Abstract
Background/Objectives
Liver disease is associated with altered serum osmolality, increased thrombin generation, and systemic inflammation, all of which may contribute to perihematomal edema (PHE) after intracerebral hemorrhage (ICH). We evaluated the association between a validated liver fibrosis index and PHE growth in a cohort of patients with primary ICH.
Methods
We performed a retrospective cohort study using data from the Virtual International Stroke Trials Archive-ICH. We included adult patients with primary ICH presenting within 6 hours of symptom onset. The exposure of interest was the Fibrosis-4 (FIB-4) score, a validated liver fibrosis index; this was modeled as a continuous variable. The primary outcome was absolute PHE growth over 96 hours. Secondary outcomes were absolute admission and 96-hour PHE volumes. We used multiple linear regression models adjusted for established determinants of PHE. In a secondary analysis, the FIB-4 score was modeled as a categorical variable to compare patients with versus without liver fibrosis.
Results
Among 354 patients with ICH, 8% had evidence of liver fibrosis based on a validated cut-off. The FIB-4 score was not associated with PHE growth in unadjusted (β, 0.03; 95% CI, −0.01–0.12) or adjusted models (β, 0.04; 95% CI, −0.03–0.13). In a secondary analysis treating FIB-4 as a categorical variable, patients with liver fibrosis did not have greater PHE growth than those without liver fibrosis. FIB-4 score was also not associated with absolute admission or 96-hour absolute PHE volumes.
Conclusions
In a multicenter cohort of patients with primary intracerebral hemorrhage, a liver fibrosis score was not associated with PHE volume or growth.
Keywords: Intracerebral hemorrhage, epidemiology, liver fibrosis, perihematomal edema
Introduction
Medical and surgical interventions targeting hematoma expansion after primary intracerebral hemorrhage (ICH) have shown modest survival benefits with no improvement in major disability.1,2 There is growing recognition that secondary injury after ICH may be a target to improve patient outcomes.2,3 A robust surrogate marker for such secondary injury is perihematomal edema (PHE); PHE growth is associated with worse functional outcomes after ICH.3,4 Changes in local osmotic gradients and an inflammatory response, for example in reaction to thrombin, are among mechanisms implicated in the evolution of PHE.3 Such aberrations are frequently seen in patients with liver disease, who can have abnormal serum osmolality,5,6 increased thrombin generation,7 and systemic inflammation.8,9 Therefore, it is biologically plausible that liver disease is associated with PHE.
Liver fibrosis, seen in up to 15% of the general population, is an often subclinical manifestation of chronic liver diseases and can be detected using non-invasive tests.10–12 There is a growing appreciation of the role of subclinical liver fibrosis in human disease.13,14 We hypothesized that subclinical liver fibrosis – liver fibrosis in a population without overt liver disease – is a potentially unrecognized contributor to PHE in patients with ICH. We evaluated the association between a validated, noninvasive liver fibrosis index and 96-hour PHE growth and absolute PHE volumes in a multicenter cohort of patients with ICH.
Methods
Data Source and Study Design
We performed a retrospective cohort study using data from the Virtual International Stroke Trials Archive ICH (VISTA-ICH).15 The VISTA-ICH database (www.vistacollaboration.org) houses anonymized, individual patient-level data from completed trials that meet the following requirements: 1) documented entry criteria into a trial with a minimum of 50 randomized patients with ICH; 2) documented consent or waiver of consent from a local ethics board; 3) baseline assessment within 24 hours of ICH; 4) baseline assessment of neurologic deficit at the time of admission; 5) confirmation of ICH by cerebral imaging within 7 days; 6) outcome assessment between 1 and 6 months with a validated stroke scale; and 7) data validation through monitoring.4 The cohort studied in this analysis was comprised of patients in the placebo arms of all trials and intervention arms of neutral non-surgical trials. Trials largely excluded patients with overt, advanced liver disease. Trials included in the VISTA-ICH cohort were performed with institutional review board and/or regulatory approval, and our analysis was approved by the Weill Cornell Medicine institutional review board. Per the terms of VISTA-ICH’s data use agreement, data used in this analysis cannot be shared directly with other investigators; however, investigators may gain access by submitting a formal application to VISTA-ICH.
