Abstract
Glacier ice is an extreme environment in which most animals cannot survive. Here we report the colonization of high elevation, climate-threatened glaciers along New Zealand’s southwestern coast by species of Arthropoda, Nematoda, Platyhelminthes, Rotifera and Tardigrada. Based on DNA barcoding and haplotype-inferred evidence for deep genetic variability, at least 12 undescribed species are reported, some of which have persisted in this niche habitat throughout the Pleistocene. These findings identify not only an atypical biodiversity hotspot but also highlight the adaptive plasticity of microinvertebrate Animalia.
Subject terms: Evolution, Zoology
Introduction
Glacier ecosystems are an inhospitable environment for most animals. The cumulative weight of overlying snow/ice compresses deep subsurface ice to densities > 900 kg/m3, effectively excluding physical space for even the smallest single-celled microbes1, 2. Prior to compression, however, upper layers of ice (i.e., weathered surface and several metres below) maintain ultrastructural spaces between crystal interfaces, forming arrays of microchannels that connect with the glacial surface3, 4. On maritime glaciers, those most threatened by our changing global climate5–7, ultrathin films of water fill these veinous aquifers and provide a microenvironment for extremophilic life. Permanently cold temperatures (0 °C and below), high UV radiation, nutrient-poor and hydrologically-limiting conditions constrain organismal diversity in this habitat to specialized psychrophilic taxa, predominantly single-celled microbes8–11.
Wright (1887) discovered the first glacially-obligate, multicellular animal—the glacier ice worm, Mesenchytraeus solifugus (phylum Annelida)–inhabiting Muir Glacier, Alaska12, 13, thereafter reported on glaciers throughout the Pacific Northwest14, 15. These worms inhabit glacier ice above the equilibrium line altitude (ELA), which separates snow accumulation and ablation zones, respectively. Ice worms also appear occasionally in meltwater pools common within the ablation zone (e.g., cryoconite holes), which support multiple trophic levels across domains of life including apex meiofauna16–18. More recently, two species of bdelloid Rotifera were discovered on maritime, Icelandic glaciers, identifying the second known animal phylum with representatives inhabiting glacier ice19.
Coastal glaciers in New Zealand’s Southern Alps are exceptional in that they descend steeply into native rainforest and experience particularly high levels of orographic precipitation20–22. Moreover, predominant oceanic westerlies channel wind up river valleys, leading to turbulent mixing of organic and inorganic debris23, 24. Significantly, glaciers in the Southern Alps advanced between 1983 and 2008 as a consequence of anthropogenic regional cooling25, but are now in rapid retreat comparable with glacial melting worldwide5, 26, 27.
The unusual climatology and geomorphology of the region, coupled with its proximity to rich sources of biodiversity in lower rainforests, prompted us to survey accessible glaciers in the region for animal life. We show here that taxa representing five animal phyla co-occur on Southern Alps, New Zealand glaciers, four of which (Arthropoda, Nematoda, Platyhelminthes, Tardigrada) are not reported previously in glacier ice.
Results and discussion
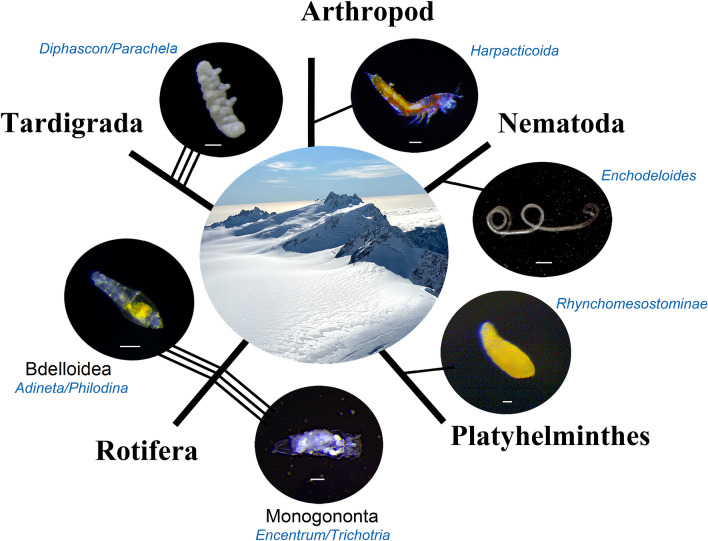
Pilot collections during mid-summer on Fox and Franz Joseph Glaciers (− 43.5319, 170.1268 and − 43.4902, 170.2408, respectively, Feb. 10, 2020) led to the identification of bdelloid Rotifera and Tardigrada populations. We returned in late autumn (April 28, 2020) following snowfalls totaling ~ 1 m, to collect ~ 80 L of surface ice from a single sampling location at three respective field sites along a NE SW transect spanning ~ 35 km: Whataroa Glacier (− 43.4002, 170.5231; 4,859 ft), Franz Joseph Glacier (− 43.6575, 170.2374; 6,890 ft) and Fox Glacier (− 43.5331, 170.1271; 6,483 ft), all above the ELA (Fig. 1). At each field site, the upper snow layer (~ 1 m) was removed to expose ~ 1 m2 of hard surface ice, corresponding to that year’s weathered crust. The upper ~ 10 cm were chipped away, collected and processed for microinvertebrates accordingly. In total, > 5,000 individual, glacier animals were observed—mostly alive–in laboratory cultures, representing five animal phyla (Fig. 2; Table S1, Suppl. Info.): Arthropoda (Crustacea), Nematoda, Platyhelminthes, Rotifera (Bdelloid and Monogononta) and Tardigrada. Animal designations were based on morphology and closest alignments with deposited GenBank sequences, with the caveat that the global database is incomplete. Nonetheless, some Antarctic ancestries can be inferred (e.g., bdelloid rotifers, nematodes, tardigrades), with a likely mechanism of passive, global dispersal (e.g., windblown, avian)28–32.
