Abstract
Deep learning (DL) has shown great potential in conversions between various imaging modalities. Similarly, DL can be applied to synthesize a high-kV computed tomography (CT) image from its corresponding low-kV CT image. This indicates the feasibility of obtaining dual-energy CT (DECT) images without purchasing a DECT scanner. In this study, we investigated whether a low-to-high kV mapping was better than a high-to-low kV mapping. We used a U-Net model to perform conversions between different kV CT images. Moreover, we proposed a double U-Net model to improve the quality of original single-energy CT images. Ninety-eight patients who underwent brain DECT scans were used to train, validate, and test the proposed DL-based model. The results showed that the low-to-high kV conversion was better than the high-to-low kV conversion. In addition, the DL-based DECT images had better signal-to-noise ratios (SNRs) than the true (original) DECT images, but at the expense of a slight loss in spatial resolution. The mean CT number differences between the true and DL-based DECT images were within 1 HU. No statistically significant difference in CT number measurements was found between the true and DL-based DECT images (p > 0.05). The DL-based DECT images with improved SNR could produce low-noise virtual monoenergetic images. Our preliminary results indicate that DL has the potential to generate brain DECT images using single-energy brain CT images.
Keywords: Deep learning, U-net, Dual-energy computed tomography
Introduction
Since its commercialization in 2006, the diagnostic value of dual-energy computed tomography (DECT) imaging has been extensively investigated in many clinical studies [1]. Several different DECT techniques including virtual non-contrast (VNC), iodine map, electron density, effective atomic number, and virtual monoenergetic image (VMI) have been proposed [1]. These DECT-based techniques have many different clinical applications such as metal artifact reduction, beam-hardening correction, contrast and noise optimization, and material differentiation [2, 3]. Moreover, DECT imaging has the potential to improve the accuracy of dose calculation in brachytherapy and proton therapy [4]. In brain, DECT has the ability to separate bone/calcification from iodine [1–3], and DECT-derived VNC imaging is able to identify intracranial hemorrhage [5]. In addition, non-contrast DECT can be used to visualize ischemic stroke [5]. Because of these various clinical applications, DECT has gained a lot of attention in recent years. Despite promising results, DECT has not gained much popularity. One possible reason is the cost of instrumentation and maintenance. In general, most DECT scanners cost between $1.6 and $2.5 million, whereas conventional multi-slice CT scanners cost roughly $0.55 million [6]. In addition, the radiation exposure of DECT remains a concern, although some previous studies demonstrated that at equal or even lower doses, DECT could provide equivalent image quality compared to conventional single-energy CT [7].
There are several different techniques which have been proposed to reduce the radiation dose of DECT. For example, DECT protocols with a low milliampere-second (mAs) value can combine with statistical iterative reconstruction algorithms [8] for reducing radiation dose while maintaining image quality. Moreover, improvements in CT hardware and software have been shown to reduce radiation dose [9]. A combination of new X-ray tubes, more sensitive detectors, and statistical iterative reconstruction algorithms can make a substantial contribution to the reduction of patient radiation exposure. Despite the great achievements in reducing radiation dose, a new way that can further reduce radiation dose while maintaining image quality is required.
In recent years, deep learning (DL) has shown many encouraging results in the field of medical image processing and analysis [10, 11]. In particular, several recent studies showed the feasibility of using convolutional neural networks (CNNs) to generate a pseudo-CT image from its corresponding magnetic resonance image [12, 13]. The pseudo-CT image can be used for the attenuation correction of positron emission tomography. Similarly, one previous study used a cascaded deep CNN to predict a basis material image directly from its corresponding single-energy CT image [14]. One recent study showed that DL could be used to generate a high-kV CT image from its corresponding low-kV CT image [15]. They first trained a CNN model for image denoising. Then, they used denoised DECT images to train a CNN model for learning the difference between the low-kV and high-kV CT images. Finally, the DL-based high-kV CT images were calculated by adding the original low-kV CT images to the predicted difference images.
