Abstract
The dental pulp, a non-mineralized connective tissue uniquely encased within the cavity of the tooth, provides a niche for diverse arrays of dental mesenchymal stem cells. Stem cells in the dental pulp, including dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs) and stem cells from apical papilla (SCAPs), have been isolated from human tissues with an emphasis on their potential application to regenerative therapies. Recent studies utilizing mouse genetic models shed light on the identities of these mesenchymal progenitor cells derived from neural crest cells (NCCs) in their native conditions, particularly regarding how they contribute to homeostasis and repair of the dental tissue. The current concept is that at least two distinct niches for stem cells exist in the dental pulp, e.g. the perivascular niche and the perineural niche. The precise identities of these stem cells and their niches are now beginning to be unraveled thanks to sophisticated mouse genetic models, which lead to better understanding on the fundamental properties of stem cells in the dental pulp and the apical papilla in humans. The new knowledge will be highly instrumental for developing more effective stem cell-based regenerative therapies to repair teeth in the future.
Keywords: Stem cell, dental pulp, mouse genetic models, dental pulp stem cells, dental mesenchymal stem cells, dental pulp stem cell niche
Introduction
Tooth development is a unique process regulated by interactions between epithelial and mesenchymal tissues (Thesleff, 2003). Dental mesenchymal cells are derived from two different origins: the cranial neural crest cells (NCCs) and the non-neural crest mesoderm (Chai, et al., 2000). NCCs of the first branchial arch are the origin of the odontogenic ectomesenchyme that develop into the dental mesenchyme, which give rise to diverse arrays of dental pulp cells, dentin-forming odontoblasts, periodontal ligament cells, cementum-forming cementoblasts, osteoblasts and chondrocytes (Bronner-Fraser, 1993, Chai, Jiang, Ito, Bringas, Han, Rowitch, Soriano, McMahon and Sucov, 2000). Various signaling molecules such as TGF-β, BMP and Wnt are involved in regulating differentiation of NCCs (Chai, et al., 2003, Trainor, et al., 2002, Tucker, et al., 1999). At the cap stage, the dental papilla is formed as a result of invagination of dental lamina epithelial cells. At the subsequent bell stage, dental epithelial cells at the apical region of the tooth bud continue to invade further apically and interact with dental papilla mesenchymal cells inside the tooth bud; as a result, dental papilla mesenchymal cells subsequently develop into dental pulp cells and odontoblasts (Nanci, 2017).
The dental pulp, a non-mineralized connective tissue uniquely encased within the cavity of the tooth, is composed of heterogeneous cell populations, such as fibroblasts, vascular cells, neural cells and lymphocytes (Goldberg and Smith, 2004). These cells play an important role in tooth homeostasis and repair of damaged dentin. Since the discovery of dental pulp stem cells (DPSCs) (Gronthos, et al., 2002, Gronthos, et al., 2000), stem cells have been isolated from diverse dental tissues, such as exfoliated deciduous teeth (Miura, et al., 2003), apical papilla (Abe, et al., 2007, Sonoyama, et al., 2006), periodontal ligament (Seo, et al., 2004), gingiva (Zhang, et al., 2012) and dental follicle (Morsczeck, et al., 2005) (Figure 1, Table 1). DPSCs, stem cells from human exfoliated deciduous teeth (SHEDs) and stem cells from apical papilla (SCAPs) all possess a property as “mesenchymal stem cells (MSCs)”, therefore present great promise for regenerative therapies (Tatullo, et al., 2015, Yamada, et al., 2019). Fundamental aspects of stem cells in the dental pulp and the apical papilla are beginning to be unraveled. Despite the accumulating knowledge on the dental stem cells (Sharpe, 2016), it is imperative to understand how these stem cells regulate odontogenesis under both physiological and regenerative conditions, because the current regenerative therapies to repair dentin and tooth structures need to be improved for better clinical outcomes. The purpose of this review is to summarize the current understanding on stem cell populations in the dental pulp and the apical papilla, and the recent findings from in vivo mouse genetic models on how these stem cells contribute to tooth homeostasis and repair.
Figure 1. Isolated tooth-derived stem cells (TDSCs).
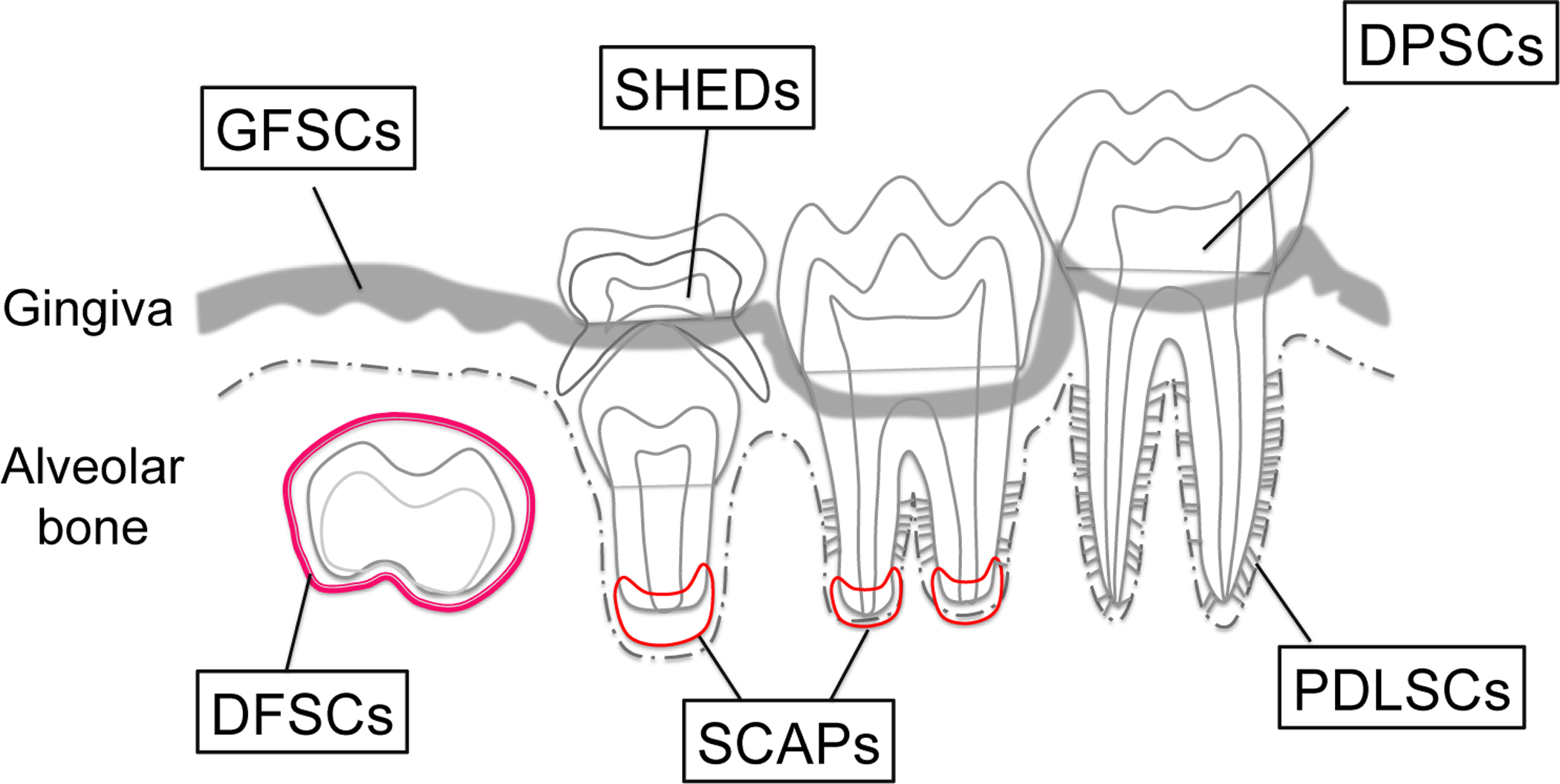
Schematic diagram of TDSCs populations. From tooth pulp, dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs) and cells from apical papilla (SCAPs) can be isolated. From supporting tissue, periodontal ligament stem cells (PDLSCs), gingival fibroblastic stem cells (GFSCs) and dental follicle stem cells (DFSCs) can be isolated.
Table 1.
Isolated tooth-derived stem cells
| Cells | Location | Ref. |
|---|---|---|
| In dental pulp | ||
| DPSCs | Dental pulp in permanent teeth | Gronthos, et al, 2000 |
| SHEDs | Immature dental pulp in deciduous teeth | Miura, et al., 2003 |
| SCAPs | Apical papilla of growing tooth roots | Sonoyama, et al., 2006 |
| Others | ||
| PDLSCs | Periodontal ligament | Seo, et al., 2004 |
| DFSCs | Dental follicle | Morsczeck et al., 2005 |
| GFSCs | Gingiva | Zhang, et al., 2012 |
Search protocol
Outcome:
To identify stem cell populations that can differentiate into odontoblasts and produce dentin in vivo.