Figure 1.
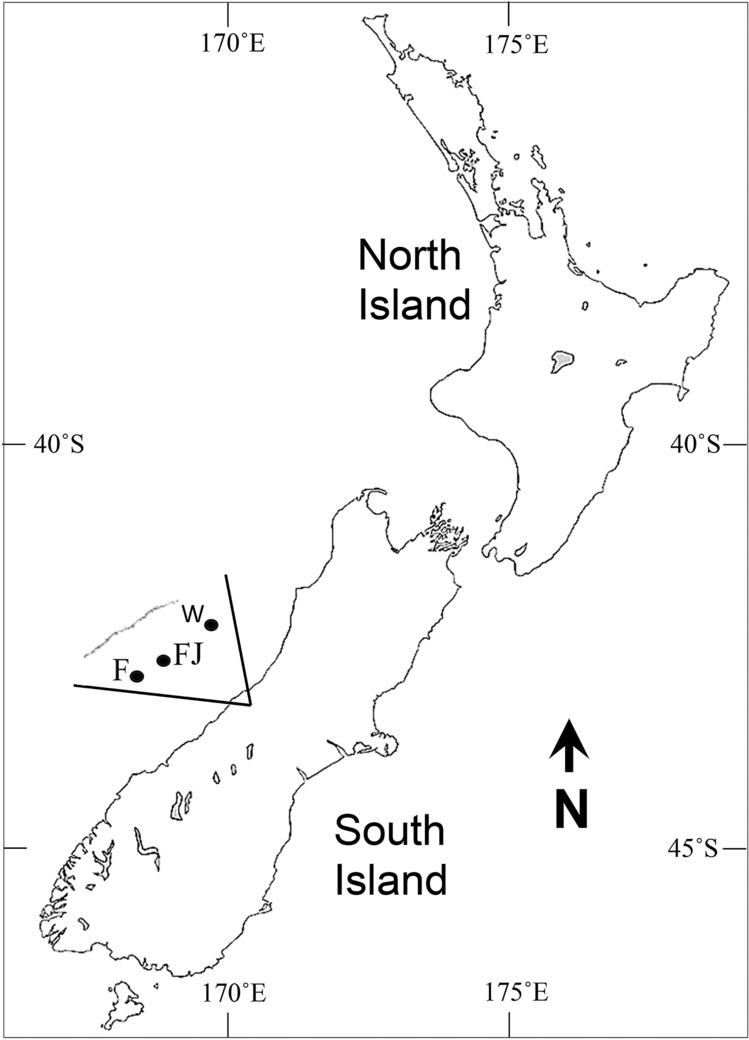
Localities of New Zealand glacier field sites. Collections were made on April 28, 2020, along a NE SW transect spanning ~ 35 km, from Whataroa (W; − 43.4002, 170.5231; 4,859 ft) to Franz Joseph (FJ; − 43.6575, 170.2374; 6,890 ft) to Fox (F; − 43.5331, 170.1271; 6,483 ft) Glaciers. North is up.
Figure 2.
Animals in glacier ice collected from New Zealand’s Southern Alps. Species from five metazoan phlya are represented: Arthropoda, Nematoda, Platyhelminthes, Rotifera (with Classes Bdelloidea and Monogononta) and Tardigrada, collected from Fox, Franz Joseph and Whataroa Glaciers, respectively. At least 12 new species were identified, indicated by lines connected to respective images (e.g., three species of Tardigrada, etc.); genera designations were estimated by nuclear and mitochondrial barcoding in comparison with closest GenBank matches (see Table 1, Suppl. Info.). Central image shows the accumulation zone at the Franz Joseph Glacier collection site, 6,890 ft asl, just west of the continental ridge. Scale bars = 50 μm.
