Abstract
Background:
Black heart transplant (HT) recipients have higher risk of mortality than White recipients. Better understanding of this disparity, including subgroups most affected and timing of highest risk, is necessary to improve care of Black recipients. We hypothesize that this disparity may be strongest among young recipients, as barriers to care like socioeconomic factors may be particularly salient in a younger population and lead to higher early risk of mortality.
Methods:
We studied 22,997 adult HT recipients using SRTR data from 1/2005–1/2017 using Cox regression models adjusted for recipient, donor, and transplant characteristics.
Results:
Among recipients aged 18–30, Black recipients had 2.05-fold (95% CI 1.67–2.51) higher risk of mortality compared to non-Black recipients (p<0.001, interaction p<0.001); however, the risk was significant only in the first year post-transplant (first year, aHR= 2.30, 95% CI 1.60–3.31, p<0.001; after first year, aHR= 0.84, 95% CI 0.54–1.29, p=0.4). This association was attenuated among recipients aged 31–40 and 41–60, in whom Black recipients had 1.53-fold (95% CI 1.25–1.89, p<0.001) and 1.20-fold (95% CI 1.09–1.33, p<0.001) higher risk of mortality. Among recipients aged 61–80, no significant association was seen with Black race (aHR= 1.12, 95% CI 0.97–1.29, p= 0.1).
Conclusions:
Young Black recipients have a high risk of mortality in the first year after heart transplant, which has been masked in decades of research looking at disparities in aggregate. In order to reduce overall racial disparities, clinical research moving forward should focus on targeted interventions for young Black recipients during this period.
Journal Subject Terms: transplantation, quality and outcomes, mortality/survival, risk factors, race and ethnicity
Keywords: transplant, epidemiology
1. INTRODUCTION
Black heart transplant recipients have higher risk of mortality compared to White recipients.1–9 Although overall post-heart transplant survival has improved over time, there has not been improvement in long-term survival among Black recipients.9 Even after adjusting for recipient, transplant, and socioeconomic factors, Black recipients had 1.34-fold (95% CI: 1.21–1.47, p<0.001) higher risk of post-transplant mortality compared to Caucasians in a national registry analysis.6 Studies of racial disparities have focused on overall differences between Black and White recipients. In order to reduce racial disparities, however, it is important to understand subgroups among Black recipients who are most at risk.
Black patients have a higher prevalence of cardiovascular diseases including hypertension, diabetes, and heart failure, and they are also affected at disproportionately younger ages with higher morbidity and mortality compared to non-Black recipients.10–15 Earlier onset of cardiovascular diseases among Black patients can progress to end-stage heart diseases at younger ages, resulting in both a different clinical presentation leading up to heart transplantation and a different set of risk factors influencing post-transplant outcomes compared to similarly aged non-Black recipients. Furthermore, most heart recipients in the US are aged 50 to 64; the risk framework, and the way Black race impacts this risk framework, is likely different for the average middle-aged recipient versus a younger recipient.16
In addition, race-associated barriers to treatments such as socioeconomic factors may be particularly salient in a younger population, who have more limited access to resources and healthcare compared to older adults.17–19 While Medicare coverage is available for older adults, insurance coverage likely varies more among younger adults.18,20 Given these factors, previous analyses of the race and post-transplant mortality association likely overestimate race-associated risk in some age categories but underestimate race-associated risk in others. It is possible that these different sets of risk factors based on age and race influence mortality at different times in the post-transplant period. Young Black recipients perhaps have higher risk in the early period when risk of graft rejection is high and close follow-up is required for immunosuppression management and optimization.4,9,21 Thus, identifying timing of highest mortality can inform more impactful surveillance and treatment of high-risk groups in order to make the greatest improvements in outcomes.
The goals of this study were (1) to determine whether the association between recipient race and post-transplant mortality varied by recipient age, (2) to assess the role of potential factors such as socioeconomic status, HLA-mismatch, PRA, and immunosuppression in explaining the disparities found, and (3) to determine whether the risk of post-transplant mortality varied over time.
2. METHODS
The data that support the findings of this study were obtained from the Scientific Registry of Transplant Recipients (SRTR) and requests for the dataset should be sent to SRTR directly. The authors declare that all analytic methods and supporting materials have been described within the article and its online supplementary files.
2.1. Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.22 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
2.2. Study population
The study population included 22,997 first-time adult (≥ 18 years) heart-only transplant recipients between January 1, 2005 and January 31, 2017. Recipients with missing waitlist status (n=1) were excluded. Race was ascertained based on reporting to the OPTN and was categorized into Black and non-Black. Black included “Black or African American.” Non-Black included Caucasian, Hispanic/Latino, Asian, American Indian or Alaska Native, Native Hawaiian, or other/unspecified. Age was categorized as 18–30, 31–40, 41–60, and 61–80 based on categories used in the literature, Martingale residuals to examine functional form, and examination to ensure sufficient observations in each category.17,23 We described clinical, demographic, and transplant characteristics of Black and non-Black recipients and their donors.
2.3. Post-transplant mortality
To determine whether post-transplant mortality varied by recipient race and age, we followed the 22,997 heart transplant recipients from date of transplant until date of death or administrative censoring on 1/31/2017. Death dates were augmented via linkage to the Social Security Master Death file. Kaplan-Meier survival estimates were used to visually compare mortality between Black and non-Black recipients in each age category at 1 year and 5 years post-transplant. We also examined mortality at 5 years post-transplant conditional on survival to 1 year post-transplant. Log-rank tests were performed to determine whether there were differences in crude survival based on race in each age category.
To assess the cumulative risk of mortality associated with Black race among all recipients, we used a Cox regression model and adjusted for recipient, donor, and transplant factors reported in the 2015 SRTR risk adjustment models. We also adjusted for additional covariates that have been identified by previous studies of the association between race and mortality.1,4,5 The model included recipient variables: age, race, sex, body mass index, heart diagnosis, waiting list status, pulmonary arterial pressure, creatinine, bilirubin, diabetes, hypertension, ventilation, VAD/TAH, ECMO, and dialysis; donor variables: age, race, sex, cancer history, INR; transplant variables: ischemic time, high center level volume (≥ 9 heart transplants/year),4 donor-recipient sex mismatch, era of transplant (2012–2017 vs. 2005–2011), and year of transplantation. Recipients with missing BMI (0.5%), pulmonary arterial pressure (7%), creatinine (0.3%), bilirubin (1.4%), diabetes (0.3%), VAD/TAH (2.1%), donor cancer history (0.4%), donor INR (1.8%) and ischemic time (1.5%) were excluded from the final overall model. We found the data was missing at random by both race and age. Donor variables excluded because they were not significant include race mismatch, CMV mismatch, weight mismatch (donor-recipient ratio <0.8), donor diabetes, donor hypertension, donor heparin, and donor infection.
To test whether the association between race and mortality varied by age, we added an interaction term between race and age categories in the above multivariable Cox regression model (Model 1). Because there was an interaction that was statistically significant, we analyzed the adjusted hazard ratios for Black race within each age category by performing a linear combination of regression parameters.
