Abstract
Background/Objective
Sleep disturbance is common among adults with osteoarthritis, but little is known about patterns over time. In this cohort study, we identified restless sleep trajectories and associated factors in adults with or at high risk for knee OA.
Methods
Longitudinal (2004–2014) restless sleep (≥ 3 nights/week) annual reports over 8 years from 4359 Osteoarthritis Initiative participants were analyzed. Group-based trajectory modeling identified heterogeneous temporal patterns. Logistic regression identified baseline health and behavioral predictors of trajectory membership.
Results
Four restless sleep trajectory groups were identified: good (69.7%, persistently low restless sleep probabilities), worsening (9.1%), improving (11.7%), and poor (9.5%, persistently high). Among 2 groups initially having low restless sleep prevalence, the worsening trajectory group had an increased likelihood of baseline cardiovascular disease (odds ratio [OR]=1.53, 95% confidence interval [CI]=1.01, 2.33), pulmonary disease (OR=1.48, 95% CI=1.07, 2.05), lower physical activity (OR=1.29, 95% CI=1.03, 1.61), knee pain (OR=1.04, 95% CI=1.00, 1.07), depressive symptoms (OR=1.03, 95% CI=1.01, 1.06), and a decreased likelihood of better mental health (OR=0.97, 95% CI=0.95, 0.98) at baseline. Among 2 groups initially having high restless sleep prevalence, the poor group had an increased likelihood of baseline depressive symptoms (OR=1.03, 95% CI=1.00, 1.05).
Conclusions
Four trajectories of restless sleep over 8 years were identified using data collected from over 4000 older adults aged 45–79 years with or at higher risk for knee OA. The presence of depressive symptoms, less physical activity, knee pain, poor mental health, cardiovascular and pulmonary disease were each associated with unfavorable trajectories.
Keywords: sleep, longitudinal studies, trajectory, osteoarthritis, risk factors
INTRODUCTION
Several recent manuscripts have highlighted the negative effects of sleep disturbances on a broad range of poor health outcomes, including respiratory infections, weight gain, and postoperative cardiovascular events.(1–3) Not only is there growing recognition of the deleterious effects of sleep disturbances, the prevalence of sleep disturbance is rising as well. Almost one in three U.S. adults reported insufficient sleep in 2012 compared to one in five in 1985.(4)
Among individuals with osteoarthritis (OA), sleep disturbance may be even more prevalent than in the general population. In the Johnston County Osteoarthritis Project, 76.4% of participants with symptomatic knee or hip OA reported insufficient sleep or insomnia.(5) Among people with OA, sleep disturbance, pain, and mental health disorders often co-exist and are intrinsically related to each other(6), forming a downward spiral and deteriorating the quality of life. Despite the frequent complaint of sleep disturbance among adults with OA (5, 7), little is known about sleep patterns over time in this population.
Traditional analytical methods generally assume a common distribution of the observed outcomes. However, sleep patterns over time are likely to be heterogeneous. Group-based trajectory modeling assumes that a population is composed of a mixture of distinct groups defined by their longitudinal trajectories.(8) Literature evaluating sleep trajectories is limited. One notable study applied group-based trajectory modeling methods to analyze sleep duration trajectories using a large national representative sample of Canadian adults. The study found four distinct and stable sleep duration trajectories over 8 years.(9) However, among adults with OA, factors contributing to sleep disturbance, such as pain and depression, are more prevalent than in the general population. Because longitudinal sleep studies have not yet been done in adults with OA, it is not clear that sleep trajectories will be the same in this population compared to other non-disease specific populations.
In this study, we examined sleep disturbance measured by the report of restless sleep among adults with or at high risk for knee OA. Our objectives were 1) to identify trajectories of restless sleep over an 8-year interval, and 2) to identify baseline health and behavioral factors associated with membership in unfavorable trajectories. There is no cure for OA and disease management is primarily symptom control. Better understanding of sleep patterns may further motivate multimodal approaches to achieve OA treatment goals. Identifying modifiable factors that predict poor sleep trajectories could potentially inform more effective intervention strategies.
