Abstract
The innate immune system is dysregulated in depression; however, less is known about the longitudinal associations of depression and inflammatory biomarkers. We investigated the prospective associations of depression and inflammatory biomarkers [interleukin-6 (IL-6), Tumor Necrosis Factor–Alpha (TNF-α), and C-reactive protein (CRP)] in community samples, both unadjusted and adjusted for covariates. The review, registered with PROSPERO, searched for published and unpublished studies via MEDLINE/PsycINFO/PsycARTICLES/EMBASE/Proquest Dissertation. Standardized Fisher transformations of the correlation/beta coefficients, both unadjusted and adjusted for covariates, were extracted from studies examining the prospective associations of depression and inflammatory biomarkers. Systematic review conducted in January, 2019 included 38 studies representing 58,256 participants, with up to 27 studies included in random-effects meta-analysis. Higher CRP/IL-6 were associated with future depressive symptoms, and higher depressive symptoms were associated with higher future CRP/IL-6 in both unadjusted and adjusted analyses – this is the first meta-analysis reporting an adjusted association of IL-6 with future depression. The adjusted prospective associations of depression with CRP/CRP with depression were substantially attenuated and small in magnitude. No significant associations were observed for TNF-α. No conclusive results were observed in studies of clinical depression. Meta-regression indicated that the association of depression and future CRP was larger in older samples and in studies not controlling for possible infection. Small, prospective associations of depression and inflammatory biomarkers are observed in both directions, particularly for IL-6; however, the strength and importance of this relationship is likely obscured by the heterogeneity in depression and profound study/methodological differences. Implications for future studies are discussed.
Introduction
Depression is a highly prevalent psychiatric disorder that typically occurs early in life, follows a relapsing, remitting course and is associated with a severe disease burden (1–3). Although the cardinal symptoms of depression are low mood and anhedonia (4), depression is accompanied by a wide range of symptoms, including disrupted appetite, sleep, and cognitive dysfunction. In fact, 227 unique symptom profiles exist by which an individual can meet criteria for a depression diagnosis (5), and in a study of 2,154 depressed individuals, 137 unique symptom profiles were observed (6). Rather than capturing a discrete disease process, it is likely that multiple subtypes of depression exist, each characterized by partially distinct etiologies, risk factors, and disruptions to neurobiological systems (7), which may explain why one in three depressed patients do not respond to conventional treatments (8). Since the early 1990s, accumulating evidence suggests that dysregulated immune functioning may characterize one subtype of depression (9, 10).
There is considerable evidence that both ‘arms’ of the immune system – the innate immune system’s rapid and non-specific response to antigens and the adaptive immune system’s slower, antibody-generating, specific response - are dysregulated in depression [see Irwin and Miller (11)]. There also is a better understanding of the humoral, neural, and cellular pathways by which peripheral immune activation can lead to the type of disruptions in neurotransmitter metabolism, neural plasticity, neuroendocrine function, and neural circuitry that are commonly observed in depression (12–14). In particular, there is strong evidence that activation of the innate immune system leads to “sickness behaviors” (e.g., anhedonia, fatigue) that are characteristic of depression (15). Inflammatory biomarkers that index the innate immune response [interleukin-6 (IL-6), Tumor Necrosis Factor – Alpha (TNF-α), and C-reactive protein (CRP)] are consistently elevated in clinical (16) and community (17) samples of depressed individuals. Moreover, when the innate immune system is activated via administration of an endogenous cytokine, interferon-α, 30-50% of medical patients develop clinical depression (18), unless prophylactically treated with an antidepressant (13, 19). Less powerful activation of the innate immune response through administration of a purified endotoxin or vaccination also reliably induces depressive symptoms (15, 20).
It has not been completely established, however, whether inflammation is a cause (15, 21), consequence (22), or correlate of depression (13). Moreover, the observed associations of inflammation and depression may be some combination of the above relationships (e.g., bidirectional) or caused by a common underlying risk factor (e.g., genotype, stress, adiposity, diet) (23, 24). Despite being well-positioned to identify the temporal relationship between inflammation and depression, longitudinal studies thus far report an inconsistent pattern of results with inflammation predicting depression (25, 26), depression predicting inflammation (27, 28), bidirectional associations (29, 30), and null results (31, 32). To date, no comprehensive meta-analysis has examined the temporal relationships between inflammatory biomarkers and depression across the lifespan, although prior work has been conducted either (i) using a very small number of studies (33) or (ii) in elderly samples (34). Equally importantly, despite widespread knowledge of the many covariates that can influence circulating levels of inflammatory biomarkers (33) and the profound differences observed across studies in (i) sample characteristics (size, age, gender, socioeconomic status), (ii) how inflammation and depression are measured (fasting versus non-fasting/finger prick versus venipuncture blood draws; depression diagnosis versus depressive symptoms), (iii) study design (time to follow-up, inclusion/exclusion criteria), and (iv) analytic approach (e.g., handling of outliers, exclusion of acute illness, statistical approach), no study has systematically investigated how these characteristics may modulate the associations of inflammation and depression.
This systematic review and meta-analysis qualitatively and quantitatively synthesize the ever increasing number of longitudinal studies investigating inflammation and depression and investigate sources of heterogeneity in effect sizes via meta-regression. We focus on three inflammatory biomarkers (IL-6/TNF-α/CRP) that closely index activation of the innate immune system because the innate immune system is hypothesized to play a causal role in depression and these biomarkers are the most commonly reported inflammatory biomarkers used in observational studies. Examining the longitudinal associations of inflammatory biomarkers and depression can contribute substantially to a theoretical understanding of the role played by the innate immune system in depression. Given the general pattern of small, bidirectional associations between depression and inflammatory biomarkers observed in prior studies, we hypothesize that inflammatory biomarkers will be associated with elevated future depression and that depression will be associated with elevated future inflammatory biomarkers.
Methods
Data Sources
The systematic review and meta-analysis followed PRISMA/MOOSE guidelines and was registered in PROSPERO (CRD42018112132) in November, 2018 prior to data collection. We searched databases (MEDLINE/PsycINFO/PsycARTICLES/EMBASE/Proquest Dissertation) in January, 2019 for theses/articles written in English since January, 1970 that indicated prospective data for depression and/or inflammatory biomarkers using the following search terms: (depress* OR mood OR affect) and (inflamm* OR interleukin OR IL-1 OR IL-6 OR Tumor Necrosis Factor OR TNF OR C-Reactive OR CRP OR cytokine) and (longitudinal OR prospective OR follow-up OR followup).
