Abstract
Purpose:
New therapies are needed to treat immune checkpoint inhibitor-resistant non- small cell lung cancer (NSCLC) and identify biomarkers to personalize treatment. Epigenetic therapies, including histone deacetylase inhibitors, may synergize with programmed cell death-1 (PD-1) blockade to overcome resistance. We report outcomes in patients with anti-PD-(L)1– resistant/refractory NSCLC treated with pembrolizumab plus entinostat in ENCORE 601.
Experimental Design:
The expansion cohort of ENCORE 601 included patients with NSCLC who previously experienced disease progression with immune checkpoint inhibitors. The primary endpoint for the phase 2 expansion cohort is overall response rate (ORR); safety, tolerability, and exploratory endpoints are described.
Results:
Of 76 treated patients, 71 were evaluable for efficacy. irRECIST-assessed ORR was 9.2% (95% CI: 3.8–18.1), which did not meet the prespecified threshold for positivity. Median DOR was 10.1 months (95% CI: 3.9–NE), PFS at 6 months was 22%, median PFS was 2.8 months (95% CI: 1.5–4.1), and median OS was 11.7 months (95% CI: 7.6–13.4). Benefit was enriched among patients with high levels of circulating classical monocytes at baseline. Baseline tumor PD-L1 expression and IFNγ gene expression were not associated with benefit. Treatment-related Grade ≥3 adverse events occurred in 41% of patients.
Conclusions:
In anti-PD-(L)1–experienced NSCLC patients, entinostat plus pembrolizumab did not achieve the primary response rate endpoint but provided a clinically meaningful benefit with objective response in 9% of patients. No new toxicities, including immune-related adverse events, were seen for either drug. Future studies will continue to evaluate the association of monocyte levels and response.
Introduction
Anti–programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) immunotherapy administered as monotherapy or combined with chemotherapy has significantly improved outcomes for patients with non-small cell lung cancer (NSCLC) and has led to the approval of nivolumab and atezolizumab in the metastatic setting, durvalumab in the locally advanced setting, and pembrolizumab in both the metastatic and locally advanced settings (1–5). Despite this success, a substantial proportion of patients do not experience a response to initial PD-1 therapy or eventually develop acquired resistance (6). Effective therapeutic approaches that circumvent resistance to PD-1 blockade and patient selection strategies to identify those who may benefit from mechanistically-driven combinations are critically needed. Although a major effort to address these clinical needs has been underway, few successes have been found to date (7,8).
To help inform rational therapeutic development to address resistance to PD-1 blockade, mechanisms of primary and acquired resistance have been explored. Resistance is likely to be multi-factorial and may be generated through genetic and epigenetic changes in the cancer cell and immune cell populations (9). Key mechanisms of immune evasion identified in preclinical studies and patient samples include neoantigen loss, poor immune cell infiltration, effector cell exhaustion and dysfunction, and upregulation of regulatory pathways that lead to an immunosuppressive microenvironment (10–13). For example, epigenetic changes in NSCLC cell lines were shown to induce aberrant activation of gene expression pathways, such as MYC signaling, and loss of antigen presentation leading to anti–PD-(L)1 resistance (8,14–16). Epigenetic repression of neoantigen expression may also be a mechanism of immune evasion (16). These data and others demonstrating impact of epigenetic factors on immune suppressive myeloid cell populations (8,14–16) suggest that the combination of immune checkpoint inhibitors plus epigenetic therapy could lead to increased activation of the interferon pathway, increased T cell attraction, and decreased proliferation of tumor cells (15).
Targeting HDACs is one approach to preventing and normalizing epigenetic changes. HDAC inhibition has been demonstrated preclinically to improve immune competency through increased MHC presentation and tumor antigen expression and reduced number and function of immunosuppressive cells (15,17–21), particularly myeloid-derived suppressor cells (MDSC) (21,22). Entinostat is a selective inhibitor of class I HDACs that has been shown to be effective in combination with PD-1 blockade in multiple tumor models, including lung carcinoma mouse models, which exhibited significant tumor growth reduction (20). Building on preclinical data, a recent correlative analysis of patients with breast cancer treated with entinostat plus an estrogen modulator (ENCORE 301) demonstrated monocytic and granulocytic MDSCs as specific targets of entinostat (22).
Based on these data, we hypothesized that the combination of entinostat plus the PD-1 inhibitor pembrolizumab could be effective for patients with NSCLC whose disease had previously progressed despite PD-1 blockade. Additionally, we predicted that evaluation of pre- treatment circulating levels of monocytic cells or their derivatives or changes in expression of selected genes in pre-treatment tumor tissue may provide a means to identify biomarkers for future selection of subjects for treatment by entinostat in combination with immunotherapy. To explore these hypotheses, ENCORE 601 was a multicenter, single-arm, open-label study designed to assess the safety and efficacy of entinostat and pembrolizumab in patients with metastatic NSCLC resistant to anti–PD-(L)1 therapy.
Methods
Study Design and Participants
ENCORE 601 is a phase 1b/2, open-label, dose escalation study of entinostat in combination with pembrolizumab in patients with NSCLC, with expansion cohorts in patients with NSCLC, melanoma, and mismatch repair-proficient colorectal cancer. This study was approved by the institutional review board or independent ethics committee at each center and conducted in accordance with Good Clinical Practice guidelines defined by the International Conference on Harmonisation. All patients provided written informed consent to participate based on the principles of the Declaration of Helsinki. This report focuses on patients with refractory NSCLC (cohort 2) enrolled in the single-arm, multi-center expansion phase (phase 2) of ENCORE-601.
Eligible patients had recurrent or metastatic NSCLC; had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; and were previously treated with at least 1 chemotherapeutic regimen for advanced or metastatic NSCLC and developed unequivocal progressive disease by either Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (23) or clinical assessment. Patients originally diagnosed with early stage disease (stage I-III) must have recurred after definitive therapy or developed distant metastatic disease in order to be eligible. Patients must also have been previously treated for at least 6 weeks with a PD-1/PD-L1 blocking antibody and experienced documented radiographic progression by Immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) (24) or similar criteria during or within 12 weeks after the last dose. Patients with epidermal growth factor receptor (EGFR) mutation-positive or anaplastic lymphoma kinase (ALK) translocation-positive disease must have previously been treated with an appropriate targeted therapy. Pre-therapy tumor biopsies were required, and on-treatment biopsies were optional. Pre-therapy PD-L1 expression was assessed retrospectively and was not used to determine eligibility for enrollment. Patients were excluded if they had immunodeficiency or active autoimmune disease or were on immunosuppressive therapy. Treatment continued up to 2 years or until confirmed disease progression, unacceptable toxicity, withdrawal of consent, or if a study investigator or sponsor decided to discontinue treatment.