Tardigrades were the dominant taxon across field sites, observed at densities between ~ 7–40 individuals/L; bdelloid rotifers and nematodes occurred at densities up to 3–4 individuals/L, while remaining taxa were less abundant (Table S2, Suppl. Info.). Additionally, an arachnid (Acari) and springtail (Collembola) were observed on the waters’ surface in laboratory cultures and likely reside on the glacial surface (Fig. S1, Suppl. Info.). All of the aforementioned animals were observed at the three respective field sites, respectively, suggesting that they comprise subpopulations along the southwestern coast, consistent with historical glacial dynamics and ice connectivity33–35.
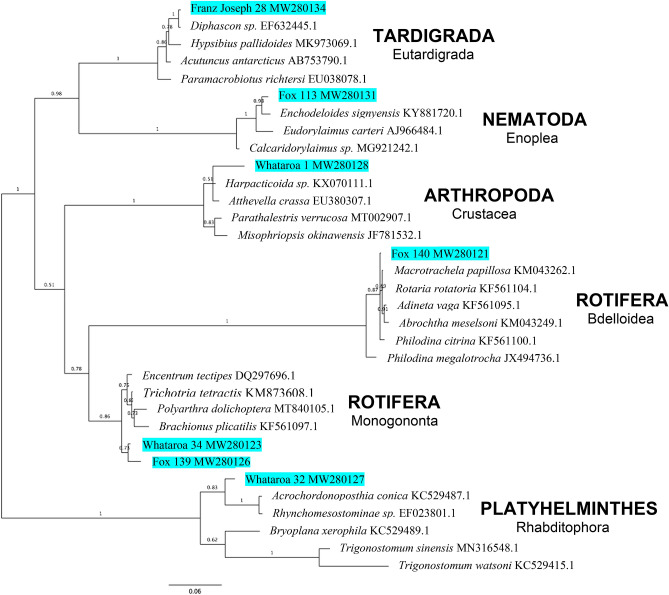
Animal specimens were captured with a fine pipet, transferred individually and DNA barcoded using nuclear 18S ribosomal RNA (rRNA)36 and mitochondrial cytochrome c oxidase subunit 1 (CO1)37 primers. More than 90 individual specimens across the five animal phyla were processed identifying at least 12 putative species (Fig. 2; Fig. S2, Suppl. Info.), all of which appear new to science and, with the exception of bdelloid Rotifera19, not previously reported in glacier ice. Species boundaries were estimated using previously proposed thresholds of sequence divergence for nuclear and mitochondrial barcoding (e.g., ~ 10% divergence at CO138, 39; 0.5–1% at 18S40) coupled with 18S rRNA Bayesian phylogeny across glacial phyla and related species (Fig. 3), collectively supporting the designation of discovered taxa as undescribed species (formal taxonomic descriptions of new taxa will be reported elsewhere).
Figure 3.
Midpoint-rooted Bayesian phylogeny across animal phyla based on 18S rRNA sequences. Blue highlighted taxa identify representative glacial specimens (designated by glacier followed by isolate number and GenBank accession) discovered in the current study. Related sequences with GenBank accession numbers appear in respective clades. Values along branches indicate node posterior probability (node support) and range from 0 to 1. Phylum and Class taxonomic designations are to the right.
The onset of New Zealand glaciation occurred in the late Pliocene41–43. By applying a mitochondrial divergence rate of approximately 2% per million years for invertebrate taxa15, 19, 44, many species identified within respective phyla diverged prior to the onset of glaciation and arrived independently upon the onset of glaciation, while other putative species pairs (often found in sympatry) are more shallowly divergent and appear to have speciated thereafter. For instance, up to seven putative species of tardigrades are recognized using mitochondrial DNA divergence thresholds of 3%45, 46 (Fig. S2, Suppl. Info.), with divergence estimates that pre- and postdate glaciation. Note that such divergence thresholds (i.e., 3–10%) do not always estimate species diversity accurately and thus detailed taxonomic treatment of specimens is required to evaluate the extent of putative new species identified here. Representative haplotype networks for single- (Nematoda) and multispecies complexes (Tardigrada) (Fig. 4), suggest that glacier animals have and continue to disperse actively between coastal glaciers over geological time; moreover, mitochondrial DNA divergence patterns (Fig. 4) (i.e., exceeding previously proposed species boundary thresholds; Table S3, Suppl. Info.) support the persistence of these glacier animals throughout the Pleistocene.