The abovementioned study has shown the feasibility of using DL to generate a high-kV CT image from its corresponding low-kV CT image [15]. This indicates that it is possible to obtain DECT imaging without using a DECT scanner. Moreover, only one single-energy CT scan is performed. As a result, there is no additional radiation exposure. Nevertheless, it remains to be shown whether a low-to-high kV mapping is better than a high-to-low kV mapping. Also, the abovementioned DL-based method requires a denoising CNN model [15] which may be difficult to obtain due to the lack of clean DECT images. In this study, we examined whether the low-to-high kV conversion was better than the high-to-low kV conversion. We also proposed a DL-based model to improve the quality of original single-energy CT images without preparing clean target images. We performed a retrospective study that included patients who underwent brain DECT scans. We compared the true (original) and DL-based DECT images in terms of the difference in CT numbers, structural similarity (SSIM) index [16] and signal-to-noise ratio (SNR). A Wilcoxon matched-pairs signed-rank test was applied to assess the differences between the true and DL-based CT numbers.
Methods and Materials
DL-Based CNN Models
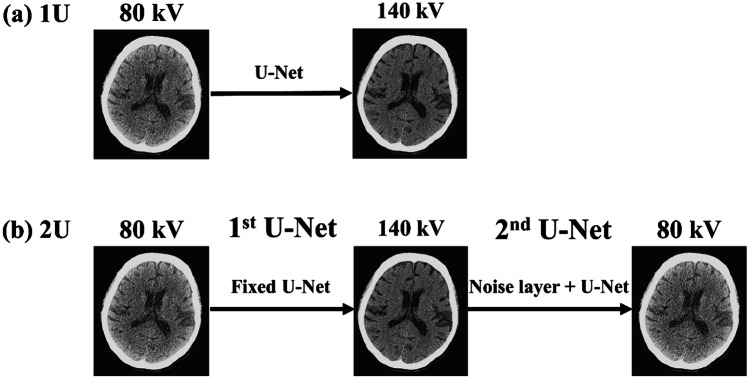
Figure 1 shows a workflow of the proposed DL-based method for predicting DECT images. As shown in Fig. 1a, we trained one U-Net (1U) model [17] for predicting 140-kV (i.e., high-kV) CT images from their corresponding 80-kV (i.e. low-kV) CT images. The architectural details for the U-Net structure are summarized in Fig. 2. Note that the results of DECT applications are easily affected by the quality of DECT images. Because image noise is generally high at 80 kV, the use of 80-kV CT images may not produce optimal results for DECT applications. Therefore, we proposed a double U-Net (2U) architecture for improving the quality of 80-kV CT images. As shown in Fig. 1b, the 2U model that consists of two U-Nets can be viewed as an autoencoder. The input and output images are 80-kV CT images. The model weights of the first U-Net were obtained from the pre-trained 1U model and fixed during the training phase. This means that the function of the first U-Net is to generate 140-kV CT images from 80-kV CT images (Fig. 1a). Then, the output of the first U-Net was connected to a noise layer before connecting the input layer of the second U-Net. The noise layer acts as a regularizer, which has been shown to improve the model’s performance [18, 19]. The function of the second U-Net is to predict 80-kV CT images from 140-kV CT images. In the 2U model, we only trained the model weights of the second U-Net. Note that the first and second U-Net architectures of the 2U model are the same as the U-Net architecture of the 1U model (Fig. 2) except that there is a noise layer connecting the first and second U-Net. The 1U model shown in Fig. 1a is illustrated for the low-to-high kV conversion. For the high-to-low kV conversion, the input and output of the 1U model are 140 kV and 80 kV CT images, respectively.
Fig. 1.
Workflow of the proposed DL-based DECT imaging method. We trained (a) one U-Net (1U) model for predicting 140-kV CT images from 80-kV CT images and (b) a double U-Net model (2U) for denoising 80-kV CT images. In the 2U model, the model weights of the first U-Net obtained from the pre-trained 1U model were fixed during training. The output of the first U-Net was connected to a noise layer before connecting the input layer of the second U-Net. The workflow shown above is illustrated for the low-to-high kV conversion. For the high-to-low kV conversion, the input and output of the 1U model are 140 kV and 80 kV CT images, respectively
Fig. 2.
