia, and cardiogenic or vasoplegic shock.6 Children with severe MIS-C cases who have either previous exposure to SARS-CoV-2 or tested positive for SARS-CoV-2 were admitted to the intensive care units for shock or acute cardiac dysfunction.7
The MIS-C features share some aspects of the Kawasaki syndrome in terms of multisystem inflammation and high levels of inflammatory biomarkers. However, MIS-C has been reported in individuals who are up to 21 years of age with a higher rate of cardiac involvement,6 whereas patients with Kawasaki syndrome have been shown to occur predominantly in infants and children under 5 years old.7 An epidemiologic feature showed an increased incidence of MIS-C in patients of African, Afro-Caribbean, and Hispanic descent and decreased incidence of MIS-C in patients of East Asian descent.8 The etiology of Kawasaki disease remains unknown, with evidence suggesting that infectious agents could trigger the initiation of this disease. The cases of previously healthy children who developed MIS-C syndrome were shown to be associated with a clinical or sub-clinical SARS-CoV-2 infection. At present, it is unclear whether the SARS-CoV-2 infection could ignite the inflammation cascade that causes MIS-C illness.
Whereas it was previously thought that SARS-CoV-2 infection shows mild to asymptomatic disease in children and those that become infected are not susceptible to pneumonia secondary to COVID-19 infection, this view has significantly changed following the documentation of MIS-C in select cases of pediatric SARS-CoV-2 infection.9 These findings have led to the conclusion that clearly more detailed studies are required to more precisely define the molecular aspects of the triggering factors of MIS-C during the COVID-19 pandemic. In this opinion, we discuss the proposed molecular basis that demonstrates the following: (a) the low susceptibility of children to SARS-CoV-2 infection than adults, and (b) how could the re-infection of children with a symptomatic COVID-19 lead to the development of the MIS-C disease.
2 |. THE LOW SUSCEPTIBILITY OF CHILDREN TO COVID-19 ILLNESS
Adults with severe COVID-19 suffer from deadly pneumonia and insufficient supply of oxygen throughout the body, while children show mild to asymptomatic COVID-19 disease with fewer death cases. A study early in the COVID-19 pandemic showed that the percentage of infected children with SARS-CoV-2 was as low as 0.9% for 0–10 years and 1.2% for 10–19 years old.10 Further analysis of the SARS-CoV-2-infected children showed that whereas there was a 4% incidence of children with asymptomatic cases, those with mild illness comprised 51%, and those with moderate illness comprised 39% of the total number of reported cases.11 The molecular basis of the differences in COVID-19 pathogenesis between children and adults has yet to be fully understood.
The expression levels of SARS-CoV-2 cellular receptors and co-receptors in the children and adults could have an impact on virus infectivity and disease severity. Angiotensin-converting enzyme-2 (ACE2) represents the primary SARS-CoV-2 receptor for viral entry, and it is co-expressed with a cluster of the transmembrane serine protease (TMPRSS2). This protein contains a type II transmembrane domain, a receptor class A domain, a scavenger receptor cysteine-rich domain, and a protease domain. It is now well known that the functional role of TMPRSS2 in terms of SARS-CoV-2 viral entry is its role in cleaving the S protein of SARS-CoV-1 and SARS-CoV-2 into two fragments S1, which is essential for virus attachment, and S2, for virus fusion into the target cells.12 Attachment of the receptor-binding domain of the virus spikes to the ACE2 initiates SARS-CoV entry into target cells.13,14 As such, lung epithelial cells represent the coronaviruses’ primary target because of the co-expression of the ACE2 receptor with TMPRSS2 protein.15
In this regard, it is important to note that children show lower levels of ACE2 expression in the lungs than adults, which could contribute to the observed differences in disease pathogenesis across different age groups.16 It has been reported that SARS-CoV infection shows slight, non-specific, and cold-like symptoms in children younger than 12 years old and these symptoms are less pronounced than it is in adolescents.17 The expression levels of the TMPRSS2 protein are regulated by the levels of androgen and androgen receptors.18 It is important to note that children younger than 12 years old have a lower level of androgen and androgen receptors than are present in adolescents and adult men. The age-based difference in the expression levels of ACE2 and TMPRSS2 could contribute to lower levels of viremia and play a potential role in the severity of COVID-19 pathogenesis.