MATERIALS AND METHODS
Study Sample
The Osteoarthritis Initiative (OAI) is a longitudinal observational cohort study of the natural history of knee OA and associated risk factors. At enrollment (2004–2006), 4796 men and women aged 45 to 79 years with or at elevated risk for knee OA were recruited from four clinical sites: Baltimore, Maryland; Pittsburgh, Pennsylvania; Pawtucket, Rhode Island; and Columbus, Ohio. Participants were clinically assessed annually. Complete inclusion and exclusion criteria and study rationale can be found at https://nda.nih.gov/oai/about-oai. Each participant provided written informed consent. All OAI sites obtained IRB approval. The present analysis conducted in 2019 used annual data from the baseline visit to 8-year follow-up (2012–2014).
Measures
Restless Sleep
OAI annually collected data on Center for Epidemiologic Studies – Depression Scale (CES-D).(10) The CES-D question regarding sleep asked “how often sleep was restless” in the past week with the following possible responses: 1) rarely or none of the time (less than 1 day), 2) some or a little of the time (1–2 days), 3) occasionally or a moderate amount of time (3–4 days), and 4) most or all of the time (5–7 days). Presence of restless sleep in this study was ascertained from the report of 3–7 nights when sleep was restless.
Baseline Factors
Baseline factors consisted of sociodemographic characteristics, health, and behavioral factors. Sociodemographic characteristics included age, sex, self-reported race (African American, White, or other race), education, and marital status. High school or lower education was defined as report of no more than 12 years of education.
Health factors included knee pain, radiographic knee OA, mental health, and comorbid health conditions known to be associated with poor sleep. Comorbid health conditions included self-report of cardiovascular disease (heart attack, heart failure, operation to unclog or bypass arteries in legs, stroke), being overweight or obese, and pulmonary disease (emphysema, chronic bronchitis, chronic obstructive lung disease, and asthma). Body mass index (BMI) was calculated from measured height and weight [weight (kg)/height (m2)]. Overweight and obesity were defined as 25 kg/m2 ≤ BMI <30 kg/m2 and BMI ≥ 30 kg/m2 respectively. Knee pain was assessed using the Western Ontario and McMaster University Osteoarthritis Index (WOMAC).(11) Participants were asked to rate pain in each knee on a 5-point Likert scale during five activities (walking, climbing stairs, in bed, sit or lie down, and standing) in the past 7 days. The WOMAC pain score ranges from 0 to 20 with higher numbers representing worse symptoms. Individual radiographic knee OA severity was evaluated using the worse Kellgren-Lawrence grade from “fixed-flexion” knee radiography protocol (12) between two knees. Presence of radiographic knee OA was identified by a Kellgren-Lawrence grade ≥ 2 in one or both knees.(13) Depressive symptoms were assessed using modified CES-D scores (14) (calculated by excluding restless sleep, using 19 of 20 questions). General mental health was assessed using short form-12 (SF-12) mental component summary (MCS) scales, ranging from 0 (best health) to 100 (worst health).(15)
Behavioral factors included smoking, alcohol assumption, caffeine intake, and physical activity. Smoking was ascertained from self-report as a current smoker of cigarettes, pipes, cigars, or cigarillos. Any alcohol consumption was confirmed based on positive answers to “During the past 12 months, how many drinks did you have in a typical week?” Daily caffeine intake was confirmed based on self-report information from the BLOCK 2000 systematic nutrition assessment.(16) Self-reported physical activity was measured using the Physical Activity Scale for the Elderly (PASE).(17) Lower physical activity level was defined as PASE scale below the median (PASE total score ≤ 151).