Study Selection
Two authors (NMG/TN) iteratively reviewed titles, then abstracts, before conducting a full text review of potentially eligible studies. At each stage, decisions between raters were compared and discrepancies resolved by consensus. Articles were included if: depression was measured via standardized self-report questionnaires/diagnostic assessment; inflammation was assessed via saliva, blood, or lumbar puncture to deliver estimates of CRP, IL-6, or TNF-α; and samples were community-based, population-representative and longitudinal in design. We excluded manuscripts if: participants were recruited on the basis of a medical/psychiatric diagnosis; data were based on non-human studies; outcome data were unavailable and could not be supplied by the authors; and results were reported in conferences, abstracts, editorials and/or letters only. When multiple manuscripts were based on a single cohort, the manuscript with the largest sample was included. Bibliographies were searched for relevant manuscripts/conference proceedings and included when appropriate. Community samples not recruited randomly were included when broadly representative of the community (e.g., convenience sample included if using a diverse sample of employees, population-based sample over-sampled minority group(s), broadly representative but risk-enriched sample). Study quality was appraised using a modification of the Newcastle Ottawa scale for cross sectional studies (35) and based on a prior meta-analysis (34) – see Supplementary Table 1 for quality assessment scores and Supplementary Table 2 for quality assessment protocol.
Data Extraction
Two authors independently extracted data. Outcomes of interest were: adjusted and unadjusted prospective linear associations (correlation/standardized beta coefficients) between depressive symptoms (A) and inflammatory biomarkers (B). Where possible, we extracted both the unadjusted/least adjusted measure of association between A and B and the most adjusted measure of association for A and B. If the appropriate statistics were not reported in the manuscript, but could be extrapolated from data presented in the manuscript (e.g., standardizing coefficients), this was undertaken. When data were not reported, manuscript authors were contacted twice via email requesting data. The following information also was extracted: author; year of publication; country; analytic sample size; baseline age; sex; race; follow-up duration; depression assessment method; inflammatory biomarker assessment method; CRP values ≥10 removed from analyses; fasting status; time of blood draw; covariates included in statistical models; exclusion criteria, and kit used to assay inflammatory biomarkers.
Statistical Analysis
Meta-analyses were conducted in R (36) using ‘metafor’ (37). Random effects models, selected to address known methodological/analytical heterogeneity, examined the unadjusted and adjusted associations of inflammatory biomarkers (CRP/IL-6/TNF-α) with subsequent depression and the unadjusted and adjusted associations of depression with subsequent inflammatory biomarkers (CRP/IL-6/TNF-α). The DerSimonian-Laird estimator was used to estimate the variance of the distribution of true effect sizes and Fisher’s z-transformation was applied to correlation coefficients and reverse transformed for forest plots. Statistical heterogeneity was assessed using Cochrane Q and the inconsistency index (I2), which tests whether there is significant variability in the magnitude of effect sizes across studies. Subgroup analyses used a fixed effects model to examine whether effect sizes differed in studies controlling or not controlling for possible acute infection (effect sizes were estimated separately in subgroups utilizing a random-effects-model). Meta-regression was performed when the number of studies was greater than 10. Publication bias was examined visually using a funnel plot and statistically with Egger’s regression intercept test. Sensitivity analyses replicated meta-analyses in: strictly defined community samples, in studies measuring inflammatory biomarkers in venous blood, when examining change in depression/inflammation, and in high quality studies.
Results
Study Selection
From 11,176 identified articles, 73 were selected for full-text review and 38 were included for systematic review (24, 25, 27–32, 38–65) and 27 for meta-analysis (see Figure 1 for complete information). Details on the excluded 45 studies can be found in Supplementary Table 3. Study characteristics (summarized in Table 1) indicate substantial variability in sample characteristics and study methodologies – see supplementary information (‘Study Characteristics’) for a complete discussion.
Figure 1.
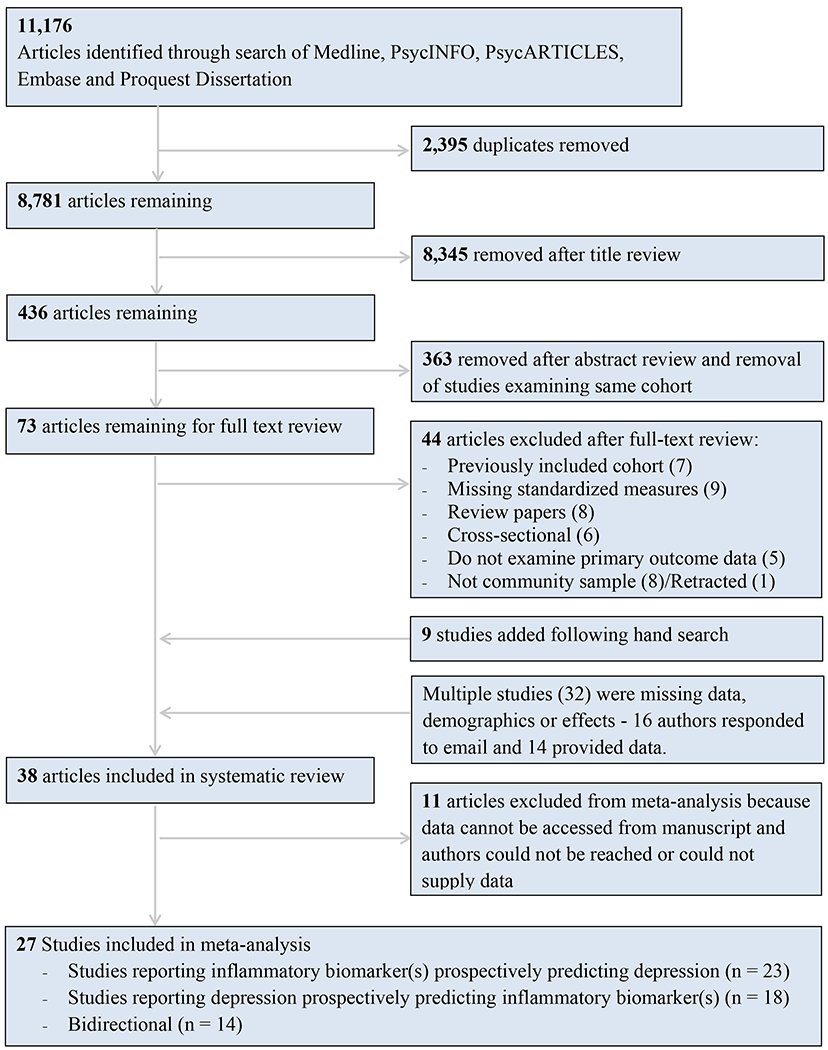
Flowchart detailing process by which studies were included in systematic review and meta-analysis.
Table 1.