Population
We included adult patients with primary ICH who presented within 6 hours of symptom onset. We excluded patients with missing liver chemistries needed to calculate our exposure variable and missing PHE data; only patients with ~96 hour CT scans were included in the study data from VISTA-ICH, so this was available for all patients in our sample. We excluded patients with known overt liver disease to minimize the impact of severe derangements seen in end-stage cirrhosis, and excluded patients with potentially secondary liver chemistry abnormalities due to self-reported alcohol use and use of potentially hepatotoxic medications (valproic acid, amiodarone, methotrexate, and tamoxifen).16 These exclusions were omitted in a post hoc analysis, described below.
Exposures
We calculated the Fibrosis-4 (FIB-4) score for each patient at the time of admission as follows:
The FIB-4 score has been validated to have good accuracy for the non-invasive detection of liver fibrosis across multiple underlying etiologies of liver disease, including nonalcoholic fatty liver disease, hepatitis B, hepatitis C, and alcoholic liver disease.17–21 Using conservative cut-offs, a FIB-4 score > 3.25 corresponds with a high probability of advanced liver fibrosis, and a score of < 1.45 corresponds to a low probability of liver fibrosis.21 We chose the FIB-4 score for this analysis because we previously demonstrated that the prevalence of liver fibrosis by this score in VISTA-ICH correlated well with population-based estimates of the prevalence of liver fibrosis.14
Outcomes
Our primary outcome was PHE growth, which was defined as the absolute difference between the PHE volume at 96 hours and the PHE volume on admission, calculated from computed tomography scans performed without contrast. We chose this as the primary outcome based on our prior work demonstrating an association between absolute PHE growth and functional outcomes.4 The PHE volume were calculated using semiautomated planimetry and were read centrally within each specific trial by a panel of radiologists blinded to outcomes.4,22 This method of PHE assessment has been shown to have a high inter-rater reliability (correlation coefficient of 0.98).23 Secondary outcomes were absolute admission PHE volume and 96-hour PHE volume.
Demographics and ICH Characteristics
Demographic and clinical data included age, sex, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, smoking, and use of antiplatelet and anticoagulant medication. Clinical markers of ICH severity (Glasgow Coma Scale score, National Institutes of Health Stroke Scale score) were obtained at pre-specified time points. Laboratory data included platelet count and standard liver chemistries. Radiological data were admission hematoma volume, 96-hour hematoma volume, location (lobar, deep, and infratentorial), and intraventricular hemorrhage.
Statistical Analyses
Patient characteristics were described using standard descriptive statistics. Discrete variables are presented as counts (percentages [%]) and continuous variables as means (standard deviation [SD]) or medians (interquartile range [IQR]), as appropriate. Pearson Chi-squared test was used for categorical variables as appropriate; the Student’s t test or Wilcoxon rank sum test were used for continuous variables depending on the normality of distribution. Fibrosis indices were treated as continuous variables. We used multiple linear regression models to assess the relationship between the continuous FIB-4 score and absolute PHE growth, which was log transformed, in addition to the secondary outcomes of absolute admission and 96-hours PHE volumes. We a priori specified the following universal confounders and potential predictors3,4,22 of PHE growth, based in part on prior work including in the VISTA-ICH dataset, as model covariates: age, sex, race, antiplatelet use, serum sodium, admission ICH volume, and ICH location. In a secondary analysis, we evaluated the association between a high FIB-4 score consistent with advanced liver fibrosis (>3.25) and PHE growth, as compared to patients with a low FIB-4 score (<1.45) consistent with absent liver fibrosis.
We performed several post hoc analyses to evaluate whether our null association findings were consistent across several additional approaches. First, we evaluated the associations between the FIB-4 score and PHE volumes and growth in a sample of all patients, without exclusion for overt liver disease, self-reported alcohol use, or hepatotoxic medications. In this analysis, we restricted models to three covariates. This was done in part to mitigate concerns regarding over-fitted models. In this model, age was excluded because age is already included in the FIB-4 score, and sodium was excluded because it may be a mediator. Second, to address the converse possibility of residual confounding, we added warfarin to existing models. Third, we added an additional outcome measure: relative PHE growth, which was defined as 96-hour PHE volume/admission PHE volume. Post hoc analyses adhered to the specification of our primary analysis: the exposure variable (FIB-4) was treated as a continuous variable in these analyses. Last, we separately evaluated univariate associations of AST and ALT with PHE growth and volumes.