2.4. Further adjustment for recipient characteristics
To determine whether socioeconomic variables and HLA mismatch might be driving the association between race and post-transplant mortality, we ran Model 1 with additional adjustment for college education and insurance (Model 2) and, separately, Model 1 with additional adjustment for HLA mismatch and PRA (Model 3). Insurance type was defined as Medicaid, Medicare, private, or other. HLA mismatch was defined as ≥ 5 antigen mismatches. We used continuous PRA (%) in the model.
To determine whether immunosuppression influenced racial disparities in post-transplant mortality, we ran Model 1 with adjustment for immunosuppression induction category (one variable with categories of no induction, thymoglobulin, IL-2, or other/multiple agents), and initial immunosuppression maintenance (three binary variables for use of steroid, tacrolimus, and/or mycophenolate mofetil, MMF) at discharge (Model 4).
2.5. Time varying hazard associated with Black race
Based on the Kaplan Meier curves showing potential non-proportional hazards at 1-year post-transplant among recipients aged 18–30, we calculated time-varying hazard ratios associated with Black race in the first year and after the first year post-transplant, with a single Cox regression model per age group. In other words, we created a person-period dataset such that any individual with >1 year of follow-up had two records, and separately modeled the association of race with mortality during the first year post-transplant and >1 year post-transplant.24 These time bins were determined based on observation of the crude mortality and non-proportional hazards at 1-year post-transplant among recipients aged 18–30. Though the true effect is not uniform over time and does not change suddenly, we chose to present it as a 1-year analysis instead of a continuous time-varying covariate for more interpretable findings. Models were adjusted for the same variables included in the overall adjusted model described in Section 2.3.
2.6. Sensitivity analysis
We performed a sensitivity analysis comparing our model to analysis restricted to transplants performed between 2012–2017 to examine whether the disparities we found persisted in the latest era.
To examine whether the differences in mortality existed between Black recipients and the sub-groups of race within the non-Black category, we performed a sensitivity analysis for an interaction between race and age after splitting the non-Black race group into Caucasian, Hispanic/Latino, Asian, and other.
We also performed a shared-frailty analysis by transplant center to consider the impact of variation in center-level management.
2.7. Cause of Death
We analyzed cause of death across race and age categories. Due to sample size and missing or unknown cause of death, we did not analyze graft failure cause (38% of values were missing).
2.8. Statistical analysis
All analyses were performed using Stata version 14.1 for Mac (Stata-Corp, College Station, Texas). All hypothesis tests were two-sided, with statistical significance defined as having a p-value <0.05. Adjusted hazard ratios (aHR) are presented with 95% confidence intervals. This study was reviewed by the institutional review board at Johns Hopkins University School of Medicine and determined to qualify for an exemption under 45 CFR 46.101(b) as study participants cannot be identified directly or through linked identifiers.
3. RESULTS
3.1. Study population
Among 22,997 first-time adult heart-only recipients transplanted between January 1, 2005 and January 31, 2017, 20% were Black (n=4,594). Non-Black (n=18,403) included Caucasian (84.8% of non-Black), Hispanic/Latino (9.9%), Asian (4.0%), American Indian or Alaska Native (0.4%), Native Hawaiian (0.4%), or other/unspecified (0.5%). Of the age categories 18–30 (n=1,788), 31–40 (n=2,087), 41–60 (n=11,665), and 61–80 (n=7,457), Black recipients made up 29%, 28%, 22%, and 12%, respectively. On average, Black recipients were younger than non-Black recipients (51 vs. 57 years; Table 1). A higher proportion of Black recipients were female (33.6% vs. 23.0%), had Medicaid for insurance (20.2% vs. 10.1%), and used dialysis (2.3% vs. 1.7%; Table 1). A lower percentage of Black recipients had college education (44.0% vs. 50.2%). A higher proportion of Black recipients had non-ischemic cardiomyopathy (72.0% vs. 44.6%) and a lower proportion had congenital heart disease (1.0% vs. 3.3%) and coronary heart disease (1.9% vs. 4.3%). Black recipients had a higher prevalence of hypertension (44.1% vs. 38.5%) and HLA mismatch (66.6% vs. 57.4%).
Table 1.
Heart Transplant Recipient Characteristics, by Race.
| Variable | Black* (n= 4,594) | non-Black† (n= 18,403) | p-value |
|---|---|---|---|
| Age, median (IQR) | 51 (18–59) | 57 (48–63) | <0.001 |
| Female (%) | 33.6 | 23.0 | <0.001 |
| Insurance | <0.001 | ||
| Medicaid (%) | 20.2 | 10.1 | |
| Medicare (%) | 36.3 | 32.5 | |
| Private (%) | 38.9 | 53.8 | |
| Other (%) | 4.6 | 3.7 | |
| Dialysis (%) | 2.3 | 1.7 | <0.05 |
| College education (%) | 44.0 | 50.2 | <0.001 |
| Diagnosis | <0.001 | ||
| Cardiomyopathy (%) | 95.0 | 90.0 | |
| Ischemic dilated (%)‡ | 19.1 | 39.6 | |
| Non-ischemic dilated (%)‡ | 72.0 | 44.6 | |
| Restrictive (%)‡ | 2.9 | 2.8 | |
| Hypertrophic (%)‡ | 1.0 | 2.7 | |
| Congenital heart disease (%) | 1.0 | 3.3 | |
| Coronary heart disease (%) | 1.9 | 4.3 | |
| Other | 2.1 | 2.8 | |
| Hypertension | 44.1 | 38.5 | <0.001 |
| Diabetes mellitus | 25.3 | 25.2 | 0.8 |
| HLA mismatch§ | 66.6 | 57.4 | <0.001 |
| Immunosuppression induction | <0.001 | ||
| None | 46.7 | 49.5 | |
| Thymoglobulin | 14.9 | 14.9 | |
| IL-2 | 31.0 | 27.7 | |
| Other, multiple agents | 7.4 | 7.9 | |
| Immunosuppression maintenance | |||
| Tacrolimus | 86.6 | 80.6 | <0.001 |
| Mycophenolate mofetil | 94.0 | 93.3 | <0.05 |
| Steroids | 91.6 | 92.0 | 0.3 |
| Era of transplant: 2012–2017 | 50.0 | 45.9 | <0.001 |
Black=Black or African American.
non-Black included Caucasian (84.8%), Hispanic/Latino (9.9%), Asian (4.0%), American Indian or Alaska Native (0.4%), Native Hawaiian (0.4%), or other/unspecified (0.5%).
Percentage of all listed diagnoses. HLA = human leukocyte antigen;
HLA mismatch defined as ≥ 5 antigen mismatches.
The most pronounced differences in insurance type, cardiomyopathy diagnosis, status 1A, and diabetes prevalence between Black and non-Black recipients were among those aged 18–30 (Table 2). Within recipients aged 18–30, a higher proportion of Black recipients had Medicaid insurance (50.0% vs. 26.8%), cardiomyopathy (93.2% vs. 78.7%), diabetes (7.6% vs. 2.8%), and hypertension (27.0% vs. 19.4%), compared to non-Black recipients (Table 2). Donor age increased with recipient age for both Black and non-Black recipients (Table 3).
