Abstract
Understanding day-to-day variations in symptoms and medication management can be important in describing patient centered outcomes for people with constipation. Patient Generated Health Data (PGHD) from digital devices is a potential solution, but its utility as a tool for describing experiences of people with frequent constipation is unknown. We conducted a virtual, 16-week prospective study of individuals with frequent constipation from an online wellness platform that connects mobile consumer digital devices including wearable monitors capable of passively collecting steps, sleep, and heart rate data. Participants wore a Fitbit monitoring device for the study duration and were administered daily and monthly surveys assessing constipation symptom severity and medication usage. A set of 38 predetermined day-level behavioral activity metrics were computed from minute-level data streams for steps, sleep and heart rate. Mixed effects regression models were used to compare activity metrics between constipation status (irregular or constipated vs. regular day), medication use (medication day vs. non-medication day) and the interaction of medication day with irregular or constipation days, as well as to model likelihood to treat with constipation medications based on daily self-reported symptom severity. Correction for multiple comparisons was performed with the Benjamini–Hochberg procedure for false discovery rate. This study analyzed 1540 enrolled participants with completed daily surveys (mean age 36.6 sd 10.0, 72.8% female, 88.8% Caucasian). Of those, 1293 completed all monthly surveys and 756 had sufficient Fitbit data density for analysis of activity metrics. At a daily-level, 22 of the 38 activity metrics were significantly associated with bowel movement or medication treatment patterns for constipation. Participants were measured to have fewer steps on irregular days compared to regular days (−200 steps, 95% CI [−280, −120]), longer periods of inactivity on constipated days (9.1 min, 95% CI [5.2, 12.9]), reduced total sleep time on irregular and constipated days (−2.4 min, 95% CI [−4.3, −0.4] and −4.0 min, 95% CI [−6.5, −1.4], respectively). Participants reported greater severity of symptoms for bloating, hard stool, difficulty passing, and painful bowel movements on irregular, constipation and medication days compared to regular days with no medication. Interaction analysis of medication days with irregular or constipation days observed small increases in severity compared to non-medication days. Participants were 4.3% (95% CI 3.2, 5.3) more likely to treat with medication on constipated days versus regular. No significant increase in likelihood was observed for irregular days. Daily likelihood to treat increased for each 1-point change in symptom severity of bloating (2.4%, 95% CI [2.0, 2.7]), inability to pass (2.2%, 95% CI [1.4, 3.0]) and incomplete bowel movements (1.3%, 95% CI [0.9, 1.7]). This is the first large scale virtual prospective study describing the association between passively collected PGHD and constipation symptoms and severity at a day-to-day granularity level. Constipation status, irregular or constipated, was associated with a number of activity metrics in steps and sleep, and likelihood to treat with medication increased with increasing severity for a number of constipation symptoms. Given the small magnitude of effect, further research is needed to understand the clinical relevance of these results. PGHD may be useful as a tool for describing real world patient centered experiences for people with constipation.
Subject terms: Digestive signs and symptoms, Outcomes research
Introduction
Constipation, a condition characterized by infrequent defecation, hard stools or straining, occurs commonly, particularly among women and the elderly1,2. While constipation can be transient, especially for people with recurrent or chronic symptoms, it negatively impacts their quality of life, and the downstream economic effects of lost workdays, decreased productivity, and increased health services use can be substantial3,4. A number of prescription and non-prescription constipation remedies exist, with non-prescription use playing an important role. In general, these medications have demonstrated efficacy in clinical trials5,6. Real world data (RWD), conventionally collected through health records, claims data or prescribing data, reflects individual or population usage in uncontrolled settings, and serves as an important tool for drug monitoring or discovery of new therapeutic uses7,8.
However, describing real-world treatment patterns of people with constipation is challenging. Over the counter medication use limits the utility of claims based or pharmacy-based data. Information on constipation management typically relies on cross sectional surveys, which are subject to recall and recency bias9,10. Validated activity surveys such as the International Physical Activity Questionnaire (IPAQ), do not capture other behavioral dimensions such as sleep activity11,12. Clinical trials for constipation agents do not provide insight into real-world treatment patterns or patient experiences, and pharmacy or prescribing data typically do not reflect management strategies in the outpatient setting13. For people with constipation, there is an opportunity for exploring new ways for generating real world evidence to better characterize patient experiences and thus understand the patient journey.
Digital Patient Generated Health Data (PGHD), a recently introduced technology, has become available with the widespread availability of digital mobile devices, and can potentially fill the information gaps left by conventional sources of real-world data14,15. Wearable devices in particular that track physical activities or physiologic parameters like heart rate allow for the passive inference of behavioral activity patterns with time granularities not possible with conventional RWD16–18. Though still in the early stages of widespread clinical use, PGHD could potentially be used to gain more nuanced data on patient centered outcomes, and serve as passive health diaries with information to guide medication approaches personalized to the individual user19.