The standard U-Net architecture. The number on the top of each arrow is the number of filters. The blue arrow means a 2D convolutional layer with a kernel size of 3 3. Each convolutional layer is followed by a nonlinear activation function (ReLu). The downward arrow is a max-pooling layer that replaces a 2 2 pixel area by its maximum. The upward arrow is a 2D transposed convolutional layer with a kernel size of 3 3 and stride of 2
In this study, we trained each CNN model (i.e., 1U and 2U) using two different loss functions (i.e., mean squared error and mean absolute error) with a batch size of 10. We took an average of all predictions from the two independent CNN models and used it as the final prediction. This ensemble learning technique acts as a variance reduction [20]. The Adam optimization algorithm was employed to minimize the loss function. The learning rate was 10–5, beta1 was 0.9, and beta2 was 0.999. The number of epochs was 500. The noise layer used in this study was employed to add zero-centered Gaussian noise (sigma = 10–4) to the input. Note that the noise layer was activated only during the training phase. The CNN model was implemented using TensorFlow, and all trainings were performed on a NVIDIA Titan XP GPU. All training, validation, and testing datasets were normalized to values between 0 and 1 before training. For qualitative and quantitative comparison, all testing datasets were multiplied by the scaling factor (= 4095) and then subtracted the results by 1024. This led to a scale running from − 1024 to 3071 Hounsfield units (HU).
DECT Data Acquisition and Reconstruction
From December 2018 to June 2019, 98 consecutive patients who underwent non-contrast brain DECT scanning were retrospectively enrolled in this study. The enrolled patients were suspected of having a stroke or head injury. The studied group included 39 men and 59 women with ages ranging from 16 to 88 years (mean, 64 18 years). The present study was approved by our institutional review board. All recruited patients were scanned using a second-generation dual-source DECT scanner (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany). DECT data were acquired using a default DE scanning protocol. Scanning parameters were as follows: 80 kV/Sn140 kV; reference mAs, 310/155 mAs; gantry rotation time, 0.5 s; pitch, 0.7; and section collimation, 0.6 mm. All raw DECT data was reconstructed with a dedicated DE medium-soft convolution kernel (D30f with filtered back projection). DECT image series were exported as axial images (512 512 pixels) with a slice thickness of 5 mm. Ninety-eight patients were randomly split into seventy-eight for training (= 2260 images), ten for validation (= 291 images), and ten for testing (= 296 images).
Data Analysis
To quantitatively compare the HU differences between the true and predicted DECT images, we analyzed four different types of tissues (i.e., brain, fat, cerebrospinal fluid (CSF), and muscle). We manually drew 100 region-of-interests (ROIs) on each tissue. Then, the mean CT number of each ROI (100 pixels per ROI) was measured. For all measurements, mean and standard deviation (SD) were derived. A Wilcoxon matched-pairs signed-rank test was used to test whether the difference in mean CT numbers between the true and predicted DECT images was significant. A p value of less than 0.05 was considered to indicate a significant difference. We also calculated the SNR for each ROI. To quantify the visual similarity between the true and predicted DECT images, the SSIM index is calculated as follows [16]:
| 1 |
where and denote the mean values of true and DL-based CT images, respectively, and denote the standard deviations of true and DL-based CT images, respectively, and is the covariance of true and DL-based CT images. c1 = (0.01 L)2 and c2 = (0.03 L)2 are constants for the luminance, contrast, and structural terms. L is the maximum value of the dynamic range between and . Finally, VMIs at 40, 70, 100, and 130 keV were reconstructed using the true and DL-based DECT images with a dedicated software application (Monoenergetic Application Class) on a multimodality workstation (Syngo MMWP VE 40A, Siemens Healthcare, Forchheim, Germany).