Statistical Analysis
Restless sleep trajectories over 8 years were identified using group-based trajectory modeling (SAS PROC TRAJ).(8, 18) Latent mixture modeling with a binary distribution identified clusters of individuals who followed similar underlying trajectories of restless sleep over time. To provide an adequate number of data points for trajectory analysis, we included 4359 participants with data on baseline restless sleep having two or more annual follow-ups in addition to baseline.
We followed standard statistical procedures to determine the number and type of different restless sleep trajectories designed for achieving parsimonious findings. Specifically, we iteratively considered between three and six trajectories, and allowed for constant, linear, quadratic, and cubic polynomial in each trajectory.(18, 19) The final number of trajectory groups was based on the Bayesian Information Criterion (BIC), the log Bayes factor (2 × ΔBIC)(18), trajectory shapes for similarity, and the proportion of participants in each trajectory being at least ≥ 5% of the study cohort(19). After identifying the optimal number of trajectories, the level of the polynomial function (i.e., cubic, quadratic, linear, and constant) for each group was reduced until a parameter estimate in the highest polynomial function had a p-value < 0.05. From this final model, we calculated the posterior predicted probability for each participant of being a member in each of the trajectory groups. Participants were assigned to the trajectory group for which they had the greatest posterior predictive probability. As suggested by Nagin(8), specific diagnostic measures were examined for trajectory model fit: average posterior probability of assignment for each trajectory group is 0.7 or higher; odds of correct classification are 5.0 or higher; and the proportion of participants assigned to each trajectory group closely approximates the proportion estimated by the trajectory model.
Baseline sociodemographic, health, and behavioral profiles of each trajectory group were summarized descriptively. Multiple logistic regression models were used to investigate distinguishing characteristics between restless sleep trajectory groups of clinical interest.
Recognizing that systematic differences between people included and excluded could influence the findings, weighted sensitivity analyses(20) conducted also produced four similar trajectory groups. For simplicity, we presented the unweighted results. We used SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) for all analyses. Statistical testing was conducted at a two-sided 5% significance level.
RESULTS
The study cohort is composed of 4359 participants who reported on restless sleep at baseline and 2 or more subsequent annual visits, representing 91% of the enrolled OAI participants (n=4796). The majority of the 4359 participants (88%) provided 5 or more restless sleep measures over time. This study cohort had a mean age of 61.2 years (SD 9.1) and mean BMI of 28.5 kg/m2 (SD 4.5) at baseline. Among them, 58.3% were women, and 16.6% reported restless sleep at baseline.
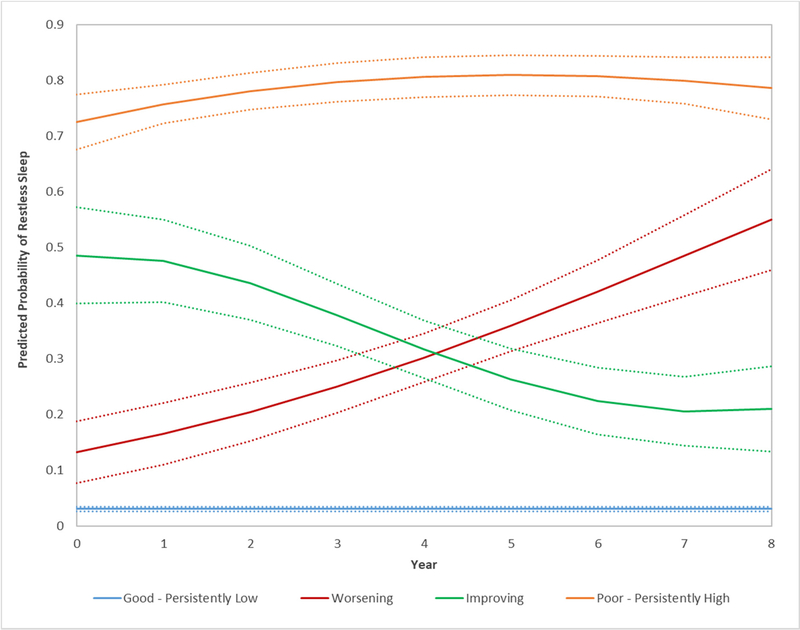
Of the 4359 participants, four distinct restless sleep trajectories were identified over an 8-year follow-up period (Figure 1). A good restless sleep trajectory group (69.7% participants) was characterized by the lowest probabilities of restless sleep over time. A worsening probability trajectory group (9.1% participants) was characterized by a low baseline probability of restless sleep followed by increasing probabilities over time. An improving probability trajectory group (11.7% participants) had decreasing probabilities of restless sleep over time. A poor restless sleep probability trajectory group (9.5% participants) was characterized by consistently having the highest probabilities of restless sleep over time.