Study characteristics for all studies examining prospective associations of inflammatory biomarkers and depression included in systematic review.
| Author | Year | Country | Analytic Sample | Baseline Age | % Female | % White | Follow-up (Years)a | In Meta-analysis? | Depression assessed via interview? | Biomarkers Assessed (Method) | CRP>10b? | Covariates? | Exclusion Criteriac | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Khandaker et al. | 2014 | UK | 4415 | 9 | 48.0 | 98 | 8.8 | Yes | No | Blood | No | a , b, c, d, e, f, s | d | 7 |
| Adriaensen et al. | 2014 | Belgium | 303 | 84.3 | 62.7 | nr | 1.7 | No | No | Blood | No | a, b, e, f, n, o, s | a | 5 |
| Deverts et al. | 2010 | USA | 2544 | 40.2 | 55.0 | 58.2 | 5.0 | Yes | No | Blood | Yes | a, b, c, d, e, f, g, h, i, j, k, l, m, n, o, s | d | 6 |
| Elovainio et al. | 2006 | Finland | 1201 | 22.5 | 59.4 | nr | 9.0 | Yes | No | Blood | No | a, b, d, e, h, i, j, k, l, s | a | 5 |
| Simanek et al. | 2014 | USA | 263 | 54 | 57.4 | 13.7 | 1.0 | Yes | No | Blood | No | h, i, o, p | c | 5 |
| Au et al. | 2015 | UK | 3397 | 64.6 | 56 | 99 | 4.0 | Yes | No | Blood | No | a, b, d, e, f, h, j, k, l, n, o, s | none | 5 |
| Copeland et al. | 2012 | USA | 1334 | 14.2 | 49 | 89.7 | 1.0 | Yes | Yes | Finger | Yes | a, b, c, d, e, f, g, h, i, o, s | none | 5 |
| Brown et al. | 2016 | USA | 3075 | 73.6 | 52 | 58.3 | 10.0 | No | No | Blood | No CRP | a, b, e, n, o, q | d | 4 |
| Hiles et al. | 2015 | Australia | 1410 | 65.6 | 50 | nr | 4.5 | Yes | No | Blood | Yes | a, b, e, f, h, i, j, n, s | a, b, d | 5 |
| Milaneschi et al. | 2009 | Italy | 550 | 75 | 56 | nr | 3.0 | No | No | Blood | No | c | 6 | |
| de Mello Franco et al. | 2017 | Brazil | 1508 | 41.31 | 19 | nr | 2.2 | No | No | Blood | Yes | a, b, e, h, j, n | a, d | 5 |
| Das et al. | 2017 | USA | 2216 | 67.11 | 51 | 80.1 | 5.0 | Yes | No | Finger | Yes* | a, b, c, d, e, f, g, n, o | none | 6 |
| Stewart et al. | 2009 | USA | 263 | 61 | 52 | 86.7 | 6.3 | Yes | No | Blood | Yes | a, b, c, d, e, f, g, h, i, j, k, l, m, n, s | a, b | 6 |
| Kern et al. | 2014 | Sweden | 86 | 72.5 | 100 | nr | 16.5 | No | Yes | CSF | No CRP | a, e, h | a | 4 |
| Zalli et al. | 2016 | Holland | 656 | 73 | 60 | nr | 5.0 | Yes | No | Blood | No | a, b, e, f, h, n, q | b, d | 6 |
| Simanek et al. | 2019 | USA | 771 | 69.4 | 55 | nr | 1.5 | No | No | Blood2 | No | a, b, c, d, e, g, h, i, o | b, c | 6 |
| Matthews et al. | 2010 | USA | 1714 | 46.2 | 100 | 51 | 1.0 | Yes | No | Blood | Yes | a, c, d, e, f, g, h, j, n, o, s | a, b, d | 6 |
| Baune et al. | 2012 | Australia | 722 | 78.8 | 55 | nr | 2.0 | Yes | No | Blood | No | a, b, d, e, h, n, o, q, s | a, c | 5 |
| Duivis et al. | 2015 | Holland | 1166 | 11.1 | 54 | nr | 3.0 | Yes | No | Blood | No* | a, b, d, e, h, j | d | 6 |
| Jonker et al. | 2017 | Holland | 1084 | 16.2 | 54 | nr | 2.7 | Yes | Yes | Blood | Yes | , b, c, d, e, h, i, s | c, d | 6 |
| Casaletto et al. | 2018 | USA | 165 | 72.6 | 49 | nr | 1.9 | Yes | No | Blood | No* | a, b, d, s | c, d | 4 |
| Kim et al. | 2018 | Korea | 610 | 72.8 | 59 | nr | 2.4 | Yes | Yes | Blood | No CRP | , b, f, g, j, n, s | c | 5 |
| Luciano et al. | 2012 | Scotland | 456 | 69.5 | 50 | nr | 3.0 | Yes | No | Blood | No* | a, b, e, j, o | a | 6 |
| Luukinen et al. | 2010 | Finland | 404 | nr | 61 | nr | 2.5 | No | No | Blood | Yes | c, d | 5 | |
| Matsushima et al. | 2015 | Japan | 64 | 72.05 | 74 | nr | 3.0 | Yes | No | Blood | No | b, c | 3 | |
| Nelson et al. | 2018 | Australia | 63 | 14.84 | 41 | 77.8 | 0.6 | Yes | No | Saliva | No | a, b, e, h, q, s | b, c | 3 |
| Niles et al. | 2018 | USA | 13375 | 67.79 | 60 | 81.61 | 4.0 | Yes | No | Finger | Yes | a, d, e, f, g, h, i, j, n, s | d | 5 |
| Pasco et al. | 2010 | Australia | 644 | 47 | 100 | nr | 10.0 | No | Yes | Blood | No | a, e, h, j, n, o | none | 6 |
| Tully et al. | 2015 | Australia | 1167 | 54.13 | 0 | nr | 4.9 | Yes | No | Blood | No | a, e, h, j, n | a, b, c | 5 |
| Walss-Bass et al. | 2018 | USA | 195 | 13.37 | 54 | 58 | 0.9 | No | No | Blood | No* | , | c, d | 3 |
| Oddy et al. | 2018 | Australia | 843 | 14 | 51 | 88 | 3.0 | Yes | No | Blood | Yes | a, c, d, e, h, i, j, s | d | 5 |
| Jones et al. | 2017 | USA | 7477 | 63.47 | 100 | 53.8 | 15.4 | Yes | No | Blood | No* | a, c, d, e, f, g, h, i, j, o, s | d | 5 |
| Chiang et al. | 2019 | USA | 187 | 16.4 | 57 | nr | 2.0 | Yes | No | Finger | Yes | a, b, d, e, h, i, n, o | d | 5 |
| van den Biggelaar et al. | 2007 | Holland | 267 | 85 | 63 | nr | 1.0 | Yes | No | Blood | No | e, f, h, n, q, s | b, c | 6 |
| Glaus et al. | 2018 | Switzerland | 2580 | 43.94 | 61 | 96.39 | 5.8 | No | Yes | Blood | Yes | a, b, c, d, e, h, j, n, o, s | d | 7 |
| Caserta et al. | 2011 | USA | 141 | 9.31 | 46 | 47 | 0.5 | No | No | Blood | No CRP | a, b, d, e, s | a | 3 |
| Mac Giollabhui et al. | 2019 | USA | 288 | 16.34 | 51 | 41 | 1.2 | Yes | No | Blood | Yes | e, f, g, q, s | a, c, d | 6 |
| Forti et al. | 2010 | Italy | 652 | 74.54 | 55 | nr | 3.9 | Yes | No | Blood | No | a, b, d, e, n, o, q, s | c | 5 |
= If more than two follow-up points of unequal length were included in the study, the average was calculated for the purpose of meta-regression.