Results
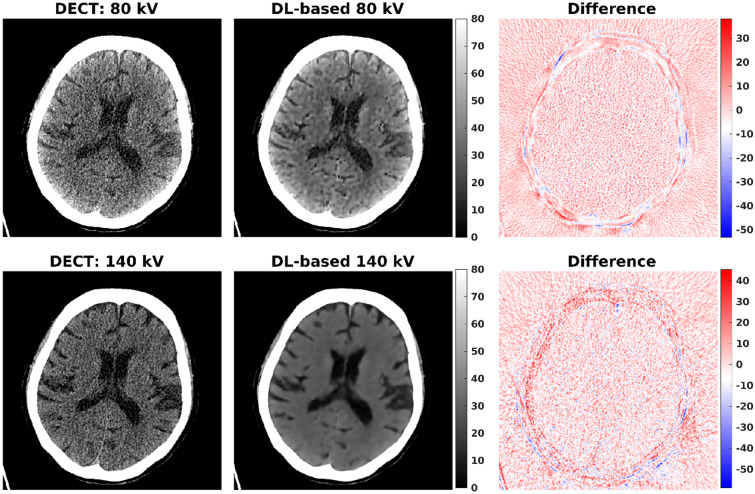
Figure 3 shows an example of true (original) and DL-based DECT images. It can be seen that the DL-based 80-kV CT image appears to be smoother than the DL-based 140-kV CT image. We calculated the mean square error (MSE) between the true and predicted CT images for all testing datasets. We found that the mean MSE ( SD) of 140-kV cases was lower than that of 80-kV cases (92 47 HU vs. 153 74 HU), indicating that the low-to-high kV conversion was better than the high-to-low kV conversion. Moreover, the DL-based 80-kV CT images obtained using the 2U model had lower mean MSE ( SD) than those obtained using the 1U model (49 19 HU vs. 153 74 HU). Therefore, all DL-based 140-kV images shown below were obtained using the 1U model with the low-to-high kV conversion, and all DL-based 80-kV images shown below were obtained using the 2U model (see Fig. 1b).
Fig. 3.
An example of true (left column) and DL-based (right column) DECT images. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. In contrast, the DL-based 80-kV image was generated from its corresponding 140-kV CT image using the 1U model with high-to-low kV conversion. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Figure 4 shows an example of true and DL-based DECT images, and difference images between the true and predicted DECT images. It can be seen that the DL-based DECT images had lower noise than the true DECT images. In Table 1, the DL-based DECT images had better mean SNR values than the true DECT images. We also observed that the overall differences between the true and predicted DECT images were small in most regions, except the regions close to the cranial bones. Figure 5 shows similar results obtained from a different axial slice. The mean SSIM index ( SD) between the true 80-kV and DL-based 80-kV CT images was 0.9981 ( 0.0003) for testing datasets, respectively. Similarly, the mean SSIM index between the true 140-kV and DL-based 140-kV CT images was 0.9966 ( 0.0007) for testing datasets, respectively.
Fig. 4.
An example of true (left column) and DL-based (middle column) DECT images, and difference images (right column) between the true and predicted DECT images. The DL-based 80-kV image was generated from the original 80-kV CT image using the 2U model. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Table 1.
Mean SNR for different types of tissues calculated using true and DL-based DECT images. For each tissue, the mean SNR value was calculated from 100 ROIs
| True 80 kV | DL-based 80 kV | True 140 kV | DL-based 140 kV | |
|---|---|---|---|---|
| Brain | 5.63 | 12.19 | 4.76 | 32.11 |
| Fat | 12.07 | 16.12 | 10.46 | 18.61 |
| CSF | 1.01 | 2.74 | 0.39 | 3.82 |
| Muscle | 7.18 | 12.76 | 7.76 | 25.41 |
Fig. 5.