Procedures
Patients were enrolled to receive entinostat 5 mg by mouth on cycle days 1, 8, and 15 in combination with pembrolizumab administered intravenously at a dose of 200 mg on cycle day 1 for a maximum of 35 21-day cycles.
Tumor response was assessed using irRECIST 1.1 criteria for evaluation of contrast- enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) performed at screening and then every 6 weeks until documented progressive disease. Complete response (CR) and partial response (PR) were initially assessed by a study investigator and confirmed by a center core radiologic laboratory.
Adverse events (AEs), including immune-related adverse events (irAEs), were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (CTCAE).
Outcomes
The primary endpoint of phase 1b (dose escalation/confirmation cohorts) was to determine the dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D) of entinostat in combination with pembrolizumab (25). The primary endpoint of phase 2 (expansion cohorts) was to evaluate the efficacy of entinostat at the RP2D in combination with pembrolizumab in patients with anti–PD-(L)1 resistant NSCLC as determined by overall response rate (ORR), per irRECIST.
The secondary endpoints were safety and the tolerability of entinostat in combination with pembrolizumab (based on clinical AEs, laboratory parameters, and electrocardiogram results). Secondary measures of efficacy included clinical benefit rate (CBR), progression-free survival (PFS) rate at 6 months, PFS, overall survival (OS), and duration of response (DOR) and time to response (TTR) in patients who experienced a response to treatment (i.e., CR or PR). These endpoints were evaluated using RECIST 1.1 and irRECIST.
Exploratory endpoints include measurement of pre- and post-treatment changes in immune cell subtypes and gene expression analysis of mandated pre-treatment tumor biopsies. Pharmacokinetics (PK) and pharmacodynamics (PD) of entinostat when given in combination with pembrolizumab were also evaluated, in addition to exploring the exposure-safety response of entinostat in combination with pembrolizumab. The correlation of clinical benefit and multiple parameters at pre-therapy were analyzed to explore potential predictive biomarkers (method further described in the Supplementary Appendix).
Pharmacodynamic analysis
Whole blood samples were collected in cell preparation tubes with sodium citrate (BD Biosciences). PBMCs were obtained by centrifugation and viably frozen until analysis. PBMCs were thawed, washed with flow buffer (5% BSA, 2 mM EDTA in PBS) and incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies), Fc receptor blocking agent (Miltenyi Biotec) and stained with surface antibodies (CD3 clone OKT3, CD4 clone RPA-T4, CD8 clone SK1, CD14 clone HCD14, CD19 clone HIB19, CD25 clone BC96, ICOS clone C398.4A, HLA-DR clone L243, PD-1 clone 29F.1A12 all from BioLegend) for 20 min at 4°C. For Foxp3 and Ki67 staining, cells were fixed and permeabilized using a Fix/Perm buffer (eBiosciences) according to the manufacturer’s instructions, then stained with anti-Foxp3 (clone 206D, BioLegend) or anti-Ki67 antibody (clone B56, BD Biosciences).
For global protein acetylation analysis, after surface staining, cells were fixed with 0.4% paraformaldehyde (Thermo Fisher Scientific), permeabilized in Triton X-100 (Sigma-Aldrich) and subsequently stained with anti-acetylated lysine antibody (clone 15G10, BioLegend).
Statistical Analysis
As previously reported, the initial phase 1b was designed to determine the DLT and the R2PD of entinostat to be given in combination with pembrolizumab (25). The expansion phase (phase 2) of the trial was designed using a Simon optimal 2-stage design; 2 responses in 20 patients were observed in Stage 1, meeting the criteria to expand to Stage 2 and enroll up to 36 patients. Before enrollment was completed, the study was further revised to accrue up to 70 patients to increase statistical power from 80% to 90% and decrease Type 1 error (1-sided significance level of 5%), allowing for more patients to be treated while making an informed decision based on an earlier analysis with appropriate control of Type 1 error. The alternative hypothesis in the NSCLC cohort was a 15% ORR, and the cohort was sized to rule out the lower bound of 5% using a single proportion binomial test.
ORR and 2-sided 95% confidence intervals (CIs) were calculated based on the proportion of patients with a CR or PR by irRECIST criteria. DOR, PFS, and OS were summarized descriptively using the Kaplan-Meier method with 95% CIs. PFS rates at 6 months and corresponding 95% CIs were estimated using the Kaplan-Meier method. The Greenwood formula was used to calculate the standard errors of the Kaplan-Meier estimates and upper and lower limits of the 95% CIs.
Gene expression data were evaluated by principal component analysis, and additional analyses were performed using R-statistical software (version 3.5.1) with Bioconductor 3.8. Differentially expressed genes with estimated fold-changes >1.5 and Benjamini-Hochberg- adjusted p-values ≤0.05 were considered significant. Pre-ranked gene set enrichment analysis (GSEA) was performed using the fast pre-ranked gene set enrichment analysis (fgsea) (1.8.0) Bioconductor package. Fold-change estimates from DESeq2 were used to calculate a rank_metric [ -log10(p.value)*sign(estimate) ], and genes were sorted and tested for enrichment against all human Molecular Signatures Database (MSigDB) collections (msigdbr, 6.2.1) using the following parameters (min_gs_size = 3, max_gs_size = 10000, permutations = 10000). Gene sets showing a positive normalized enrichment score and a bh-adjusted p-value ≤0.05 were considered to be significantly enriched for a gene set. Sub-selected heatmaps (pheatmap, 1.0.12) displaying normalized enrichment scores were generated from filtered results of interest, including Hallmark, c2 curated, or c7 immunologic gene sets. Some further sub-selected heatmaps were specifically filtered to show only gene sets that contained the MYC gene as a defined leading-edge member.
High/low monocyte levels were defined based on the median percentage (8.64%) of Human Leukocyte Antigen-DR isotype high (HLA-DRhi) classical monocytes among total peripheral blood mononuclear cells (PBMCs) from available samples.
Role of the funding source
The funders provided the study drugs and worked with the investigators to design the study and to collect, analyze, and interpret the data. All authors, including those employed by the sponsor of the study, contributed to the interpretation of the data. All drafts of the report were prepared by the corresponding author with input from all co-authors and editorial assistance from professional medical writers, funded by the sponsor. The corresponding author had final responsibility for the decision to submit for publication.