= Were CRP values greater than or equal to 10mg/L excluded. Where alternative cut-offs were used they are highlighted with “*” and marked “Yes” if they use a more conservative cut-off and “No” if they use a more liberal cut-off.
= Represents a selection of the most important and common exclusion criteria used across studies.
= Median age reported.
= 32.4% assessed via venipuncture.
nr = not reported; Finger = Blood drawn via finger prick; CSF = cerebrospinal fluid;
Covariates: a = age, b = sex, c = race, d = socio-economic status, e = index of body mass/body fat, f = baseline depression, g = baseline inflammatory biomarker, h = smoking status, i = alcohol use, j = physical activity, k = triglycerides, l = cholesterol, m = glucose, n = medical diagnosis, o = medication use, p = stress, q = cognitive functioning, r = other
Exclusion criteria (non-exhaustive list): a = medical diagnosis, b = medication use, c = psychiatric diagnosis, d = acute infection.
Inflammatory Biomarkers and Subsequent Depression
There were 32 studies that examined the association between at least one inflammatory biomarker (CRP: 25; IL-6: 17; TNF-α: 9) and subsequent depression. Baseline CRP was associated with future depressive symptoms in 52% (k=12) of all studies, which reduced to 20% (k=5) following adjustment for covariates. Baseline IL-6 was associated with future depressive symptoms in 25% of studies (k=4), which reduced to 13% (k=2) when adjusting for covariates. Baseline TNF-α was associated significantly with future depressive symptoms in 0% of studies, which increased to 11% (k=1) when adjusting for covariates.
Meta-analysis:
Random effects meta-analysis reported a significant association of baseline unadjusted CRP [f(r)=.058, p < .0001, k=18] and adjusted CRP [(f(r)=.022, p=.002, k=19)] with future depression – see Figure 2. Baseline unadjusted IL-6 [f(r)=.053, p=.002, k=11] and adjusted IL-6 [f(r)=.043, p < .0001, k=10] were associated significantly with future depression – see Figure 3. Neither unadjusted baseline TNF-α [f(r)=-.008, p=.83, k=5] nor adjusted TNF-α, [f(r)=.013, p=.64, k=5] were associated with future depression.
Figure 2. Forest Plots of Baseline CRP and Future Depressive Symptoms.
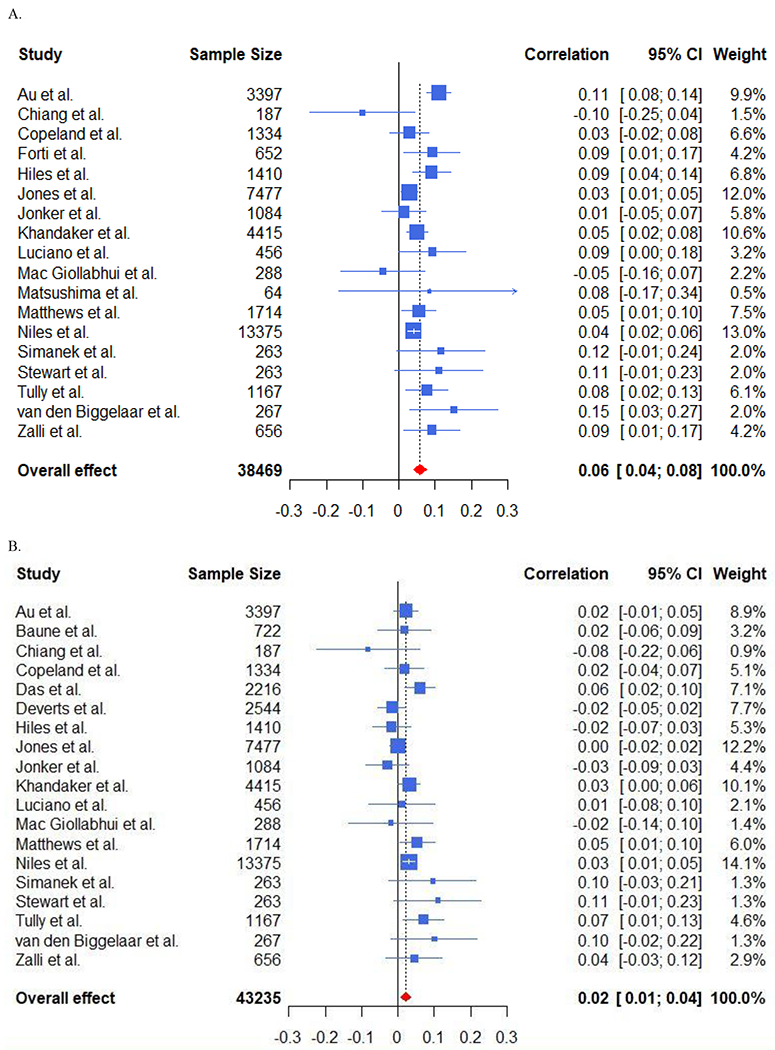
A. Forest Plot Displaying Unadjusted Associations of Baseline CRP and Future Depressive Symptoms.
B. Forest Plot Displaying Adjusted Associations of Baseline CRP and Future Depressive Symptoms.
Figure 3. Forest Plots of Baseline IL-6 and Future Depressive Symptoms.