3 |. THE POSSIBLE TRIGGERING OF MIS-C VIA ANTIBODY-DEPENDENT ENHANCEMENT
Antibody-dependent enhancement (ADE) is a phenomenon by which the complex consisting of the antigen and non-neutralizing virus-specific antibodies binds via the Fc portion of the Ig to the Fc receptors on the cellular membrane of the immune cells thereby enhancing virus entry. The ADE-dependent virus entry is independent of the conventional pathway of entry of the SARS-CoV-2 virus mediated by binding of the spike proteins of the virus to the ACE2 receptor. Such ADE mechanisms are well described in other viral diseases such as dengue and Zika virus infections.19 A number of reports documented the finding that most of the individuals presenting with MIS-C have significant levels of SARS-CoV-2 antibodies in their sera but they are negative for SARS-CoV-2 by RT-PCR. We reasoned that there may be a potential role of the ADE that could trigger the MIS-C syndromes whereby the pathogen-specific antibodies can promote pathology.
It has been observed that the severe disease caused by SARS-CoV-1 infection is associated with the peak of neutralizing antibody response, suggesting that antibody responses that potentially contain ADE antibodies may also be related to disease outcome in SARS-CoV-1 infection.20 The spike protein of SARS-CoV-2 contains various epitopes that could induce neutralizing and non-neutralizing antibody production. The neutralizing antibodies afford a protective effect against virus entry into the host cells. On the other hand, the antibodies generated against the non-neutralizing epitopes could enhance virus entry leading to severe disease outcomes.
We hypothesize that the initial exposure of children to the SARS-CoV-2 induces both neutralizing and non-neutralizing antibodies production by immune cells. However, over time, it is possible that those children with predominantly virus-neutralizing antibodies progress to asymptomatic COVID-19 illness. However, a select number of those that shift to producing predominantly non-neutralizing antibodies progress to severe disease due to ADE. This is exemplified by the finding that at low dilutions, anti-sera against SARS-CoV neutralized SARS-CoV infection, while highly diluted anti-sera significantly increased SARS-CoV infection and induced higher levels of apoptosis.21 While low levels of neutralizing antibodies may be insufficient to inhibit virus entry, these low levels of non-neutralizing antibodies could on the other hand enhance virus entry and worsen the disease outcome. Thus, the complex of antibody and Fc receptor functionally mimics viral receptor in mediating MERS-CoV entry into the target cells.22
Previous in vitro (using cell lines) and in vivo studies (utilizing the murine model) of SARS-CoV suggest the potential role of ADE in enhancing viral infection and pathogenesis. The ADE was implicated in a respiratory syncytial virus (RSV) vaccine trial when the vaccinated children carried high titers of non-neutralizing antibodies. Approximately, 80% of the children immunized against RSV ended up hospitalized, and 2 children died, while only 5% of the children in the control group required hospitalization.23 Macaques received vaccinia virus expressing SARS-CoV-1 spike exhibit acute lung injury upon viral challenge than controls and the blockade of FcγR reduced such effects.24 Hamsters vaccinated with SARS-CoV-1 spike protein were potentially protected from SARS-CoV-1 infection but showed evidence of developing anti-sera that facilitated ACE2-independent virus entry.25 Cats vaccinated with spike protein against feline coronavirus died much faster than unvaccinated cats and carried more anti-spike antibodies, implicating ADE.23 Four different SARS-CoV-1 vaccines developed for human use were tested in mice. All vaccines induced immune response and protection against virus infection but Th2-type immunopathology suggesting hypersensitivity to SARS-CoV components was observed.26 Other studies on mice also showed similar effects when the animals vaccinated with SARS-CoV nucleocapsid or inactivated MERS-CoV.27,28
It is important to note the delay between the recognized beginning of the SARS-CoV-2 pandemic in the population and the recent emergence of MIS-C illness in children. It is possible that COVID-19-induced pathology changed since the beginning of the pandemic, after the virus began circulating within the general population due to varied levels of background generational immunity of hosts. The severe or mild and symptomatic or asymptomatic infections produce a different ratio of neutralizing and non-neutralizing antibodies that will affect the pathology of the subsequent second infection with SARS-CoV-2 (Figure 1).