Population and intervention:
Our focus was on pre-clinical studies using mouse genetic models to define the lineage of stem cells in the dental pulp and the apical papilla. We included animal (mouse) studies using conditional knockout models based on the cre-loxP system, but excluded animal studies using conventional (global) knockout models.
Search strategy:
For searching in vivo stem cells in the dental pulp and the apical papilla, a literature search was conducted using PubMed, Web of Science and Google Scholar by the first author (M.N.). A search strategy for the database was performed to find studies that matched the following terms: (dental pulp stem cell OR dental pulp) AND (cre OR creER). The electronic search aimed to obtain all the studies that identified in vivo stem cells in the dental pulp and the apical papilla using mouse genetic models. Animal studies using the cre-loxP system was eligible for inclusion in this review, while animal studies using only conventional knockout mice were excluded. No restriction regarding the original language of the article or publication status were set. Databases were searched up to January 2020, with no limit on the year of publication. To complement the search, the reference list of the main articles related to this narrative review were also assessed. A total of 25 in vivo mouse genetic studies were included. Two authors (M.N. and W.O.) independently assessed the quality of the identified studies and resolving any disagreements through discussion.
In vitro MSCs isolated from the dental pulp
Dental pulp stem cells (DPSCs)
Smith et al. reported that the odontoblast layer can be stimulated to form reactionary dentin (otherwise known as tertiary dentin, which is formed in response to aggression) in response to enamel or dentin damage, suggesting that the dental pulp may contain MSCs that are responsible for tooth repair by producing newly formed dentin (Smith, et al., 1995, Smith and Lesot, 2001). MSCs have been isolated from dental mesenchymal tissues including the dental pulp (Sharpe, 2016). DPSCs, a postnatal stem cell population resident in the dental pulp, were initially isolated from extracted human third molars by Gronthos et al.; these cells possess high clonogenic abilities, rapid proliferative rates, and capabilities to produce mineralized tissues both in vitro and in vivo (Gronthos, Mankani, Brahim, Robey and Shi, 2000). Subsequent studies identified that DPSCs are capable of differentiating into adipocytes, osteoblasts, chondrocytes, melanocytes and endotheliocytes in vitro and after transplantation (d’Aquino, et al., 2007, Gronthos, Brahim, Li, Fisher, Cherman, Boyde, DenBesten, Robey and Shi, 2002, Hilkens, et al., 2013, Koyama, et al., 2009, Stevens, et al., 2008, Zhang, et al., 2008). Importantly, DPSCs present enhanced odontogenic capacity compared with bone marrow mesenchymal stem cells (BM-MSCs), indicating that DPSCs may represent a suitable source for repairing the dentin (Yu, et al., 2007).
Moreover, the dental pulp may play an important role for defense against dentin-invading bacteria by inducing immune response (Farges, et al., 2015). Dental pulp cells express a diverse array of chemokines and cytokines (Farges, et al., 2009), particularly CXCL12 (chemokine C-X-C motif ligand 12), which is abundantly expressed by mesenchymal stromal cells in the bone marrow stromal compartment (Matsushita, et al., 2020, Sugiyama, et al., 2006). Indeed, DPSCs ameliorate inflammation-related tissue injuries by suppressing T cell proliferation, therefore possessing immunomodulatory properties (Pierdomenico, et al., 2005, Tang and Ding, 2011, Zhao, et al., 2012). Therefore, DPSCs might also possess inherent capabilities to coordinate development and homeostasis of the dental pulp, in addition to their potential for regenerative medicine.
Stem cells from exfoliated deciduous teeth (SHEDs)
Deciduous teeth similarly possess a variety of immature cells within their dental pulp, thus considered as an ideal source of stem cells. SHEDs were first isolated by Miura et al. (Miura, Gronthos, Zhao, Lu, Fisher, Robey and Shi, 2003). These cells have a potential to differentiate into a variety of cell types including osteoblasts, adipocytes, chondrocytes, endothelial cells, neural cells and odontoblasts (Casagrande, et al., 2010, Cordeiro, et al., 2008, Miura, Gronthos, Zhao, Lu, Fisher, Robey and Shi, 2003, Sakai, et al., 2010, Wang, et al., 2012). After in vivo transplantation, SHEDs can undergo osteogenesis and dentinogenesis (Miura, Gronthos, Zhao, Lu, Fisher, Robey and Shi, 2003, Sakai, Zhang, Dong, Neiva, Machado, Shi, Santos and Nor, 2010, Shi, et al., 2005). SHEDs possess higher proliferation rates and osteo-inductive capacities after in vivo transplantation than those of DPSCs, suggesting that these cells demonstrate a great potential for mineralized tissue regeneration (Miura, Gronthos, Zhao, Lu, Fisher, Robey and Shi, 2003, Wang, Sha, Li, Yang, Ji, Wen, Liu, Chen, Ding and Xuan, 2012). In a recent human clinical trial, implantation of autologous SHEDs into necrotic immature permanent incisors of pediatric patients generated a functional dental pulp associated with blood vessels and sensory nerves (Xuan, et al., 2018). SHEDs have a unique advantage of being retrievable from exfoliated teeth, which otherwise had been considered as a disposable human tissue. SHEDs have been applied not only to pulp regeneration but also to extraoral application such as kidney (Hattori, et al., 2015), brain (Fujii, et al., 2015, Mita, et al., 2015), spinal cord (Nicola, et al., 2017, Taghipour, et al., 2012), liver (Yamaza, et al., 2015) and bone (Ma, et al., 2012, Novais, et al., 2019) in pre-clinical animal models (Shi, et al., 2020).
Stem cells from apical papilla (SCAPs)
The apical papilla, the apical portion of the dental papilla, is loosely attached to the apex of the developing tooth root in erupting permanent teeth. A cell-rich zone exists between the apical papilla and the dental pulp. The apical papilla has been considered to play an important role in tooth root formation as it contains an MSC-like cell population (Abe, Yamaguchi and Amagasa, 2007, Abe, et al., 2008, Huang, et al., 2008, Sonoyama, Liu, Fang, Yamaza, Seo, Zhang, Liu, Gronthos, Wang, Wang and Shi, 2006, Sonoyama, et al., 2008). SCAPs were initially isolated by Sonoyama et al. (Sonoyama, Liu, Fang, Yamaza, Seo, Zhang, Liu, Gronthos, Wang, Wang and Shi, 2006). These cells can differentiate into osteoblasts, odontoblasts and adipocytes in vitro with a higher proliferation rate than that of DPSCs, while expressing typical markers for stem cells (Sonoyama, Liu, Fang, Yamaza, Seo, Zhang, Liu, Gronthos, Wang, Wang and Shi, 2006). In addition, SCAPs have the ability to differentiate into other non-native cell types such as neural cells (Abe, Yamaguchi and Amagasa, 2007, Kim, et al., 2017, Sonoyama, Liu, Yamaza, Tuan, Wang, Shi and Huang, 2008), chondrocytes (Abe, Yamaguchi and Amagasa, 2007, Dong, et al., 2013, Patil, et al., 2014) and hepatocytes (Kumar, et al., 2017, Patil, Kumar, Lee, Jeon, Jang, Lee, Park, Byun, Ahn, Kim and Rho, 2014) after transplantation. Interestingly, cultured SCAPs express several neural markers without neurogenic stimulation, such as Nestin, Musashi1 and TrkA (Abe, Yamaguchi and Amagasa, 2007). Additional neural markers are also expressed in SCAPs after neurogenic stimulation, such as glutamic acid decarboxylase (GAD), neuronal nuclear antigen (NeuN), neurofilament M (NFM), neuron-specific enolase (NSE), and glial markers 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) (Sonoyama, Liu, Yamaza, Tuan, Wang, Shi and Huang, 2008). Indeed, SCAPs and DPSCs participate in nerve regeneration upon transplantation (Kolar, et al., 2017). Compared with other dental MSCs such as DPSCs or periodontal ligament stem cells (PDLSCs), SCAPs has a higher ability for nerve regeneration in a rat sciatic nerve injury model (Kolar, Itte, Kingham, Novikov, Wiberg and Kelk, 2017). As SCAPs have the ability to differentiate into multilineage cells, they can be considered as a promising cell source for stem cell-based therapies (Kang, et al., 2019). In fact, SCAPs are applied clinically in the “cell-homing techniques” to support the root development of immature teeth that previously experienced pulp necrosis and periapical periodontitis (Palma, et al., 2019). Consistently, SCAPs demonstrate the capacity to promote mineralized tissue regeneration inside the root of previously necrotic teeth or in the periapical tissue after severe infection is resolved (Palma, et al., 2017).