Figure 4.
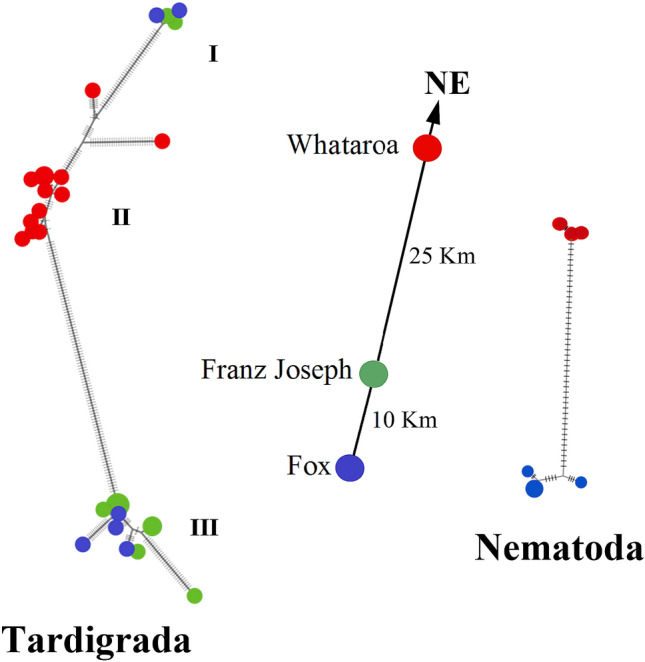
Haplotype networks depicting evolutionary relationships within Tardigrada and Nematoda populations. Each coloured circle represents a haplotype (i.e., a unique DNA sequence in a population) with radius proportional to number of individuals, collected along a NE transect at Fox, Franz Joseph and Whataroa Glaciers, accordingly (distance between glacier field sites indicated). The mitochondrial CO1 locus was successfully amplified in 29 tardigrades (with 25 haplotypes; GenBank accessions MW262004-MW262032) and eight nematodes (with six haplotypes; GenBank accessions MW262759-MW262766). DNA sequences were aligned in MEGA655 and analyzed by HaplowebMaker59. Ticks along connector lines (edges) indicate mutational steps between individuals. Tardigrade clusters I and III represent separate species by delimitation criteria (~ 10% divergence at CO1 from cluster II; ~ 20% divergence between clusters I and III) that co-occur on Fox and Franz Joseph Glaciers, and appear to disperse actively between these two glaciers. Nematodes on Fox and Whataroa Glaciers, 35 km apart, displayed ~ 7% divergence at CO1, with no apparent gene flow between populations.
The unexpected discovery of such animal diversity in New Zealand’s Southern Alps raises two important evolutionary questions. First, does this habitat represent an anomalous ecosystem that is driven by rainforest proximity and turbulent climatic winds, or does comparable animal diversity occur in glaciers worldwide? Limited data is available to assess this question, but to date North American and Icelandic glaciers appear restricted to monophylum animal representatives (Annelida and Rotifera, respectively) above the ELA15, 19. Secondly, the independent evolution of disparate animal phyla to the harsh and physiologically challenging conditions of glacial life above the ELA highlights the adaptive plasticity among microinvertebrate Animalia, raising the question of whether convergent mechanism(s) and/or novel biological strategies have facilitated their respective transitions into glacier ice. Previous studies show that glacier residents across domains of life8, 47, and particularly the North American glacier ice worm8, 48, 49, display enhanced purine anabolism that may compensate for cold temperature stress and lethargy50–52; this putative metabolic contribution to other glacial fauna remains an intriguing unknown, but now a testable hypothesis.
Methods
Specimen collection
Ice samples were taken from the top ~ 10 cm of glacier surfaces, chipped away and collected with EtOH-sterilized field equipment (shovels, picks) that were washed thoroughly between collections. Glacier ice was stored in 20 L plastic containers, transported to the University of Otago and thawed slowly at 4 °C over several days. To observe microinvertebrate specimens, melted glacier water was gravity filtered through Whatman 1 paper employing a Bückner funnel, viewed by stereomicroscopy and sorted into phylogroups based on morphology. Images were captured with a Leica M205 C stereomicroscope using LAS software.