We report the first virtual large-scale prospective study to evaluate the utility of digitally collected active and passive PGHD for describing and linking symptom characteristics and medication use patterns for people with frequent constipation. For our primary objective, we hypothesized that differences in behavior as recorded from wearable activity trackers may be used to discern differences in these parameters between days with reported constipation, and days where individuals report regular bowel movement patterns. As a secondary objective, we also explored digital daily diaries for describing symptom severity and its relationship to medication use and treatment preferences for constipation episodes.
Results
Study population descriptive characteristics
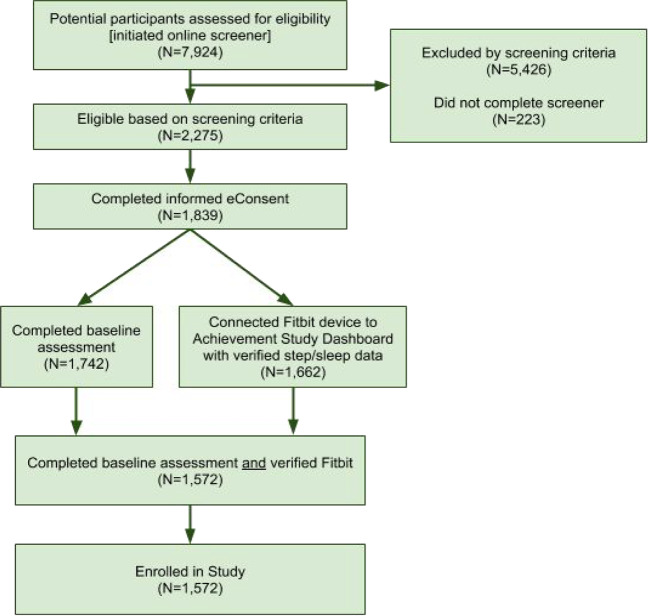
Figure 1 shows the flow of participants from recruitment to enrollment. A total of 7924 individuals were screened for study eligibility, of whom 5426 individuals (68.5%, 5426/7924) did not meet screening criteria, 223 individuals (2.8%, 223/7924) did not complete the screening questionnaire, which resulted in 2275 potential participants (28.7%, 2275/7924) who were eligible based on screening criteria. Among those eligible, 1839 individuals (80.8%, 1839/2275) completed eConsent procedures. After completing informed consent, 1572 participants (85.5%, 1572/1839) completed the final steps required for enrollment, completion of baseline assessment, and connection of a Fitbit device, and were then enrolled into the study. Of those, 32 participants did not complete any of the daily surveys, and were removed, leaving 1540 enrolled persons in the analysis. Analysis of management approaches for constipation was conducted on those individuals who completed all the monthly surveys (n = 1293 or 82% of enrolled), while the behavioral activity analysis was performed on those individuals who satisfied the previously described data density criteria (n = 756 or 48% of enrolled).
Fig. 1. Flow diagram of participants from recruitment to study enrollment.
Flow of participants (starting at top) from initial eligibility screening to informed consent to to enrollment completion is shown. Participants were considered enrolled if they completed the baseline assessment and connected a Fitbit device after consenting to the study.
Study participants were relatively young (mean age 36.6), and mostly female (72.8%), Caucasian (88.8%) and well educated (69.6% with at least a college degree). In terms of general management approaches, most participants (34%) reported a combination of lifestyle changes with waiting to see if symptoms improved. Only a small proportion of participants regularly used constipation medications (7.7%) (Table 1). Fitbit data coverage was generally high for the enrolled participants (mean coverage 82.2% of 112 study days for steps), and even higher for the Behavioral Populations subcohort. Not all participants had Fitbit devices capable of heart rate monitoring, which explains the lower coverage rates for heart rate features (67.4% in the enrolled population).
Table 1.