In vivo stem cell markers of the dental pulp
Stem cell niches in the dental pulp
The dentin-pulp complex possesses the inherent capability for regeneration in order to protect itself against external insults such as caries and mechanical trauma (Smith, Cassidy, Perry, Begue-Kirn, Ruch and Lesot, 1995). The dental pulp contains niches for stem/progenitor cells, which provide precursor cells for odontoblasts and other types of mesenchymal cells (Sloan and Smith, 2007, Sloan and Waddington, 2009). Recently, two distinct niches for stem cells derived from NCCs have been identified in the dental pulp in mice; namely, the perivascular niche and the perineural niche (Feng, et al., 2011, Kaukua, et al., 2014, Vidovic, et al., 2017, Zhao, et al., 2014).
Cranial neural crest cells (NCCs)
The proto-oncogene Wnt1 is expressed exclusively during early development of the central nervous system, and Wnt1-cre can mark a pre-migratory neural crest cell population. (Baggiolini, et al., 2015, Echelard, et al., 1994, Hari, et al., 2012, McMahon, et al., 1992, Wilkinson, et al., 1987). Wnt1-cre has been widely used to map the fate of NCCs during craniofacial development. Chai et al. reported that NCCs contribute to all dental mesenchymal tissue including the dental pulp using Wnt1-cre-based fate mapping experiments (Chai, Jiang, Ito, Bringas, Han, Rowitch, Soriano, McMahon and Sucov, 2000). Wnt1-cre-marked dental pulp cells exhibit multi-lineage differentiation into osteoblasts, adipocytes, neurons, and smooth muscles in vitro (Janebodin, et al., 2011). Upon in vivo subcutaneous transplantation with a hydroxyapatite/tricalcium phosphate carrier, Wnt1-cre-marked dental pulp cells differentiate into odontoblast-like cells and produce a dentin-like structure (Janebodin, Horst, Ieronimakis, Balasundaram, Reesukumal, Pratumvinit and Reyes, 2011). Moreover, implantation of Wnt1-cre-marked dental pulp cells contribute to repair of critical-size calvarial bone defects (Collignon, et al., 2019). Transplanted Wnt1-cre-marked dental pulp cells are present up to 3 months after transplantation in the calvarial defect and they rapidly differentiate into chondrocyte-like cells (Collignon, Castillo-Dali, Gomez, Guilbert, Lesieur, Nicoletti, Acuna-Mendoza, Letourneur, Chaussain, Rochefort and Poliard, 2019). In fact, various signaling pathways in Wnt1-cre-marked dental pulp cells can modulate dentin formation. Oka et al. reported that dentin thickness and tooth size were reduced in Wnt1-cre; Tgfbr2fl/fl mutant mice associated with decreased Col1a1 and Dspp expression, suggesting that the TGF-β signaling pathway controls odontoblast maturation and dentin formation (Oka, et al., 2007). A transcriptional factor Kruppel-like factor 4 (KLF4) is expressed by the polarizing odontoblast layer, and dentin mineralization is impaired in Wnt1-cre; KLF4fl/fl mutant mice (Tao, et al., 2019). KLF4 directly activates the TGF-β signaling pathway at the beginning of odontoblast differentiation associated with upregulation of dentin matrix protein (Dmp1) and Osterix (Osx/Sp7).
Protein 0 (P0) is expressed by migrating neural crest cells. As a result, P0-cre transgenic line represents a useful tool as a neural crest cell-specific cre driver (Komada, et al., 2012, Yamauchi, et al., 1999). P0-cre marks cells in the dental mesenchyme that contribute to development of the dentin-pulp complex. However, P0-cre also marks cells in the enamel organ (Wang, et al., 2011). Indeed, P0-cre marks cell populations that are different from Wnt1-cre-marked NCCs, especially in the midbrain and hindbrain region (Chen, et al., 2017); therefore, P0-cre and Wnt1-cre transgenic lines mark non-identical cell populations. Similarities and differences between P0-cre marked and Wnt1-cre marked NCCs remains to be studied.
Perivascular stem cell niche
Neural/glial antigen 2 (NG2) is a proteoglycan commonly used as a marker for pericytes that maintain hematopoietic stem cell quiescence in bone marrow (Chan-Ling and Hughes, 2005, Kunisaki, et al., 2013, Murfee, et al., 2005). Lineage-tracing experiments using NG2-creER mice revealed that pericytes can differentiate into odontoblasts during tooth root formation as well as in response to injuries in mouse incisors (Feng, Mantesso, De Bari, Nishiyama and Sharpe, 2011). Moreover, the tip of the mouse incisor pulp also contains a population of NG2+ pericytes that differentiate into odontoblast-like cells, suggesting that rapid and continuous mineralization occurs at the tip of the incisor to protect the dental pulp tissue from external stimuli (Pang, et al., 2016).
Glioma-associated oncogene homolog 1 (Gli1), a canonical transcriptional target of hedgehog (Hh) signaling, is expressed by both dental mesenchymal cells and epithelial cells in HERS (Feng, et al., 2017, Liu, et al., 2015). Gli1 marks perivascular MSC-like cells in vivo, and Gli1+ cells express typical MSC markers and possess an ability for trilineage differentiation in vitro (Kramann, et al., 2015). Gli1+ mesenchymal cells are slow-cycling and function as mesenchymal stem cells in developing mouse incisors (Zhao, Feng, Seidel, Shi, Klein, Sharpe and Chai, 2014). Gli1+ cells are located surrounding small arteries and mobilized in response to injuries. In addition, Gli1+ cells give rise to all NG2+ or CD146+ perivascular cells in vivo, indicating that NG2+ cells are a subset of MSC subpopulations derived from Gli1+ cells. Importantly, in contrast to incisors, adult mouse molars do not contain Gli1+ cells surrounding the small arteries in the dental pulp, whereas NG2+ pericytes surround all vasculatures in the molars (Zhao, Feng, Seidel, Shi, Klein, Sharpe and Chai, 2014). These results indicate that Gli1+ cells may behave as transient root progenitor cells for the growth of the molar. Indeed, during tooth root development, several signaling pathways in Gli1+ cells affect tooth root formation. Constitutive activation of Hh signaling in Gli1+ root progenitor cells using Gli1-creER; SmoM2 mice leads to shortened roots in molars; however, odontoblast marker gene Dspp expression is unaffected (Liu, Feng, Li, Zhao, Ho and Chai, 2015). Feng et al. reported that disruption of Bmp signaling in Gli1+ root progenitor cells (Gli1-creER; Bmpr1αfl/fl) results in impaired root development associated with lack of Dspp expression in most of the apical region of molars (Feng, Jing, Li, Zhao, Punj, Zhang, Xu and Chai, 2017). Furthermore, overactivation of a transcriptional factor Klf4, which is a downstream target of BMP signaling, promotes odontogenic differentiation of MSCs, suggesting that BMP signaling specifically regulates Klf4 in odontogenesis.
Alpha-smooth muscle actin (αSMA) is one of the markers for perivascular cells (Crisan, et al., 2009, Crisan, et al., 2011, Crisan, et al., 2008). Vidovic et al. reported that αSMA+ perivascular cells in the dental pulp differentiate into odontoblasts during tooth root formation (Vidovic, Banerjee, Fatahi, Matthews, Dyment, Kalajzic and Mina, 2017). After tooth root formation, αSMA+ perivascular cells lie dormant in the intact dental pulp. However, when the dental pulp is injured in an experimental pulp exposure model, αSMA+ perivascular cells rapidly proliferate and contribute to reparative dentinogenesis (Vidovic, Banerjee, Fatahi, Matthews, Dyment, Kalajzic and Mina, 2017). Furthermore, the delivery of exogenous fibroblast growth factor 2 (FGF2) leads to proliferation of αSMA+ dental pulp cells and accelerated differentiation into odontoblasts (Vidovic-Zdrilic, et al., 2018). Consistently, mild injuries leading to reactionary dentinogenesis activate αSMA+ perivascular cells to give rise to pulp cells as well as a few odontoblasts that are integrated into the pre-odontoblastic layer (Vidovic-Zdrilic, et al., 2019). These findings suggested that the perivascular niche harbors various MSC populations such as NG2+, Gli1+ and αSMA+ cells which contribute to homeostasis and injury repair in the dental pulp.
Perineural stem cell niche
Another subset of dental stem cells has been identified among peripheral nerve-associated glial cells (Kaukua, Shahidi, Konstantinidou, Dyachuk, Kaucka, Furlan, An, Wang, Hultman, Ahrlund-Richter, Blom, Brismar, Lopes, Pachnis, Suter, Clevers, Thesleff, Sharpe, Ernfors, Fried and Adameyko, 2014). Schwann cell precursors associated with innervating nerves are identified as a cellular origin of melanocytes in the skin, although Schwann cells and melanocytes were considered to be generated through entirely distinct pathways from NCCs (Adameyko and Lallemend, 2010, Adameyko, et al., 2009, Adameyko, et al., 2012). Lineage-tracing analyses of Schwann cell precursors using PLP1-creER and Sox10-creER demonstrates that neural crest-derived Schwann cells and Schwann cell precursors give rise to odontoblasts and dental pulp cells in mouse growing incisor (Kaukua, Shahidi, Konstantinidou, Dyachuk, Kaucka, Furlan, An, Wang, Hultman, Ahrlund-Richter, Blom, Brismar, Lopes, Pachnis, Suter, Clevers, Thesleff, Sharpe, Ernfors, Fried and Adameyko, 2014). Moreover, Schwann cell-derived odontoblasts contribute to reparative dentinogenesis after mouse incisor injuries. Importantly, these Schwann cells do not express a pericyte marker, indicating that pericytes and Schwann cells represent two distinct subsets of dental pulp stem cell populations. These findings suggest that the dental pulp contains several distinct stem cell niches in the perivascular and neural-vascular bundle area.