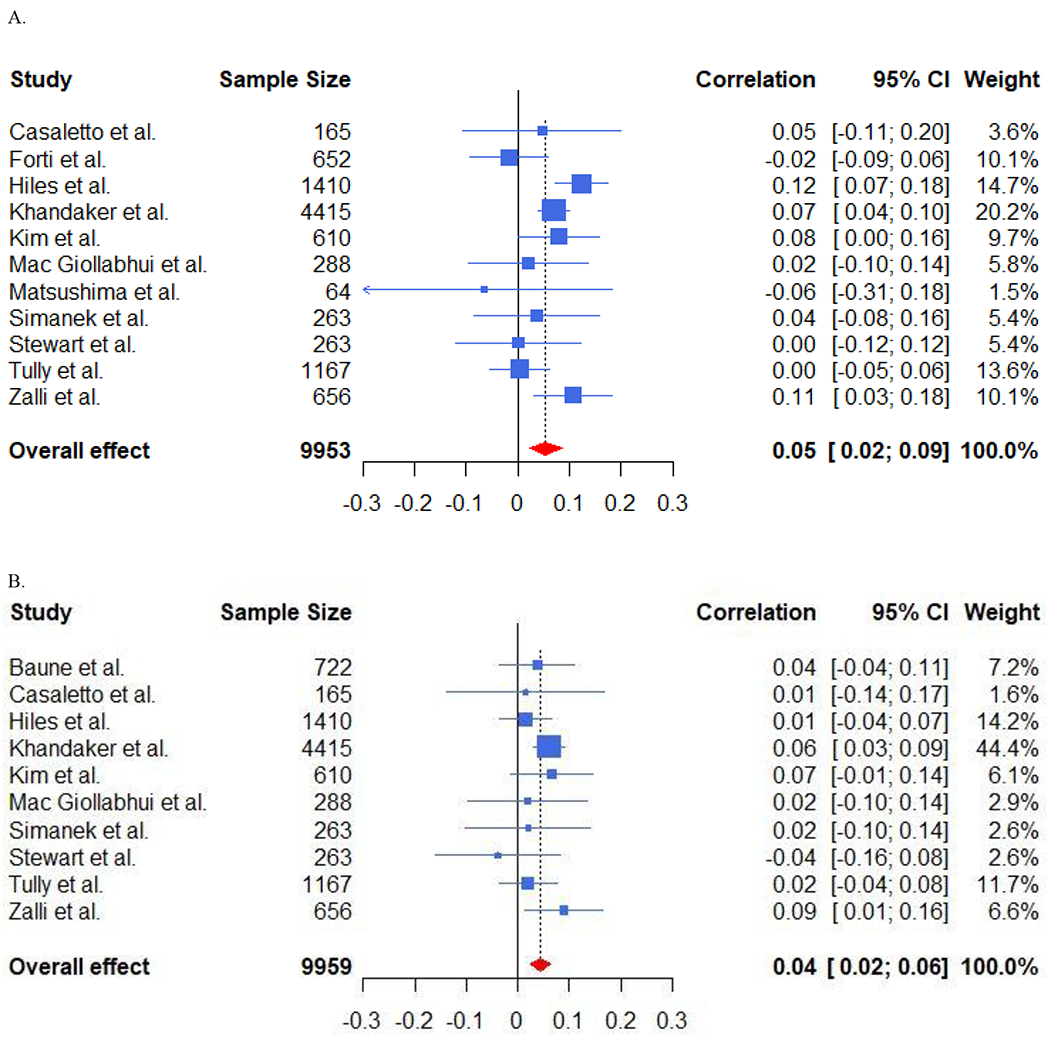
A. Forest Plot Displaying Unadjusted Associations of Baseline IL-6 and Future Depressive Symptoms.
B. Forest Plot Displaying Adjusted Associations of Baseline IL-6 and Future Depressive Symptoms.
Meta-regression:
The association between baseline CRP and future depression was greater in older samples (SD=25.48 years; b=.026, p=.016, k=18), but not for IL-6 (p=.99). Likewise, the association of baseline CRP (but not IL-6) and future depression was significantly greater in studies that did not remove participants with extreme CRP values (CRP: b=.032, p=.049, k=18). The size of the association of baseline CRP with future depression was smaller when baseline inflammation was controlled for (difference = 0.038, p = 0.007, k = 18), but not for IL-6 (p = 0.36). Neither sex, race, nor time to follow-up predicted the association of CRP/IL-6 with future depression.
Heterogeneity/Publication bias:
There was substantial heterogeneity in the association of inflammatory biomarkers and future depression, which typically decreased in adjusted associations. There was no indication of publication bias for CRP, IL-6 or TNF-α – complete details provided as supplementary information (‘Heterogeneity and Publication Bias’) and in Supplementary Figure 1.
Sensitivity analyses:
Results did not differ when analyses were replicated in: community samples alone, in studies using venous blood draws, when controlling for baseline depression, and in high quality studies. An exception to this was that higher TNF-α predicted higher depressive symptoms in studies controlling for baseline depression, [f(r)=0.061, p=.049, k=3]. See Supplementary Table 4 for complete information.
Depression and Subsequent Inflammatory Biomarkers
Twenty-two studies examined the association between depression and at least one future inflammatory biomarker (CRP: 17; IL-6: 8; TNF-α: 5). Baseline depression was associated with future CRP in 65% (k=11) of studies, which reduced to 6% (k=1) after adjusting for confounding factors. Depression was associated with future IL-6 in 38% (k=3) of studies, which increased to 60% (k=3) when adjusting for confounding factors. Baseline depression was not associated with TNF-α in unadjusted (k=5) or adjusted analyses (k=3).
Meta-analysis:
Baseline depression was associated with future CRP in unadjusted [f(r)=.051, p < .0001, k=14] and adjusted analyses [f(r)=.011, p=.038, k=14] and for IL-6 in unadjusted [f(r)=.090, p < .0001, k=6] and adjusted [f(r)=.094, p=.016, k=5] analyses – see Figure 4. Baseline depression was not associated significantly with future TNF-α in either unadjusted [f(r)=.015, p=0.49, k=5] or adjusted [f(r)=0.022, p=.48, k=5] analyses.
Figure 4. Forest Plots of Baseline Depressive Symptoms and Future CRP and Future IL-6.