Table 2. Clinical and Demographic Characteristics of Heart Transplant Recipients, by Age and Race.
Cells include # (%) unless otherwise specified.
| Black Recipients | Non-Black Recipients | All Recipients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age categories | 18–30 | 31–40 | 41–60 | 61–80 | 18–30 | 31–40 | 41–60 | 61–80 | |
| n | 514 | 593 | 2,559 | 928 | 1,274 | 1,494 | 9,106 | 6,529 | 22,997 |
| Age* | 25 (21, 28) | 36 (34, 39) | 52 (47, 56) | 64 (62, 67) | 24 (21, 27) | 36 (34, 39) | 54 (49, 57) | 65 (63, 67) | 56 (46, 62) |
| Female | 201 (39.1) | 215 (36.3) | 825 (32.2) | 304 (32.8) | 489 (38.4) | 522 (34.9) | 2,103 (23.1) | 1,112 (17.0) | 5,771 (25.1) |
| Insurance Type | |||||||||
| Medicaid insurance | 257 (50.0) | 182 (30.7) | 430 (16.8) | 60 (6.5) | 342 (26.8) | 270 (18.1) | 997 (10.9) | 246 (3.8) | 2,784 (12.1) |
| Medicare insurance | 70 (13.6) | 168 (28.3) | 907 (35.4) | 521 (56.1) | 126 (9.9) | 260 (17.4) | 2,171 (23.8) | 3,423 (52.4) | 7,646 (33.2) |
| Private insurance | 162 (31.5) | 213 (35.9) | 1,105 (43.2) | 308 (33.2) | 719 (56.4) | 902 (60.4) | 5,623 (61.8) | 2,649 (40.6) | 11,681 (50.8) |
| Other | 25 (4.9) | 30 (5.1) | 117 (4.6) | 39 (4.2) | 87 (6.8) | 62 (4.1) | 315 (3.5) | 211 (3.2) | 886 (3.9) |
| College education | 186 (36.2) | 261 (44.0) | 1,128 (44.1) | 448 (48.3) | 554 (43.5) | 788 (52.7) | 4,359 (47.9) | 3,533 (54.1) | 11,257 (48.9) |
| Body mass index* | 26.3 (22.2, 31.4) | 28.4 (24.2, 32.7) | 27.7 (24.1, 31.6) | 26.4 (23.4, 29.7) | 23.9 (20.6, 28.6) | 26.2 (22.6, 30.5) | 27.3 (24.0, 30.9) | 26.6 (23.8, 29.7) | 26.9 (23.6, 30.5) |
| Diagnosis | |||||||||
| Cardiomyopathy | 479 (93.2) | 575 (97.0) | 2,445 (95.5) | 866 (93.3) | 1,003 (78.7) | 1,269 (84.9) | 8,255 (90.7) | 5,959 (91.3) | 20,851 (90.7) |
| Ischemic dilated† | 17 (3.3) | 39 (6.6) | 531 (20.8) | 288 (31.0) | 32 (2.5) | 170 (11.4) | 3,520 (38.7) | 3,564 (54.6) | 8,161 (35.5) |
| Non-ischemic dilated† | 444 (86.4) | 523 (88.2) | 1,828 (71.4) | 514 (55.4) | 853 (67.0) | 990 (66.3) | 4,208 (46.2) | 2,149 (32.9) | 11,509 (50.0) |
| Restrictive† | 5 (1.0) | 4 (0.7) | 68 (2.7) | 58 (6.2) | 37 (2.9) | 37 (2.5) | 266 (2.9) | 169 (2.6) | 644 (2.8) |
| Hypertrophic† | 13 (2.5) | 9 (1.5) | 18 (0.7) | 6 (0.6) | 81 (6.4) | 72 (4.8) | 261 (2.9) | 77 (1.2) | 537 (2.3) |
| Congenital heart disease | 21 (4.1) | 7 (1.2) | 17 (0.7) | 2 (0.2) | 231 (18.1) | 154 (10.3) | 190 (2.1) | 33 (0.5) | 655 (2.8) |
| Coronary heart disease | 1 (0.2) | 2 (0.3) | 39 (1.5) | 45 (4.8) | 8 (0.6) | 27 (1.8) | 386 (4.2) | 368 (5.6) | 876 (3.8) |
| Other | 13 (2.5) | 9 (1.5) | 58 (2.3) | 15 (1.6) | 32 (2.5) | 44 (2.9) | 275 (3.0) | 169 (2.6) | 615 (2.7) |
| Status 1A (%) | 337 (65.6) | 366 (61.7) | 1,470 (57.4) | 515 (55.5) | 774 (60.8) | 864 (57.8) | 4,968 (54.6) | 3,436 (52.6) | 12,730 (55.4) |
| PAP,* mmHg | 29 (21, 36) | 30 (22, 37) | 29 (22, 36) | 28 (22, 35) | 27 (19, 35) | 26 (19, 35) | 27 (20, 35) | 26 (20, 34) | 27 (20, 35) |
| Creatinine,* mg/dL | 1.0 (0.8, 1.3) | 1.2 (1.0, 1.4) | 1.3 (1.0, 1.6) | 1.3 (1.1, 1.6) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.4) | 1.2 (1.0, 1.5) | 1.2 (0.9, 1.5) |
| Bilirubin,* mg/dL | 0.9 (0.5, 1.5) | 0.8 (0.5, 1.3) | 0.7 (0.5, 1.2) | 0.7 (0.5, 1.1) | 0.8 (0.5, 1.4) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) |
| Diabetes mellitus | 39 (7.6) | 77 (13.0) | 716 (28.0) | 332 (35.8) | 36 (2.8) | 164 (11.0) | 2,327 (25.6) | 2,102 (32.2) | 5,793 (25.2) |
| Hypertension | 139 (27.0) | 235 (39.6) | 1,180 (46.1) | 472 (50.9) | 247 (19.4) | 387 (25.9) | 3,612 (39.7) | 2,847 (43.6) | 9,119 (39.7) |
| Ventilation | 11 (2.1) | 9 (1.5) | 29 (1.1) | 11 (1.2) | 42 (3.3) | 34 (2.3) | 150 (1.6) | 109 (1.7) | 395 (1.7) |
| VAD/TAH | 242 (47.1) | 294 (49.6) | 1,212 (47.4) | 417 (44.9) | 519 (40.7) | 644 (43.1) | 3,753 (41.2) | 2,500 (38.3) | 9,581 (41.7) |
| ECMO | 7 (1.4) | 4 (0.7) | 12 (0.5) | 2 (0.2) | 30 (2.4) | 10 (0.7) | 60 (0.7) | 16 (0.2) | 141 (0.6) |
| Dialysis | 14 (2.7) | 13 (2.2) | 55 (2.1) | 22 (2.4) | 32 (2.5) | 25 (1.7) | 178 (2.0) | 81 (1.2) | 420 (1.8) |
| Transfusion | 78 (15.2) | 85 (14.3) | 406 (15.9) | 185 (19.9) | 259 (20.3) | 269 (18.0) | 1,711 (18.8) | 1,307 (20.0) | 4,300 (18.7) |
Median (IQR).