FIGURE 1.
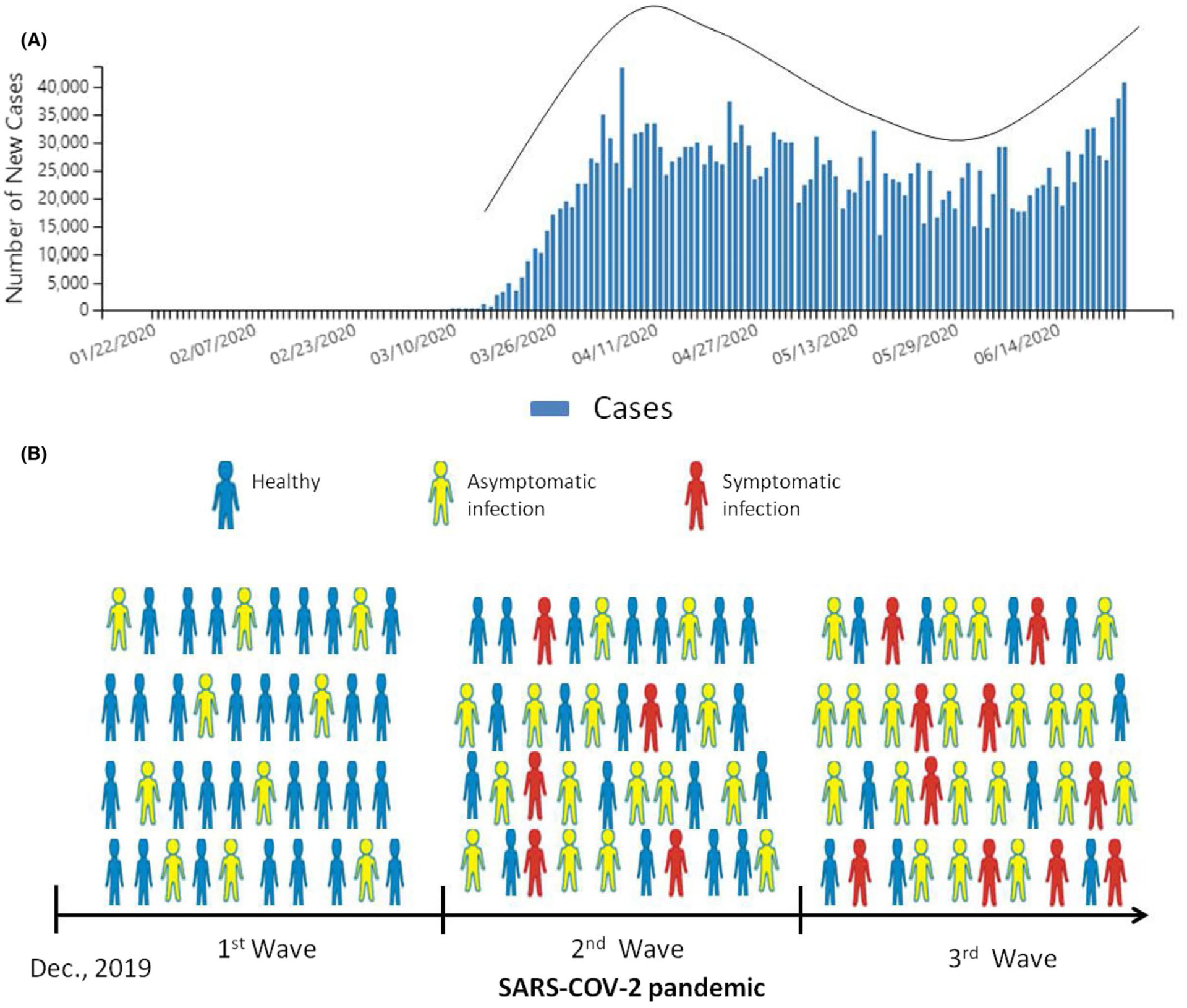
The MIS-C cases could increase through the SARS-CoV-2 waves of infection. (A) The number of new COVID-19 cases reported by CDC each day in the United States since the beginning of the outbreak shows wave-like patterns.39 (B) The hypothetical scheme shows that the susceptibility of children to re-infection with SARS-CoV-2 via ACE2-independent pathway will increase when the accumulated number of children with asymptomatic disease is increasing in the population during the waves of SARS-CoV-2 pandemic
4 |. MACROPHAGE ACTIVATION VIA ANTIBODY-DEPENDENT ENHANCEMENT
A growing body of evidence suggests the host’s innate immune response to SARS-CoV-2 infection triggers the inflammation cascade that causes severe tissue damage.29 Macrophages are the main tissue-resident immune cells that produce high levels of inflammatory cytokines upon infection. The virus-specific antibodies increase the uptake of virus by macrophages in the tissues that lead to the synthesis of high levels of pro-inflammatory cytokines, also known “cytokine storm.” In select cases, COVID-19 is characterized by a cytokine storm resembling that of macrophage activation seen in viral-induced lymphohistiocytosis and hemophagocytosis.30 Macrophages and dendritic cells are the key immune cells which express ACE2 receptor and susceptible to SARS-CoV-2 infection in the lung and other tissues. Previous studies showed that coronaviruses infect different immune cells with or without ACE2 receptors and the infected immune cells mediate virus entry into lung cells.31,32 Macrophages expressing CD68+CD169+ in the spleen and lymph nodes contain SARS-CoV-2 nucleoprotein