Figure 1.
Trajectories of restless sleep over 8 years among adults with or at high risk of knee OA (n=4359). *
* Each solid line represents a distinct trajectory of predicted probability of restless sleep. Dotted lines indicate 95% confidence intervals.
Table 1 shows the baseline characteristics by the four trajectory groups. People with low probabilities of baseline restless sleep tended to be older and were less likely to have high depressive symptoms and pain interference than those with higher baseline probabilities of restless sleep.
Table 1.
Baseline characteristics by restless sleep trajectories (n=4359)
| Baseline Characteristics | Good - Persistently Low n=3036 |
Worsening n=398 |
Improving n=511 |
Poor - Persistently High n=414 |
|---|---|---|---|---|
| Mean (SD) or % a | Mean (SD) or % a | Mean (SD) or % a | Mean (SD) or % a | |
| Restless sleep | 3.0 | 7.5 | 57.9 | 73.9 |
| Sociodemographic factors | ||||
| Age, mean (SD) | 61.7 (9.1) | 60.2 (9.4) | 59.8 (8.8) | 60.3 (9.1) |
| Female | 55.8 | 64.3 | 66.5 | 61.1 |
| Race | ||||
| White | 81.1 | 82.9 | 78.5 | 80.7 |
| AA | 16.8 | 13.8 | 19.0 | 15.7 |
| Other race | 2.2 | 3.3 | 2.5 | 3.6 |
| High school or lower education | 86.3 | 85.7 | 81.4 | 77.8 |
| Not married | 20.4 | 24.1 | 23.9 | 24.9 |
| Health Factors | ||||
| Comorbid conditions | ||||
| Cardiovascular disease | 5.8 | 8.0 | 6.3 | 10.4 |
| Overweight b | 40.3 | 36.7 | 37.7 | 38.2 |
| Obesity b | 33.7 | 38.4 | 41.4 | 45.4 |
| Pulmonary disease | 8.5 | 14.3 | 13.1 | 13.3 |
| Knee specific factors | ||||
| K/L grade c | ||||
| 0–1 | 44.2 | 40.2 | 45.2 | 41.8 |
| 2 | 30.2 | 29.2 | 33.1 | 28.0 |
| 3–4 | 25.6 | 30.7 | 21.7 | 30.2 |
| WOMAC pain, mean (SD) d | 2.9 (3.3) | 3.5 (3.8) | 4.2 (4.0) | 4.8 (4.3) |
| Mental health | ||||
| CES-D, mean (SD) e | 4.3 (5.1) | 6.8 (6.6) | 8.5 (7.9) | 10.3 (8.8) |
| SF-12 Mental Health Score, mean (SD) f | 50.3 (8.0) | 48.6 (9.7) | 47.4 (9.6) | 44.7 (10.8) |
| Behavioral factors | ||||
| Smoking | 7.9 | 10.1 | 10.4 | 12.8 |
| Alcohol consumption | 81.6 | 80.9 | 80.4 | 81.6 |
| Caffeine consumption | 94.7 | 95.2 | 96.1 | 96.4 |
| PASE total score, ≤ median g | 50.5 | 43.7 | 48.5 | 50.2 |
Column % of each characteristic reported by individuals belonging to each restless sleep trajectory.
n=4356 for overweight and obese.