Results
Patients
Between 17 May 2015 and 13 December 2017, 110 patients from 14 centers and hospitals within the United States were screened for eligibility. In total, 77 patients were enrolled, 76 patients were treated (one patient had anemia prior to treatment on Cycle 1 Day 1 that limited eligibility), and 71 were evaluable for efficacy (5 patients discontinued due to unrelated adverse events or withdrew consent before the Week 6 scan assessment).
The median age of patients was 66 years (range, 29–85 years), 53% were male, 54 (71%) had an ECOG Status of 1, and 88% were either current or former smokers (Table 1). PD- L1 expression data from pre-treatment biopsies were available for 62/76 patients: 25 (33%) were PD-L1 negative, 37 (49%) were positive (Table 1).
Table 1.
Patient Baseline Demographics and anti-PD-1 Treatment History
| Demographics | N=76 |
|---|---|
| Male, n (%) | 40 (52.6) |
| Median age (range) | 66 yrs (29–85) |
| ECOG PS, n (%) | |
| 0 | 21 (27.6) |
| 1 | 54 (71.1) |
| Missing | 1 (1.3) |
| Current/former smoker, n (%) | 67 (88.2) |
| Race, n (%) | |
| Asian | 2 (2.6) |
| Black or African American | 3 (3.9) |
| Other | 5 (6.6) |
| White | 66 (86.8) |
| PD-L1 expression, n (%) | |
| Negative | 25 (32.9) |
| Positive | 37 (48.7) |
| Not Evaluable | 8 (10.5) |
| Not Done | 6 (7.9) |
| Visceral Metastases, n (%) | |
| Yes | 66 (86.8) |
| No | 8 (10.5) |
| Missing | 2 (2.6) |
| Metastatic Sites, n (%) | |
| Abdomen | 1 (1.3) |
| Adrenal/Kidney | 6 (7.9) |
| Bone | 22 (28.9) |
| CNS | 14 (18.4) |
| Liver | 13 (17.1) |
| Lung | 43 (56.6) |
| Lymph Nodes | 40 (52.6) |
| Pancreas | 1 (1.3) |
| Pleura | 9 (11.8) |
| Other | 12 (15.8) |
| Stage at Metastatic Diagnosis, n (%) | |
| I | 0 (0.0) |
| II | 0 (0.0) |
| IIIA | 5 (6.6) |
| IIIB | 7 (9.2) |
| IIIC | 0 (0.0) |
| IV | 64 (84.2) |
| Baseline LDH (%> ULN), n (%) | |
| No | 49 (64.5) |
| Missing | 1 (1.3) |
| ALK Result, n (%) | |
| Negative | 54 (71.1) |
| Missing | 22 (28.9) |
| EGFR Result, n (%) | |
| Positive | 5 (6.6) |
| Negative | 52 (68.4) |
| Missing | 19 (25.0) |
| Best response on prior anti-PD-(L)1, n (%) | |
| Complete Response | 1 (1) |
| Partial Response | 7 (9) |
| Stable Disease | 42 (55) |
| Disease Progression | 22 (29) |
| Unknown | 2 (3) |
| Duration on latest anti-PD-(L)1 | |
| Median | 5.6 months |
| Time from prior anti-PD-(L)1 to study therapy | |
| Median | 2.3 months |
| PD-(L)1 as immediate prior therapy, n (%) | 47 (62) |
All patients had received ≥1 chemotherapy regimen and anti-PD-(L)1 therapy at some point prior to enrollment. Anti–PD-(L)1 therapy was the most recent systemic treatment in 47 patients (62%). Median duration of prior anti–PD-(L)1 therapy was 5.6 months (range, 0.6–27.6 months), and best response (per local investigator assessment) to prior anti–PD-(L)1 therapy was 1% CR, 9% PR, 55% stable disease (SD), and 29% progressive disease (PD). The median time between the last dose of prior anti–PD-(L)1 therapy and the start of study therapy was 2.3 months (range, 0.3–53.0 months) (Table 1).
At the time of data freeze on October 2, 2019, the median duration of follow-up was 5.95 months (range, 0.4–27.0). Three patients continued to receive entinostat and pembrolizumab beyond that date. The median number of cycles started was 3 (range, 1–35).
Efficacy
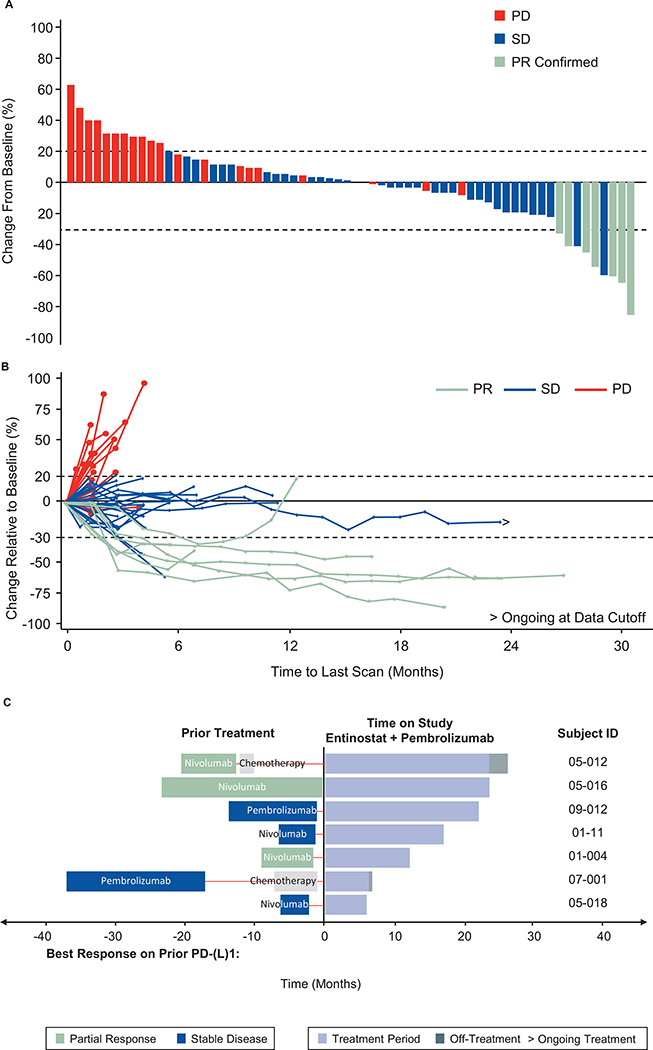
The primary endpoint, irRECIST-assessed ORR, was 7 of 76 (9.2%, 95% CI: 3.8– 18.1%) (Figure 1). This primary endpoint did not reach the prespecified threshold for the lower bound of the 95% confidence interval. Median DOR was 10.1 months, based on the Kaplan- Meier method (95% CI: 3.9–not estimable) (Figure 1B, 1C). PFS rate at 6 months was 22%, and median PFS was 2.8 months (95% CI: 1.5–4.1). Median OS was 11.7 months (95% CI: 7.6– 13.4). Median time between last dose of prior PD-1/PD-L1 therapy and first of dose of therapy in ENCORE 601 was comparable between responders and non-responders [median 1.41 months (range, 0.69–11.20) vs 1.64 months (range, 0.03–53.03).