Percentage of all listed diagnoses.
PAP = pulmonary arterial pressure.
Table 3. Donor and Transplant Characteristics.
Cells include # (%) unless otherwise specified.
| Black Recipients | Non-Black Recipients | All Recipients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recipient Age Category | 18–30 | 31–40 | 41–60 | 61–80 | 18–30 | 31–40 | 41–60 | 61–80 | |
| Donor Traits | |||||||||
| Age* | 26 (20, 36) | 29 (22, 37) | 29 (22, 40) | 32 (23, 43) | 25 (19, 33) | 27 (21, 38) | 30 (22, 41) | 31 (23, 43) | 30 (22, 41) |
| Race | |||||||||
| Caucasian | 322 (62.6) | 372 (62.7) | 1,600 (62.5) | 588 (63.4) | 833 (65.4) | 957 (64.1) | 5,912 (64.9) | 4,307 (66.0) | 14,891 (64.8) |
| Black | 116 (22.6) | 124 (20.9) | 516 (20.2) | 176 (19.0) | 201 (15.8) | 221 (14.8) | 1,365 (15.0) | 1,014 (15.5) | 3,733 (16.2) |
| Hispanic | 66 (12.8) | 79 (13.3) | 387 (15.1) | 140 (15.1) | 212 (16.6) | 268 (17.9) | 1,584 (17.4) | 1,037 (15.9) | 3,773 (16.4) |
| Other | 10 (1.9) | 18 (3.0) | 56 (2.2) | 24 (2.6) | 28 (2.2) | 48 (3.2) | 245 (2.7) | 171 (2.6) | 600 (2.6) |
| Female | 155 (30.2) | 155 (26.1) | 696 (27.2) | 282 (30.4) | 431 (33.8) | 481 (32.2) | 2,544 (27.9) | 1,851 (28.4) | 6,595 (28.7) |
| Anti-CMV positive | 319 (62.1) | 380 (64.1) | 1,575 (61.5) | 563 (60.7) | 781 (61.3) | 931 (62.3) | 5,621 (61.7) | 3,975 (60.9) | 14,145 (61.5) |
| Cancer history | 6 (1.2) | 5 (0.8) | 34 (1.3) | 18 (1.9) | 13 (1.0) | 20 (1.3) | 135 (1.5) | 128 (2.0) | 359 (1.6) |
| Diabetes | 15 (2.9) | 19 (3.2) | 69 (2.7) | 36 (3.9) | 29 (2.3) | 29 (1.9) | 313 (3.4) | 247 (3.8) | 757 (3.3) |
| Hypertension | 72 (14.0) | 67 (11.3) | 386 (15.1) | 174 (18.8) | 109 (8.6) | 186 (12.4) | 1,332 (14.6) | 1,086 (16.6) | 3,412 (14.8) |
| INR* | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) | 1.3 (1.1, 1.4) |
| Transplant variables | |||||||||
| Ischemic time,* min | 192 (149, 231) | 188 (142, 233) | 187 (143, 225) | 187 (142, 228) | 197 (151, 238) | 190 (148, 230) | 193 (149, 232) | 193 (148, 232) | 192 (148, 231) |
| Center level volume* | 23 (15, 37) | 24 (16, 40) | 24 (15, 43) | 28 (17, 46) | 24 (16, 43) | 27 (17, 46) | 26 (16, 45) | 27 (18, 50) | 26 (16, 45) |
| HLA mismatch† | 312 (65.0) | 349 (65.4) | 1,562 (67.3) | 554 (66.0) | 633 (57.1) | 756 (57.7) | 4,595 (57.2) | 3,378 (57.7) | 12,139 (59.3) |
| PRA ≥ 10% | 131 (27.5) | 163 (29.7) | 588 (25.4) | 184 (21.7) | 248 (21.8) | 297 (21.9) | 1,480 (18.0) | 980 (16.6) | 4,071 (19.5) |
| Sex mismatch | 162 (31.5) | 150 (25.3) | 659 (25.8) | 234 (25.2) | 386 (30.3) | 393 (26.3) | 2,239 (24.6) | 1,557 (23.8) | 5,780 (25.1) |
| Era of transplant, 2012–2017 | 214 (41.6) | 286 (48.2) | 1,259 (49.2) | 539 (58.1) | 564 (44.3) | 678 (45.4) | 3,939 (43.3) | 3,273 (50.1) | 10,752 (46.8) |
Median (IQR). Anti-CMV = anti-cytomegalovirus antibodies in donor. HLA = human leukocyte antigen;
HLA mismatch defined as ≥ 5 antigen mismatches. PRA = panel reactive antigen
3.2. Model of post-transplant mortality
Post-transplant mortality associated with Black race varied by recipient age (p-interaction <0.001). There was a significant difference in risk of crude mortality between Black and non-Black recipients in each age category (Figure 1). Among recipients aged 18–30, mortality for Black vs. non-Black recipients was 14.7% vs. 7.2% one year post-transplant and 39.0% vs. 22.8% five years post-transplant (Figure 1a, log-rank p<0.001). Among recipients aged 31–40, mortality for Black vs. non-Black recipients was 11.3% vs. 7.8% one year post-transplant and 27.8% vs. 17.9% five years post-transplant (Figure 1b, log-rank p<0.001). Among recipients aged 41–60, mortality for Black vs. non-Black recipients was 9.1% vs. 9.2% one year post-transplant and 24.0% vs. 19.4% five years post-transplant (Figure 1c, log-rank p<0.001). Among recipients aged 61–80, mortality for Black vs. non-Black recipients was 14.1% vs. 11.7% one year post-transplant and 26.2% vs. 22.7% five years post-transplant (Figure 1d, log-rank p<0.05).
Figure 1. Racial Disparities in Post-heart Transplant Survival within Age Categories.