An example of true (left column) and DL-based (middle column) DECT images, and difference images (right column) between the true and predicted DECT images. The DL-based 80-kV image was generated from the original 80-kV CT image using the 2U model. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
The mean CT numbers for different types of tissues are reported in Table 2. For all tissue types, the mean CT numbers between the true and DL-based DECT images did not differ significantly (p > 0.05). Table 3 presents a comparison of the mean CT number differences of four different tissue types on true and DL-based DECT images. For all tissue types, the mean CT number differences did not exceed 1 HU. For 80-kV CT images, the mean CT number differences ranged from − 6 to 6 HU. For 140-kV CT images, the mean CT number differences ranged from − 6 to 7 HU. Overall, the proposed DL-based method has better performance in predicting low-kV CT images. Figures 6 and 7 show the true and DL-based VMIs obtained using DECT images shown in Figs. 4 and 5, respectively. Compared to the true DECT imaging, the proposed DL-based DECT imaging could provide VMIs with less noise.
Table 2.
Mean CT number and SD (HU) for different types of tissues calculated using true and DL-based DECT images. We drew 100 ROIs for each tissue and measured the mean CT number of each ROI. Means and SDs were presented
| True 80 kV | DL-based 80 kV | True 140 kV | DL-based 140 kV | |
|---|---|---|---|---|
| Brain | 51.55 7.46 | 51.70 7.13 (p value = 0.15) | 34.91 2.87 | 35.13 2.22 (p value = 0.41) |
| Fat | − 115.79 14.67 | − 115.59 13.68 (p value = 0.19) | − 84.68 7.76 | − 84.84 7.34 (p value = 0.48) |
| CSF | 8.55 2.06 | 8.73 1.55 (p value = 0.09) | 3.04 2.07 | 3.33 1.15 (p value = 0.10) |
| Muscle | 57.25 7.21 | 57.34 7.04 (p value = 0.65) | 52.87 3.85 | 52.51 3.58 (p value = 0.34) |
Table 3.
Mean CT number differences (HU) between the true and DL-based DECT images. We measured 100 mean CT number differences for each tissue. Mean values (minimum~maximum values) were presented
| True 80 kV minus DL-based 80 kV (min~max) | True 140 kV minus DL-based 140 kV (min~max) | |
|---|---|---|
| Brain | − 0.16 (− 3.78~4.03) | − 0.23 (− 4.73~4.04) |
| Fat | − 0.20 (− 5.71~5.92) | − 0.17 (− 5.04~6.60) |
| CSF | − 0.18 (− 2.62~1.97) | − 0.29 (− 3.25~3.75) |
| Muscle | − 0.09 (− 5.34~4.08) | − 0.36 (− 5.89~6.33) |
Fig. 6.
An example of DECT-based (upper row) and DL-based (lower row) VMIs images. All VMIs are displayed in a window width of 80 HU and a window level (center) of 40 HU
Fig. 7.
An example of DECT-based (upper row) and DL-based (lower row) VMIs images. All VMIs are displayed in a window width of 80 HU and a window level (center) of 40 HU
Finally, some abnormal cases from our testing datasets were presented. Note that all abnormal cases were not included in the training and validation datasets. Figure 8 shows the DECT images from one patient who underwent cranioplasty. Figure 9 shows the DECT images from one patient who had the presence of intracranial air. Figure 10 shows the DECT images from one patient with brain hemorrhage. As also shown in Figs. 8, 9, and 10, the SSIM values calculated within the abnormal regions were reported.
Fig. 8.
An example of true (left column) and DL-based (middle column) DECT images, and difference images (right column) between the true and predicted DECT images. The DL-based 80-kV image was generated from the original 80-kV CT image using the 2U model. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. All CT images are displayed in a window width of 1500 HU and a window level (center) of 300 HU. The SSIM value was calculated within the local window (i.e., dash box)
Fig. 9.
An example of true (left column) and DL-based (middle column) DECT images, and difference images (right column) between the true and predicted DECT images. The DL-based 80-kV image was generated from the original 80-kV CT image using the 2U model. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU. The SSIM value was calculated within the local window (i.e., dash box)
Fig. 10.