Other progenitor cell populations of the dentin–pulp complex
Studies utilizing mouse genetics models have shed light on the mechanism of odontoblast differentiation. An transcriptional factor Osx, also known as Sp7, is broadly expressed in the dental mesenchyme by dental papilla cells, dental follicle cells, odontoblasts and alveolar bone osteoblasts during tooth development, and regulates cementogenesis (Cao, et al., 2012, Chen, et al., 2009). Osx plays a critical role in proliferation and differentiation of both ameloblasts and odontoblasts (Bae, et al., 2018). Major signaling pathways play important regulatory roles in Osx+ root progenitor cells and dentinogenesis. Rakian et al. reported that conditional deletion of Bmp2 in Osx+ cells by Osx-cre; Bmp2fl/fl results in loss of tooth root formation with reduction of odontoblast marker genes such as Dmp1, Col1a1 and Sp7 (Rakian, et al., 2013). In addition, Wang et al. reported that conditional deletion of Tgfbr2 in Osx+ cells (Osx-cre; Tgfrb2fl/fl) results in failure of tooth root formation and tooth eruption associated with reduction in dentin matrix density in molars (Wang, et al., 2013). Li et al. reported that conditional deletion of intraflagellar transport 140 (IFT140), which is specialized for retrograde transportation in the cilia, in Osx+ cells (Osx-cre; IFT140fl/fl) reduces dentin thickness and delays dentin formation (Li, et al., 2018). Moreover, mTOR signaling plays an important role in odontoblast differentiation in dental pulp cells (GEORGE and Eapen, 2015, Kim, et al., 2011), as conditional deletion of Raptor/mTORC1 in Osx+ cells (Osx-cre; Raptorfl/fl) causes dentinogenesis imperfecta associated with a smaller tooth volume (Xie, et al., 2019).
We previously reported that conditional deletion of parathyroid hormone (PTH) / parathyroid hormone-related protein (PTHrP) receptor (PPR) signaling in Osx+ cells (Osx-cre; PPRfl/fl) disrupts tooth root formation and causes severe failure of tooth eruption (Ono, et al., 2016). As PTHrP is a locally acting autocrine/paracrine ligand that is mainly expressed by the dental follicle, Osx+ root progenitor cells may functionally overlap with dental follicle cells during tooth root formation (Nagata, et al., 2019, Ono, Sakagami, Nishimori, Ono and Kronenberg, 2016, Takahashi, et al., 2019). Moreover, Osx-creER+ dental root progenitor cells differentiate into all relevant cell types contributing to formation of the tooth root and periodontal tissues, such as odontoblasts, cementoblasts, alveolar bone osteoblasts and periodontal ligament (PDL) cells; these progenitor cells continue to provide these cells even after root formation is complete, suggesting that Osx+ cells also play important roles in tooth root maintenance (Takahashi, et al., 2017).
Transcription factor paired-related homeobox gene 1 (Prrx1) regulates early mesenchymal cell fates in myogenesis, osteoblastogenesis, chondrogenesis and tooth morphogenesis; as a result, Prrx1-cre is widely used to mark MSCs (Elefteriou and Yang, 2011, Hu, et al., 1998, Lu, et al., 1999, Lu, et al., 2011, Martin, et al., 1995, Mitchell, et al., 2006, Peterson, et al., 2005, ten Berge, et al., 1998). In tooth development, Prrx1 is exclusively expressed by mesenchymal cells. Prrx1−/−Prrx2−/− embryos that do not survive postnatally exhibit molar malformation with cuspal changes and ectopic epithelial projection, suggesting that Prrx1 plays a role in molar tooth morphogenesis (Mitchell, Hicklin, Doughty, Hicklin, Dickert Jr, Tolbert, Peterkova and Kern, 2006). Wang et al. reported that Prrx1+ mesenchymal cells and their descendants were located in the dental pulp, particularly in the pulp horn using Prrx1-cre; R26RmTmG mice, whereas overexpression of Sirtuin 1 (Sirt1) in Prrx1+ cells (Prrx1-cre; Sirt1TG) increases tooth size (Wang, et al., 2018). Furthermore, overexpression of Sirt1 in Prrx1+ cells rescues the reduced cell proliferation and differentiation as well as the increased cell apoptosis induced by deficiency of B-lymphoma Mo-MLV insertion region 1 (Bmi1). Consistently, overexpression of Sirt1 in Prrx1+ cells increases alveolar bone mass associated with enhanced MSC proliferation and osteogenic differentiation (Wang, et al., 2019).
CD90/Thy1 is one of the putative cell surface markers of MSCs that is widely used to isolate MSCs in vitro (Dominici, et al., 2006, Lin, et al., 2013). An et al. reported that a sub-population of MSCs characterized by expression of CD90/Thy1 is located in the dental pulp between the labial and lingual cervical loop of mouse incisors and contributes to formation of the dental pulp and the dentin (An, et al., 2018b). Interestingly, CD90/Thy1+ cell populations are quiescent and barely detectable in adulthood when homeostasis is established; however, these cells reappear under regenerative conditions and provide a source of cells for tissue repair. During tissue regeneration stimulated by clipping of the mouse incisor, a population of Celsr+ quiescent cells in the dental pulp become mitotic and replenish the CD90/Thy1+ MSCs. These results support the evidence that MSCs in the dental pulp can be re-activated during injury repair.
Wnt/β-catenin signaling plays an essential role in osteogenic differentiation of MSCs (Glass II, et al., 2005, Logan and Nusse, 2004, Matsushita, Nagata, Kozloff, Welch, Mizuhashi, Tokavanich, Hallett, Link, Nagasawa and Ono, 2020) as well as in tooth root formation (Lohi, et al., 2010). Axin2, also known as axis inhibition protein 2, is one of the major Wnt target genes (Jho, et al., 2002, Lustig, et al., 2002). An et al. reported that Axin2+ cells were located in the odontoblast layer and the dental pulp in the proximal region of the mouse incisor (An, et al., 2018a). Lineage-tracing analysis of Axin2+ cells with Axin2-creER; R26RmTmG mice revealed that Axin2+ cells and their descendants demonstrate a transiently increased contribution to dental pulp cells and odontoblast by 2 weeks after pulse, indicating that Axin2+ cells mark transit-amplifying cells (TACs) that temporally give rise to dental pulp cells. Moreover, polycomb repressive complex 1 (PRC1), a mitotic spindle-associated cyclin-dependent kinases (CDKs) essential for cell cleavage, is crucial for coordinating TACs phenotype by regulating Wnt/β-catenin signaling activities, suggesting that the Wnt/β-catenin activity downstream of PRC1 is required for stem cell maintenance. Similarly, Axin2+ dental pulp cells in molars differentiate into new odontoblast-like cells that secret the reparative dentin via Wnt/β-catenin signaling in response to injuries (Babb, et al., 2017). Disruption of Wntless (Wls) in osteocalcin-expressing odontoblasts (Osteocalcin-cre; Wlsco/co) leads to reduction of dentin thickness and shorter roots with decreased Wnt10a and Axin2 expression in odontoblasts (Bae, et al., 2015). These results suggest that Wnt-responsible gene Axin2 is crucial for odontoblast maturation and dentin formation.
Platelet-derived growth factor (PDGF) is one of the most abundant growth factors in platelets and promote tissue regeneration after injury (Andrew, et al., 1995, Antoniades, et al., 1979, Pierce, et al., 1988). PDGF-BB and its receptor PDGF receptor beta (PDGFRβ) pathway has been demonstrated to play an important role for odontoblast differentiation in dental pulp cells (Yokose, et al., 2004). PDGF signaling is also critical for differentiation of bone marrow (BM) MSCs into multilineage cells such as osteoblasts, adipocytes and chondrocytes (Ng, et al., 2008). Walker et al. reported that PDGFRβ+ cells mark most of MSCs in the cervical loop (CL-MSCs) and mesenchymal TACs (MTACs) in the mouse incisor and differentiate into odontoblasts using Pdgfrb-creER mice (Walker, et al., 2019). When incisors are fully erupted and functioning, PDGFRβ+ cells remain as CL-MSCs and MTACs in the cervical loop, indicating that PDGFRβ+ mesenchymal cells represent distinct MSC populations from those maintained in the neuro-vascular niches (Kaukua, Shahidi, Konstantinidou, Dyachuk, Kaucka, Furlan, An, Wang, Hultman, Ahrlund-Richter, Blom, Brismar, Lopes, Pachnis, Suter, Clevers, Thesleff, Sharpe, Ernfors, Fried and Adameyko, 2014, Zhao, Feng, Seidel, Shi, Klein, Sharpe and Chai, 2014). Cai et al. reported that isolated PDGFRβ+/c-kit+ dental pulp cells possess stem cell properties with high proliferative and osteogenic differentiating abilities in vitro. Moreover, in an emptied rat incisor root canal therapy model, transplanted PDGFRβ+/c-kit+ dental pulp cells produce globular dentin-like structures and pulp-like tissue, suggesting that a subset of PDGFRβ+/c-kit+ dental pulp cells are capable of regenerating dentin/pulp (Cai, et al., 2016).