Demographic and descriptive characteristics of the population.
| Characteristic | Daily population | Behavioral population | Monthly population |
|---|---|---|---|
| Number | 1540 | 756 | 1293 |
| Mean age in years (SD) | 36.6 (10.0) | 37.5 (10.0) | 36.8 (10.0) |
| Mean BMI in kg/m2 (SD) | 29.7 (6.8) | 29.9 (6.8) | 29.6 (6.7) |
| Female gender, n (%) | 1121 (72.8) | 516 (68.3) | 959 (74.2) |
| Education, n (%)a | |||
| ≥College degree | 1070 (69.5) | 540 (71.4) | 914 (70.7) |
| Some college | 308 (20.0) | 142 (18.8) | 253 (19.6) |
| Trade or vocational | 68 (4.4) | 28 (3.7) | 57 (4.4) |
| High school graduate | 88 (5.7) | 42 (5.6) | 66 (5.1) |
| No high school | 5 (0.3) | 3 (0.4) | 2 (0.2) |
| Race, n (%) | |||
| White | 1367 (88.8) | 683 (90.3) | 1155 (89.3) |
| African American | 105 (6.8) | 49 (6.5) | 83 (6.4) |
| Asian | 71 (4.6) | 25 (3.3) | 58 (4.5) |
| American Indian or Alaska Native | 29 (1.9) | 11 (1.5) | 23 (1.8) |
| Native Hawaiian or Pacific Islander | 10 (0.6) | 5 (0.7) | 7 (0.5) |
| Other | 36 (2.3) | 14 (1.9) | 28 (2.2) |
| Mean Pct of study days with data (%) | |||
| Survey | 67.2% | 70.8% | 76.1% |
| Steps | 82.2% | 96.0% | 84.2% |
| Sleep | 75.9% | 88.7% | 78.5% |
| Heart rate | 67.4% | 74.0% | 68.7% |
| Management approaches, n (%) | |||
| Lifestyle changers | – | – | 97 (8%) |
| Immediate treaters | – | – | 207 (16%) |
| Lifestyle + Waiting | – | – | 438 (34%) |
| Waiters | – | – | 279 (22%) |
| Other | – | – | 272 (21%) |
| Medication approaches, n (%) | |||
| As needed | – | – | 367 (28.4) |
| Regular | – | – | 99 (7.7) |
| Non-user | – | – | 403 (31.2) |
| Other | – | – | 424 (32.8) |
Daily population refers to individuals who completed the daily surveys. Behavioral population refers to a subset of the daily population who satisfied data density requirements for the analysis of wearable activity metrics. Monthly population refers to individuals who completed at least one monthly survey for the analysis of medication and management approaches.
aEducation categories are missing one participant who did not complete this question in the baseline questionnaire.
Relation of daily behavioral activity to constipation symptoms and medication use
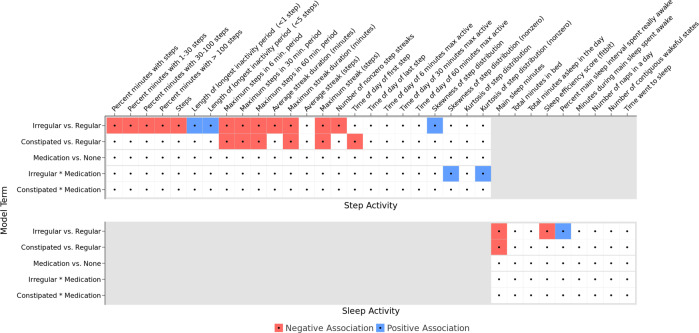
22 of the 38 daily activity features were found to be significantly associated with different patterns of bowel movements. Most of these associations centered around constipation status, where participants tended to be less active (in daily steps or sleep activity) on irregular or constipated days versus regular days. Only two steps features (skewness and kurtosis of step distribution) were found to be associated with the interaction of irregular days with medication use, otherwise medication days were not associated with activity features. Active heart rate, computed as the 95th percentile of heart rate for the day, was associated with irregular and constipated BM days when compared to regular BM days. The remaining heart rate features were not significant after accounting for multiple comparisons (Fig. 2).
Fig. 2. Regression model results for steps and activity features.
Overview of the regression model results for the five different comparisons of bowel movement or medication use patterns to steps and sleep activity features. Significant results are shown in color, with red denoting a negative association and blue denoting a positive association. Among the models for heart rate features (not shown) significant associations were only observed for 95th percentile heart rate, which was negatively associated with both irregular and constipated days.
Compared with regular days, days with irregular BM patterns saw fewer steps (−200 steps per day, 95% CI [−280, −120]) and longer periods of inactivity (9.1 min additional minutes, 95% CI [5.2, 12.9]). There was a small reduction in sleep efficiency, defined as the proportion of time asleep to time in bed, on irregular days compared to regular BM days (−0.1% 95% CI [−.19%, −.01%]). Participants also had a small reduction in total minutes asleep on irregular and constipation BM days compared to regular BM days (−2.4 min, 95% CI [−4.3, −0.4] and −4 min, 95% CI [−6.5, −1.4], respectively). Active heart rate, a proxy of the time a person spends engaged in physical activity, was also lower on irregular and constipation BM days when compared to regular days—consistent with the findings of decreased steps activity (Table 2).
Table 2.