Statistical analyses were performed using Stata (version 14.0, College Station, TX). All analyses were two-tailed with the threshold of statistical significance allowing for an alpha error of 0.05.
Results
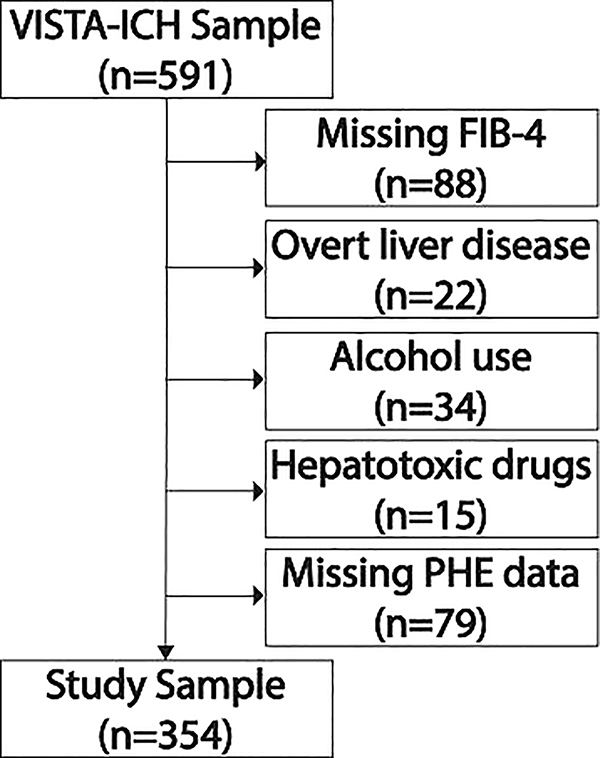
The VISTA-ICH dataset consisted of 591 patients of whom 354 were eligible for our study (Figure 1). The mean age was 65 years (SD, 12), and 66% were men. Warfarin use was noted in 6% and antiplatelet use in 19% of patients. Based on validated cut-offs, 8.1% had a FIB-4 score consistent with a high probability of advanced liver fibrosis, and 44.1% had a low probability of any liver fibrosis. The median admission ICH volume was 14.8 milliliters (IQR, 7.5–26.3), the median admission PHE volume was 8.6 milliliters (IQR, 4.7–14.3), and the median PHE volume at ~96 hours was 23.3 milliliters (IQR, 11.7–37.2).
Figure 1.
Patient selection flow diagram.
Patients with missing laboratory data, known overt liver disease, alcohol use, use of potentially hepatotoxic medications, and missing perihematomal edema data were excluded. Some patients had multiple reasons for exclusion. Abbreviations: VISTA-ICH, Virtual International Stroke Trials Archive–Intracerebral Hemorrhage; FIB-4, Fibrosis-4; PHE, perihematomal edema.
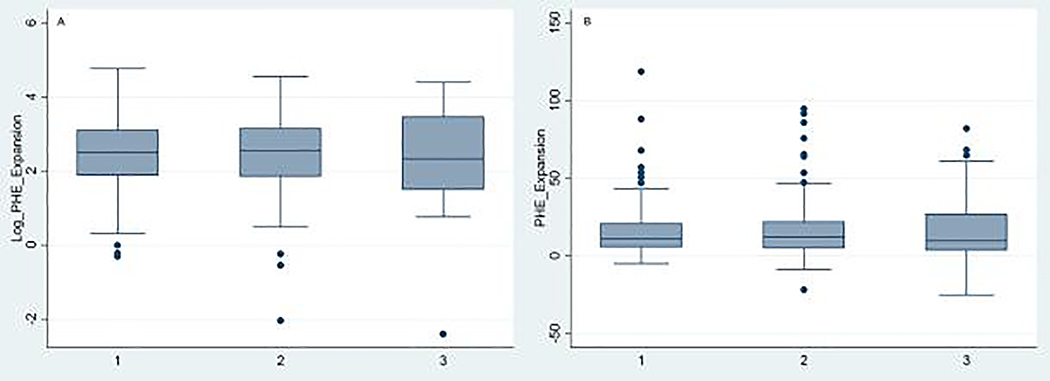
In the unadjusted model, FIB-4 score was not associated with PHE growth (β = 0.03, 95% confidence interval [CI], −0.01–0.12). FIB-4 score was also not associated with PHE growth in models after adjusting for age, sex, race, serum sodium, antiplatelet use, admission ICH volume, and ICH location (β = 0.04, 95% CI, −0.03–0.13) (Table 2). In a secondary analysis, we evaluated the association between a high FIB-4 score and PHE growth, as compared to patients with a low FIB-4 score. Crude or log transformed PHE expansion volumes did not differ by liver fibrosis group (high probability, indeterminate probability, low probability) (Figure 2). Patients with high FIB-4 scores did not have greater PHE growth than patients with low scores in unadjusted (β = 0.03, 95% CI, −0.46–0.39) or adjusted models (β = 0.12, 95% CI, −0.32–0.55) (Table 2).