Challenges and future directions
We now have better understanding of stem cells in the dental pulp and the apical papilla and their characteristics both in vitro and in vivo. Particularly, use of cell type-specific cre-loxP transgenic systems has facilitated in-depth characterization of mesenchymal progenitor cells in the dental pulp (Figure 2, Table 2). The majority of dental pulp cells are derived from NCCs. Various markers for NCCs and their descendants have been utilized to identify stem cells in the dental pulp in vivo. However, the limitation of these approaches is that these stem cell populations tend to be overestimated due to rather broad expression patterns of these utilized markers. To identify the regulatory mechanism of these stem cells, it is important to identify specific markers for a bona fide mesenchymal progenitor cell population in the dental pulp. Additionally, a majority of lineage-tracing experiments of dental pulp cells have been performed exclusively in the mouse incisor model, which is characterized by a life-long continuous growth. The relevance of these studies to more static molars, as well as human incisors and molars, remains largely unknown. Further characterization of mesenchymal progenitor cells in the dental pulp and the apical papilla of molars are required to define the function of these tooth-derived stem cells in an environment similar to the human dental pulp.
Figure 2. Stem cell populations in the dental pulp.
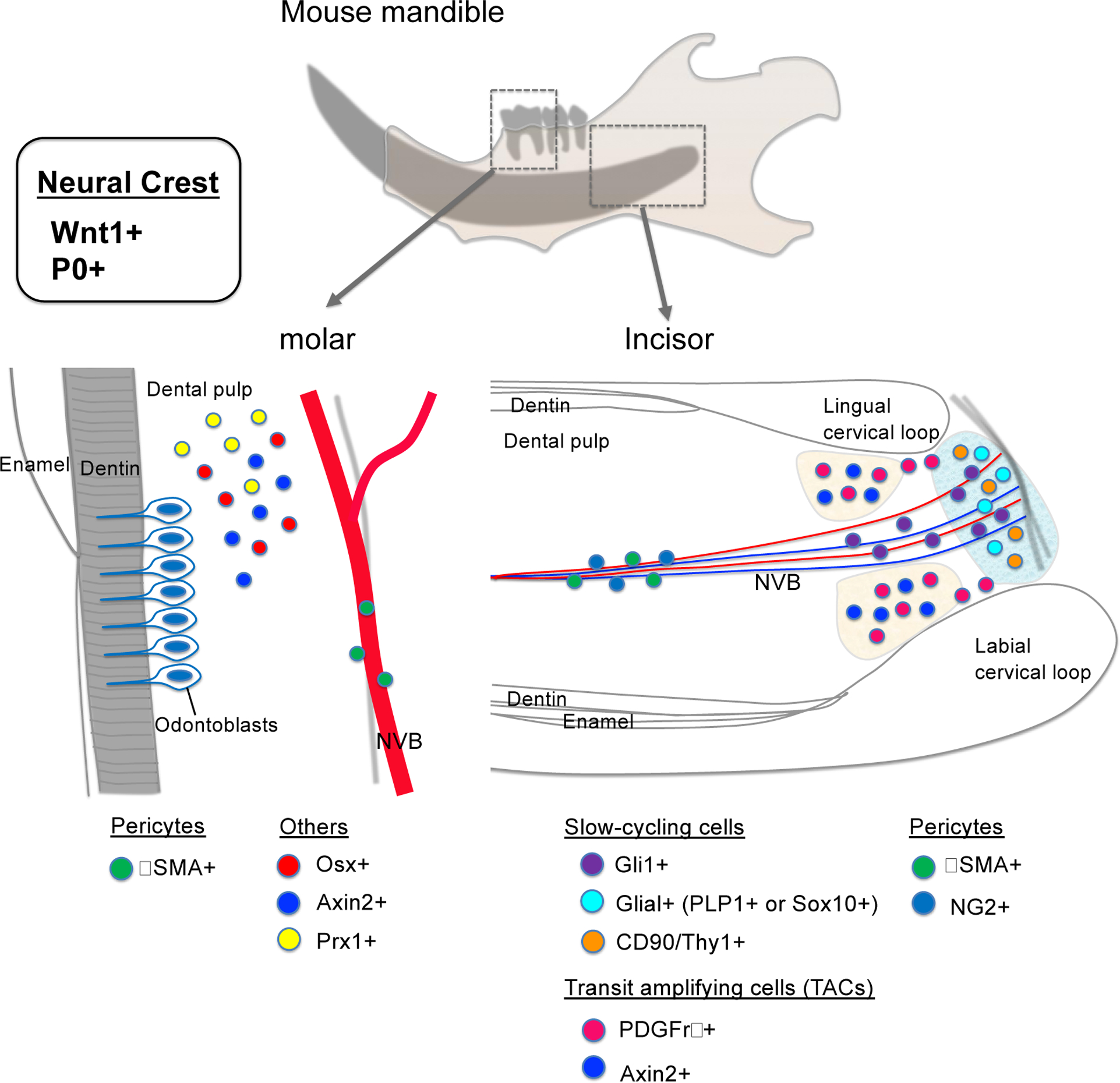
Schematic diagrams of stem cell populations both in mice. During tooth development, dental pulp cells are derived from NCCs such as Wnt1+ or P0+. In the molar, Osx+, Axin2+ and Prx1+ mesenchymal progenitor cells and αSMA+ pericytes differentiate into odontoblasts. In the incisor, Gli1+, PLP1+ or Sox10+ glial, and CD90/Thy1+ slow cycling cells, PDGFRβ+ and Axin2+ TACs, αSMA+ and NG2+ pericytes differentiate into odontoblasts and contribute to dentin formation. NVB: Neurovascular bundle
Table 2.