Activity features and patterns of bowel movement or treatment approach.
| Intercept | Irregular – Regular | Constipated –Regular | Medication –No Medication | Irregular x Medication | Constipation x Medication | |
|---|---|---|---|---|---|---|
| Severity on regular days, no medication | Change on irregular days | Change on constipated days | Change on medication days | Change on irregular days with medication | Change on constipated days with medication | |
| Regression coefficient estimates [95% CI] | ||||||
| Total steps | 8887 [8627, 9147] | −200* [−280, −120] | −96 [−200, 8] | −102 [−375, 171] | −56 [−396, 284] | −96 [−452, 260] |
| Maximum consecutive steps | 1764 [1665, 1863] | −57* [−92, −22] | −94* [−139, −48] | 95 [−24, 214] | −46 [−194, 103] | −64 [−219, 91] |
| Longest inactive period (minutes) | 533 [525, 541] | 9.1* [5.2, 12.9] | 0.1 [−5.0, 5.084] | 9.871 [−3.2, 23.0] | −7.423 [−23.9, 9.1] | 11.239 [−5.9, 28.4] |
| Nightly sleep duration (minutes) | 410 [404, 415] | −2.4* [−4.3, −0.4] | −4.0* [−6.5, −1.4] | −0.15 [−6.7, 6.4] | 3 [−5.2, 11.3] | 2.1 [−6.5, 10.8] |
| Sleep efficiency score (%) | 91.12 [90.39, 91.85] | −0.10* [−0.19, −0.01] | 0.033 [−0.08, 0.15] | 0.023 [−0.27, 0.31] | 0.08 [−0.29, 0.44] | −0.16 [−0.54, 0.22] |
| Active HR (95 percentile of daily HR; BPM) | 103.9 [102.3, 103.7] | −0.27* [−0.48, −0.07] | −0.46* [−0.73, −0.19] | 0.23 [−0.47, 0.93] | −0.83 [−1.68, 0.02] | −0.73 [−1.63, 0.18] |
Magnitude of changes in selected activity features estimated from regression models for each comparison type of bowel movement or treatment pattern. Each row presents the results of a different model, the dependent variable of the model (an activity feature) is shown in the first cell of that corresponding row. Each column presents a different regression coefficient. The second column, Intercept, shows the model intercept or the baseline measurement, which is the activity observed at the reference level of the predictor variables (regular days, no medication). Columns 3-7 reflect changes with respect to the baseline measure.
*Statistically significant with q-value < 0.05.
As expected, participants reported significant changes in symptom severity on irregular and constipated BM days when compared to regular BM days. Medication days compared to non-medication days were also associated with significant increases in symptom severity. Medication days during irregular stool days were not associated with significant changes in bloating and painful BM severity (Table 3).
Table 3.
Symptom severity and patterns of bowel movement or treatment approach.
| Intercept | Irregular–regular | Constipated–regular | Medication–no medication | Irregular × medication | Constipation × medication | |
|---|---|---|---|---|---|---|
| Severity on regular days, no medication | Change on irregular days | Change on constipated days | Change on medication days | Change on irregular days with medication | Change on constipated days with medication | |
| Regression coefficient estimates [95% CI] | ||||||
| Bloating severity | 0.47 [0.45, 0.5] | 0.38* [0.37, 0.39] | 0.70* [0.69, 0.72] | 0.30* [0.27, 0.33] | 0.00004 [−0.04, 0.04] | 0.14* [0.10, 0.18] |
| Hard stool severity | 0.21 [0.19, 0.22] | 0.35* [0.34, 0.36] | 1.5* [1.48, 1.52] | 0.11* [0.08, 0.15] | 0.11* [0.06, 0.15] | 0.06* [0.01, 0.12] |
| Painful BM severity | 0.21 [0.19, 0.23] | 0.38* [0.37, 0.39] | 0.79* [0.78, 0.81] | 0.17* [0.13, 0.20] | 0.03 [−0.008, 0.07] | 0.14* [0.09, 0.18] |
| Difficulty passing severity | 0.41 [0.39, 0.44] | 0.47* [0.45, 0.48] | 1.36* [1.35, 1.38] | 0.18* [0.14, 0.21] | 0.10* [0.06, 0.15] | 0.14* [0.08, 0.19] |
Magnitude of changes in self-reported daily symptom severity estimated from regression models for each comparison type of bowel movement or treatment pattern. Each row presents the results of a different model, the dependent variable of the model (a symptom) is shown in the first cell of that row. Each column presents a different regression coefficient. The second column, Intercept, shows the model intercept or the baseline measurement, which is the activity observed at the reference level of the predictor variables (regular days, no medication). Columns 3–7 reflect changes with respect to the baseline measure. Symptom severity was rated on a 0–4 scale.