Table 2.
Association* between Fibrosis-4 Liver Fibrosis Score and Perihematomal Edema Growth after Primary Intracerebral Hemorrhage
| Primary analysis: Fibrosis-4 score, continuous | |||
| Beta | 95% Confidence Interval | P value | |
| Unadjusted | 0.03 | −0.01 – 0.12 | 0.48 |
| Model 2 | 0.07 | −0.01 – 0.18 | 0.10 |
| Model 3 | 0.04 | −0.04 – 0.12 | 0.36 |
| Model 4 | 0.04 | −0.03 – 0.13 | 0.26 |
| Secondary analysis: High Fibrosis-4 versus Low Fibrosis-4† | |||
| Beta | 95% Confidence Interval | P value | |
| Unadjusted | 0.03 | −0.46 – 0.39 | 0.88 |
| Model 2 | 0.24 | −0.25 – 0.73 | 0.34 |
| Model 3 | 0.07 | −0.36 – 0.50 | 0.75 |
| Model 4 | 0.12 | −0.32 – 0.55 | 0.60 |
Multiple linear regression was used to model the association between the Fibrosis-4 score and absolute 96-hour perihematomal edema (PHE) growth, which was defined as: absolute difference between 96-hour PHE volume and admission PHE volume. Model 2 was adjusted for age, sex, and race. Model 3 was additionally adjusted for serum sodium, antiplatelet use, and admission hematoma volume. Model 4 was additionally adjusted for intracerebral hemorrhage location (lobar versus deep versus infratentorial). PHE growth was log transformed.
Patients with a high Fibrosis-4 score (>3.25) were compared to patients with a low Fibrosis-4 score (<1.45).
Figure 2.
Box-whisker plot of perihematomal edema volume growth by category of Fibrosis-4 liver fibrosis score.
Log transformed (A) and absolute [milliliters] (B) 96-hour perihematomal edema volume growth, defined as the difference between the 96-hour and admission volumes, categorized by Fibrosis-4 liver fibrosis score category: (1) low probability of liver fibrosis [Fibrosis-4 < 1.45], (2) intermediate probability [Fibrosis-4 1.45–3.25], (3) high probability [Fibrosis-4 > 3.25]. Abbreviations: PHE, perihematomal edema.
Secondary outcomes were absolute admission PHE volume and 96-hour PHE volume. FIB-4 score was not associated with admission PHE volume in the unadjusted model or after adjusting for confounders (β = −0.03, 95% CI, −0.04–0.11) (Table 3). Similarly, there was also no association between FIB-4 and 96-hour PHE volume in any model (Table 3).
Table 3.