Figure 1.
Maximal tumor burden and change in tumor volume over time. A, Waterfall plot and B, Spider plot of change in lesions over time. C, Prior treatment and duration of treatment for responders. Responses were observed regardless of prior treatment history or PD-L1 status.
Prior treatment and duration of treatment are shown in detail for responders. Responses were observed regardless of prior treatment history (Figure 1C). All 7 responders were among the 67 former or current smokers enrolled in the trial, but no significant correlation between response and smoking history was identified in multi-variate subset analyses. Of the 7 responders, 5 were negative for PD-L1 expression, 1 was weak positive, and 1 was strong positive.
Safety
Treatment-emergent adverse events (TEAEs) related to entinostat and/or pembrolizumab occurred in 82% of patients. Grade ≥3 TEAEs occurred in 44 (58%) of patients; 31 (41%) had Grade ≥3 TEAEs related to entinostat and/or pembrolizumab. TEAEs that led to entinostat and/or pembrolizumab discontinuation occurred in 25% (19) of patients; 15% (11) of patients had a drug-related TEAE that led to entinostat and/or pembrolizumab discontinuation. Fifty-three percent of patients had at least one entinostat dose modification (dose decreased, dose delayed, dose skipped or discontinued), and 37% of patients required pembrolizumab dose modification at least once. TEAEs not related to treatment led to death in 3 patients.
The most common any-grade TEAEs related to entinostat and/or pembrolizumab treatment were fatigue (42%), diarrhea (21%), anemia (20%), and decreased appetite (20%). Grade ≥3 TEAEs related to either treatment that occurred in 4 or more patients included fatigue (11%), hypophosphatemia (9%), anemia (7%), and hyponatremia (5%). Seven patients (9%) experienced a Grade ≥3 immune-related AE (3 events of pneumonitis, 3 events of colitis, 1 each of encephalitis, pancreatitis, and hyperthyroidism).
Pharmacodynamics
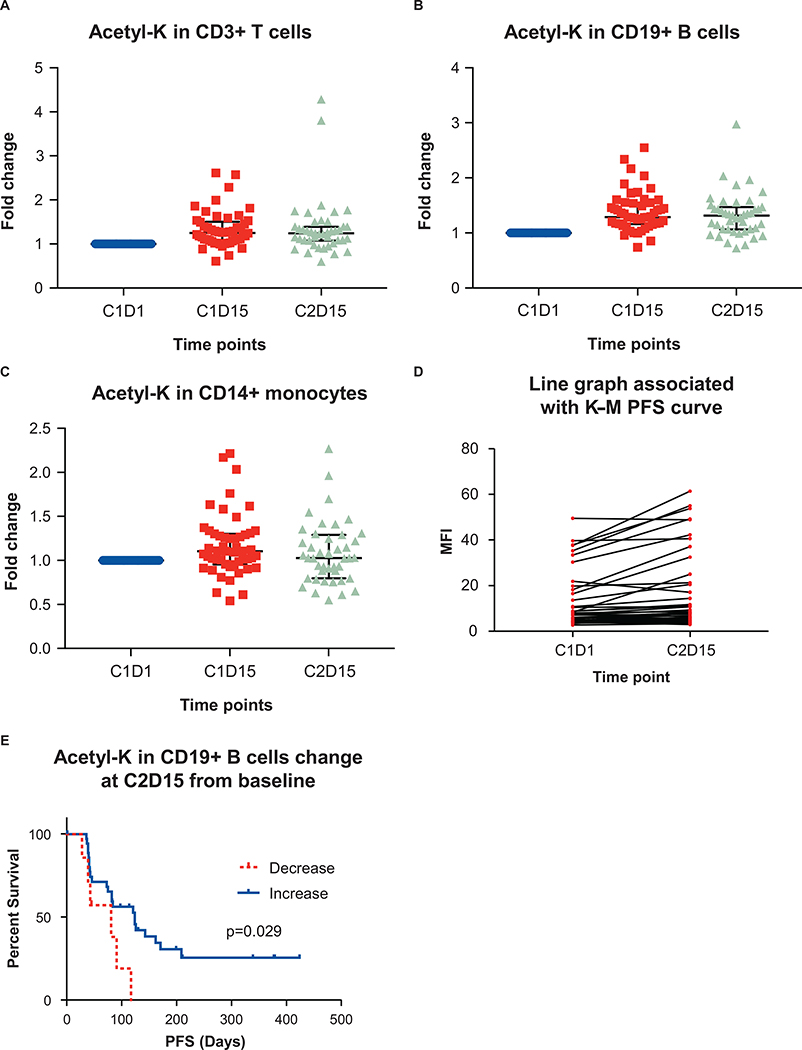
The expected pharmacodynamic (PD) impact of entinostat was observed as an increase in lysine acetylation across peripheral immune cells, including CD3+ T cells, CD19+ B cells and CD14+ monocytes (Figure 2A–C). Increased acetylation at Cycle 2 Day 15 (C2D15) in CD19+ B cells, as compared to pre-treatment C1D1 values, was associated with improved PFS, and decreased acetylation was associated with an inferior PFS (Figure 2D, 2E). Increased acetylation in T cells and monocytes was not associated with improved PFS. It is unclear why only acetylation in B cells would translate to a PFS difference, although acetylation in B cells was also found to exhibit the most robust PFS difference in the previously reported phase 2 ENCORE 301 study of entinostat combined with exemestane in ER+ breast cancer (26). Additional observations associated with an activated immune response, and potentially related to the immunomodulatory activity of entinostat combined with pembrolizumab, included post- treatment C1D15 and C2D15 increases in human leukocyte antigen-DR isotype (HLA-DR) expression in CD8+ and CD4+ T cells (Supplemental Figure 1A–D), increased inducible T cell costimulatory (ICOS) expression in CD4+ T cells (Supplemental Figure 1E), an increase in the proportion of Ki67+/PD-1+ T cells, a decrease in FoxP3 expression and intensity in CD4+ regulatory T cells (Tregs) (Supplemental Figure 1F), and a decrease in the ratio of Tregs to CD8+ T cells (Supplementary Figure 1G). Further, while the baseline value of T cells with a reinvigorated phenotype (Ki67+/PD1+ in CD4+ T cells and Ki67+/PD1+ in CD8+ T cells) did not correlate with duration of treatment, the percent of reinvigorated T cells at C1D15 correlated with duration of treatment, suggesting an association between treatment-induced reinvigorated T cells and a favorable duration of treatment (Supplemental Figure 1H and 1I).