Transgenic mice line used for stem cell population in the dental pulp
| Gene | Marked cell population | Cre transgenic line | Gene manipulation | Findings | Ref. |
|---|---|---|---|---|---|
| Wnt1 | All neural crest cell lineage | Wnt1-cre | - | Clonal Wnt1-cre cells differentiate into neural crest cell lineage in vitro culture | Janebodin et al. 2011 |
| Wnt1-cre | - | Implanted Wnt1-cre cells promote bone healing in critical-size calvaria bone defect | Collignon et al. 2019 | ||
| Wnt1-cre | Wnt1-cre; Tgfr b2fl/fl | Dentin thickness and tooth size are decreased with reduction of Col1a1 and Dspp expression | Oka et al. 2007 | ||
| Wnt1-cre | Wnt1-cre; KLF 4fl/fl | Dentin mineralization is impaired and root pulp/root canal are enlarged | Tao et al. 2019 | ||
| Gli1 | Perivascular cells in the incisor | Gli1-creER | - | Differentiate to all mesenchymal cells in the dental pulp and located at reparative dentin after tooth injury | Zhao et al. 2014 |
| All root progenitor cells in the molar | Gli1-creER | Gli1-creER;Bmpr1αfl/fl | Failure of tooth root formation with lack of Dspp expression in the periapical region | Feng et al. 2017 | |
| Gli1-creER | Gli1-creER;SmoM2 | Shorter root with reduction of Ki67+ proliferative cells | Liu et al. 2015 | ||
| NG2 | Pericytes | NG2-creER | - | Differentiate into Dspp+ odontoblasts after mandible damage | Feng et al. 2011 |
| NG2-creER | - | Differentiate into Dspp+ odontoblasts at the tip of incisors | Pang et al. 2016 | ||
| αSMA | Pericytes | aSMA-creER | - | Differentiate into Col2.3-GFP+ cells composed Dspp+ odontoblasts and Bsp+ osteoblasts in reparative dentin after pulp exposure | Vidovic et al. 2017 |
| aSMA-creER | - | FGF2 promotes aSMA+ cells to differentiate into Col2.3-GFP+ odontoblasts in reparative dentin after pulp exposure | Vidovic et al. 2018 | ||
| aSMA-creER | - | Proliferate in dental pulp and differentiate into some odontoblasts after mild dentin injury | Vidovic et al. 2019 | ||
| PLP | Schwann cell precursor cells | PLP-creER | - | Differentiate into pulp cells and odontoblasts during tooth development and regeneration after tooth damage | Kaukua et al. 2014 |
| Sox10 | Sox10-creER | - | |||
| Osx | Mesenchym al root progenitor cells in the molar | Osx-cre | Osx-cre;Bmp2fl/fl | Failure of tooth root formation with reduction of Dmpl, Col1al and Osx expression | Rakian et al. 2013 |
| Osx-cre |
Osx-cre; Tgfrbf l/fl |
Failure of tooth root formation and delayed tooth eruption with reduction of Dspp and Bglap expression | Wang et al. 2013 | ||
| Osx-cre | Osx-cre;IFT140fl/fl | Lead to shorter root and thin dentin with loss of primary cilia and impair reparative dentin formation in a tooth drilling model | Li et al. 2018 | ||
| Osx-cre | Osx-cre;Partorfl/fl | Lead to small tooth volume and reduce of proliferation, differentiation of odontoblasts and Dspp expression | Xie et al. 2019 | ||
| Osx-cre | Osx-cre;PPRfl/fl | Disrupt tooth root formation and failure of tooth eruption with reduction of EdU+ proliferative cells | Ono et al. 2016 | ||
| Osx-creER | Differentiate into odontoblasts, cementoblasts, PDL cells and alveolar cryptal bone osteoblasts | Takahashi et al. 2017 | |||
| Prrx1 | Mesenchym al cells in the region of pulp horns | Prrx1-cre | Prrx1-cre;Sirt1TG | Increase tooth size with upregulation of Dspp, Dmpl and Ocn expression | Wang et al. 2018 |
| CD90/Thy1 | Slow cycling cells in the incisor | CD90/Thy1-cre | - | CD90/Thyl+ cells are quiescent in adulthood but proliferate rapidly and differentiate into pulp cells and odontoblasts after clipping of the incisor | An et al. 2018a |
| Axin2 | TACs in the incisor | Axin2-creER | Axin2-creER;Wlsfl/fl | Axin2+ cells located in the proximal region of the incisor, transiently proliferate and differentiate into dental pulp cells and odontoblasts by 2 weeks | An et al. 2018b |
| Odontoblasts at the periphery of the pulp | Axin2-creER | Axin2-creER;Wlsfl/fl | Proliferate rapidly and differentiate into Dspp+ odontoblasts in reparative dentin after pulp exposure | Babb et al. 2017 | |
| PDGFRβ | TACs in the incisor | Pdgfrb-creER | - | PDGFRβ+ cells mark most of CL-MSCs and MTACs in the incisor and differentiate into odontoblasts | Walker et al. 2019 |
Stem cells are important factors of tissue engineering, and the combinational use of various MSCs, signaling molecules and nanostructures has been developed in the dental field in recent decades (Chieruzzi, et al., 2016). However, the functional and complete regeneration of the dental tissue, i.e. the dentin-pulp complex and periodontal apparatus, is still difficult in a clinical setting. Identifying the dental stem cell population in vivo will facilitate our understanding on the regulatory mechanism of tissue repair, which may lead to the predictable regeneration of the functional dental tissue in the future.
Conclusion
In the last decade, in vitro studies have identified stem cells in the dental pulp and the apical papilla as a promising MSC source for regenerative medicine. Thanks to lineage-tracing analyses of dental pulp cells using mouse genetics model, diversity of stem cell populations in the dental pulp and the apical papilla in vivo is beginning to be unraveled. For much better understanding of the fundamental biological property of these important stem cells, we need to develop more specific genetic tools that allow functional analysis of specific groups of dental mesenchymal progenitor cells in the dental pulp. These technological advances will shed light the regulatory mechanism of repairing teeth and could be applied for effective stem cell-based regenerative therapies in the future.
Table 3.
Abbreviations of gene or cytokine
| Abbreviation | Definition |
|---|---|
| αSMA | Alpha-smooth muscle actin |
| Axin2 | Axis inhibition protein 2 |
| BMP | Bone morphogenetic protein |
| Bmp2 | Bone morphogenetic protein 2 |
| Bmpr1 | Bone morphogenetic protein receptor type 1 |
| CD90/Thy1 | Cluster of differentiation 90 |
| CDKs | Cyclin-dependent kinases |
| CNPase | Glial markers 2’, 3’-cyclic nucleotide 3’-phosphodiesterase |
| Col1a1 | Collagen, type I, alpha 1 |
| CXCL12 | Chemokine C-X-C motif ligand 12 |
| Dmp1 | Dentin matrix acidic phosphoprotein 1 |
| Dspp | Dentin sialophosphoprotein |
| FGF2 | Fibroblast growth factor 2 |
| GAD | Glutamic acid decarboxylase |
| Gli1 | Glioma-associated oncogene homolog 1 |
| Hh | Hedgehog |
| IFT140 | Intraflagellar Transport 140 |
| KFL4 | Kruppel-like factor 4 |
| NeuN | Neuronal nuclear antigen |
| NFM | Neurofilament M |
| NG2 | Neural/glial antigen 2 |
| NSE | Neuron-specific enolase |
| Ocn/Bglap | Osteocalcin |
| Osx/Sp7 | Osterix |
| P0 | Protein 0 |
| Pdgfrβ | Platelet derived growth factor receptor beta |
| PLP1 | Proteolipid protein 1 |
| PPR | Parathyroid hormone / Parathyroid hormone-related protein receptor |
| PRC1 | Polycomb repressive complex 1 |
| Prrx1 | Paired related homeobox 1 |
| Prrx2 | Paired related homeobox 2 |
| PTHrP | Parathyroid hormone-related protein |
| Raptor/mTORC1 | Regulatory-associated protein of mTOR /Mammalian target of rapamycin complex 1 |
| Sirt1 | Sirtuin 1 |
| Sox10 | SRY-box transcription factor 10 |
| TGFβ | Transforming growth factor beta |
| TrkA | Tropomyosin receptor kinase A |
| Wls | Wntless |
| Wnt1 | Wnt family member 1 |
Funding
This work was supported by grants from the National Institutes of Health grants (DE027421 to W.O., DE026666 to N.O.,) and American Association of Orthodontists Foundation Postdoctoral Research Award (to W.O.)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
References
- Abe S, Yamaguchi S, Amagasa T (2007) Multilineage cells from apical pulp of human tooth with immature apex. Oral Science International 4:45–58 [Google Scholar]
- Abe S, Yamaguchi S, Watanabe A, Hamada K, Amagasa T (2008) Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun 371:90–93 [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F (2010) Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes. Cell Mol Life Sci 67:3037–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P (2009) Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139:366–379 [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Furlan A, Zinin N, Aranda S, Kitambi SS, Blanchart A, Favaro R, Nicolis S, Lubke M, Muller T, Birchmeier C, Suter U, Zaitoun I, Takahashi Y, Ernfors P (2012) Sox2 and Mitf cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z, Akily B, Sabalic M, Zong G, Chai Y, Sharpe PT (2018a) Regulation of mesenchymal stem to transit-amplifying cell transition in the continuously growing mouse incisor. Cell reports 23:3102–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z, Sabalic M, Bloomquist RF, Fowler TE, Streelman T, Sharpe PT (2018b) A quiescent cell population replenishes mesenchymal stem cells to drive accelerated growth in mouse incisors. Nature communications 9:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew JG, Hoyland JA, Freemont AJ, Marsh DR (1995) Platelet-derived growth factor expression in normally healing human fractures. Bone 16:455–460 [DOI] [PubMed] [Google Scholar]
- Antoniades HN, Scher CD, Stiles CD (1979) Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A 76:1809–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb R, Chandrasekaran D, Carvalho Moreno Neves V, Sharpe PT (2017) Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/beta-catenin signaling in response to tooth damage. Sci Rep 7:3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae CH, Kim TH, Ko SO, Lee JC, Yang X, Cho ES (2015) Wntless regulates dentin apposition and root elongation in the mandibular molar. J Dent Res 94:439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JM, Clarke JC, Rashid H, Adhami MD, McCullough K, Scott JS, Chen H, Sinha KM, de Crombrugghe B, Javed A (2018) Specificity Protein 7 Is Required for Proliferation and Differentiation of Ameloblasts and Odontoblasts. J Bone Miner Res 33:1126–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini A, Varum S, Mateos JM, Bettosini D, John N, Bonalli M, Ziegler U, Dimou L, Clevers H, Furrer R, Sommer L (2015) Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell 16:314–322 [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M (1993) Segregation of cell lineage in the neural crest. Curr Opin Genet Dev 3:641–647 [DOI] [PubMed] [Google Scholar]