An example of true (left column) and DL-based (middle column) DECT images, and difference images (right column) between the true and predicted DECT images. The DL-based 80-kV image was generated from the original 80-kV CT image using the 2U model. The DL-based 140-kV image was generated from its corresponding 80-kV CT image using the 1U model with the low-to-high kV conversion. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU. The SSIM value was calculated within the local window (i.e., dash box)
Discussion
In this study, we showed that DL has the potential to predict 140-kV brain CT images from 80-kV brain CT images. Moreover, reducing image noise in 80-kV CT could be achieved by using the proposed 2U model. Our results showed that the DL-based DECT images had improved SNR and high SSIM (> 0.99). More importantly, no significant difference in CT number measurements was observed between the true and DL-based DECT images. These preliminary results indicate that it is feasible to obtain DECT imaging without using a DECT scanner. Only one single-energy CT scan is required. Our results also showed that the VMIs derived from the proposed DL-based DECT images had lower image noise than those derived from the true DECT images (Figs. 6 and 7). The reduced image noise may increase the diagnostic value of VMIs [21, 22]. Other DECT applications including electron density and effective atomic number will be investigated using the proposed DL-based DECT images.
Although the results of our study were consistent with those of one recent study that showed the feasibility of using DL to produce DECT images [15], we proposed a different DL-based approach for generating DECT images. First, we trained a CNN model that directly learns a non-linear mapping function to convert a low-kV CT image to its corresponding high-kV CT image. The previous study proposed a CNN model to learn the differences between the low-kV and high-kV CT images [15]. Second, we proposed a double U-Net architecture to improve the quality of original low-kV CT images. We do not need to train a CNN model for denoising DECT images. Additionally, we showed that the low-to-high kV conversion was better than the high-to-low kV conversion. One possible reason is that attenuation differences between tissues are relatively small at high kV compared to low kV. Small attenuation differences in the presence of noise interference make it difficult to provide reliable mapping. Finally, our low-kV CT images were acquired at 80 kV instead of 100 kV [15]. We implemented the DL-based method using 80-kV CT images simply because DECT imaging with 80/Sn140 kV has larger spectrum separation than that with 100/Sn140 kV. The greater the spectrum separation, the better the DE contrast [23].
Despite some encouraging results, the present study has some limitations. First, the CNN model trained with brain datasets may not apply to other body sections. Similarly, the CNN model trained with non-contrast DECT images may not produce optimal results for contrast-enhanced DECT images. Moreover, the trained CNN model may not be robust to DECT images obtained with different scanners or reconstructed with different algorithms and reconstruction kernels. These effects will be the subject of further investigation. Second, we did not evaluate the diagnostic accuracy of the proposed DL-based DECT images. Further validation of the proposed method on abnormal cases is necessary. Third, the U-Net architecture that includes down-scaling (i.e., maximum pooling) and up-scaling operations may not completely preserve object details (e.g., cerebral sulci and fissures). As a result, a loss of spatial resolution is unavoidable. However, compared to the true DECT images denoised using a 2D Gaussian filter (Figs. 11 and 12), the proposed DL-based DECT images did not have a severe degradation in the spatial resolution. Moreover, a similar degradation in the spatial resolution was observed when comparing the proposed DL-based DECT images with the true DECT images denoised using a block-matching and three-dimensional filtering (Figs. 13 and 14) [24]. These findings were consistent with the results of the k‐space (i.e., frequency) energy density [25] of the true and DL-based DECT images as a function of the distance to the k‐space center (Fig. 15). Using unscaled CNN architectures may reduce the loss of image details but would increase the number of training parameters. The increased training parameters increase the memory requirement and computation time for training and predicting. It would also be worth investigating whether the performance of the proposed DL-based method can be further improved by using other powerful deep CNN models such as generative adversarial networks [26].
Fig. 11.
An example of true (left column) and filtered (middle column) DECT images, and difference images (right column) between the true and filtered DECT images. The filtered DECT images were the true DECT images with a 2D Gaussian filter (GF) and sigma = 1. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Fig. 12.