The worse Kellgren-Lawrence (K/L) grade between the two knees.
Western Ontario and McMaster University Osteoarthritis Index pain score, range 0–20, worse knee reported.
n=4347, Center for Epidemiological Studies Scale.
n=4343 for SF-12 mental health component scale, 0 (best health) to 100 (worst health).
Physical Activity Scale for the Elderly. The median of baseline PASE total score is 151.
For clinical interest, we investigated baseline characteristics associated with unfavorable restless sleep patterns over time. First, the worsening and good restless sleep trajectory groups shared an initially low prevalence of restless sleep reports (worsening: 7.5% reported baseline restless sleep; good: 3% reported baseline restless sleep), but the worsening group experienced an unfavorable pattern of elevated prevalence over 8 years. Compared to the good trajectory group, the worsening trajectory group had an increased likelihood of baseline cardiovascular disease (OR=1.53, 95% CI=1.01, 2.33), pulmonary disease (OR=1.48, 95% CI=1.07, 2.05), lower physical activity (OR=1.29, 95% CI=1.03, 1.61), knee pain (OR=1.04, 95% CI=1.00, 1.07), depressive symptoms (OR=1.03, 95% CI=1.01, 1.06), and a decreased likelihood of better mental health (OR=0.97, 95% CI=0.95, 0.98), adjusting for sociodemographic and other health and behavioral factors (Table 2).
Table 2.
Multiple regression analyses investigating characteristics related to unfavorable versus favorable trajectories (i.e., worsening versus good and poor versus improving trajectory groups). Odds ratio (OR) and 95% confidence interval (CI) relative to reference trajectory adjusting for other covariates (n=4327)*.
| Trajectory Group Baseline Status: Restless Sleep Infrequent** N=3410 |
Trajectory Group Baseline Status: Restless Sleep Frequent N=917 |
|
|---|---|---|
|
| ||
| Baseline Characteristics | Worsening versus Good (Reference) | Poor versus Improving (Reference) |
| OR (95% CI) | OR (95% CI) | |
| SOCIODEMOGRAPHIC FACTORS | ||
| Age | 0.98 (0.97, 0.99) | 1.01 (0.99, 1.02) |
| Female | 1.41 (1.11, 1.79) | 0.82 (0.61, 1.09) |
| AA | 0.50 (0.36, 0.72) | 0.67 (0.45, 1.01) |
| Other race | 1.17 (0.63, 2.20) | 1.25 (0.58, 2.71) |
| High school or lower education | 0.94 (0.68, 1.30) | 1.10 (0.78, 1.57) |
| Not Married | 1.17 (0.92, 1.49) | 1.06 (0.78, 1.43) |
| HEALTH FACTORS | ||
| Cardiovascular disease | 1.53 (1.01, 2.33) | 1.63 (0.99, 2.69) |
| CES-D a | 1.03 (1.01, 1.06) | 1.03 (1.00, 1.05) |
| Knee OA presence b | 1.24 (0.99, 1.57) | 1.04 (0.78, 1.38) |
| WOMAC pain c | 1.04 (1.00, 1.07) | 1.03 (0.99, 1.06) |
| Mental health d | 0.97 (0.95, 0.98) | 1.00 (0.98, 1.02) |
| Overweight or obesity | 1.06 (0.82, 1.39) | 1.37 (0.95, 1.96) |
| Pulmonary disease | 1.48 (1.07, 2.05) | 1.05 (0.71, 1.57) |
| BEHAVIORAL FACTORS | ||
| Alcohol consumption | 1.04 (0.78, 1.38) | 1.18 (0.83, 1.70) |
| Caffeine consumption | 0.95 (0.58, 1.57) | 0.78 (0.38, 1.60) |
| PASE total score, ≤ median e | 1.29 (1.03, 1.61) | 0.91 (0.69, 1.21) |
| Smoking | 1.30 (0.89, 1.90) | 1.12 (0.72, 1.75) |
n=4327, 32 participants with incomplete baseline data were excluded.