- Cai S, Zhang W, Chen W (2016) PDGFRbeta(+)/c-kit(+) pulp cells are odontoblastic progenitors capable of producing dentin-like structure in vitro and in vivo. BMC Oral Health 16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, Xiao Y, De Crombrugghe B, Somerman MJ, Feng JQ (2012) Genetic evidence for the vital function of Osterix in cementogenesis. Journal of Bone and Mineral Research 27:1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nor JE (2010) Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res 89:603–608 [DOI] [PubMed] [Google Scholar]
- Chai Y, Ito Y, Han J (2003) TGF-β signaling and its functional significance in regulating the fate of cranial neural crest cells. Critical Reviews in Oral Biology & Medicine 14:78–88 [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127:1671–1679 [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S (2005) NG2 can be used to identify arteries versus veins enabling the characterization of the different functional roles of arterioles and venules during microvascular network growth and remodeling. Microcirculation (New York, NY: 1994) 12:539. [DOI] [PubMed] [Google Scholar]
- Chen G, Ishan M, Yang J, Kishigami S, Fukuda T, Scott G, Ray MK, Sun C, Chen SY, Komatsu Y, Mishina Y, Liu HX (2017) Specific and spatial labeling of P0-Cre versus Wnt1-Cre in cranial neural crest in early mouse embryos. Genesis 55: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Gluhak-Heinrich J, Wang Y, Wu Y, Chuang H, Chen L, Yuan G, Dong J, Gay I, MacDougall M (2009) Runx2, osx, and dspp in tooth development. Journal of dental research 88:904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieruzzi M, Pagano S, Moretti S, Pinna R, Milia E, Torre L, Eramo S (2016) Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials (Basel) 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon AM, Castillo-Dali G, Gomez E, Guilbert T, Lesieur J, Nicoletti A, Acuna-Mendoza S, Letourneur D, Chaussain C, Rochefort GY, Poliard A (2019) Mouse Wnt1-CRE-Rosa(Tomato) Dental Pulp Stem Cells Directly Contribute to the Calvarial Bone Regeneration Process. Stem Cells 37:701–711 [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969 [DOI] [PubMed] [Google Scholar]
- Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Péault B (2009) Perivascular multipotent progenitor cells in human organs. Annals of the New York Academy of Sciences 1176:118–123 [DOI] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen CW, Peault B (2011) Multilineage stem cells in the adult: a perivascular legacy? Organogenesis 7:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14:1162–1171 [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- Dong R, Yao R, Du J, Wang S, Fan Z (2013) Depletion of histone demethylase KDM2A enhanced the adipogenic and chondrogenic differentiation potentials of stem cells from apical papilla. Exp Cell Res 319:2874–2882 [DOI] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP (1994) Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development 120:2213–2224 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Yang X (2011) Genetic mouse models for bone studies—strengths and limitations. Bone 49:1242–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges J-C, Alliot-Licht B, Renard E, Ducret M, Gaudin A, Smith AJ, Cooper PR (2015) Dental pulp defence and repair mechanisms in dental caries. Mediators of inflammation 2015: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges JC, Keller JF, Carrouel F, Durand SH, Romeas A, Bleicher F, Lebecque S, Staquet MJ (2009) Odontoblasts in the dental pulp immune response. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 312:425–436 [DOI] [PubMed] [Google Scholar]
- Feng J, Jing J, Li J, Zhao H, Punj V, Zhang T, Xu J, Chai Y (2017) BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development 144:2560–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT (2011) Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A 108:6503–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Matsubara K, Sakai K, Ito M, Ohno K, Ueda M, Yamamoto A (2015) Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res 1613:59–72 [DOI] [PubMed] [Google Scholar]
- GEORGE A, Eapen A (2015) Dentin phosphophoryn in the matrix activates AKT and mTOR signaling pathway to promote preodontoblast survival and differentiation. Frontiers in physiology 6:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass II DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental cell 8:751–764 [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ (2004) Cells and Extracellular Matrices of Dentin and Pulp: A Biological Basis for Repair and Tissue Engineering. Crit Rev Oral Biol Med 15:13–27 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Miescher I, Shakhova O, Suter U, Chin L, Taketo M, Richardson WD, Kessaris N, Sommer L (2012) Temporal control of neural crest lineage generation by Wnt/beta-catenin signaling. Development 139:2107–2117 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kim H, Tsuboi N, Yamamoto A, Akiyama S, Shi Y, Katsuno T, Kosugi T, Ueda M, Matsuo S, Maruyama S (2015) Therapeutic Potential of Stem Cells from Human Exfoliated Deciduous Teeth in Models of Acute Kidney Injury. PLoS One 10:e0140121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I, Bronckaers A (2013) Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell and tissue research 353:65–78 [DOI] [PubMed] [Google Scholar]
- Hu YS, Zhou H, Kartsogiannis V, Eisman JA, Martin TJ, Ng KW (1998) Expression of rat homeobox gene, rHOX, in developing and adult tissues in mice and regulation of its mRNA expression in osteoblasts by bone morphogenetic protein 2 and parathyroid hormone-related protein. Molecular Endocrinology 12:1721–1732 [DOI] [PubMed] [Google Scholar]
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janebodin K, Horst OV, Ieronimakis N, Balasundaram G, Reesukumal K, Pratumvinit B, Reyes M (2011) Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PloS one 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho E-h, Zhang T, Domon C, Joo C-K, Freund J-N, Costantini F (2002) Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and cellular biology 22:1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Fan W, Deng Q, He H, Huang F (2019) Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed Res Int 2019:6104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513:551–554 [DOI] [PubMed] [Google Scholar]
- Kim BC, Jun SM, Kim SY, Kwon YD, Choe SC, Kim EC, Lee JH, Kim J, Suh JF, Hwang YS (2017) Engineering three dimensional micro nerve tissue using postnatal stem cells from human dental apical papilla. Biotechnol Bioeng 114:903–914 [DOI] [PubMed] [Google Scholar]
- Kim J-K, Baker J, Nor JE, Hill EE (2011) mTor plays an important role in odontoblast differentiation. Journal of endodontics 37:1081–1085 [DOI] [PubMed] [Google Scholar]
- Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P (2017) The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep 7:12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada Y, Yamane T, Kadota D, Isono K, Takakura N, Hayashi S, Yamazaki H (2012) Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS One 7:e46436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama N, Okubo Y, Nakao K, Bessho K (2009) Evaluation of pluripotency in human dental pulp cells. Journal of Oral and Maxillofacial Surgery 67:501–506 [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16:51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kumar V, Rattan V, Jha V, Pal A, Bhattacharyya S (2017) Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci Rep 7:15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liu M, Zhang S, Wan H, Zhang Q, Yue R, Yan X, Wang X, Wang Z, Sun Y (2018) Essential role of IFT140 in promoting dentinogenesis. Journal of dental research 97:423–431 [DOI] [PubMed] [Google Scholar]
- Lin C-S, Xin Z-C, Dai J, Lue TF (2013) Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histology and histopathology 28:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Feng J, Li J, Zhao H, Ho T-V, Chai Y (2015) An Nfic-hedgehog signaling cascade regulates tooth root development. Development 142:3374–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810 [DOI] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT (2010) Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Developmental dynamics: an official publication of the American Association of Anatomists 239:160–167 [DOI] [PubMed] [Google Scholar]
- Lu M-F, Cheng H-T, Kern MJ, Potter SS, Tran B, Diekwisch T, Martin JF (1999) prx-1 functions cooperatively with another paired-related homeobox gene, prx-2, to maintain cell fates within the craniofacial mesenchyme. Development 126:495–504 [DOI] [PubMed] [Google Scholar]
- Lu X, Beck GR Jr, Gilbert LC, Camalier CE, Bateman NW, Hood BL, Conrads TP, Kern MJ, You S, Chen H (2011) Identification of the homeobox protein Prx1 (MHox, Prrx-1) as a regulator of osterix expression and mediator of tumor necrosis factor α action in osteoblast differentiation. Journal of Bone and Mineral Research 26:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and cellular biology 22:1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Makino Y, Yamaza H, Akiyama K, Hoshino Y, Song G, Kukita T, Nonaka K, Shi S, Yamaza T (2012) Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS One 7:e51777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JF, Bradley A, Olson EN (1995) The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes & development 9:1237–1249 [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, Hallett SA, Link DC, Nagasawa T, Ono W (2020) A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nature Communications 11:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA (1992) The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell 69:581–595 [DOI] [PubMed] [Google Scholar]
- Mita T, Furukawa-Hibi Y, Takeuchi H, Hattori H, Yamada K, Hibi H, Ueda M, Yamamoto A (2015) Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav Brain Res 293:189–197 [DOI] [PubMed] [Google Scholar]
- Mitchell J, Hicklin D, Doughty P, Hicklin J, Dickert J Jr, Tolbert S, Peterkova R, Kern M (2006) The Prx1 homeobox gene is critical for molar tooth morphogenesis. Journal of dental research 85:888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24:155–165 [DOI] [PubMed] [Google Scholar]