Association* between Fibrosis-4 Liver Fibrosis Score and Admission and 96-hour Perihematomal Edema Volumes in Primary Intracerebral Hemorrhage
| Admission PHE volume | |||
| Beta | 95% Confidence Interval | P value | |
| Unadjusted | 0.03 | −0.04–0.11 | 0.36 |
| Model 2 | 0.03 | −0.05–0.11 | 0.41 |
| Model 3 | −0.03 | −0.08–0.02 | 0.23 |
| Model 4 | −0.03 | −0.08–0.02 | 0.28 |
| 96-hour PHE volume | |||
| Beta | 95% Confidence Interval | P value | |
| Unadjusted | 0.02 | −0.06–0.94 | 0.64 |
| Model 2 | 0.04 | −0.36–0.13 | 0.28 |
| Model 3 | −0.01 | −0.07–0.05 | 0.78 |
| Model 4 | −0.002 | −0.06–0.06 | 0.94 |
Multiple linear regression was used to model the association between the Fibrosis-4 score (continuous measure) and absolute admission and 96-hour perihematomal edema volumes. Model 2 was adjusted for age, sex, and race. Model 3 was additionally adjusted for serum sodium, antiplatelet use, and admission hematoma volume. Model 4 was additionally adjusted for intracerebral hemorrhage location (lobar versus deep versus infratentorial). PHE volumes were log transformed.
Results were consistent across several post hoc analyses (Supplemental File). First, FIB-4 was not associated with PHE volume or growth even when including patients with overt liver disease, alcohol use, and use of hepatotoxic medications, and results were consistent in models limited to three covariates (e.g., sex, race, and hematoma volume). Conversely, results were also consistent when adding warfarin to the most adjusted models treating FIB-4 as a continuous variable. Last, the outcome of relative PHE growth was separately examined; an association of FIB-4 with relative PHE growth was not observed. Univariate associations between AST and ALT and PHE parameters were not seen.”
Discussion
In a multicenter, retrospective cohort of patients with primary intracerebral hemorrhage, a liver fibrosis score was not associated with PHE volume or increased PHE growth.
Multiple prior studies have demonstrated that chronic liver disease is associated with worse outcomes after ICH.14,24,25 PHE growth is one determinant of outcomes after ICH,4,22 and our data provide insufficient evidence to support the hypothesis that liver fibrosis contributes to worse outcomes through an association with PHE volume or growth. Rather, as we previously demonstrated in the VISTA-ICH cohort, an independent association between liver fibrosis and hematoma volume and growth14 may underlie the link between liver disease and outcomes after ICH. Other data suggest that non-neurological factors such as susceptibility to infection and liver-related complications are responsible for poor outcomes.25–27 An improved understanding of mechanistic factors in the association between liver disease and outcomes after ICH may yield novel opportunities for intervention. This is especially the case for liver fibrosis because it is both common and frequently subclinical,10–12 and its role in cerebrovascular disease is largely unrecognized.
Our null findings must be considered in light of several caveats. First, our focus was to understand the role of subclinical liver fibrosis in PHE volume and growth. The VISTA-ICH cohort was well-suited for this because randomized trials contributing data to VISTA-ICH largely excluded patients with overt, advanced liver disease, and we further excluded the few patients with overt liver disease. Based on our data, there is insufficient evidence of an association between subclinical liver fibrosis and PHE growth; however, our data are not intended to address the impact of end-stage, clinically overt liver disease on PHE. With that said, our results did not meaningfully change with inclusion of patients with overt liver disease and alcohol-use. Second, hematoma volumes were small in VISTA-ICH; patients contributing data to VISTA-ICH trials had clinically and radiographically mild ICH. Because ICH volume is a known determinant of PHE volume and growth,4 our data do not address the possibility that liver fibrosis is associated with PHE growth in patients with more severe hematomas. Third, while the FIB-4 liver fibrosis score is validated, it is possible that misclassification of liver fibrosis biased our findings towards the null. Specifically, the performance of the FIB-4 score may be suboptimal in unselected patients, e.g., patients without known chronic liver disease who may have a lower pre-test probability of liver fibrosis. However, the concordance of the prevalence of liver fibrosis in VISTA-ICH defined by the FIB-4 with population-based estimates10–12 partially mitigates this concern. Additionally, in primary analyses, we used the FIB-4 score as a continuous variable in part because the cut-off used to exclude liver fibrosis may vary with age;28 therefore, the results of our secondary analysis comparing high versus low FIB-4 scores must be interpreted with caution.
Conclusions
In conclusion, there is insufficient evidence of an association between a liver fibrosis score and PHE volume or growth after ICH. Future studies should investigate alternate mechanisms by which liver fibrosis may impact outcomes in ICH.
Supplementary Material
Table 1.