Figure 2.
Expression of acetylated lysine in peripheral immune cells after therapy. Flow cytometry was used to analyze levels of acetylated lysine in A, CD3+ T cells; B, CD19+ B cells; and C, CD14+ monocytes at Cycle 1, Day 1 (C1D1), Cycle 1, Day 15 (C1D15) and Cycle 2, Day 15 (C2D15). Error bars represent mean ± standard deviation. D, Acetyl-lysine expression in CD19+ B cells at C2D15 compared to baseline C1D1 values. E, Kaplan-Meier curve of PFS by increase or decrease in acetyl-lysine expression in CD19+ B cells from baseline to C2D15.
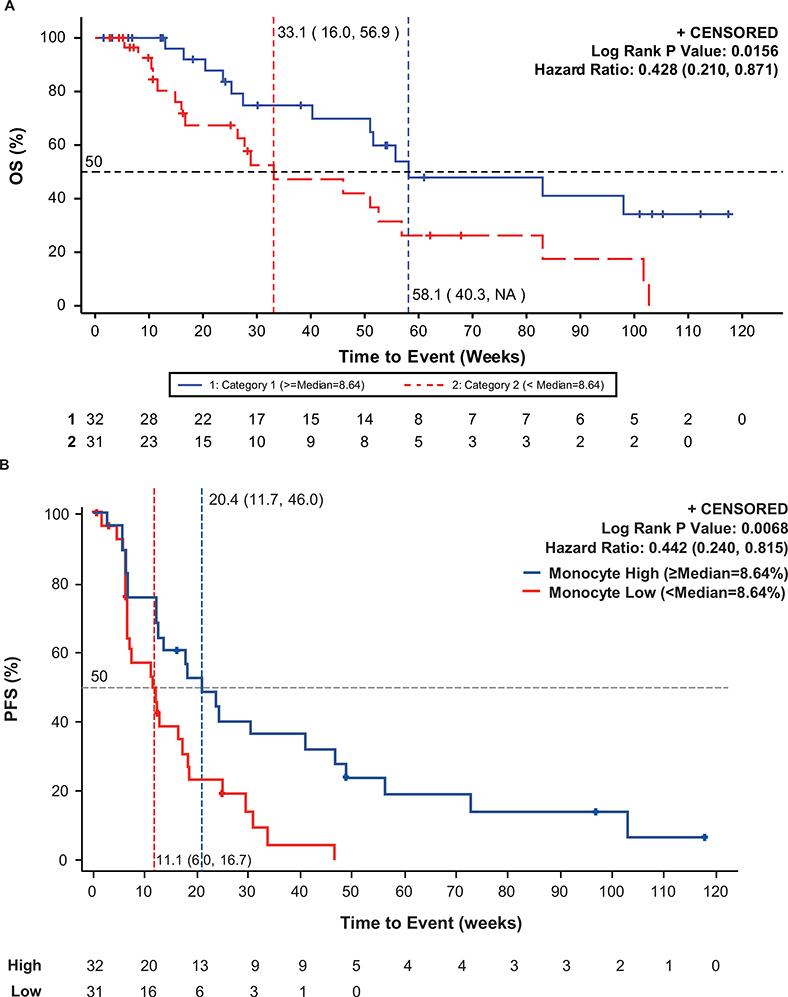
We found that responses were enriched among patients with high levels of classical monocytes (n=32; defined as CD14+/CD16-/HLA-DRhi and split high vs low around the median of 8.6%) [ORR 14.7% (95% CI:5.0–31.1)] compared to those with lower levels of monocytes (n=31; <8.6%) [ORR, 5.9% (95% CI: 0.7–19.7)]. Patients with high monocytes also had longer progression-free survival [HR, 0.44 (95% CI: 0.24–0.82); mPFS, 4.1 months (95% CI: 1.4–9.3) vs 2.7 months (95% CI: 1.4–3.7)] and overall survival [HR = 0.43 (95% CI: 0.21–0.87); mOS 58.1 weeks (95% CI: 40.3–not reached) vs 33.1 weeks (95% CI: 16.0–56.9)] (Figure 3A,B).
Figure 3.
Kaplan-Meier curve of PFS (A) and OS (B) by proportion of classical monocytes (CD14+/CD16-/HLA-DRhi). Median PFS and median OS were increased in the monocyte high group (≥8.64%) versus the monocyte low group (<8.64%).