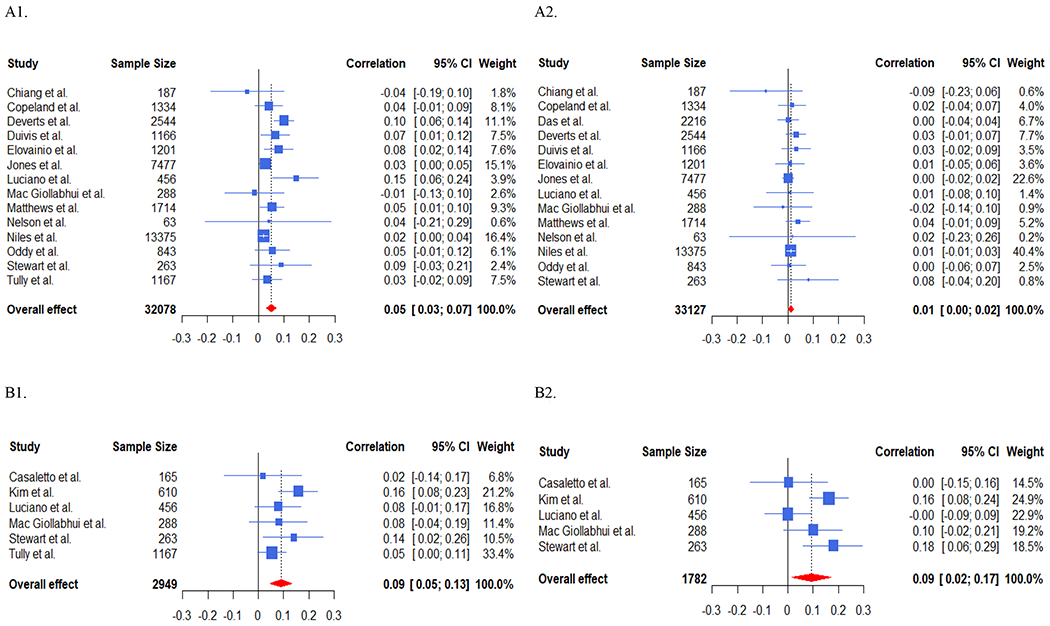
A1. Forest Plot Displaying Unadjusted Associations of Baseline Depressive Symptoms and Future CRP.
A2. Forest Plot Displaying Adjusted Associations of Baseline Depressive Symptoms and Future CRP.
B1. Forest Plot Displaying Unadjusted Associations of Baseline Depressive Symptoms and Future IL-6.
B2. Forest Plot Displaying Adjusted Associations of Baseline Depressive Symptoms and Future IL-6.
Meta-regression:
The unadjusted association of baseline depressive symptoms and future CRP was not dependent on age (p=.733), sex (p=.716), the percentage of the sample who identified as Caucasian (p=.935), time to follow-up (p=.746) or whether extreme CRP values were removed (p=.639). Insufficient studies existed to perform meta-regression for IL-6/TNF-α.
Heterogeneity/Publication bias:
There was substantial heterogeneity in the association of depression and future inflammatory biomarkers. There was no indication of publication bias for CRP, IL-6 or TNF-α. Complete details provided as supplementary information (‘Heterogeneity and Publication Bias’) and in Supplementary Figure 2.
Sensitivity analyses:
Replication of analyses in community samples alone, in studies using venous blood draws, when controlling for baseline inflammation, and in high quality studies did not yield substantially differing results, except that depression did not predict higher CRP assayed in venous blood, [f(r)=0.015, p=.076, k=9]. See Supplementary Table 5 for complete information.
Inflammatory Biomarkers and Clinical Depression
Six studies examined the prospective associations of inflammatory biomarkers and a future depression diagnosis (assessed via clinical interview). Considerable heterogeneity in sample characteristics and methodologies were evident. Although CRP was reported to be associated with first onset of depression in a sample of middle-aged women (56), no association was observed in other studies (27, 49). Similarly, inflammatory cytokines (IL-6/TNF-α) did not predict onset of future clinical depression in other studies (44, 51, 63). Three studies examining whether a depression diagnosis predicted subsequent inflammation found that clinical depression predicted higher future CRP. In a prospective study of 1,420 children followed through adolescence, only the cumulative number of episodes predicted future CRP following adjustment for covariates (27). Incident depression, independent of relevant covariates, was significantly associated with increased IL-1β, IL-6, and IL-8 levels in 732 Koreans aged 65+ assessed at two-year follow-up (51). Finally, current, but not remitted depression, was associated with higher levels of future CRP (but not IL-6 or TNF-α) in a representative sample of 3,118 individuals at 5 year follow-up (63).
Discussion
This is the largest, most comprehensive meta-analysis examining the prospective associations of depressive symptoms and inflammatory biomarkers. Based on systematic review of 38 studies containing 58,256 participants and meta-analysis of 27 studies of 47,999 individuals, both CRP and IL-6 were associated with future depressive symptoms – importantly, this is the first meta-analysis to demonstrate a prospective association of IL-6 with depression when controlling for covariates. Depression also was associated with future CRP and IL-6 in both systematic review and meta-analysis. However, the associations of CRP and depression as well as depression and CRP were substantially attenuated following adjustment for covariates and the size of adjusted associations were small (r ≤ .02). Importantly, the association between depressive symptoms and future CRP was larger in older samples and in studies that did not control for possible acute infection. For studies measuring clinical depression, conclusive associations between depression and inflammatory biomarkers were not observed. There was no evidence for prospective relationships between TNF-α and depression.
Longitudinal studies are crucial in understanding whether inflammatory biomarkers prospectively predict future depression (supporting a causal role), are a correlate of depression, whether they are risk markers of other risk factors (e.g., BMI), or whether inflammation is a consequence of depression (or some combination of the above relationships). Both CRP and IL-6 were associated significantly with future depression in unadjusted and adjusted analyses; these results differ from previous meta-analyses insofar as this is the first study reporting that adjusted IL-6 is associated with future depression (33, 34). This result is unsurprising because IL-6 is arguably the inflammatory biomarker most consistently associated with depression in humans (66) and, instead, likely reflects the greater number of studies examining IL-6 that were included in this review. Moreover, this finding is consistent with a recent Mendelian randomization study which found that the genetic variants responsible for heightened circulating CRP/IL-6 are causally involved in the etiology of depression in a large, population-based sample (67).
There was a small, but consistent, association linking depression with future IL-6. This may reflect the pro-inflammatory effect of multiple behaviors that often accompany depression (e.g., substance use/sedentary behavior/poor diet) (22). However, although statistically significant, depression was not substantially associated with future CRP (r = .01) once relevant covariates were included, a finding observed previously (34). It is particularly notable that the number of studies reporting significant associations for CRP in systematic review dropped from 65% to 6% of studies once covariates were included. In particular, multiple studies identified BMI, a known risk factor for depression reliably associated with CRP (r≈.4), as a confounding (26, 27, 39) or mediating (24, 42) variable. Although the association of CRP and depression may be spurious, inflammatory biomarkers also may play a mechanistic role linking the known association of BMI and depression (24). Thus, care is needed when interpreting the association of depression and inflammatory biomarkers in adjusted analyses.