Infrequent: baseline restless sleep group prevalence <10%.
Center for Epidemiological Studies Scale
The worse Kellgren-Lawrence (K/L) grade between the two knees ≥ 2.
Western Ontario and McMaster University Osteoarthritis Index pain score, range 0–20, worse knee reported.
SF-12 mental health component scale, 0 (best health) to 100 (worst health)
Physical Activity Scale for the Elderly. The median of baseline PASE total score is 151.
Second, the poor and improving restless sleep trajectory groups shared an initially high prevalence of restless sleep (poor: 74% reported baseline restless sleep; improving: 58% reported baseline restless sleep), but the improving group showed a favorable pattern of reduced restless prevalence over 8 years. Compared to the improving trajectory group, the poor group had an increased likelihood of depressive symptoms (OR=1.03, 95% CI=1.00, 1.05), controlling for sociodemographic and other health and behavioral factors.
Sensitivity analyses further examined baseline prescription medications (pain relievers, sleep inducers, and medications for mood disorders) as potential predictors for trajectory membership. None were significantly associated with distinguishing membership in either the good vs worsening and the improving vs poor sleep trajectory comparisons.
DISCUSSION
Sleep pattern evaluation using group-based trajectory analysis is valuable because it explicitly explores the heterogeneity of sleep experience over time. To our knowledge, this is the first study that describes distinct trajectories of restless sleep. Based on the 8-year sleep experience of more than 4000 older adults aged 45–79 years with or at higher risk for knee OA, four distinctive patterns of self-reported restless sleep were identified: 1) good (69.7%), 2) worsening (9.1%), 3) improving (11.7%), and 4) poor (9.5%). Overall, the majority of individuals who had low probabilities of restless sleep at baseline (the good and worsening groups) continued to have low probabilities of restless sleep over time, and over half of those who started with high probabilities of restless sleep (the improving and poor groups) had lower probabilities of restless sleep over time.
Nonetheless almost one in five adults followed unfavorable sleep trajectories. Identifying characteristics related to unfavorable longitudinal sleep patterns is important, because such factors may signal opportunities for potential clinic and public health interventions to increase the proportion of patients in the persistently good and improving trajectories. Notably, lower baseline physical activity level distinguished the worsening trajectory group from the good trajectory group. Lack of physical activity is a known risk factor for developing insomnia among older adults.(21) Compared to older inactive adults, persons who exercised at least once a week were less likely to report sleep problems.(22) A 2012 meta-analysis of randomized trials in middle-aged and older adults with sleep problems showed a moderate but statistically significant positive effect of exercise on sleep quality.(23) Despite this positive evidence, the assessment and encouragement of physical activity are not common among physicians who take care of people with knee OA.(24) Our observational study findings support the potential benefits of physical activity to improve sleep quality even for people with knee symptoms and pain, which is consistent with the general advice of recommending exercises to help prevent and treat sleep disorders.(25) At the very least, our findings support healthcare provider assessment of lifestyle habits (e.g., physical activity and sleep quality) as part of routine office encounters to raise awareness.