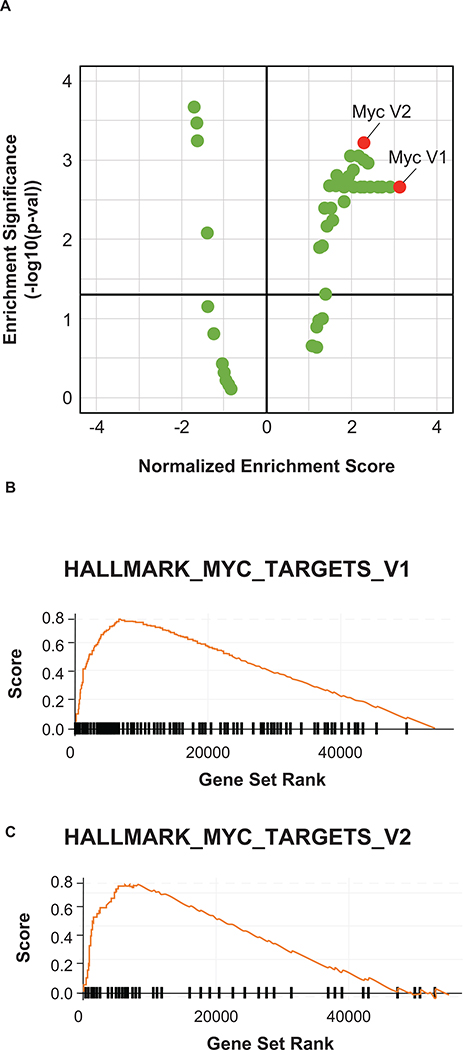
In addition to the peripheral blood, we also examined bulk transcriptional profiles of tumors (n=43; 4 responders and 39 non-responders) to evaluate tumor-specific features associated with response. Two post-treatment biopsies were obtained, both in non-responders, and these samples were not evaluated further. Unlike previous results in anti–PD-(L)1–naïve patients, IFNγ expression was not associated with response to pembrolizumab plus entinostat in patients with prior anti–PD-(L)1 treatment (27,28). Instead, MYC was identified as the top pathway associated with response (Supplementary Table 1; Figure 4). This observation is consistent with preclinical data showing that combination of MYC depletion with epigenetic therapy reversed immune evasion, potentially allowing for more effective NSCLC treatment (15).
Figure 4.
Enrichment of MYC Hallmark gene sets V1 and V2 in pretreatment tumors from anti–PD-1 pretreated NSCLC patients responding to pembrolizumab plus entinostat.
Discussion
We report on the safety, efficacy, and PD results from a Phase 2 expansion cohort of a Phase 1b/2 trial of the combination of entinostat plus pembrolizumab in patients with NSCLC who were previously treated with and progressed on anti–PD-(L)1-based therapy. Treatment was associated with a response rate of 9.2% (95% CI: 3.8%–18.1%). The lower bound of the 95% confidence interval did not exceed 5%, which was considered the lowest threshold for antitumor activity that would warrant continued development in this setting. The median duration of response (10.1 months; 95% CI: 3.9–not estimable) and percentage of patients with PFS at 6 months (22%) were nevertheless notable in demonstrating a meaningful clinical benefit for some patients. Of note, evaluation of PD-L1 expression in pre-treatment biopsies indicated that tumors from 5 of the 7 responding patients were negative for PD-L1 expression. While the sample size is too small to associate lack of PD-L1 expression with response, particularly with the lack of response in the majority of patients with negative PD-L1 expression, these results may reflect the potential for the combination to elicit responses in a subset of patients with non- immunogenic tumors. The response rate remains of clinical interest, particularly if correlative data can predict which patients could benefit the most from this combination. In multivariate analyses, we observed a positive correlation between high baseline HLA-DRhi classical monocytes and clinical benefit in patients with NSCLC previously treated with anti–PD-(L)1 therapy. ORR was 14.7% in the monocyte high population, and mPFS was 4.1 months, and mOS was 58.1 weeks (Figure 3A,B).
HLA-DR is a primary antigen-presenting molecule of the major histocompatibility complex class II (MHC-II) pathway that is primarily expressed on antigen-presenting cells, including monocytes and, in some cases, tumor cells. The expression of HLA-DR can be upregulated by cytotoxic T cell-secreted IFNγ, and elevation of HLA-DR may be an indication of activated T cells and IFNγ-induced responses that usually mediate sensitivity to anti–PD-(L)1 therapy. Indeed, the presence of HLA-DR–expressing tumor cells in melanoma patient tumor biopsies is strongly correlated with response to immunotherapy (29), and elevated numbers of HLA-DR classical monocytes in the peripheral blood were shown to be predictive of response to anti–PD-1 therapy in melanoma patients previously untreated with anti–PD-(L)1 therapy (30). In the present study, the patients had either never responded to or had progressed after responding initially to prior immunotherapy, suggesting that HLA-DRhi classical monocyte levels may also indicate sensitivity to the entinostat–pembrolizumab combination. A phase 2/3 study has been designed to further explore clinical outcomes of the combination of entinostat and pembrolizumab in patients with metastatic NSCLC stratified by high/low HLA-DRhi monocyte count as measured by an HLA-DRhi monocyte assay. This trial will evaluate the hypothesis that patients with metastatic NSCLC with elevated HLA-DRhi monocytes (CD14+/CD16-/HLA-DRhi≥9%) whose disease progressed on approved standard of care platinum-based therapy and within 12 weeks of checkpoint inhibitor therapy will have an improved PFS with the combination of entinostat and pembrolizumab in comparison to docetaxel.
In addition to the observation that elevated levels of circulating classical monocytes were associated with clinical benefit, this study provided an opportunity to explore gene expression pathways tied to immune therapy resistance in a sizeable population of anti–PD-(L)1 therapy relapsed/refractory NSCLC patients. Interestingly, the top five enriched pathways included MYC and E2F signaling, both of which have recently and independently been shown to coincide with an immune-suppressed tumor microenvironment and immune checkpoint therapy resistance in preclinical studies and in patient samples (15,31–34). Entinostat has previously been shown to decrease MYC activity (34–37), which may suggest a potential mechanism for overcoming resistance to anti–PD-(L)1 therapy in addition to its ability to reduce the immunosuppressive function of MDSCs.
Toxicities with entinostat plus pembrolizumab were comparable to other studies with pembrolizumab alone or combined with chemotherapy or another HDAC inhibitor (1–3,38–40). No new toxicities, including irAEs, were seen for either drug. Immune-related AEs of interest that were Grade ≥3 included colitis (n=3), pneumonitis (n=3), encephalitis (n=1), pancreatitis (n=1), and hyperthyroidism (n=1), none of which were unexpected.
Other anti–PD-(L)1 combinations with immunotherapy in patients already treated with immune checkpoint inhibitors have also shown some activity. In patients with prior immune checkpoint inhibitor therapy, sitravatinib in combination with nivolumab resulted in PRs in 7/25 patients (4 confirmed) (41). Another HDAC inhibitor, vorinostat, has also been examined in combination with pembrolizumab in NSCLC patients previously treated with immune checkpoint inhibitors, and 3 of 24 patients had PRs (1 confirmed) (40). Similar to the results of this study, outcomes and treatment duration in patients treated with vorinostat plus pembrolizumab were not associated with PD-L1 expression.