The longitudinal associations of inflammatory biomarkers and depression observed in this review, particularly in the case of IL-6, suggest that the immune system may be implicated in the etiology of depression; however, caution is needed when interpreting results. First, whereas inflammatory biomarkers are reliably elevated in depression (16), it is less clear whether they remain elevated when depression enters remission. There is some evidence that treatments (pharmacological/cognitive-behavioral) decrease concentrations of inflammatory biomarkers, that decreased concentrations of biomarkers are correlated with reductions in depressive symptoms, and that, following treatment, inflammatory biomarkers do not differ between controls and depressed individuals (68, 69). Thus, it may be that elevated inflammatory biomarkers are not elevated outside of a depressive episode, thereby reducing their detectability in longitudinal studies. Instead, the innate immune system may be ‘primed’ towards an exaggerated inflammatory response, which may only lead to depression in the context of an immune challenge (15). Second, elevated inflammatory biomarkers typically are observed in a subset (25-30%) of depressed individuals, reducing the ability to detect mean level associations (10, 70). It is noteworthy that the association of CRP and future depression was significantly higher in older samples – it may be that dysregulated inflammatory processes indexed by CRP are more important in older populations, in line with existing theories of geriatric depression (71). Finally, there is growing appreciation that elevated levels of IL-6 may not necessarily reflect a state of chronic, low-grade inflammation (72), and thus, may index other pathological processes implicated in depression. More work is needed to understand for whom inflammatory processes play a role in the etiology of depression as well as a more nuanced understanding of the role played by inflammatory biomarkers in immune functioning.
This review highlights the profound methodological differences observed across studies, which may contribute to weak/inconsistent findings. Inflammatory biomarkers fluctuate according to both (largely) fixed (sex/SES/race) and (largely) varying factors [kit used(details on kit used to measure inflammatory biomarkers provided in Supplementary Table 6)/exercise/substance-use/medication-use/medical status] (73). Although some variability may be difficult to avoid, such as worse health status in elderly samples or the known effect of kit selection on concentrations of inflammatory biomarkers (74), greater effort is needed to produce comparable results. For example, there was remarkable variability in how researchers handled CRP values ≥10. CRP values ≥10 may not capture acute illness [see Mac Giollabhui et al. for a review (75)] and implementation of standardized approaches to assess acute illness is needed in addition to greater efforts to follow existing guidelines when controlling for covariates (73). At the very least, reporting sensitivity analyses as supplementary material when different analytic strategies were pursued would improve comparability of studies.
Results should be interpreted within the limitations of this review. A meta-analysis draws conclusions based on the pooled results of comparable studies and it is abundantly clear that studies included in this review differ substantially based on a wide range of factors that influence how we interpret results. Likewise, adjusted estimates were based on variables that clearly differed across studies and were limited to studies reported in English. However, this systematic review provides the first comprehensive picture of longitudinal research linking depression and inflammatory biomarkers, which allows us to evaluate and draw conclusions based on a comprehensive review of the literature and may prove useful in the design of future studies and informing our understanding of the etiology of depression.
There is growing consensus that innate immune system functioning is disrupted in clinical depression; however, a clear understanding of its role in the etiology of depression is lacking. The prospective associations of inflammation with future depression and depression with future inflammation (particularly in the case of IL-6) that were observed in this systematic review suggest that a complex relationship exists. For some, inflammation probably plays a causal role in the development of depression and, conversely, depressogenic behaviors likely lead to increases in future inflammation. Importantly, it is probable that both the strength and importance of this relationship is obscured by the heterogeneity inherent in depression as well as profound differences in study designs. Greater efforts to reduce variability in how we assess inflammatory biomarkers and include/exclude participant data are needed. Although the last two decades have seen enormous progress in identifying the biological mechanisms linking immune system dysregulation with depression, further work, particularly theoretical work, is needed to describe how the immune system relates to depression outside of depressive episodes. In particular, fine-grained longitudinal studies that assess inflammatory biomarkers and depression on multiple occasions over relatively short periods of time prior to first onset of depression are needed to disentangle the temporal relationship between depression and inflammatory biomarkers.
Supplementary Material
Acknowledgements
This research was supported by National Institute of Mental Health Grants MH079369 and MH101168 to Lauren Alloy, National Institute of Mental Health Grants MH118545 and MH096478 to Lauren Ellman and National Research Service Award F31MH118808 as well as an American Psychological Foundation grant to Naoise Mac Giollabhui.
Footnotes
Disclosures
No disclosures or conflicts of interest to report.
References
- 1.Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G, et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med. 2015;45(07):1551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–105. [DOI] [PubMed] [Google Scholar]
- 3.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Pub: Arlington, 2013. [Google Scholar]
- 5.Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry. 2015;56:29–34. [DOI] [PubMed] [Google Scholar]
- 6.Olbert CM, Gala GJ, Tupler LA. Quantifying heterogeneity attributable to polythetic diagnostic criteria: theoretical framework and empirical application. J Abnorm Psychol. 2014;123(2):452. [DOI] [PubMed] [Google Scholar]
- 7.Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015;69(10):597–608. [DOI] [PubMed] [Google Scholar]
- 8.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry. 2006;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 9.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35(4):298–306. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21(4):374–83. [DOI] [PubMed] [Google Scholar]
- 12.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. [DOI] [PubMed] [Google Scholar]
- 17.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–86. [DOI] [PubMed] [Google Scholar]
- 18.Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128–38. [DOI] [PubMed] [Google Scholar]
- 19.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the Prevention of Depression Induced by High-Dose Interferon Alfa. N Engl J Med. 2001;344(13):961–6. [DOI] [PubMed] [Google Scholar]
- 20.Schedlowski M, Engler H, Grigoleit J-S. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain Behav Immun. 2014;35:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Chiu W, Su Y, Su K, Chen P. Recurrence of depressive disorders after interferon-induced depression. Transl Psychiatry. 2017;7(2):e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco Ja, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, et al. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med. 2009;71(2):152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mac Giollabhui N, Swistun D, Murray S, Moriarity DP, Kautz MM, Ellman LM, et al. Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol Med. 2020;50(4):683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au B, Smith KJ, Gariépy G, Schmitz N. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). Int J Geriatr Psychiatry. 2015;30(9):976–84. [DOI] [PubMed] [Google Scholar]