antigen and upregulate of IL-6 levels.33 It is also suggested that CD169+ macrophages could have a role in viral dissemination, inflammation, and cell death during SARS-CoV-2 infection.33 Further studies are required to address the relationship between Th2 cell–mediated pathology during coronavirus infection and the occupancy of ADE.
The ACE2-independent pathway of virus entry depends on the expression of IgG Fc receptors (FcR) on the cellular membrane of the immune cells. Binding of virus-antibody complex to FcR induces cellular endocytosis21 (Figure 2). The existing SARS-CoV-2-specific antibodies in MIS-C patients may thus promote viral entry into immune cells resulting in immune cell activation and subsequent acute inflammation. The macrophages are activated when the endosomal Toll-like receptors TLR3, TLR7, and TLR8 start sensing viral RNA and induce a downstream cascade of pro-inflammatory cytokines such as TNF and IL-6.21 The elevated levels of TNF are the leading cause of septic shock, and multi-organ failure may result in myocardial damage and circulatory failure observed in some COVID-19 patients.34 Furthermore, it has been observed that the majority of the children with MIS-C do have gastrointestinal symptoms and in a recent study of pediatric Crohn’s disease in a cohort of MIS-C children linked to the COVID-19 infection was successfully treated with TNF-α blockade.35 Therefore, it was suggested that local inflammation and accumulation of pathological macrophage populations in the tissues could be among the leading causes of MIS-C syndrome. Further investigation is required to illustrate the role of macrophage populations in MIS-C syndrome.
FIGURE 2.