DNA extraction and PCR
Individual microinvertebrates were captured in 1–3 μl of glacier meltwater using a fine pipet and transferred into 7 μl of 70% EtOH for storage. To extract DNA, EtOH was evaporated on a 65 °C heat block for ~ 5 min with lid open, and 10 μl of a solution containing 25 mM Tris pH 8.5, 50 mM KCl, 5 mM MgCl2, Proteinase K (20 μg/μl) was added. Following incubation at 55 °C for 20 min, Proteinase K was inactivated by heating at 95 °C for 2 min and 1 μl was removed for polymerase chain reaction (PCR) analysis. DNA samples representing individual glacier specimens are archived in the laboratory of PKD. PCR reactions contained 1X Takara mix (Takara, Japan), 0.4 μM respective barcode primers [18S rRNA36; cytochrome c oxidase subunit 1 (CO1)37], 1 μl template in a total reaction volume of 25 μl balanced with H2O. Primers were: 18S2a-GATCCTTCCGCAGGTTCACC, 18S11b-GTCAGAGGTTCGAAGGCG36; HCO-TAAACTTCAGGGTGACCAAAAAATCA, LCO-GGTCAACAAATCATAAAGATATTGG37, respectively. Conditions for PCR were 95 °C for 2 min, then 94 °C (20 s)/45 °C for CO1, 54 °C for 18S rRNA (40 s)/72 °C (45 s) for 35 cycles, then 72 °C for 5 min. Aliquots were run on 0.8% agarose gels with EtBr and visualized by UV light. Positive samples were sequenced on both strands with respective PCR primers at the Genetics Services Facility (University of Otago, Dunedin).
DNA sequence and data analyses
Sanger-sequenced DNA chromatograms were assembled and trimmed to remove primer sequence and low-quality base reads using 4 Peaks software53. BLASTn searches of assembled and cleaned sequences against the GenBank non-redundant nucleotide database were performed in 4 Peaks. New multi-sequence alignments were created within each phylum by combining new sequences with existing sequences drawn from Genbank (Suppl. Info., Table S1) using MAFFT v7.45054 employing default parameters. Using these alignments pairwise genetic distances were calculated by the Kimura 2-parameter correction in MEGA655. MrBayes v3.2.7a56 was used to infer phylogenetic relationships among animal taxa by the General Time Reversible model of molecular evolution with invariant sites and a gamma distribution of rates (GTR + I + G). MrBayes was run on the CIPRES Science Gateway57 for 100 million Metropolis-coupled Markov Chain Monte Carlo (MCMCMC) generations with one cold and three heated chains, sampling every 10,000 generations. The R package RWTY v1.0.158 was used to assess convergence of MCMCMC runs ensuring that the posterior sample was stationary and that the posterior sample of trees in independent runs recovered similar posterior probabilities for nodes. After evaluation, the first 50% of trees were removed as burn-in and the remaining sample was retained to infer a majority rule consensus tree. Haplotypes, as defined by unique CO1 sequences within a population, were created for each phylum by HaplowebMaker59 using default parameters (delimiter, mask error, radius proportion), and TCS software60, a Java program to estimate gene genealogies including multifurcations and/or reticulations by statistical parsimony61.
Supplementary Information
Acknowledgements
We thank Heather Purdie for logistic assistance. Collections were made under DOC concession number CA-31615-OTH, the project was supported by Rutgers Global Grant 302945 to DHS and PKD.
Author contributions
DHS and PKD conceived the study, DHS conducted field and laboratory work, PMN conducted initial fieldwork, KZ and AJG contributed to data analysis, ACG and PKD facilitated laboratory analyses. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83256-3.
References
- 1.Price PB. A habitat for psychrophiles in deep Antarctic ice. Proc. Natl. Acad. Sci. USA. 2000;97:1247–1251. doi: 10.1073/pnas.97.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mader HM, Pettitt ME, Wadham JL, Wolff EW, Parkes RJ. Subsurface ice as a microbial habitat. Geology. 2006;34:169–172. doi: 10.1130/G22096.1. [DOI] [Google Scholar]
- 3.Patterson WSB. The Physics of Glaciers. 3. Oxford: Elsevier; 1994. p. 480. [Google Scholar]