An example of true (left column) and filtered (middle column) DECT images, and difference images (right column) between the true and filtered DECT images. The filtered DECT images were the true DECT images with a 2D Gaussian filter (GF) and sigma = 1. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Fig. 13.
An example of true (left column) and filtered (middle column) DECT images, and difference images (right column) between the true and filtered DECT images. The filtered DECT images were the true DECT images with a block-matching and three-dimensional filtering (BM3D) and sigma = 20. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Fig. 14.
An example of true (left column) and filtered (middle column) DECT images, and difference images (right column) between the true and filtered DECT images. The filtered DECT images were the true DECT images with a block-matching and three-dimensional filtering (BM3D) and sigma = 20. All CT images are displayed in a window width of 80 HU and a window level (center) of 40 HU
Fig. 15.
k‐Space energy density of true and DL-based a 80-kV and b 140-kV CT images (Fig. 5) as a function of the distance to the k‐space center. The results indicated that the spatial resolution of DL-based DECT images was slightly degraded as compared to that of true DECT images. However, the loss of spatial resolution observed in the DL-based DECT images was less severe than that observed in the true DECT images with a 2D Gaussian filter (GF) and sigma = 1. Moreover, a similar degradation in the spatial resolution was observed when comparing the proposed DL-based DECT images with the true DECT images denoised using a block-matching and three-dimensional filtering (BM3D) and sigma = 20
Conclusions
In this study, we proposed a DL-based method to perform a conversion between two different kV CT images. The results obtained from clinical DECT data showed that the low-to-high kV conversion was better than the high-to-low kV conversion. In addition, the DL-based DECT images had improved SNR, but at the expense of a slight loss in the spatial resolution. For the mean CT number, no significant difference was found between the true and the DL-based DECT images. The DL-based DECT images with improved SNR could reduce noise in the DECT-derived VMIs. Our study indicates that it is feasible to generate brain DECT images based on single-energy brain CT images using the proposed DL-based method.
Appendix
Funding
This work was supported by MOST 109-2221-E-002-049 from Ministry of Science Technology, Taiwan.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Disclosure
All authors have approved the manuscript for submission. The content of the manuscript has not been published, or submitted for publication elsewhere.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, Grasruck M, Stierstorfer K, Krauss B, Raupach R, Primak AN, Küttner A, Achenbach S, Becker C, Kopp A, Ohnesorge BM. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–268. doi: 10.1007/s00330-005-2919-2. [DOI] [PubMed] [Google Scholar]
- 2.McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276:637–653. doi: 10.1148/radiol.2015142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goo HW, Goo JM. Dual-energy CT: new horizon in medical imaging. Korean J Radiol. 2017;18:555. doi: 10.3348/kjr.2017.18.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Elmpt W, Landry G, Das M, Verhaegen F. Dual energy CT in radiotherapy: Current applications and future outlook. Radiother Oncol. 2016;119:137–144. doi: 10.1016/j.radonc.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Naruto N, Itoh T, Noguchi K. Dual energy computed tomography for the head. Jpn J Radiol. 2018;36:69–80. doi: 10.1007/s11604-017-0701-4. [DOI] [PubMed] [Google Scholar]
- 6.Freiherr G: Do Community Hospitals Need Dual-Energy CT? HitachimedCom 2–14, 2016
- 7.Henzler T, Fink C, Schoenberg SO, Schoepf UJ. Dual-energy CT: radiation dose aspects. Am J Roentgenol. 2012;199:S16–25. doi: 10.2214/AJR.12.9210. [DOI] [PubMed] [Google Scholar]