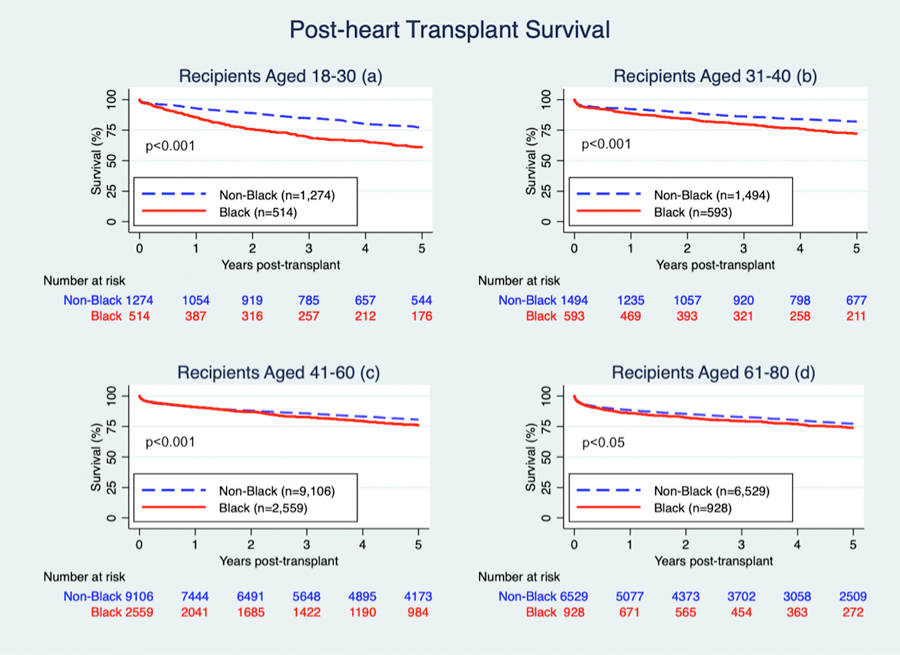
Among recipients aged 18–30, mortality for Black vs. non-Black recipients was 14.7% vs. 7.2% one year post-transplant and 39.0% vs. 22.8% five years post-transplant (a). Among recipients aged 31–40, mortality for Black vs. non-Black recipients was 11.3% vs. 7.8% one year post-transplant and 27.8% vs. 17.9% five years post-transplant (b). Among recipients aged 41–60, mortality for Black vs. non-Black recipients was 9.1% vs. 9.2% one year post-transplant and 24.0% vs. 19.4% five years post-transplant (c). Among recipients aged 61–80, mortality for Black vs. non-Black recipients was 14.1% vs. 11.7% one year post-transplant and 26.2% vs. 22.7% five years post-transplant (d).
Next, we examined crude five-year post-transplant survival conditional on survival to the first year post-transplant. Among recipients who survived to one year post-transplant, crude five-year mortality for Black vs. non-Black recipients was 28.4% vs. 16.8% for ages 18–30 (log-rank p<0.001), 18.6% vs. 11.0% for ages 31–40 (log-rank p<0.001), 16.3% vs. 11.3% for ages 41–60 (log-rank p<0.001), and 14.1% vs. 12.5% for ages 61–80 (log-rank p=0.2).
After adjustment for donor, recipient, and transplant characteristics, Black recipients had 29% higher cumulative risk of mortality compared to non-Black recipients overall (aHR = 1.29, 95% CI 1.20–1.38, p<0.001). When a race-age interaction term was added in the overall adjusted Cox regression model that included all covariates, the interaction term for Black recipients aged 18–30 was statistically significant (p<0.001). Black recipients had higher cumulative risk of mortality compared to non-Black recipients in the three youngest age categories (Table 4, Model 1). The highest disparity was among recipients aged 18–30, with Black recipients having over two times the risk of mortality compared to non-Black recipients in this age category (aHR = 2.05, 95% CI 1.67–2.51, p<0.001). Among recipients aged 31–40 and 41–60, Black recipients had 1.53 (95% CI 1.25–1.89, p<0.001) and 1.20 (95% CI 1.09–1.33, p<0.001) times higher risk of mortality compared to non-Black recipients, respectively (Table 4). Among recipients aged 61–80, there was no significant difference in risk of mortality between Black and non-Black recipients (aHR = 1.12, 95% CI 0.97–1.29, p=0.1). The aHRs for other variables in the model are reported in Supplemental Table 1.
Table 4. Adjusted Hazard Ratios (aHR) for Post-Heart Transplant Mortality.
We report the aHR (95% CI) associated with Black race in each age category and the aHR for the additional variables that were added for each given model.
| Variables | Model 1* | p-value | Model 2 = Model 1 + college and insurance | p-value | Model 3 = Model 1 + HLA and PRA | p-value | Model 4 = Model 1 + IS | p-value |
|---|---|---|---|---|---|---|---|---|
| Ages 18–30, Black vs. non-Black | 2.05 (1.67–2.51)† | <0.001 | 1.94 (1.58–2.37) | <0.001 | 2.13 (1.72–2.64) | <0.001 | 2.08 (1.69–2.55) | <0.001 |
| In first year post-transplant | 2.30 (1.60–3.31)† | <0.001 | 2.18 (1.51–3.15) | <0.001 | 2.52 (1.70–3.73) | <0.001 | 2.24 (1.52–3.31) | <0.001 |
| After first year post-transplant | 0.84 (0.54–1.29)† | 0.4 | 0.84 (0.54–1.29) | 0.4 | 0.77 (0.49–1.22) | 0.3 | 0.85 (0.54–1.34) | 0.5 |
| Ages 31–40, Black vs. non-Black | 1.53 (1.25–1.89) | <0.001 | 1.45 (1.18–1.79) | <0.001 | 1.55 (1.24–1.93) | <0.001 | 1.59 (1.28–1.98) | <0.001 |
| Ages 41–60, Black vs. non-Black | 1.20 (1.09–1.33) | <0.001 | 1.16 (1.05–1.28) | <0.01 | 1.18 (1.06–1.32) | <0.05 | 1.23 (1.11–1.36) | <0.001 |
| Ages 61–80, Black vs. non-Black | 1.12 (0.97–1.29) | 0.1 | 1.10 (0.95–1.27) | 0.2 | 1.11 (0.95–1.30) | 0.2 | 1.19 (1.03–1.38) | <0.05 |
| College education | 0.90 (0.85–0.96) | <0.01 | ||||||
| Insurance, Medicaid vs. private | 1.28 (1.17–1.41) | <0.001 | ||||||
| Insurance, Medicare vs. private | 1.19 (1.11–1.27) | <0.001 | ||||||
| HLA mismatch (0–1 vs. 2–6 matches) | 1.04 (0.97–1.10) | 0.3 | ||||||
| PRA | 1.002 (1.000–1.003) | <0.05 | ||||||
| Induction IS | ||||||||
| Thymoglobulin vs. none | 1.08 (0.99–1.18) | 0.1 | ||||||
| IL-2 vs. none | 1.12 (1.05–1.20) | <0.01 | ||||||
| Others/multiple vs. none | 0.91 (0.82–1.02) | 0.1 | ||||||
| Maintenance IS | ||||||||
| Steroid | 0.59 (0.53–0.65) | <0.001 | ||||||
| Tacrolimus | 0.70 (0.66–0.76) | <0.001 | ||||||
| Mycophenolate mofetil | 0.59 (0.53–0.64) | <0.001 | ||||||
Model 1 included recipient age, race, interaction term for age and race, sex, body mass index, heart diagnosis, waiting list status, pulmonary arterial pressure, creatinine, bilirubin, diabetes, hypertension, ventilation, VAD/TAH, ECMO, and dialysis; donor age, race, sex, cancer history, INR; ischemic time, high center level volume (≥ 9 heart transplants/year), donor-recipient sex mismatch, era of transplant (2012–2017 vs. 2005–2011), and year of transplantation. A linear combination of regression parameters was used to calculate the adjusted hazard ratios in each age category.