- 4.Nye JF. Lecture notes on water in ice: microscopic and geophysical scales. In: Wettlaufer JS, Dash JG, Untersteiner N, editors. Ice Physics and the Natural Environment. Berlin: Springer; 1999. pp. 273–279. [Google Scholar]
- 5.Zemp M, Frey H, Gärtner-Roer I, Nussbaumer SU, Hoelzle M, Paul F, Haeberli W, Denzinger F, Ahlstrøm AP, Anderson B, Bajracharya S. Historically unprecedented global glacier decline in the early 21st century. J. Glaciol. 2015;61:745–762. doi: 10.3189/2015JoG15J017. [DOI] [Google Scholar]
- 6.Milner AM, Khamisa K, Battinc TJ, Brittaind JE, Barranda NE, Füredere L, Cauvy-Frauni S, Gíslasonh G, Jacobseni D, Hannaha DM, Hodsonj AJ, Hoodk E, Lencionil V, Olafsson JS, Robinsonn CT, Trantero M, Brown LE. Glacier shrinkage driving global changes in downstream systems. Proc. Natl. Acad. Sci. USA. 2017;114:9970–9978. doi: 10.1073/pnas.1619807114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zawierucha K, Shain DH. Disappearing Kilimanjaro snow—are we the last generation to explore equatorial glacier biodiversity? Ecol. Evol. 2019;9:8911–8918. doi: 10.1002/ece3.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Napolitano MJ, Shain DH. Four kingdoms on glacier ice: convergent energetic processes boost energy levels as temperatures fall. Proc. R. Soc. B. (Suppl.) 2004;271:S273–S276. doi: 10.1098/rspb.2003.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Maayer P, Anderson D, Cary G, Cowan DA. Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep. 2014;15:508–517. doi: 10.1002/embr.201338170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boetius A, Anesio A, Deming J, Mikucki JA, Rapp JZ. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat. Rev. Microbiol. 2015;13:677–690. doi: 10.1038/nrmicro3522. [DOI] [PubMed] [Google Scholar]
- 11.Anesio AM, Lutz S, Chrismas N, Benning LG. The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes. 2017;3:10. doi: 10.1038/s41522-017-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright GF. The Muir Glacier. Am. J. Sci. 1887;33:5. [Google Scholar]
- 13.Emery C. Diagnosi di un nuovi genere e nuova specie di annelidi della famiglia degli Enchytraeidae. Atti R. Accademei Lincei. 1898;5:100–111. [Google Scholar]
- 14.Tynen MJ. The geographical distribution of ice worms (Oligochaeta: Enchytraeidae) Can. J. Zool. 1970;48:1363–1367. doi: 10.1139/z70-233. [DOI] [Google Scholar]
- 15.Dial CR, Dial RJ, Saunders R, Lang SA, Tetreau MD, Lee B, Wimberger P, Dinapoli MS, Egiazarov AS, Gipple SL, Maghirang MR, Swartley-McArdle DJ, Yudkovitz SR, Shain DH. Historical biogeography of the North American glacier ice worm, Mesenchytraeus solifugus (Annelida: Oligochaeta: Enchytraeidae) Mol. Phylogenet. Evol. 2012;63:577–584. doi: 10.1016/j.ympev.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Wharton RA, McKay CP, Simmons GM, Parker BC. Cryoconite holes on glaciers. Bioscience. 1985;35:499–503. doi: 10.2307/1309818. [DOI] [PubMed] [Google Scholar]
- 17.Zawierucha K, Kolicka M, Takeuchi N, Kaczmarek L. What animals can live in cryoconite holes? A faunal review. J. Zool. 2015;295:159–169. doi: 10.1111/jzo.12195. [DOI] [Google Scholar]
- 18.Zawierucha K, Porazinska DL, Ficetola GF, Ambrosini R, Baccolo G, Buda J, Ceballos JL, Devetter M, Dial R, Franzetti A, Fuglewicz U, Gielly L, Łokas E, Janko K, Jaromerska T, Kościński A, Kozłowska A, Ono M, Parnikoza I, Pittino F, Poniecka E, Sommers P, Schmidt PK, Shain DH, Sikorska S, Uetake J, Takeuchi N. A hole in the nematosphere: tardigrades and rotifers dominate the cryoconite hole environment, whereas nematodes are missing. J. Zool. 2020 doi: 10.1111/jzo.12832. [DOI] [Google Scholar]
- 19.Shain DH, Halldórsdóttir K, Pálsson F, Aðalgeirsdóttir G, Gunnarsson A, Jónsson T, Lang SA, Pálsson HS, Steinþórssson S, Arnason E. Colonization of maritime glacier ice by bdelloid Rotifera. Mol. Phylog. Evol. 2016;98:280–287. doi: 10.1016/j.ympev.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Henderson, R.D. Extreme storm rainfalls in the Southern Alps, New Zealand. Proc., Joint IAMAP-IAHS Symp. on Extreme Hydrological Events: Precipitation, Floods and Droughts, Yokohama, Japan. International Association for Hydrological Sciences Publ.213, 113–120 (1993).