*Indicates statistically significant result (q-values < 0.05).
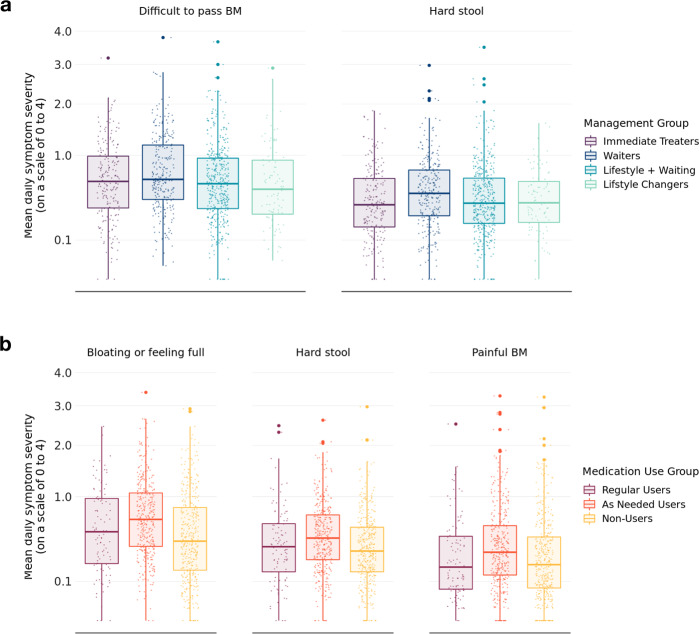
From the monthly surveys, we observed more severe symptoms among people who tended to wait to manage their symptoms. Waiters reported greater severity of difficulty passing and hard stools when compared to Lifestyle changers and Immediate treaters, while people who used medications as needed had greater severity of bloating, hard stool, and painful bowel movements (Fig. 3).
Fig. 3. Boxplots of symptom severity.
a A boxplot of symptom severity on self-reported management approach from the monthly surveys. b A boxplot of symptom severity on medication use approach from the monthly surveys. Symptoms scores in each group are on a 0–4 scale with 4 as the most severe symptom. Each boxplot shows the median (center line) and inter-quartile range (box bounds) and the largest (top whisker) and smallest (bottom whisker) values within 1.5* interquartile. Only significant results are shown.
Upper edge represents the 75th percentile, while the lower edge shows the 25th percentile. Only significant results are shown. Symptom severity is based on a 4-point scale. In a) Waiters differed significantly from Lifestyle changers in terms of symptom severity for difficulty passing and hard stool. In b) As needed users differed significantly from non-users in terms of bloating, hard stool and painful BM.
The coefficients in Table 4 show the increase in likelihood of treating for each 1-point increase in symptom severity score. The largest increase in treatment likelihood was due to participants reporting constipated BM (4.3% increase compared to regular days, 95% CI [3.2, 5.3]), and on days with increasing severity of bloating, difficulty passing stool, or pain. For all symptom and bowel movement parameters, the estimated coefficients were directionally larger for females as compared to males. This indicates that generally increased symptoms, as well as self-reported bowel patterns of constipation directionally increase the probability of treatment more for females as compared to males. The magnitude of this difference appeared to be especially large for bloating (2.6% likelihood to treat vs 1.5% for women vs. men) as well as self-reported constipation bowel movement pattern (4.5% likelihood to treat vs. 3.2% likelihood to treat for women vs. men).
Table 4.
Estimated percent increase in likelihood of using a constipation medication.
| % Likelihood increase** [95% CI] | |||
|---|---|---|---|
| Parameter | Full cohort | Female only | Male only |
| Bowel pattern: none of the above | −3.3 [−6.7, 0.1] | – | – |
| Bowel pattern: irregular | -0.2 [−1.0, 0.5] | −0.2 [−1.1, 0.6] | 0.3 [−0.8, 1.4] |
| Gas | 0.1 [−0.3, 0.5] | 0.2 [−0.3, 0.7] | −0.2 [−0.8, 0.4] |
| Nausea | 0.2 [−0.5, 0.9] | 0.1 [−0.7, 0.9] | 0.3 [−1, 1.5] |
| Hard stool | 0.3 [−0.2, 0.7] | 0.2 [−0.4, 0.8] | 0.4 [−0.3, 1.2] |
| Painful BM | 0.4 [−0.1, 0.9] | 0.4 [−0.2, 1] | 0.4 [−0.6, 1.5] |
| Difficulty passing | 0.6* [0.1, 1.0] | 0.6* [0, 1.2] | 0.6 [−0.1, 1.2] |
| Cramps | 0.6* [0, 1.3] | 0.6 [−0.2, 1.3] | 0.6 [−0.5, 1.7] |
| Pain | 0.9* [0.2, 1.6] | 1* [0.2, 1.8] | 0.1 [−1.2, 1.4] |
| Incomplete BM | 1.3* [0.9, 1.7] | 1.4* [0.9, 1.8] | 1* [0.2, 1.8] |
| Unable to pass | 2.2* [1.4, 3.0] | 2.1* [1.2, 3] | 2.3* [0.9, 3.7] |
| Bloating | 2.4* [2.0, 2.7] | 2.6* [2.2, 3.1] | 1.5* [0.8, 2.1] |
| Bowel pattern: constipated | 4.3* [3.2, 5.3] | 4.5* [3.3, 5.7] | 3.2* [1.1, 5.3] |
The percent increase in likelihood of using a constipation medication for each unit change in daily symptom severity is shown for the full daily survey cohort, and then based on self-reported gender. 95% confidence limits are shown in the brackets.