Limitations of this phase 2 study include a small sample size and lack of direct comparison to the standard of care. In addition, the pre-treated patient population enrolled in this study may be more susceptible to increased or cumulative toxicities. Another limitation was the lack of prospective incorporation of biomarkers for patient selection.
The treatment landscape of NSCLC is changing quickly, and the advent of immunotherapy has led to increased options for patients. However, patients progressing on, or not responding to, currently available immune checkpoint therapy-based regimens require novel approaches to overcome resistance. The results of this study in patients with NSCLC relapsed or refractory to prior anti–PD-(L)1 therapy identify no new toxicities, including irAEs, for either drug and suggest that entinostat combined with pembrolizumab provides significant clinical benefit to a subset comprising 9% of patients that may be identified through measurement of circulating baseline classical monocytes.
Supplementary Material
Table 2.
Treatment-Related Adverse Events
| Treatment-Related Adverse Event (N=76)* | Any Grade | Grade ≥ 3 |
|---|---|---|
| Any | 62 (81.6) | 31 (40.8) |
| Fatigue | 32 (42.1) | 8 (10.5) |
| Diarrhea | 16 (21.1) | 2 (2.6) |
| Anemia | 15 (19.7) | 5 (6.6) |
| Decreased appetite | 15 (19.7) | 1 (1.3) |
| Platelet count decreased | 12 (15.8) | 1 (1.3) |
| Hypophosphatemia | 11 (14.5) | 7 (9.2) |
| Nausea | 10 (13.2) | 1 (1.3) |
| Blood alkaline phosphatase increased | 8 (10.5) | 1 (1.3) |
| Hypoalbuminemia | 7 (9.2) | 0 (0.0) |
| Hyponatremia | 7 (9.2) | 4 (5.3) |
| Vomiting | 7 (9.2) | 1 (1.3) |
| Weight decreased | 7 (9.2) | 0 (0.0) |
| Pneumonitis | 6 (7.9) | 3 (3.9) |
| Colitis | 3 (3.9) | 3 (3.9) |
| Edema | 3 (3.9) | 2 (2.6) |
Treatment-related adverse events occurring in ≥ 9% of patients for all grade or ≥ 2 patient for grade ≥ 3
Translational Relevance.
Despite improved outcomes for patients with non–small cell lung cancer (NSCLC) with single-agent anti–PD-1 immunotherapy, some patients do not respond to treatment, and resistance occurs in other patients. New therapeutic strategies to overcome resistance are needed, as well as biomarkers to drive insight and personalize treatment. To help address these obstacles, we performed a clinical trial of the anti–programmed cell death-1 (anti–PD-1) inhibitor pembrolizumab combined with the histone deacetylase (HDAC) inhibitor entinostat in patients with PD-1 axis inhibitor-resistant NSCLC and examined predictive biomarkers for response. Preclinical results suggest that HDAC inhibition may enhance the efficacy of immunotherapy through multiple mechanisms. Here we show that this combination provides clinical benefit in a subset comprising 9% of patients. No new toxicities, including immune-related adverse events, were seen for either drug. We also report that benefit is enriched in patients with increased circulating baseline classical monocytes.
Acknowledgments
The authors thank all the patients who participated in this trial. Writing assistance for this manuscript was provided by Kristen Evaul, PhD of Brightly Network, LLC, with funding from Syndax Pharmaceuticals, Inc. We acknowledge prior contributions to this study by Leena Gandhi, MD, at New York University. M.D. Hellmann is supported by a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation Grant No. CI-98-18, by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30- CA008748, and is a member of the Parker Institute for Cancer Immunotherapy. D. Gabrilovich used core facilities at Wistar Institute supported by NIH grant P30 CA010815. J.B. Trepel was supported with funding from the Intramural Research Program of the National Cancer Institute. M. Opyrchal acknowledges Ashley Jackson, study coordinator for the Roswell Park Comprehensive Cancer Center site.
(Funded by Syndax Pharmaceuticals, Inc.; ClinicalTrials.gov number, NCT02437136)
Disclosure of Potential Conflicts of Interest
M.D. Hellmann. receives research support from Bristol-Myers Squibb; has been a compensated consultant for Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax Pharmaceuticals, Inc., Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles, and Arcus; received travel support/honoraria from AstraZeneca, Eli Lilly, and Bristol-Myers Squibb; has options from Shattuck Labs, Immunai, and Arcus; has a patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. P.A. Jänne has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, LOXO Oncology, Daiichi Sankyo, Syndax Pharmaceuticals, Inc, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals and Biocartis; receives post-marketing royalties from DFCI owned intellectual property on EGFR mutations licensed to Lab Corp; has sponsored research agreements with AstraZeneca, Daichi-Sankyo, PUMA, Boehringer Ingelheim, Eli Lilly and Company, Revolution Medicines and Astellas Pharmaceuticals; and has stock ownership in LOXO Oncology and Gatekeeper Pharmaceuticals. M. Opyrcha receives research support Pfizer and Bayer. Advisory Board participant for Novartis and Astra-Zeneca. L.E. Raez receives research support from Bristol-Myers Squibb, Genentech/Roche, Pfizer, Nanthealth, MSD, Merck Serono, Lilly Oncology, Boheringer-Ingelheim, Syndax Pharmaceuticals, Inc, Novartis, Heat Biologics, Astra- Zeneca, Exomes DX, Liquid Genomics, and Loxo Oncology. D. Gabrilovich is currently an employee of AstraZeneca. Work for the study was supported by Syndax Pharmaceuticals, Inc. research grant. J.B. Trepel receives research funding to her institution from Syndax Pharmaceuticals, Inc. S. Sankoh, is an employee of Syndax Pharmaceuticals, Inc. and has equity in the company. D. Tamang is an employee of Syndax Pharmaceuticals, Inc. and has equity in the company. L. Wang was an employee in Syndax Pharmaceuticals, Inc. during contribution to this manuscript. E. Schmidt is employed by Merck and Co., Inc. and is a stockholder Merck and Co., Inc. M. Meyers is the CMO of Syndax Pharmaceuticals, Inc. S.S. Ramalingam has served on scientific advisory board meetings and received honoraria from Amgen, Astra Zeneca, Bristol Myers Squibb, Merck, Glaxo SmithKline, Takeda, Genentech, Daichii Sankyo and Puma. S.S. Ramalingam’s institution has received research support from Syndax Pharmaceuticals, Inc., Merck, Astra Zeneca and Bristol-Myers Squibb. E. Shum receives consulting fees from AstraZeneca and Boehringer-Ingleheim. P. Ordentlich is the CSO and has ownership interest (including stocks and patents) in Syndax Pharmaceuticals, Inc. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;21372:2018–28. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 4.Duma N, Santana-Davila R, Molina JR. Non–Small Cell Lung Cancer: Epidemiology, screening, diagnosis, and treatment Mayo Clin Proc. Mayo Foundation for Medical Education and Research; 2019;94:1623–40. [DOI] [PubMed] [Google Scholar]