- 21.Chamberlain CP, Poage MA, Craw D, Reynolds RC. Topographic development of the Southern Alps recorded by the isotopic composition of authigenic clay minerals, South Island, New Zealand. Chem. Geol. 1999;155:279–294. doi: 10.1016/S0009-2541(98)00165-X. [DOI] [Google Scholar]
- 22.Sturman A, Wanner H. A comparative review of the weather and climate of the Southern Alps of New Zealand and the European Alps. Mt. Res. Dev. 2001;21:359–369. doi: 10.1659/0276-4741(2001)021[0359:ACROTW]2.0.CO;2. [DOI] [Google Scholar]
- 23.Sturman AP, Fitzsimmons SJ, Holland LM. Local winds in the Southern Alps, New Zealand. J. Climatology. 1985;5:145–150. doi: 10.1002/joc.3370050203. [DOI] [Google Scholar]
- 24.Knudson KP, Hendy IL, Neil HL. Re-examining Southern Hemisphere westerly wind behavior: Insights from a late Holocene precipitation reconstruction using New Zealand fjord sediments. Quatern. Sci. Rev. 2011;30:3124–3138. doi: 10.1016/j.quascirev.2011.07.017. [DOI] [Google Scholar]
- 25.Mackintosh A, Anderson B, Lorrey A, Renwick JA, Frei P, Dean SM. Regional cooling caused recent New Zealand glacier advances in a period of global warming. Nat. Commun. 2017;8:14202–14215. doi: 10.1038/ncomms14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putnam AE, Schaefer JM, Denton GH, Barrell DJA, Andersen BG, Koffman TNB, Rowan AV, Finkel RC, Rood DH, Schwartz R, Vandergoes MJ, Plummer MA, Brocklehurst SH, Kelley SE, Ladig KL. Warming and glacier recession in the Rakaia valley, Southern Alps of New Zealand, during Heinrich Stadial 1. Earth Planet. Sci. Lett. 2013;382:98–110. doi: 10.1016/j.epsl.2013.09.005. [DOI] [Google Scholar]
- 27.Purdie H, Anderson B, Chinn T, Owens I, Mackintosh A, Lawson W. Franz Josef and Fox Glaciers, New Zealand: Historic length records. Global Planet. Change. 2014;121:41–52. doi: 10.1016/j.gloplacha.2014.06.008. [DOI] [Google Scholar]
- 28.Maguire B. The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water. Ecol Monogr. 1963;33:161–185. doi: 10.2307/1948560. [DOI] [Google Scholar]
- 29.Figuerola J, Green AJ. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw. Biol. 2002;47:483–494. doi: 10.1046/j.1365-2427.2002.00829.x. [DOI] [Google Scholar]
- 30.Ptatscheck C, Gansfort B, Traunspurger W. The extent of wind-mediated dispersal of small metazoans, focusing nematodes. Sci. Rep. 2018;8:6814–6824. doi: 10.1038/s41598-018-24747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontaneto D. Long-distance passive dispersal in microscopic aquatic animals. Mov. Ecol. 2019;7:10–20. doi: 10.1186/s40462-019-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotaling S, Shain DH, Lang SA, Bagley RK, Tronstad LM, Weisrock DW, Kelley JL. Long-distance dispersal, ice sheet dynamics, and mountaintop isolation underlie the genetic structure of glacier ice worms. Proc. R. Soc. B. 2019;286:20190983. doi: 10.1098/rspb.2019.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzsimons SJ. Late-glacial and early Holocene glacier activity in the Southern Alps, New Zealand. Quatern. Int. 1997;38–39:69–76. doi: 10.1016/S1040-6182(96)00022-5. [DOI] [Google Scholar]
- 34.Suggate RP, Almond PC. The Last Glacial Maximum (LGM) in western South Island, New Zealand: implications for the global LGM and MIS 2. Quatern. Sci. Rev. 2005;24:1923–1940. doi: 10.1016/j.quascirev.2004.11.007. [DOI] [Google Scholar]
- 35.Schaefer JM, Putnam AE, Denton GH, Kaplan MR, Birkel S, Doughty AM, Kelley S, Barrell DJA, Finkel RC, Winckler G, Anderson RF, Ninneman US, Barker S, Schwartz R, Andersen BG, Schlüchter C. The Southern Glacial Maximum 65,000 years ago and its Unfinished Termination. Quatern. Sci. Rev. 2015;114:52–60. doi: 10.1016/j.quascirev.2015.02.009. [DOI] [Google Scholar]
- 36.Horton DJ, Kershner MW, Blackwood CB. Suitability of PCR primers for characterizing invertebrate communities from soil and leaf litter targeting metazoan 18S ribosomal or cytochrome oxidase I (COI) genes. Eur. J. Soil Biol. 2017;80:43–48. doi: 10.1016/j.ejsobi.2017.04.003. [DOI] [Google Scholar]
- 37.Folmer O, Hoeh WR, Black MB, Vrijenhoek RC. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Mar. Biol. Biotech. 1994;3:294–299. [PubMed] [Google Scholar]