*Indicates statistically significant result (q-values < 0.05).
**Increases are for a change in 1-point of symptom severity.
Discussion
Despite some non-interventional studies suggesting that constipation and irregularity were associated with less active lifestyles20,21, empirical investigations into this claim do not always produce consistent evidence. For example, whereas one survey-based study reported no relationship between physical activity and self-reported constipation among 1069 respondents22, two other large-scale survey studies of similar design but comprising nearly 40,000 and over 60,000 women found lower prevalence of constipation among women who reported some degree of physical activity versus a sedentary lifestyle23,24.
Although there have been previous studies of activity and constipation, this is the first study that utilized commercially available activity trackers to quantify the association between self-reported bowel patterns, laxative utilization, and patterns of behavior related to activity and sleep at a daily level25,26. The 4-month longitudinal nature of this study, in combination with its scale of more than 1500 participants with approximately 750 wearing activity tracking devices recording minute level step, sleep, and heart rate measures, allowed for the first time the opportunity to examine how constipation symptoms as well as constipation treatment patterns were associated with daily behavior.
This body of research revealed several associations of note. The baseline steps activity in our cohort is similar to baseline steps in other settings27. Days with self-reported constipation and irregularity were negatively associated with many variables that quantified the level and distribution of daily activity. Although the magnitudes of the behavioral changes with regards to activity observed in this study are small, their trend is consistent with other research that shows that more severe disease states are associated with fewer steps per day28,29. The smaller effect of our findings may be explained by the focus on constipation episodes that are of shorter duration. Additionally, the study participants, who reflect more real world experiences, may have more heterogeneity in constipation symptoms than those who participate in clinical trials. Constipation and irregularity showed significant association in 22 of 38 models with decreased sleep and step activity. Specifically, activity features capturing sleep quality and duration, as well as activity features capturing the quantity and timing of daily steps showed association on days with constipation and irregularity. Given the small magnitude of the effects observed in this study, further research is needed to understand the clinical relevance of the results. More stringent inclusion and exclusion criteria might lead to more pronounced effects in future studies using RWD.
It was also found that individuals reported increased symptom severity on days with constipation or irregularity and use of laxative medications compared to days with constipation status being constipated or irregular but abstinence from medication. This finding may be interpreted as evidence that on average individuals opt for treatment once their symptoms surpass an internal symptom threshold, rather than treating as soon as any symptoms manifest. The results of the daily self-reported behaviors are supported by participants’ perceptions of their own behaviors from the monthly surveys—“Waiters” and people who took medications “As-needed” noted more symptom severity, and likelihood to take medications increased with increasing symptom severity.
In line with previous reports, positive associations between self-reported daily constipation and individual’s likelihood to treat were observed. Furthermore, a positive association of laxative treatment and bloating has been seen. Bloating has been known to be a symptom of constipation but has not been widely associated with an increased likelihood for laxative treatment. This observation may suggest that individuals deem laxative medications more appropriate for bloating relief than previously known. Further research is needed to better understand the factors that motivate patients to use laxative medications.
The study has limitations. The study cohort consists predominantly of well educated, comparably young Caucasian women with access to mobile wearable devices, which limits generalizability to other populations. To reduce the participant burden, we used shorter daily surveys with questions customized to assess constipation severity and medication use instead of known validated surveys, which may have reduced the observable effect size. One early study has suggested that constipation in elderly people is associated with lack of activity, which may also be important when looking at the small magnitude of effects observed in this trial29. Another limitation is that we did not specifically exclude participants for using older versions of the Fitbit device. However, the data requirement to have minute-level granular data for steps and sleep would have removed from analysis people with older devices that did not have this capability. The requirement for high density data from the Fitbit devices may have selected participants who are more engaged in wellness behaviors. Fitbit devices may also be inaccurate when compared to conventional gold standards for steps and sleep, though the devices themselves have been shown to be internally reliable and consistent30–32. Fitbit devices may measure steps or sleep differently depending on the location of use33, but this fixed effect of location is accounted for in the mixed-effects models used for the studies. Finally, the use of only three categories to describe BM days makes it difficult to translate the results to prior studies that use standard diagnostic criteria like the Rome criteria34.