In Model 1, among recipients aged 18–30, Black recipients had a 2.05-fold higher cumulative risk of death compared to non-Black recipients but this risk was time-varying; in the first year post-transplant, there was a 2.30-fold higher risk of death, while after the first year, there was no significant difference in risk of mortality (p=0.4). All other aHRs outside the denoted 2 rows are cumulative risk. HLA=human leukocyte antigen, PRA=panel reactive antigen, IS=immunosuppression at discharge.
3.3. Further adjustment for recipient characteristics
After further adjustment for recipient college and insurance type, the cumulative aHR of mortality among Black recipients aged 18–30 was 1.94 (95% CI 1.58–2.37, p<0.001; Table 4, Model 2 = Model 1 + college and insurance). For recipients aged 31–40, 41–60, and 61–80, the cumulative aHR of mortality associated with Black race was 1.45 (95% CI 1.18–1.79, p<0.001), 1.16 (95% CI 1.05–1.28, p<0.01), and 1.10 (95% CI 0.95–1.27, p=0.2), respectively (Table 4, Model 2). After adjustment of the overall model for HLA and PRA, the aHR of mortality among Black recipients aged 18–30 was 2.13 (95% CI 1.72–2.64, p<0.001), while the aHR of mortality for recipients aged 31–40, 41–60, and 61–80 was 1.55 (95% CI 1.24–1.93, p<0.001), 1.18 (95% CI 1.06–1.32, p<0.05), and 1.11 (95% CI 0.95–1.30, p=0.2), respectively (Table 4, Model 3 = Model 1 + HLA and PRA).
When immunosuppression variables were added to the original overall model, the aHR did not change significantly for recipients aged 18–30 (aHR = 2.08, 95% CI 1.69–2.55, p<0.001), 31–40 (aHR = 1.59, 95% CI 1.28–1.98, p<0.001), nor 41–60 (aHR = 1.23, 95% CI 1.11–1.36, p<0.001), but the aHR among Black recipients aged 61–80 increased to 1.19 (95% CI 1.03–1.38) and became statistically significant (p<0.05; Table 4, Model 4 = Model 1 + immunosuppression at discharge).
3.4. Time varying hazard associated with Black race in each age category
The aHR of mortality associated with Black race among recipients aged 18–30 was time-varying (p-interaction race/time post-transplant <0.01). Young Black recipients aged 18–30 had 2.30 (95% CI 1.60–3.31, p<0.001) times higher risk of mortality in the first year post-transplant, but the difference was not statistically significant after the first year (aHR= 0.84, 95% CI 0.54–1.29, p=0.4; Table 4). There were no significant interactions between race and time after transplant for ages 31–40 (p=0.5), 41–60 (p=0.07), and 61–80 (p=0.4) consistent with Kaplan Meier curves; thus time-varying hazard ratios were not calculated for these age categories.
3.5. Sensitivity analysis
When the overall model was restricted to transplants performed in 2012–2017, the interaction between age and race among recipients aged 18–30 remained significant (p<0.01). The aHR of mortality for Black race among recipients aged 18–30, 31–40, 41–60, and 61–80 were 1.89 (95% CI 1.23–2.88, p<0.01), 0.91 (95% CI 0.59–1.41, p=0.7), 1.09 (95% CI 0.90–1.31, p=0.4), and 0.92 (95% CI 0.71–1.18, p=0.5).
For the sensitivity analysis based on further categorization of the non-Black race groups, there continued to be an interaction between race and age among Black recipients aged 18–30, but no interaction between age and race for any of the other subgroups.
For a sensitivity analysis of center-level variation in management, a shared-frailty analysis resulted in the interaction term remaining significant (p<0.001) for Black race and age category 18–30. The aHR for recipients aged 18–30, 31–40, 41–60, and 61–80 was 1.96 (95% CI 1.59–2.41, p<0.001), 1.44 (95% CI 1.17–1.78, p<0.001), 1.14 (95% CI 1.03–1.26, p<0.05), and 1.07 (95% CI 0.97–1.24, p=0.3).
3.6. Cause of Death
Among recipients aged 18–30, the most common known causes of death were cardiovascular and graft failure for both Black (29.5% and 24.6%, respectively) and non-Black recipients (28.3% and 22.8%, respectively, Table 5). In contrast, among Black recipients aged 61–80, though 21.0% of causes of death were unknown, cardiovascular and graft failure made up only 14.5% and 12.5% of causes of death, respectively. Overall, 15.4% of deaths of recipients had a missing or unknown cause.
Table 5. Cause of post-heart transplant mortality, by Age and Race.
Cells include # (%) unless otherwise specified.
| Black Recipients | Non-Black Recipients | All Recipients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Category | 18–30 | 31–40 | 41–60 | 61–80 | 18–30 | 31–40 | 41–60 | 61–80 | |
| n of deaths | 207 | 177 | 663 | 248 | 307 | 306 | 2,112 | 1,743 | 5,763 |
| Cardiovascular | 61 (29.5%) | 50 (28.2%) | 165 (24.9%) | 36 (14.5%) | 87 (28.3%) | 69 (22.5%) | 338 (16.0%) | 156 (9.0%) | 962 (16.7%) |
| Graft failure | 51 (24.6%) | 47 (26.6%) | 103 (15.5%) | 31 (12.5%) | 70 (22.8%) | 59 (19.3%) | 286 (13.5%) | 158 (9.1%) | 805 (14.0%) |
| Infection | 8 (3.9%) | 12 (6.8%) | 75 (11.3%) | 42 (16.9%) | 28 (9.1%) | 29 (9.5%) | 267 (12.6%) | 295 (16.9%) | 756 (13.1%) |
| Malignancy | 1 (0.5%) | 0 (0.0%) | 25 (3.8%) | 20 (8.1%) | 4 (1.3%) | 4 (1.3%) | 197 (9.3%) | 224 (12.9%) | 475 (8.2%) |
| Pulmonary | 7 (3.4%) | 2 (1.1%) | 27 (4.1%) | 14 (5.6%) | 7 (2.3%) | 13 (4.2%) | 110 (5.2%) | 149 (8.5%) | 329 (5.7%) |
| Renal Failure | 1 (0.5%) | 3 (1.7%) | 6 (0.9%) | 3 (1.2%) | 0 (0.0%) | 3 (1.0%) | 52 (2.5%) | 37 (2.1%) | 105 (1.8%) |
| Cerebrovascular | 4 (1.9%) | 4 (2.3%) | 29 (4.4%) | 14 (5.6%) | 9 (2.9%) | 11 (3.6%) | 96 (4.5%) | 92 (5.3%) | 259 (4.5%) |
| Hemorrhage | 2 (1.0%) | 5 (2.8%) | 11 (1.7%) | 3 (1.2%) | 5 (1.6%) | 14 (4.6%) | 34 (1.6%) | 35 (2.0%) | 109 (1.9%) |
| Accident | 1 (0.5%) | 2 (1.1%) | 1 (0.2%) | 2 (0.8%) | 3 (1.0%) | 2 (0.7%) | 21 (1.0%) | 11 (0.6%) | 43 (0.7%) |
| Non-compliance | 3 (1.4%) | 6 (3.4%) | 7 (1.1%) | 1 (0.4%) | 5 (1.6%) | 3 (1.0%) | 9 (0.4%) | 5 (0.3%) | 39 (0.7%) |
| Unknown | 33 (15.9%) | 19 (10.7%) | 79 (11.9%) | 52 (21.0%) | 37 (12.1%) | 40 (13.1%) | 322 (15.2%) | 303 (17.4%) | 885 (15.4%) |
4. DISCUSSION
In this national registry study of adult heart transplant recipients, we found that the risk of mortality associated with Black race in aggregate, indiscriminate of age, was 1.29 (95% CI 1.20–1.38), which is consistent with the population-based data reported in the literature.1,2,5,6,9 However, focusing on aggregate data, as has been done in the literature, masks the highest-risk subgroup of young Black recipients, aged 18–30 years, who we found had over two times the risk of mortality relative to comparable young non-Black recipients after adjustment for recipient, donor, and transplant characteristics. Adjusting for socioeconomic variables college and education, HLA and PRA, and immunosuppression variables did not account for the racial disparity.