- 38.Erséus C, Gustaffson D. Cryptic speciation in clitellate model organisms. In: Shain DH, editor. Annelids in Modern Biology. Hoboken: Wiley Blackwell; 2009. pp. 31–46. [Google Scholar]
- 39.DeSalle R, Goldstein P. Review and interpretation of trends in DNA barcoding. Front. Ecol. Evol. 2019;7:302. doi: 10.3389/fevo.2019.00302. [DOI] [Google Scholar]
- 40.Tang CQ, Leasi F, Obertegger U, Kieneke A, Barraclough TG, Fontaneto D. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proc. Natl. Acad. Sci. 2012;109:16208–16212. doi: 10.1073/pnas.1209160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suggate RP. Late pliocene and quaternary glaciations of New Zealand. Quatern. Sci. Rev. 1990;9:175–197. doi: 10.1016/0277-3791(90)90017-5. [DOI] [Google Scholar]
- 42.McCulloch GA, Wallis GP, Waters JM. Onset of glaciation drove simultaneous isolation of alpine insects in New Zealand. Evolution. 2010;64:2033–2043. doi: 10.1111/j.1558-5646.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 43.Craw D, Upton P, Waters J, Wallis G. Biological memory of the first Pleistocene glaciation in New Zealand. Geology. 2017;45:595–598. doi: 10.1130/G39115.1. [DOI] [Google Scholar]
- 44.Knowlton N, Weigt LA. New date and new rate for divergence across the isthmus of Panama. Proc. R. Soc. Lond. B. 1998;265:2257–2263. doi: 10.1098/rspb.1998.0568. [DOI] [Google Scholar]
- 45.Bertolani R, Biserov V, Rebecchi L, Cesari M. Taxonomy and biogeography of tardigrades using an integrated approach: new results on species of the Macrobiotus hufelandi group. Invertebr. Zool. 2011;8:23–36. doi: 10.15298/invertzool.08.1.05. [DOI] [Google Scholar]
- 46.Cesari M, Guidetti R, Rebecchi L, Giovannini I, Bertolani R. A DNA barcoding approach in the study of tardigrades. J. Limnol. 2013;72:182–198. doi: 10.4081/jlimnol.2013.s1.e23. [DOI] [Google Scholar]
- 47.Amato P, Christner BC. Energy metabolism response to low-temperature and frozen conditions in Psychrobacter cryohalolentis. Appl. Environ. Microbiol. 2009;75:711–718. doi: 10.1128/AEM.02193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Napolitano MJ, Nagele RO, Shain DH. The ice worm, Mesenchytraeus solifugus, elevates adenylate levels at low physiological temperature. Comp. Biochem. Physiol. Part A. 2004;137:227–235. doi: 10.1016/j.cbpb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Lang SA, McIlroy PJ, Shain DH. Structural evolution of the glacier ice worm F1Fo ATP Synthase complex. Protein. J. 2020;39:152–159. doi: 10.1007/s10930-020-09889-x. [DOI] [PubMed] [Google Scholar]
- 50.Morrison BA, Shain DH. An AMP nucleosidase gene knockout in Escherichia coli elevates intracellular ATP levels and increases cold tolerance. Biol. Lett. 2008;4:53–56. doi: 10.1098/rsbl.2007.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parry BR, Shain DH. Manipulations of AMP metabolic genes increase growth rate and cold tolerance in Escherichia coli: Implications for psychrophilic evolution. Mol. Biol Evol. 2011;28:2139–2145. doi: 10.1093/molbev/msr038. [DOI] [PubMed] [Google Scholar]
- 52.Kotchoni SO, Gachomo EW, Slobodenko K, Shain DH. AMP Deaminase suppression increases biomass, cold tolerance and oil content in green algae. Algal Res. 2016;16:473–480. doi: 10.1016/j.algal.2016.04.007. [DOI] [Google Scholar]
- 53.Griekspoor, A., Groothuis, T. 4peaks. Available: mekentosj. Com (2006).
- 54.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller, M. A., Pfeiffer, W., Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA, 1–8.
- 58.Warren DL, Geneva AJ, Lanfear R, Rosenberg M. RWTY (R We There Yet): An R package for examining convergence of Bayesian phylogenetic analyses. Mol. Biol. Evol. 2017;34:1016–1020. doi: 10.1093/molbev/msw279. [DOI] [PubMed] [Google Scholar]
- 59.Spöri Y, Flot J. HaplowebMaker and CoMa: two web tools to delimit species using haplowebs and conspecificity matrices. Methods Ecol. Evol. 2020 doi: 10.1111/2041-210X.13454. [DOI] [Google Scholar]
- 60.Clement M, Posada D, Crandall KA. TCS: A computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 61.Templeton AR, Crandall KA, Sing CF. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics. 1992;132:619–633. doi: 10.1093/genetics/132.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.