Characteristics of Patients with Primary Intracerebral Hemorrhage in VISTA-ICH.
| Characteristic* | Study Sample (N=354) |
|---|---|
| Patient Characteristics | |
| Age, mean (SD), years | 64.6 (11.5) |
| Male sex | 232 (66) |
| Race | |
| White | 291 (82) |
| Black | 16 (5) |
| Other | 47 (13) |
| Hypertension | 293 (83) |
| Hyperlipidemia | 53 (15) |
| Diabetes mellitus | 66 (19) |
| Atrial fibrillation | 28 (8) |
| Warfarin use | 21 (6) |
| Antiplatelet use | 68 (19) |
| Admission Laboratory Data | |
| Platelet count†, ×103 per microliter | 217 (182–259) |
| International Normalized Ratio† | 1.0 (0.9–1.1) |
| aPTT†, seconds | 24.6 (21.5–29.2) |
| AST†, units/liter | 23 (2–38) |
| ALT†, units/liter | 20 (15–29) |
| Albumin†, gram/dL | 41 (39–44) |
| Total bilirubin†, milligram/dL | 0.5 (0.3–0.7) |
| Admission Intracerebral Hemorrhage Data | |
| Glasgow Coma Scale† | 15 (14–15) |
| National Institutes of Health Stroke Scale† | 13 (9–17) |
| Intraventricular hemorrhage | 107 (30) |
| Hematoma location | |
| Lobar | 73 (21) |
| Deep | 274 (77) |
| Infratentorial | 7 (2) |
| Time to admission CT scan†, hours | 1.8 (1.1–2.5) |
| Hematoma volume at admission†, mL | 14.8 (7.5–26.3) |
| Perihematoma edema volume at admission†, mL | 8.6 (4.7–14.3) |
| 96-hour Intracerebral Hemorrhage Imaging Data | |
| Time to 96-hour CT scan†, hours | 87.6 (83.2–98.2) |
| Hematoma volume at 96 hours†, mL | 17.1 (8.2–33.9) |
| Perihematoma edema volume at 96 hours†, mL | 23.3 (11.7–37.2) |
Abbreviations; VISTA-ICH, Virtual International Stroke Trials Archive-Intracerebral Hemorrhage; SD, standard deviation; aPPT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; dl, deciliter; mL, milliliters; μL, microliter.
Data reported as n (%) unless otherwise specified.
Data reported as median (interquartile range).
Acknowledgments
Sources of Funding: Dr. Parikh was supported by the Leon Levy Foundation, the New York State Department of Health Empire Clinical Research Investigator Program, and the Florence Gould Foundation. Dr. Jesudian has funding from the Society of Interventional Radiology Foundation. Dr. Kamel is supported by NINDS grants R01NS097443 and U01NS095869 and the Michael Goldberg Research Fund. Dr. Hanley is supported by the NIH (1U01NS080824, U24TR001609). Dr. Ziai is supported by NIH 1U01NS080824. Dr. Murthy is funded by NINDS grant K23NS105948 and the Leon Levy Foundation.
5. Conflicts of interest: Dr. Parikh, Dr. Jesudian, Dr. Murthy: none. Hooman Kamel serves as the co-PI for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Hanley reports personal fees from Op2Lysis, personal fees from BrainScope, personal fees from Neurotrope, and non-financial support from Genentech outside the submitted work. Dr, Ziai receives consulting fees from C.R. Bard, Inc. and Portola Pharmaceuticals, Inc outside of the area of work commented on here.
APPENDIX
*VISTA-ICH Steering Committee
VISTA-ICH Steering Committee Collaborators: D.F. Hanley (Chair), K. Butcher, S. Davis, B. Gregson, K.R. Lees, P. Lyden, S. Mayer, K. Muir, and T. Steiner.
Footnotes
1. This manuscript complies with all instruction to authors.
2. Authorship requirements were met, and the final manuscript was approved by all authors.
3. This manuscript has not been published elsewhere and is not under consideration by another journal.
4. Ethical guidelines were followed. Trials included in the VISTA-ICH cohort were performed with institutional review board and/or regulatory approval, and our analysis was approved by the Weill Cornell Medicine institutional review board.