Among the older three age categories, there was no interaction between race and age. There were racial differences in the 31–40 and 41–60 age categories with Black recipients having higher risk of mortality, but no difference between Black and non-Black recipients in the 61–80 age category after adjustment. Of note, racial differences in survival in the 31–40 and 41–60 age categories was no longer found to be significant in the recent era (2012–2017), but the difference persisted among recipients aged 18–30. Improvements over time in transplantation, technique and/or management, may have benefited mostly older recipients.
Clinical decision-making can be flawed when assumptions regarding outcomes are generalized from population-based studies instead of tailoring to subgroups who may have fundamentally different risk factors, including severity of disease and response to immunosuppression, as well as access to resources. Overall, Black recipients are not only at higher risk of advancing to end-stage heart disease, requiring transplant at earlier ages, but disparities in cardiovascular health may also be contributing to higher risk of post-transplant mortality at younger ages than previously recognized.10–15,25 In our study, young Black recipients aged 18–30 had a clinical picture very distinct from young non-Black recipients, with the former having higher prevalence of non-ischemic dilated cardiomyopathy as indication for transplant and higher prevalence of hypertension and diabetes. While adjustment for co-morbidities and indication for transplant did not explain the elevated risk of mortality among young Black recipients, severity of disease may not be fully captured in these variables.
Liu et al. analyzed cause of death data using UNOS and found that Black recipients were more likely to die from graft failure or cardiovascular causes and less likely to die from infection or malignancy compared to white recipients.6 However, the analysis was not performed by age categories. By analyzing based on age of recipients, we were able to find a stark contrast in the cause of death between young and older Black recipients with a higher proportion of young recipients dying due to cardiovascular and graft failure causes. Unfortunately, the level of missing/unknown cause of death warrants cautious interpretation. Given graft failure made up a higher proportion of causes of death in young compared to older Black recipients, a key area for further research to improve graft survival is optimizing immunosuppression management and regimens especially in the first year post-transplant for young Black recipients.
We not only identified a high-risk subgroup, but we also illuminated a “window of opportunity” in the first year post-transplant, when the disparity was most prominent for recipients aged 18–30 and when better surveillance and further research have the potential for significant improvement in outcomes. The first year post-transplant furthermore has been found to be a period when the main causes of death for transplant recipients have been characterized as primary graft failure, infections, and rejections; graft failure and rejections have also been found to be more common causes of death among younger recipients in the literature.17 A smaller study of 7,013 heart recipients by Tallaj et al. (2015) briefly touched upon both race and age when assessing heart transplant outcomes, finding that the interaction of age and ethnicity was significant in the “late phase,” defined by the study as “relatively low, gradually increasing risk” five years after transplant, while in our study, the risk was significantly higher for young Black vs. non-Black recipients aged 18–30 in the first year post-transplant. Our study expands on the prior work as we were able to quantify these effects in finer detail due to our use of national registry data.26 Specifically, we were able to analyze the interaction of age and race for cumulative mortality as well as time-varying mortality, and we did so in a more recent era of transplantation, 2005–2017 (with sensitivity analysis of 2012–2017) vs. 1990–2008. Our analysis provides more generalizable findings for the existing nature of the disparity and the timeline of risk among young Black recipients in order to design more targeted interventions and research.
Possible mechanisms driving the overall increased risk we observed among young Black recipients might also explain the time-varying risk we observed within this subgroup compared to the moderate but consistent risk in the 31–40 and 41–60 age groups. More severe illness and co-morbidities prior to transplant may result in a higher early risk of mortality from surgical complications for Black recipients aged 18–30. More financial limitations in the youngest age category may also influence ability to remain adherent to follow-up visits and medications in the post-transplant period where risk of immune response is otherwise high.7,17 This registry-based study does not allow for granular consideration of potential mechanisms, but we hope this work will spur further research to investigate the mechanisms underlying this disparity.
We utilized the United Network of Organ Sharing (UNOS) data collection form classification for race and grouped our study recipients into Black and non-Black recipients. We recognize that race is a social, not biological, construct. We have attempted to identify subgroups within racial categories at higher risk of post-transplant mortality to highlight the need for targeted interventions and improved care within these populations. We are not ascribing high risk to Black race, but forced to use registry-defined race as a proxy for the systemic racism and oppression that is associated with healthcare disparities we observed in the current study.6
Strengths of our study include a large national database and twelve years of data, which allowed us to reliably detect an interaction between race and age for post-transplant mortality among young recipients. Unfortunately, we were limited in not having immunosuppression levels nor immunosuppression changes after discharge, as well as having missing data for cause of death and rate of graft loss. However, we were able to link the data to the Social Security Death Master File for our primary outcome of cumulative mortality, and this is considered a reliable outcome from this registry. In addition, we had access to data for analyzing co-morbidities, indication for transplant, and transplant characteristics that allowed us to examine clinical differences by race, to adjust for these characteristics in order to get a better estimate of the racial disparities by age, and to analyze potential mediators.
In conclusion, this study identified young Black recipients as a high-risk subgroup in the first year after transplant, which has been masked in decades of research looking at the disparities between Black and non-Black recipients in aggregate. Clinical decision-making and clinical trials should be better individualized based on the unique set of risk factors mediating higher risk of mortality among young Black recipients in order to reduce long-standing disparities in heart transplant outcomes.
Supplementary Material
SUPPLEMENTAL TABLE I
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
SOURCES OF FUNDING
This work was supported by grant numbers K24DK101828 (Segev) and K01DK101677 (Massie) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Nonstandard Abbreviations and Acronyms
- aHR
Adjusted Hazard Ratio
- CI
Confidence Interval
- ECMO
Extracorporeal Membrane Oxygenation
- HLA
Human Leukocyte Antigen
- HRSA
Health Resources and Services Administration
- HT
Heart Transplant
- IS
Immunosuppression
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel reactive antibody
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
- VAD/TAH
Ventricular assist device/Total artificial heart
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by Circulation: Heart Failure.