In our study, worse mental health and more depressive symptoms at baseline increased the odds of following the worsening versus the good restless sleep trajectory. Depressive symptoms also increased the odds of following the poor versus the improving trajectory. Sleep disturbance is often concurrent with depression and the relationship is bidirectional.(6, 26) Clinical and epidemiological samples have shown that depression is the most frequent cause of chronic insomnia and sleep disturbance, which are among the most consistent symptoms associated with major depressive disorder.(26, 27) About 90% of patients suffering from depression will have sleep quality complaints.(26) Literature also supports insomnia as a risk factor for developing depression. A 2011 meta-analysis of longitudinal epidemiology studies showed an overall odds ratio of 2.6 for baseline insomnia to predict depression at follow-up.(28)
In the present study, we found that people with more severe knee pain at baseline were more likely to follow the worsening versus the good trajectories. Intuitively, it seems reasonable that individuals with OA have pain that may disrupt their sleep. In a cross-sectional, weighted sample of adults in the Canadian Community Health Survey, arthritis was significantly associated with insomnia and unrefreshing sleep and those associations were partly reduced by pain.(29) Inclusion of pain in the multivariable models reduced the association between arthritis and insomnia by 53% and the association between arthritis and unrefreshing sleep by 64%. This study, however, was cross-sectional, so causality could not be determined. In fact, the association between sleep disturbance and pain is likely reciprocal, as systems that regulate arousal and pain share common pathways.(6, 30),(31) Sleep problems have been associated with heightened pain sensitivity at sites distant to the OA-affected joint, suggesting a component of central nervous system augmentation of pain. (30, 32, 33)
Our study showed that baseline cardiovascular disease increased the likelihood of having worsening over good restless sleep trajectories over time. While sleep disturbance is a recognized risk factor for cardiovascular disease, less has been published about cardiovascular disease as a risk factor for restless sleep. Individuals with cardiovascular conditions, such as congestive heart failure, may find it difficult to lie flat, and therefore, have trouble sleeping, which could, in turn, worsen cardiovascular disease by increasing cortisol levels, sympathetic activity and vascular endothelial dysfunction.(34) A large prospective cohort study of US men found that insomnia symptoms of difficulty initiating sleep and non-restorative sleep were associated with a 55% and 32% increased risk of cardiovascular disease mortality compared to those without sleep complaints.(35)
Finally, our study identified baseline pulmonary disease as a strong predictor for following the worsening over good restless sleep trajectory. This was consistent with the literature. Data from general population surveys indicated that adults with asthma and COPD were more likely to report sleep disturbance (e.g., difficulty in initiating and maintaining sleep, early-morning awakening) than those without such pulmonary dysfunctions.(36) Another study using similar data additionally found that odds ratios for insomnia increased with asthma severity, measured by the number of asthma-related symptoms.(37)
This study has important strengths. The OAI is a unique, valuable cohort for examining longitudinal sleep disturbance patterns because it is a large, well-characterized, comprehensively-assessed sample of men and women with or at high risk for knee OA, followed annually over an extended period of time. We leveraged the rich, longitudinal OAI dataset and the robust statistical tool of group-based trajectory modeling to identify heterogeneous patterns of sleep disturbance over 8 years in more than 4000 participants. Although not all OAI participants had restless sleep information available at every follow-up visit, group-based trajectory modeling utilized all available observations in contrast to other methods which exclude individuals with partial longitudinal data.
Several limitations should be mentioned. The assessment of restless sleep in this study was based on a single question regarding frequency of nights with restless sleep in the past week. Future studies are needed to evaluate trajectories of objectively measured parameters of sleep quality. Even though the OAI is a multi-center study and recruited participants with a diverse distribution, the trajectory groups identified may not be generalizable to broader populations. Finally, causation cannot be inferred from these observational data.
In conclusion, we identified four distinctive restless sleep trajectories among people with or at high risk of knee OA. Modifiable health factors including less physical activity, depressive symptoms, and greater knee pain were related to subsequent unfavorable trajectories of restless sleep. Future interventions which target these health risk factors may improve sleep quality. Our findings add to the importance of identifying and treating these factors to stop a downward spiral, which may lead to more severe health problems.
Acknowledgments
Source of Funding
Dr. Yvonne Lee has received grant from Pfizer and has stock in Cigna; This work was supported in part by National Institute for Arthritis and Musculoskeletal Diseases (grant no. P30-AR072579, R01-AR064850, R01-AR054155, P60-AR064464). This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
No other financial disclosures were reported by the authors of this paper. No author has any potential conflict of interest as related to this manuscript.