6. The STROBE checklist for observational research is uploaded along with the manuscript.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Cusack TJ, Carhuapoma JR, Ziai WC. Update on the Treatment of Spontaneous Intraparenchymal Hemorrhage: Medical and Interventional Management. Curr Treat Options Neurol 2018;20:1. [DOI] [PubMed] [Google Scholar]
- 2.Gross BA, Jankowitz BT, Friedlander RM. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA : the journal of the American Medical Association 2019;321:1295–303. [DOI] [PubMed] [Google Scholar]
- 3.Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage--perihaematomal oedema. Nature Reviews Neurology 2015;11:111–22. [DOI] [PubMed] [Google Scholar]
- 4.Murthy SB, Moradiya Y, Dawson J, et al. Perihematomal Edema and Functional Outcomes in Intracerebral Hemorrhage: Influence of Hematoma Volume and Location. Stroke 2015;46:3088–92. [DOI] [PubMed] [Google Scholar]
- 5.Liotta EM, Romanova AL, Lizza BD, et al. Osmotic Shifts, Cerebral Edema, and Neurologic Deterioration in Severe Hepatic Encephalopathy. Crit Care Med 2018;46:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Córdoba J, García-Martinez R, Simón-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis 2010;25:73–80. [DOI] [PubMed] [Google Scholar]
- 7.Bos S, van den Boom B, Kamphuisen PW, et al. Haemostatic Profiles are Similar across All Aetiologies of Cirrhosis. Thromb Haemost 2019;119:246–53. [DOI] [PubMed] [Google Scholar]
- 8.Yoneda M, Mawatari H, Fujita K, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 2007;42:573–82. [DOI] [PubMed] [Google Scholar]
- 9.Khoury T, Mari A, Nseir W, Kadah A, Sbeit W, Mahamid M. Neutrophil-to-lymphocyte ratio is independently associated with inflammatory activity and fibrosis grade in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2019;31:1110–5. [DOI] [PubMed] [Google Scholar]
- 10.Caballería L, Pera G, Arteaga I, et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin Gastroenterol Hepatol 2018;16:1138–45.e5. [DOI] [PubMed] [Google Scholar]
- 11.Watt GP, Lee M, Pan JJ, et al. High Prevalence of Hepatic Fibrosis, Measured by Elastography, in a Population-Based Study of Mexican Americans. Clin Gastroenterol Hepatol 2019;17:968–75.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011;60:977–84. [DOI] [PubMed] [Google Scholar]
- 13.Ostovaneh MR, Ambale-Venkatesh B, Fuji T, et al. Association of Liver Fibrosis With Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Imaging 2018;11:e007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh NS, Kamel H, Navi BB, et al. Liver Fibrosis Indices and Outcomes After Primary Intracerebral Hemorrhage. Stroke 2020; 51:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali M, Bath P, Brady M, et al. Development, expansion, and use of a stroke clinical trials resource for novel exploratory analyses. Int J Stroke 2012;7:133–8. [DOI] [PubMed] [Google Scholar]
- 16.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112:581–7. [DOI] [PubMed] [Google Scholar]
- 17.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- 18.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
- 19.Naveau S, Gaudé G, Asnacios A, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 2009;49:97–105. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 22.Murthy SB, Urday S, Beslow LA, et al. Rate of perihaematomal oedema expansion is associated with poor clinical outcomes in intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2016;87:1169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urday S, Beslow LA, Goldstein DW, et al. Measurement of perihematomal edema in intracerebral hemorrhage. Stroke 2015;46:1116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh NS, Merkler AE, Schneider Y, Navi BB, Kamel H. Discharge Disposition After Stroke in Patients With Liver Disease. Stroke 2017;48:476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoya K, Tanaka Y, Uchida T, et al. Intracerebral hemorrhage in patients with chronic liver disease. Neurol Med Chir (Tokyo) 2012;52:181–5. [DOI] [PubMed] [Google Scholar]
- 26.Morotti A, Marini S, Lena UK, et al. Significance of admission hypoalbuminemia in acute intracerebral hemorrhage. Journal of neurology 2017;264:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Napoli M, Behrouz R, Topel CH, et al. Hypoalbuminemia, systemic inflammatory response syndrome, and functional outcome in intracerebral hemorrhage. J Crit Care 2017;41:247–53. [DOI] [PubMed] [Google Scholar]
- 28.McPherson S, Hardy T, Dufour JF, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol 2017;112:740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.