- 27.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression–inflammation relationship. Brain Behav Immun. 2009;23(7):936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deverts DJ, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley M, et al. Depressive symptoms, race, and circulating C-reactive protein: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2010;72(8):734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun. 2010;24(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology. 2014;50:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niles AN, Smirnova M, Lin J, O’Donovan A. Gender differences in longitudinal relationships between depression and anxiety symptoms and inflammation in the health and retirement study. Psychoneuroendocrinology. 2018;95:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–44. [DOI] [PubMed] [Google Scholar]
- 34.Smith KJ, Au B, Ollis L, Schmitz N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp Gerontol. 2018;102:109–32. [DOI] [PubMed] [Google Scholar]
- 35.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute: Ottawa. 2011. [Google Scholar]
- 36.R Core Team. R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing: Vienna; 2019. [Google Scholar]
- 37.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 38.Adriaensen W, Matheï C, Vaes B, Van Pottelbergh G, Wallemacq P, Degryse J-M. Interleukin-6 predicts short-term global functional decline in the oldest old: results from the BELFRAIL study. Age. 2014;36(6):9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elovainio M, Keltikangas-Järvinen L, Pulkki-Råback L, Kivimäki M, Puttonen S, Viikari L, et al. Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychol Med. 2006;36(6):797–805. [DOI] [PubMed] [Google Scholar]
- 40.Au B, Smith KJ, Gariepy G, Schmitz N. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). Int J Geriatr Psychiatry. 2015;30(9):976–84. [DOI] [PubMed] [Google Scholar]
- 41.Brown PJ, Roose SP, Zhang J, Wall M, Rutherford BR, Ayonayon HN, et al. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J. Gerontol. A Biol. Sci. Med. Sci 2016;71(2):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiles SA, Baker AL, de Malmanche T, McEvoy M, Boyle M, Attia J. Unhealthy lifestyle may increase later depression via inflammation in older women but not men. J Psychiatr Res. 2015;63:65–74. [DOI] [PubMed] [Google Scholar]
- 43.Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65(11):973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kern S, Skoog I, Börjesson-Hanson A, Blennow K, Zetterberg H, Östling S, et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav Immun. 2014;41:55–8. [DOI] [PubMed] [Google Scholar]
- 45.Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology (Berl). 2016;233(9):1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simanek AM, Zheng C, Yolken R, Haan M, Aiello AE. A Longitudinal Study of the Association Between Persistent Pathogens and Incident Depression Among Older US Latinos. J. Gerontol: Series A. 2018;74(5):634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, et al. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37(9):1521–30. [DOI] [PubMed] [Google Scholar]
- 48.Duivis HE, Kupper N, Vermunt JK, Penninx BW, Bosch NM, Riese H, et al. Depression trajectories, inflammation, and lifestyle factors in adolescence: The TRacking Adolescents’ Individual Lives Survey. Health Psychol. 2015;34(11):1047. [DOI] [PubMed] [Google Scholar]
- 49.Jonker I, Rosmalen J, Schoevers R. Childhood life events, immune activation and the development of mood and anxiety disorders: the TRAILS study. Transl Psychiatry. 2017;7(5):e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casaletto KB, Staffaroni AM, Elahi F, Fox E, Crittenden PA, You M, et al. Perceived stress is associated with accelerated monocyte/macrophage aging trajectories in clinically normal adults. Am. J. Geriatr. Psychiatry 2018;26(9):952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J-M, Stewart R, Kim J-W, Kang H-J, Bae K-Y, Kim S-W, et al. Changes in pro-inflammatory cytokine levels and late-life depression: a two year population based longitudinal study. Psychoneuroendocrinology. 2018;90:85–91. [DOI] [PubMed] [Google Scholar]
- 52.Luciano M, Mõttus R, Starr JM, McNeill G, Jia X, Craig LC, et al. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun. 2012;26(5):717–20. [DOI] [PubMed] [Google Scholar]
- 53.Luukinen H, Jokelainen J, Hedberg P. The relationships between high-sensitivity C-reactive protein and incident depressed mood among older adults. Scand J Clin Lab Invest. 2010;70(2):75–9. [DOI] [PubMed] [Google Scholar]
- 54.Matsushima J, Kawashima T, Nabeta H, Imamura Y, Watanabe I, Mizoguchi Y, et al. Association of inflammatory biomarkers with depressive symptoms and cognitive decline in a community-dwelling healthy older sample: a 3-year follow-up study. J Affect Disord. 2015;173:9–14. [DOI] [PubMed] [Google Scholar]
- 55.Nelson BW, Byrne ML, Simmons JG, Whittle S, Schwartz OS, O’Brien-Simpson NM, et al. Adolescent temperament dimensions as stable prospective risk and protective factors for salivary C-reactive protein. Br J Health Psychol. 2018;23(1):186–207. [DOI] [PubMed] [Google Scholar]
- 56.Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197(5):372–7. [DOI] [PubMed] [Google Scholar]
- 57.Tully PJ, Baumeister H, Bengel J, Jenkins A, Januszewski A, Martin S, et al. The longitudinal association between inflammation and incident depressive symptoms in men: the effects of hs-CRP are independent of abdominal obesity and metabolic disturbances. Physiol Behav. 2015;139:328–35. [DOI] [PubMed] [Google Scholar]
- 58.Walss-Bass C, Suchting R, Olvera RL, Williamson DE. Inflammatory markers as predictors of depression and anxiety in adolescents: statistical model building with component-wise gradient boosting. J Affect Disord. 2018;234:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oddy WH, Allen KL, Trapp GS, Ambrosini GL, Black LJ, Huang R-C, et al. Dietary patterns, body mass index and inflammation: pathways to depression and mental health problems in adolescents. Brain Behav Immun. 2018;69:428–39. [DOI] [PubMed] [Google Scholar]
- 60.Jones SM, Weitlauf J, Danhauer SC, Qi L, Zaslavsky O, Wassertheil-Smoller S, et al. Prospective data from the Women’s Health Initiative on depressive symptoms, stress, and inflammation. J Health Psychol. 2017;22(4):457–64. [DOI] [PubMed] [Google Scholar]
- 61.Chiang JJ, Park H, Almeida DM, Bower JE, Cole SW, Irwin MR, et al. Psychosocial stress and C-reactive protein from mid-adolescence to young adulthood. Health Psychol. 2019;38(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Biggelaar AH, Gussekloo J, de Craen AJ, Frölich M, Stek ML, van der Mast RC, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42(7):693–701. [DOI] [PubMed] [Google Scholar]
- 63.Glaus J, von Känel R, Lasserre A, Strippoli M-P, Vandeleur C, Castelao E, et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 2018;48(6):961–73. [DOI] [PubMed] [Google Scholar]
- 64.Caserta MT, Wyman PA, Wang H, Moynihan J, O’Connor TG. Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Dev Psychopathol. 2011;23(4):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forti P, Rietti E, Pisacane N, Olivelli V, Mariani E, Chiappelli M, et al. Blood inflammatory proteins and risk of incident depression in the elderly. Dement Geriatr Cogn Disord. 2010;29(1):11–20. [DOI] [PubMed] [Google Scholar]
- 66.Baumeister D, Russell A, Pariante CM, Mondelli V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Soc Psychiatry Psychiatr Epidemiol. 2014;49(6):841–9. [DOI] [PubMed] [Google Scholar]
- 67.Khandaker GM, Zuber V, Rees JM, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Vives AH, Cleare A. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. 2015;25(10):1532–43. [DOI] [PubMed] [Google Scholar]
- 69.Hiles S, Baker A, De Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol Med. 2012;42(10):2015–26. [DOI] [PubMed] [Google Scholar]
- 70.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49(12):1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. [DOI] [PubMed] [Google Scholar]
- 73.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. A Biol. Sci. Med. Sci 2008;63(8):879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB. To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