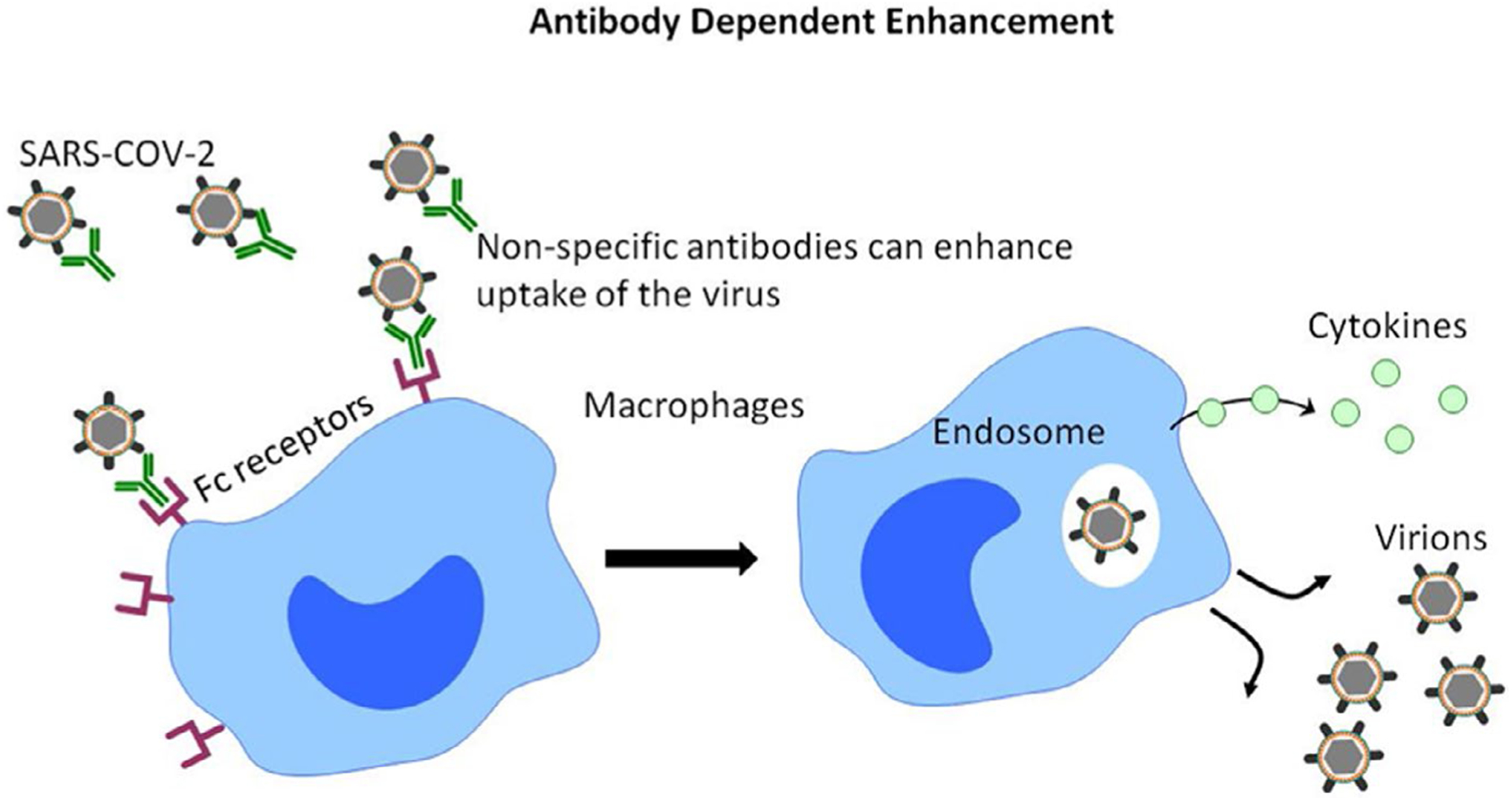
The ACE2-independent pathway of virus entry depends on the expression of Fc receptors on the cellular membrane of the immune cells. Binding of virus-antibody complex to Fc receptors induces cellular endocytosis resulting in immune cell activation and subsequent acute inflammation
In conclusion, a few months after the COVID-19 pandemic, children with MIS-C syndrome linked to SARS-CoV-2 infection were reported in different countries. To the best of our knowledge, there was a paucity of reports from Asian countries that addressed the issue of pediatric cases of COVID-19 until recently.36 As reported, there were only 14 cases of children under the age of 10 among the total of 8866 cases of COVID-19 in one study from China.36 In addition, the number of cases in patients aged from 10 to 19 was only 1% or less. It is of interest to note that the incidence of Kawasaki disease is significantly higher in Asian populations37 but the incidence of systemic inflammation secondary to COVID-19 in pediatric cases has been rarely observed.38 The precise reasons for this finding have yet to be established but we suspect that the low number of pediatric COVID-19 patients may be a contributing factor. There is an apparent correlation between the percentage of asymptomatic SARS-CoV-2 infection in children and the incidence of MIS-C. The absence of SARS-CoV-2 RNA in seropositive individuals suggests the possible role of SARS-CoV-2 non-specific antibodies in the development of MIS-C disease via ADE.