REFERENCES
- 1.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, Shah AS. The Impact of Race on Survival After Heart Transplantation: An Analysis of More Than 20,000 Patients. The Annals of Thoracic Surgery. 2010;89:1956–1964. [DOI] [PubMed] [Google Scholar]
- 2.Higgins RSD, Fishman JA. Disparities in Solid Organ Transplantation for Ethnic Minorities: Facts and Solutions. American Journal of Transplantation. 2006;6:2556–2562. [DOI] [PubMed] [Google Scholar]
- 3.Jarcho J, Naftel DC, Shroyer TW, Kirklin JK, Bourge RC, Barr ML, Pitts DG, Starling RC. Influence of HLA mismatch on rejection after heart transplantation: a multiinstitutional study. The Cardiac Transplant Research Database Group. The Journal of Heart and Lung Transplantation. 1994;13:583–596. [PubMed] [Google Scholar]
- 4.Kilic A, Weiss ES, George TJ, Arnaoutakis GJ, Yuh DD, Shah AS, Conte JV. What predicts long-term survival after heart transplantation? An analysis of 9,400 ten-year survivors. The Annals of Thoracic Surgery. 2012;93:699–704. [DOI] [PubMed] [Google Scholar]
- 5.Kilic A, Higgins RSD, Whitson BA, Kilic A. Racial Disparities in Outcomes of Adult Heart Transplantation. Circulation. 2015;131:882–889. [DOI] [PubMed] [Google Scholar]
- 6.Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent Racial Disparities in Survival After Heart Transplantation. Circulation. 2011;123:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehra MR, Uber PA, Scott RL, Park MH. Ethnic disparity in clinical outcome after heart transplantation is abrogated using tacrolimus and mycophenolate mofetil-based immunosuppression. Transplantation. 2002;74:1568–73. [DOI] [PubMed] [Google Scholar]
- 8.Morris AA, Kransdorf EP, Coleman BL, Colvin M. Racial and ethnic disparities in outcomes after heart transplantation: A systematic review of contributing factors and future directions to close the outcomes gap. The Journal of Heart and Lung Transplantation. 2016;35:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh TP, Almond C, Givertz MM, Piercey G, Gauvreau K. Improved survival in heart transplant recipients in the United States: racial differences in era effect. Circulation: Heart Failure. 2011;4:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JE, Ferdinand KC, Watson KE, Wenger NK, Watkins LO, Flack JM, Gavin JR, Reed JW, Saunders E, Wright JT. Treatment of Heart Failure in African Americans— A Call to Action. Journal of the National Medical Association. 2011;103. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawar D, Yancy C. Racial Differences in Heart Failure Therapeutics. Heart Failure Clinics. 2010;6:65–74. [DOI] [PubMed] [Google Scholar]
- 12.Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Social Science & Medicine. 2014;118:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CM, Hawkins NM, Jhund PS, MacDonald MR, Solomon SD, Granger CB, Yusuf S, Pfeffer MA, Swedberg K, Petrie MC, et al. Clinical Characteristics and Outcomes of Young and Very Young Adults With Heart Failure: The CHARM Programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). Journal of the American College of Cardiology. 2013;62:1845–1854. [DOI] [PubMed] [Google Scholar]
- 14.Kishi S, Teixido-Tura G, Ning H, Venkatesh BA, Wu C, Almeida A, Choi EY, Gjesdal O, Jacobs DR, Schreiner PJ, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. Journal of the American College of Cardiology. 2015; 65:2679–2687. [DOI] [PubMed] [Google Scholar]
- 15.Falkner B, DeLoach S, Keith SW, Gidding SS. High Risk Blood Pressure and Obesity Increase the Risk for Left Ventricular Hypertrophy in African-American Adolescents. The Journal of Pediatrics. 2013;162:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colvin M, Smith JM, Skeans MA, Edwards LB, Uccellini K, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Heart. American Journal of Transplantation. 2017;17:286–356. [DOI] [PubMed] [Google Scholar]
- 17.George JF, Pamboukian SV, Tallaj JA, Naftel DC, Myers SL, Foushee MT, Brown RN, Pajaro OE, McGiffin DC, Kirklin JK. Balancing rejection and infection with respect to age, race, and gender: clues acquired from 17 years of cardiac transplantation data. The Journal of Heart and Lung Transplantation. 2010;29:966–72. [DOI] [PubMed] [Google Scholar]
- 18.Van Arendonk KJ, King EA, Orandi BJ, James NT, Smith JM, Colombani PM, Magee JC, Segev DL. Loss of pediatric kidney grafts during the “high-risk age window”: insights from pediatric liver and simultaneous liver-kidney recipients. American Journal of Transplantation. 2015;15:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wayda B, Clemons A, Givens RC, Takeda K, Takayama H, Latif F, Restaino S, Naka Y, Farr MA, Colombo PC, et al. Socioeconomic Disparities in Adherence and Outcomes After Heart Transplant. Circulation: Heart Failure. 2018;11:e004173. [DOI] [PubMed] [Google Scholar]
- 20.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumin D, McConnell PI, Galantowicz M, Tobias JD, Hayes D. Reported Nonadherence to Immunosuppressive Medication in Young Adults After Heart Transplantation. Transplantation. 2017;101:421–429. [DOI] [PubMed] [Google Scholar]
- 22.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. American Journal of Transplantation. 2014;14:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sponga S, Deroma L, Sappa R, Piani D, Lechiancole A, Spagna E, Tursi V, Nalli C, Livi U. Recipient age impact on outcome after cardiac transplantation: should it still be considered in organ allocation? Interactive Cardiovascular and Thoracic Surgery. 2016;23:573–9. [DOI] [PubMed] [Google Scholar]
- 24.Bolard P, Quantin C, Esteve J, Faivre J, Abrahamowicz M. Modelling time-dependent hazard ratios in relative survival: Application to colon cancer. Journal of Clinical Epidemiology. 2001;54:986–996. [DOI] [PubMed] [Google Scholar]
- 25.Kalogeropoulos AP, Samman-Tahhan A, Hedley JS, McCue AA, Bjork JB, Markham DW, Bhatt KN, Georgiopoulou VV, Smith AL, Butler J. Progression to Stage D Heart Failure Among Outpatients With Stage C Heart Failure and Reduced Ejection Fraction. JACC: Heart Failure. 2017;5:528–537. [DOI] [PubMed] [Google Scholar]
- 26.Tallaj JA, Pamboukian SV, George JF, Kirklin JK, Brown RN, McGiffin DC, Acharya D, Loyaga-Rendon R, Melby SJ, Bourge RC, et al. Have risk factors for mortality after heart transplantation changed over time? Insights from 19 years of Cardiac Transplant Research Database study. The Journal of Heart and Lung Transplantation. 2014;33:1304–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE I