REFERENCES
- 1.Prather AA, Leung CW. Association of Insufficient Sleep With Respiratory Infection Among Adults in the United States. JAMA Intern Med. 2016;176(6):850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Depner CM, Melanson EL, Eckel RH, et al. Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Curr Biol. 2019;29(6):957–67 e4. [DOI] [PubMed] [Google Scholar]
- 3.Chan MTV, Wang CY, Seet E, et al. Association of Unrecognized Obstructive Sleep Apnea With Postoperative Cardiovascular Events in Patients Undergoing Major Noncardiac Surgery. JAMA. 2019;321(18):1788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep. 2015;38(5):829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen KD, Renner JB, Devellis B, et al. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35(6):1102–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken). 2015;67(3):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox S, Brenes GA, Levine D, et al. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48(10):1241–51. [DOI] [PubMed] [Google Scholar]
- 8.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 9.Gilmour H, Stranges S, Kaplan M, et al. Longitudinal trajectories of sleep duration in the general population. Health Rep. 2013;24(11):14–20. [PubMed] [Google Scholar]
- 10.Radloff L The CES-D Scale: A Self-Report Depression Scale for Research in the General Population Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 11.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 12.Peterfy C, Li J, Zaim S, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. [DOI] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandya R, Metz L, Patten SB. Predictive value of the CES-D in detecting depression among candidates for disease-modifying multiple sclerosis treatment. Psychosomatics. 2005;46(2):131–4. [DOI] [PubMed] [Google Scholar]
- 15.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–35. [DOI] [PubMed] [Google Scholar]
- 17.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation 2. J Clin Epidemiol. 1993;46:153–62. [DOI] [PubMed] [Google Scholar]
- 18.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–93. [Google Scholar]
- 19.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classess in latent class analysis and growth mixture modeling: a Monte Carlo Simulation Study. Struct Equ Model. 2007;14(4):535–69. [Google Scholar]
- 20.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23(9):1455–97. [DOI] [PubMed] [Google Scholar]
- 21.Morgan K Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12(3):231–8. [DOI] [PubMed] [Google Scholar]
- 22.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. [DOI] [PubMed] [Google Scholar]
- 23.Yang PY, Ho KH, Chen HC, et al. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–63. [DOI] [PubMed] [Google Scholar]
- 24.Selten EMH, Vriezekolk JE, Nijhof MW, et al. Barriers Impeding the Use of Non-pharmacological, Non-surgical Care in Hip and Knee Osteoarthritis: The Views of General Practitioners, Physical Therapists, and Medical Specialists. J Clin Rheumatol. 2017;23(8):405–10. [DOI] [PubMed] [Google Scholar]
- 25.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–65, xi. [DOI] [PubMed] [Google Scholar]
- 26.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy MJ, Peterson MJ. Sleep Disturbances in Depression. Sleep Med Clin. 2015;10(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–9. [DOI] [PubMed] [Google Scholar]
- 29.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53(6):911–9. [DOI] [PubMed] [Google Scholar]
- 30.Campbell CM, Buenaver LF, Finan P, et al. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care Res (Hoboken). 2015;67(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman SF, Finan PH, Smith MT, et al. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 2017;158(11):2189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MT, Quartana PJ, Okonkwo RM, et al. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–54. [DOI] [PubMed] [Google Scholar]
- 34.Khan MS, Aouad R. The Effects of Insomnia and Sleep Loss on Cardiovascular Disease. Sleep Med Clin. 2017;12(2):167–77. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhang X, Winkelman JW, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation. 2014;129(7):737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mindus S, Malinovschi A, Ekerljung L, et al. Asthma and COPD overlap (ACO) is related to a high burden of sleep disturbance and respiratory symptoms: Results from the RHINE and Swedish GA2LEN surveys. PLoS One. 2018;13(4):e0195055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundbom F, Lindberg E, Bjerg A, et al. Asthma symptoms and nasal congestion as independent risk factors for insomnia in a general population: results from the GA(2)LEN survey. Allergy. 2013;68(2):213–9. [DOI] [PubMed] [Google Scholar]