Key Message.
Multisystem inflammatory syndrome in children (MIS-C) is associated with SARS-CoV-2 infection and a correlation was found between the percentage of asymptomatic SARS-CoV-2 infection in children and the incidence of MIS-C. Further, there is a possible role of SARS-CoV-2 non-specific antibodies in the development of MIS-C disease via antibody-dependent enhancement (ADE).
ACKNOWLEDGMENTS
We thank Dr. Aftab A. Ansari for critical reading of this manuscript. This work was partially supported by the National Institute of Allergy and Infectious Diseases Grant R01 AI129745 and Frances E. Lageschulte and Evelyn B. Weese New Frontiers in Medical Research Fund to SNB.
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: AI129745
Footnotes
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists.
REFERENCES
- 1.Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). https://emergency.cdc.gov/han/2020/han00432.asp [Google Scholar]
- 2.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Medicine. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin M Childhood Multisystem Inflammatory Syndrome - A New Challenge in the Pandemic. N Engl J Med. 2020;383(4):393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiotos K, Bassiri H, Behrens EM, et al. Multisystem Inflammatory Syndrome in Children during the COVID-19 pandemic: a case series. J Pediatr Infect Dis Soc. 202069(32):1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennon TR, Penque MD, Abdul-Aziz R, et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. 2020;57:101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 8.American College of Rheumatology Clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Published June 17, 2020 Accessed July 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novel Coronavirus Pneumonia Emergency Response Epidemiology T. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(2):145–151. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Mo X, Hu Y, et al. Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China. Pediatrics. 2020;146(3):e20200702. [Google Scholar]
- 12.Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J Virol. 2020;94:e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaimes JA, Millet JK, Stout AE, Andre NM, Whittaker GR. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquinet E, Rao NV, Rao GV, Hoidal JR. Cloning, genomic organization, chromosomal assignment and expression of a novel mosaic serine proteinase: epitheliasin. FEBS Lett. 2000;468(1):93–100. [DOI] [PubMed] [Google Scholar]
- 16.Schouten LR, van Kaam AH, Kohse F, et al. Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intens Care. 2019;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denison MR. Severe acute respiratory syndrome coronavirus pathogenesis, disease and vaccines: an update. Pediatr Infect Dis J. 2004;23(11 suppl):S207–214. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Yu J, Mani RS, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17(5):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothan HA, Bidokhti MRM, Byrareddy SN. Current concerns and perspectives on Zika virus co-infection with arboviruses and HIV. J Autoimmun. 2018;89:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho MS, Chen WJ, Chen HY, et al. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11(11):1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SF, Tseng SP, Yen CH, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94(5):e02015–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber K Coronavirus vaccine developers wary of errant antibodies. Nat Biotechnol. 2020. 10.1038/d41587-020-00016-w [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kam YW, Kien F, Roberts A, et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25(4):729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Medicine. 2006;3(12):e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Antiga L Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26(6):832–834. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Wo J, Shao J, et al. SARS-coronavirus replicates in mononu-clear cells of peripheral blood (PBMCs) from SARS patients. J Clin Virol. 2003;28(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020;46(5):846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolinger MT, Person H, Smith R, et al. Pediatric Crohn’s Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated with Infliximab. J Pediatr Gastroenterol Nutr. 2020;71(2):153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.She J, Liu L, Liu W. COVID-19 epidemic: Disease characteristics in children. J Med Virol. 2020;92(7):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Zhu H, Yuan C, et al. Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Network Open. 2020;3(6):e2010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.United States COVID-19 Cases and Deaths by State. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html [Google Scholar]