- 5.FDA. FDA expands pembrolizumab indication for first-line treatment of NSCLC (TPS ≥1%) [Internet]. 2019. [cited 2020 Aug 5]. Available from: https://www.fda.gov/drugs/fda-expands-pembrolizumab-indication-first-line-treatment-nsclc-tps-1
- 6.Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non–Small Cell Lung Cancer. J Thorac Oncol. Elsevier Inc; 2018;13:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Pearce L, O’donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno- oncology landscape Nat Rev Drug Discov [Internet]. Nature Publishing Group; 2018;17:783–4. Available from: 10.1038/nrd.2018.167 [DOI] [PubMed] [Google Scholar]
- 8.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017;7:1420–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors Cancer Cell [Internet]. Elsevier; 2020. [cited 2020 Jul 9];37:443–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32289269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44:1255–69. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell JS, Long G V., Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition Cancer Treat Rev [Internet]. Elsevier Ltd; 2017;52:71–81. Available from: 10.1016/j.ctrv.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy Cell [Internet]. Elsevier Inc; 2017;168:707–23. Available from: 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topper MJ, Vaz M, Chiappinelli KB, DeStefano Shields CE, Niknafs N, Yen RWC, et al. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer Cell [Internet]. Elsevier; 2017;171:1284–1300.e21. Available from: 10.1016/j.cell.2017.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal R, Cadieux EL, Salgado R, Bakir M Al, Moore DA, Hiley CT, et al. Neoantigen- directed immune escape in lung cancer evolution. Nature. 2019;567:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly RM, Rudek MA, Piekarz R. Entinostat: A promising treatment option for patients with advanced breast cancer. Futur Oncol. 2017;13:1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansari J, Shackelford RE, El-Osta1 H. Epigenetics in non-small cell lung cancer: From basics to therapeutics. Transl Lung Cancer Res. 2016;5:155–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I Histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res. 2017;23:5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomita Y, Lee MJ, Lee S, Tomita S, Chumsri S, Cruickshank S, et al. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor- positive breast cancer: Correlative analysis of ENCORE 301, a randomized, placebo- controlled phase II trial of exemestane with or without entinostat Oncoimmunology [Internet]. Taylor & Francis; 2016;5:1–12. Available from: 10.1080/2162402X.2016.1219008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours - Revised RECIST Guideline (version 1.1) Eur J Cancer. Elsevier Ltd; 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 24.Seymour PL, Cancer C, Group T, Bogaerts J, Perrone A, Medicine T, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M, Adjei A, Opyrchal M, Ramalingam S, Janne P, Dominguez G, et al. Dose escalation/confirmation results of ENCORE 601, a phase Ib/II, open-label study of entinostat (ENT) in combination with pembrolizumab (PEMBRO) in patients with non- small cell lung cancer (NSCLC). J Immunother Cancer [Internet]. 2016;4:73 Available from: https://www.bertelsmann-stiftung.de/fileadmin/files/BSt/Publikationen/GrauePublikationen/MT_Globalization_Report_2018.pdf%0Ahttp://eprints.lse.ac.uk/43447/1/India_globalisation%2Csocietyandinequalities%28lsero%29.pdf%0Ahttps://www.quora.com/What-is-the [Google Scholar]
- 26.Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromata. J Clin Oncol. 2013;31:2128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy Science (80- ). American Association for the Advancement of Science; 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ- related mRNA profile predicts clinical response to PD-1 blockade J Clin Invest. American Society for Clinical Investigation; 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson DB, Estrada M V., Salgado R, Sanchez V, Doxie DB, Opalenik SR, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy Nat Commun [Internet]. Nature Publishing Group; 2016;7:1–10. Available from: 10.1038/ncomms10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High- dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–53. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y, et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting Nat Med. Nature Publishing Group; 2019;25:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell. 2018;175:984–997.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, et al. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression Cell [Internet]. Elsevier; 2017;171:1301–1315.e14. Available from: 10.1016/j.cell.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons JK, Michalowski AM, Gamache BJ, DuBois W, Patel J, Zhang K, et al. Cooperative targets of combined mTOR/HDAC inhibition promote MYC degradation. Mol Cancer Ther. 2017;16:2008–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nebbioso A, Carafa V, Conte M, Tambaro FP, Ciro A, Martens J, et al. C-Myc modulation and acetylation is a key HDAC inhibitor target in cancer. Clin Cancer Res. 2017;23:2542–55. [DOI] [PubMed] [Google Scholar]
- 36.Merino VF, Cho S, Nguyen N, Sadik H, Narayan A, Talbot C, et al. Induction of cell cycle arrest and inflammatory genes by combined treatment with epigenetic, differentiating, and chemotherapeutic agents in triple-negative breast cancer. Breast Cancer Res. 2018;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanioka M, Mott KR, Hollern DP, Fan C, Darr DB, Perou CM. Identification of Jun loss promotes resistance to histone deacetylase inhibitor entinostat through Myc signaling in luminal breast cancer Genome Med. Genome Medicine; 2018;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csöszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 39.Lisberg A, Andrew Tucker D, Goldman JW, Wolf B, Carroll J, Hardy A, et al. Treatment- related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018;6:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray JE, Saltos AN, Tanvetyanon T, Haura EB, Creelan BC, Antonia SJ, et al. Phase 1/1b study of pembrolizumab plus vorinostat in advanced/metastatic non-small cell lung cancer. Clin Cancer Res [Internet]. 2019;clincanres.1305.2019. Available from: http://clincancerres.aacrjournals.org/lookup/doi/10.1158/1078-0432.CCR-19-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leal T, Spira A, Blakely C, He K, Berz D, Richards D, et al. Stage 2 enrollment complete: Sitravatinib in combination with nivolumab in NSCLC patients progressing on prior checkpoint inhibitor therapy. Ann Oncol. 2018;29:viii400–viii441. [Google Scholar]
- 42.Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, et al. Development of a Companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non–small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.