In summary, this study is the largest virtual study of activity and constipation using passively and actively collected PGHD with more than 100 million digital activity data points. The study allowed us to measure activity based associations with constipation and medication use at minute-level granularity over extended periods of time, which is not possible with conventional real-world approaches35. The study findings were also consistent with prior research on the effects of physical activity on symptoms of constipation.
Methods
Study design and Platform
A 16-week prospective and completely virtual study was conducted with a novel study platform (Achievement Studies, Evidation Health Inc., San Mateo, CA). The Achievement study platform is available as a mobile application for both Android and iOS operating systems, with functionality to connect consumer mobile devices and third-party digital diary apps, as well as enable users to participate and engage in clinical research efforts36,37. The Achievement community, which numbers more than 3 million, consists of adults (age ≥ 18) residing in the United States with members in all 50 states.
Study population
Study participants were recruited from the Achievement platform with an email or within-app offer to participate in the study, targeting adults (age ≥ 18) residing in the United States. Additional inclusion criteria for participation were:
Self-reported constipation, irregular bowel movements, and/or hard dry stool at least once every 2 months.
Self-reported use of at least one over-the-counter constipation medication in the past 3 months.
Access to a Fitbit activity tracker capable of collecting steps, heart rate or sleep data that was connected to the Achievement platform and willing to wear the tracker daily during the day and at night for the entire study.
Willingness to respond to daily short questionnaires and report whether they experienced constipation symptoms the previous day.
Potential participants were excluded from participation if they self-reported a diagnosis of Inflammatory Bowel Disease (Ulcerative Colitis and Crohn’s Disease), irritable bowel syndrome, gastrointestinal malignancies, or chronic constipation. Potential enrollees into the study were determined by sending a screening questionnaire to interested participants. Individuals who satisfied inclusion and exclusion criteria were then confirmed to have functioning Fitbit devices that were connected to the Achievement platform. All enrolled participants completed an online informed consent form agreeing to study protocols. The study was approved by Solutions Institutional Review Board (Yarnell, AZ, USA).
Survey data variables
Participants enrolled in the study completed three survey questionnaire types, each with different time frequencies. As described above, a single baseline survey was administered to potential participants to determine eligibility for the study, and to gather baseline demographic data as well as information about medication use and constipation symptoms. A monthly survey (every 4 weeks) was administered to enrolled participants (four surveys total per participant) asking them to describe self-reported management patterns for constipation and quality of life metrics. Lastly, a daily survey asking about constipation symptoms and medication use, started within 24 h of enrollment, was administered to every participant for the duration of the study (16 weeks or 112 days total).
Demographic data captured in the baseline survey include date-of-birth, gender, race and ethnicity, highest educational level, and comorbid conditions. Body mass index (weight in kg per meter2) is computed from self-reported height and weight data. Age was determined by the date of birth as reported at the time of enrollment.
In the monthly survey, participants were asked about management approaches on two dimensions in the prior month for symptoms of constipation/irregularity. The first dimension focused on general management approaches, which were categorized as Treat with medication immediately, Treat with lifestyle/dietary changes, Watchful waiting, or No treatment done. The second addressed the frequency of medication use, with options for Regular use of medications, As-needed use, and no-use of medications. For both of these dimensions, the 4 monthly responses were aggregated over the entire study period for each participant to assign him/her to treatment categories. General management groups were: Waiters (all four responses Watchful waiting), Lifestyle Changers (all four responses lifestyle/dietary changes), Lifestyle + Waiting (any combination of Watchful waiting and lifestyle/dietary changes), or Immediate Treaters (any response of medication immediately). Medication frequency groups were: Regular user (all four responses regular), As needed user (minimum one response As-needed) or Non-user (all four responses no-use). People who did not fit into one of the aggregated categories were labeled as “Other”.