- 8.Padole A, Ali Khawaja RD, Kalra MK, Singh S. CT radiation dose and iterative reconstruction techniques. Am J Roentgenol. 2015;204:W384–392. doi: 10.2214/AJR.14.13241. [DOI] [PubMed] [Google Scholar]
- 9.Lee T-Y, Chhem RK. Impact of new technologies on dose reduction in CT. Eur J Radiol. 2010;76:28–35. doi: 10.1016/j.ejrad.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Shen D, Wu G, Suk H-I. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;19:221–248. doi: 10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier A, Syben C, Lasser T, Riess C. A gentle introduction to deep learning in medical image processing. Z Med Phys. 2019;29:86–101. doi: 10.1016/j.zemedi.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Jang H, Kijowski R, Bradshaw T, McMillan AB. Deep learning MR imaging–based attenuation correction for PET/MR imaging. Radiology. 2018;286:676–684. doi: 10.1148/radiol.2017170700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leynes AP, Yang J, Wiesinger F, Kaushik SS, Shanbhag DD, Seo Y, Hope TA, Larson PEZ. Zero-echo-time and Dixon deep pseudo-CT (ZeDD CT): direct generation of pseudo-CT images for pelvic PET/MRI attenuation correction using deep convolutional neural networks with multiparametric MRI. J Nucl Med. 2018;59:852–858. doi: 10.2967/jnumed.117.198051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Wang Y, Li S, He J, Zeng D, Bian Z, Ma J: Pseudo dual energy CT imaging using deep learning-based framework: basic material estimation. Med. Imaging 2018 Phys. Med. Imaging, vol. 10573, SPIE; 2018, p. 172
- 15.Zhao W, Lv T, Gao P, Shen L, Dai X, Cheng K, Jia M, Chen Y, Xing L: A deep learning approach for dual-energy CT imaging using a single-energy CT data. 15th Int. Meet. Fully Three-Dimensional Image Reconstr. Radiol. Nucl. Med., vol. 11072, SPIE; 2019, p. 27
- 16.Wang Z, Bovik A C, Sheikh H R, Simoncelli E P: Image quality assessment: from error visibility to structural similarity. IEEE Trans Image Process 13:600–612, 2004 [DOI] [PubMed]
- 17.Ronneberger O, Fischer P, Brox T: U-Net: Convolutional networks for biomedical image segmentation. Proc Int Conf Med Image Comput Comput Interv 234‐241, 2015
- 18.Bishop C M: Training with noise is equivalent to Tikhonov regularization. Neural Comput 7:108–116, 1995
- 19.Rifai S, Glorot X, Bengio Y, Vincent P: Adding noise to the input of a model trained with a regularized objective. ArXiv 1104.3250, 2011
- 20.Breiman L: Bias, variance, and arcing classifiers. Tech Rep 460 Dep Stat Univ California, Berkeley, CA 1996
- 21.Li J-H, Tsai C-Y, Huang H-M. Assessment of hepatic fatty infiltration using dual-energy computed tomography: a phantom study. Physiol Meas. 2014;35:597–606. doi: 10.1088/0967-3334/35/4/597. [DOI] [PubMed] [Google Scholar]
- 22.Böning G, Feldhaus F, Adelt S, Kahn J, Fehrenbach U, Streitparth F. Clinical routine use of virtual monochromatic datasets based on spectral CT in patients with hypervascularized abdominal tumors - evaluation of effectiveness and efficiency. Acta Radiol. 2019;60:425–432. doi: 10.1177/0284185118786077. [DOI] [PubMed] [Google Scholar]
- 23.Primak A N, Giraldo J C R, Eusemann C D, Schmidt B, Kantor B, Fletcher J G, McCollough C H: Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in vivo. AJR Am J Roentgenol 195:1164–1174, 2010 [DOI] [PMC free article] [PubMed]
- 24.Dabov K, Foi A, Katkovnik V, Egiazarian K. Image denoising by sparse 3D transform-domain collaborative filtering. IEEE Trans on Image Process. 2007;16:2080–2095. doi: 10.1109/TIP.2007.901238. [DOI] [PubMed] [Google Scholar]
- 25.Veraart J, Fieremans E, Jelescu IO, Knoll F, Novikov DS. Gibbs ringing in diffusion MRI. Magn Reso Med. 2016;76:301–314. doi: 10.1002/mrm.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodfellow I J, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y: Generative adversarial networks. Adv Neural Inf Process Syst 2672–2680, 2014