- Murfee WL, Skalak TC, Peirce SM (2005) Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12:151–160 [DOI] [PubMed] [Google Scholar]
- Nagata M, Ono N, Ono W (2019) Mesenchymal Progenitor Regulation of Tooth Eruption: A View from PTHrP. Journal of Dental Research 0022034519882692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanci A (2017) Ten Cate’s Oral Histology-E-Book: Development, Structure, and Function. Elsevier Health Sciences [Google Scholar]
- Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V (2008) PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112:295–307 [DOI] [PubMed] [Google Scholar]
- Nicola FDC, Marques MR, Odorcyk F, Arcego DM, Petenuzzo L, Aristimunha D, Vizuete A, Sanches EF, Pereira DP, Maurmann N, Dalmaz C, Pranke P, Netto CA (2017) Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res 1663:95–105 [DOI] [PubMed] [Google Scholar]
- Novais A, Lesieur J, Sadoine J, Slimani L, Baroukh B, Saubamea B, Schmitt A, Vital S, Poliard A, Helary C, Rochefort GY, Chaussain C, Gorin C (2019) Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. Stem Cells Transl Med 8:844–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Oka K, Xu X, Sasaki T, Bringas P Jr., Chai Y (2007) Cell autonomous requirement for TGF-beta signaling during odontoblast differentiation and dentin matrix formation. Mech Dev 124:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono W, Sakagami N, Nishimori S, Ono N, Kronenberg HM (2016) Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nature communications 7:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma PJ, Martins J, Diogo P, Sequeira D, Ramos JC, Diogenes A, Santos JM (2019) Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl Sci-Basel 9: [Google Scholar]
- Palma PJ, Ramos JC, Martins JB, Diogenes A, Figueiredo MH, Ferreira P, Viegas C, Santos JM (2017) Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J Endod 43:1279–1287 [DOI] [PubMed] [Google Scholar]
- Pang YW, Feng J, Daltoe F, Fatscher R, Gentleman E, Gentleman MM, Sharpe PT (2016) Perivascular Stem Cells at the Tip of Mouse Incisors Regulate Tissue Regeneration. J Bone Miner Res 31:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil R, Kumar BM, Lee WJ, Jeon RH, Jang SJ, Lee YM, Park BW, Byun JH, Ahn CS, Kim JW, Rho GJ (2014) Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp Cell Res 320:92–107 [DOI] [PubMed] [Google Scholar]
- Peterson RE, Hoffman S, Kern MJ (2005) Opposing roles of two isoforms of the Prx1 homeobox gene in chondrogenesis. Developmental dynamics: an official publication of the American Association of Anatomists 233:811–821 [DOI] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Senior RM, Reed J, Griffin GL, Thomason A, Deuel TF (1988) In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med 167:974–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836–842 [DOI] [PubMed] [Google Scholar]
- Rakian A, Yang W-C, Gluhak-Heinrich J, Cui Y, Harris MA, Villarreal D, Feng JQ, MacDougall M, Harris SE (2013) Bone morphogenetic protein-2 gene controls tooth root development in coordination with formation of the periodontium. International journal of oral science 5:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nor JE (2010) SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89:791–796 [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155 [DOI] [PubMed] [Google Scholar]
- Sharpe PT (2016) Dental mesenchymal stem cells. Development 143:2273–2280 [DOI] [PubMed] [Google Scholar]
- Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 8:191–199 [DOI] [PubMed] [Google Scholar]
- Shi X, Mao J, Liu Y (2020) Concise review: Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan AJ, Smith AJ (2007) Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13:151–157 [DOI] [PubMed] [Google Scholar]
- Sloan AJ, Waddington RJ (2009) Dental pulp stem cells: what, where, how? Int J Paediatr Dent 19:61–70 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H (1995) Reactionary dentinogenesis. Int J Dev Biol 39:273–280 [PubMed] [Google Scholar]
- Smith AJ, Lesot H (2001) Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med 12:425–437 [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Zuliani T, Olejnik C, LeRoy H, Obriot H, Kerr-Conte J, Formstecher P, Bailliez Y, Polakowska RR (2008) Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem cells and development 17:1175–1184 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T (2006) Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25:977–988 [DOI] [PubMed] [Google Scholar]
- Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, Nasr-Esfahani MH, Baharvand H (2012) Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev 21:1794–1802 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Nagata M, Gupta A, Matsushita Y, Yamaguchi T, Mizuhashi K, Maki K, Ruellas AC, Cevidanes LS, Kronenberg HM (2019) Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proceedings of the National Academy of Sciences 116:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Ono N, Ono W (2017) The fate of Osterix-expressing mesenchymal cells in dental root formation and maintenance. Orthodontics & craniofacial research 20:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Ding G (2011) Swine dental pulp stem cells inhibit T-cell proliferation. Transplant Proc 43:3955–3959 [DOI] [PubMed] [Google Scholar]
- Tao H, Lin H, Sun Z, Pei F, Zhang J, Chen S, Liu H, Chen Z (2019) Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-beta Signaling Pathway and Interaction With Histone Acetylation. J Bone Miner Res 34:1502–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatullo M, Marrelli M, Shakesheff KM, White LJ (2015) Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med 9:1205–1216 [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brouwer A, Korving J, Martin JF, Meijlink F (1998) Prx1 and Prx2 in skeletogenesis: roles in the craniofacial region, inner ear and limbs. Development 125:3831–3842 [DOI] [PubMed] [Google Scholar]
- Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116:1647–1648 [DOI] [PubMed] [Google Scholar]
- Trainor PA, Ariza-McNaughton L, Krumlauf R (2002) Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science 295:1288–1291 [DOI] [PubMed] [Google Scholar]
- Tucker AS, Yamada G, Grigoriou M, Pachnis V, Sharpe PT (1999) Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development 126:51–61 [DOI] [PubMed] [Google Scholar]
- Vidovic I, Banerjee A, Fatahi R, Matthews BG, Dyment NA, Kalajzic I, Mina M (2017) alphaSMA-Expressing Perivascular Cells Represent Dental Pulp Progenitors In Vivo. J Dent Res 96:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovic-Zdrilic I, Vijaykumar A, Mina M (2019) Activation of alphaSMA expressing perivascular cells during reactionary dentinogenesis. Int Endod J 52:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovic-Zdrilic I, Vining KH, Vijaykumar A, Kalajzic I, Mooney DJ, Mina M (2018) FGF2 Enhances Odontoblast Differentiation by alphaSMA(+) Progenitors In Vivo. J Dent Res 97:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JV, Zhuang H, Singer D, Illsley CS, Kok WL, Sivaraj KK, Gao Y, Bolton C, Liu Y, Zhao M (2019) Transit amplifying cells coordinate mouse incisor mesenchymal stem cell activation. Nature communications 10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu Z, Wu J, Mei Y, Zhang Q, Zhang H, Miao D, Sun W (2019) Sirt1 Promotes Osteogenic Differentiation and Increases Alveolar Bone Mass via Bmi1 Activation in Mice. J Bone Miner Res 34:1169–1181 [DOI] [PubMed] [Google Scholar]
- Wang H, Lv C, Gu Y, Li Q, Xie L, Zhang H, Miao D, Sun W (2018) Overexpressed Sirt1 in MSCs Promotes Dentin Formation in Bmi1-Deficient Mice. Journal of dental research 97:1365–1373 [DOI] [PubMed] [Google Scholar]
- Wang SK, Komatsu Y, Mishina Y (2011) Potential contribution of neural crest cells to dental enamel formation. Biochem Biophys Res Commun 415:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sha XJ, Li GH, Yang FS, Ji K, Wen LY, Liu SY, Chen L, Ding Y, Xuan K (2012) Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol 57:1231–1240 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cox MK, Coricor G, MacDougall M, Serra R (2013) Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Developmental biology 382:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Bailes JA, McMahon AP (1987) Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell 50:79–88 [DOI] [PubMed] [Google Scholar]
- Xie F, Dai Q, Liu X, Wang J (2019) Conditional knockout of Raptor/mTORC1 results in dentin malformation. Frontiers in physiology 10:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y (2018) Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med 10: [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nakamura-Yamada S, Kusano K, Baba S (2019) Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci 20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, Hitoshi Y, Suzuki M, Osuzu F, Kuratani S, Yamamura K (1999) A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol 212:191–203 [DOI] [PubMed] [Google Scholar]
- Yamaza T, Alatas FS, Yuniartha R, Yamaza H, Fujiyoshi JK, Yanagi Y, Yoshimaru K, Hayashida M, Matsuura T, Aijima R, Ihara K, Ohga S, Shi S, Nonaka K, Taguchi T (2015) In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res Ther 6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokose S, Kadokura H, Tajima N, Hasegawa A, Sakagami H, Fujieda K, Katayama T (2004) Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res 315:375–384 [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y (2007) Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell 99:465–474 [DOI] [PubMed] [Google Scholar]
- Zhang QZ, Nguyen AL, Yu WH, Le AD (2012) Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. J Dent Res 91:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Van Kuppevelt TH, Daamen WF, Van Damme PA, Bian Z, Jansen JA (2008) In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. Journal of tissue engineering and regenerative medicine 2:117–125 [DOI] [PubMed] [Google Scholar]
- Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y (2014) Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14:160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang L, Jin Y, Shi S (2012) Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. Journal of dental research 91:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]