For the daily surveys, participants were asked about bowel habits for the previous day, which were labeled into mutually exclusive categories of normal, irregular, constipated, or none of the above. All participants were also asked to rate on a 4-point severity scale symptom dimensions of bloating, hard stool, painful bowel movement, or difficulty passing stool. Those who reported Irregular or Constipated days were asked to rate five additional symptoms of incomplete bowel movements, periumbilical cramps, sub-umbilical cramps, flatulence and nausea. Finally, participants were also asked about medication use to manage constipation symptoms for the previous day. A list of common over-the-counter (OTC) medications, including stool-softeners, laxatives, suppositories, stool bulk, and fiber supplements was provided for the participant to label with binary Yes or No responses if the medication(s) were used. Daily survey questions on constipation severity and medication use were drawn from a bank of questions that had been used by Sanofi-Aventis for consumer-focused research. Participant days for which any medication was used was labeled a medication day (binary Yes or No) for that participant.
Patient generated activity tracker data
The Achievement platform uses the Fitbit application programming interface (API) to pull data on steps, heart rate and sleep for each participant at minute level granularity (e.g. steps per minute, heart beats per minute, sleep states of asleep, restless or awake). The Fitbit API includes for each sleep state a binary (True/False) designation for the “main” sleep period of the day.
Each activity data stream (steps, heart rate, and sleep) was then aggregated into a set of daily activity features engineered in collaboration with a medical domain expert. The features were designed to capture behavioral episodes that reflected a priori beliefs about the effect that constipation might have on these different types of activity. Table 5 shows a summary of the activity features along with a brief description. A total of 38 daily activity features were engineered (24 steps, 9 sleep, and 5 heart rate features). Skewness and kurtosis features for steps and heart rate were computed for each daily distribution. Features over a specific duration (e.g., 5, 6, 30, or 60 min) were determined with a sliding window of that duration over the entire day. The Sleep Efficiency score is provided from the Fitbit API and is determined by the proportion of time asleep (main sleep at night) to the time spent in bed.
Table 5.
Fitbit derived behavioral activity features.
| Steps activity features | Sleep activity features | Heart rate features | ||
|---|---|---|---|---|
| Percent minutes with steps | Maximum steps in 30-min period | Time of day of last step | Main sleep minutes | Resting heart rate (beats per minute) |
| Percent minutes with 1–30 steps | Maximum steps in 60-minute period | Time of day of 6 min max active | Total minutes spent in bed | Skewness of heart rate |
| Percent minutes with 30–100 steps | Average streak duration (minutes) | Time of day of 30 min max active | Total minutes asleep in day | Kurtosis of heart rate |
| Percent minutes with >100 steps | Maximum streak duration (minutes) | Time of day of 60 min max active | Sleep efficiency score (Fitbit) | Max rolling 5-min average |
| Steps taken per day | Average streak (steps) | Skewness of step distribution (all values) | Percent main sleep interval spent really awake | 95th percentile of heart rate |
| Length of longest inactivity period (<1 step) | Maximum streak (steps) | Skewness of nonzero step distribution | Minutes during main sleep spent awake | |
| Length of longest inactivity period (<5 step) | Number of nonzero step streaks | Kurtosis of step distribution (all values) | Number of naps in a day | |
| Maximum steps in 6-min period | Time of day of first step | Kurtosis of nonzero step distribution | Number of contiguous wakeful states | |
| Time went to sleep |
Data streams from the Fitbit API were downloaded at minute-level granularity, and then aggregated into day-level features with input from a medical domain expert.
Analysis using PGHD (activity data and daily survey responses) was conducted on a subgroup of the study cohort with high density activity tracker data (the Behavioral Analysis Population). To define this subgroup, we followed a three-step process that has been previously used:38,39
Fitbit wear time for each participant computed based on available minute-level data,
Any day with wear time <10 h was labeled as an invalid day, and
Removal of all individuals with ≥7 consecutive invalid days.
Statistical methods
Linear mixed-effects regression was used to estimate the amount of variance in daily activities or constipation severity that could be explained by bowel movement (BM) patterns and medication use. The regression models took the form:
| 1 |
where,
Yi,t is a day level activity feature or day level self-report of constipation severity for individual i (for i = 1, 2, 3, …, n; where n is the number of participants in the Behavioral Analysis Population), on day t (for t = 1, 2, 3, …, 112th day of study)
medicationi,t is a binary variable that is 1 for individual i on day t if a laxative is reported taken, else 0
BMpatternj,i,t is a dummy-coded variable indicating if individual i reported being constipated or irregular as opposed to regular on day t, with regular BM pattern being the reference state.
ui is a random intercept which is allowed to vary for each participant.
For each dependent variable, significance of association was determined on the β-coefficient for five comparisons—irregular vs. regular days, constipated vs. regular days, medication vs. no-medication days, and the interaction terms for irregular × medication days and constipation × medication days. Daily observations in the model were excluded for both the activity feature and symptom severity models if the participant reported their BM pattern to be “None of the above”, failed to respond to the symptom severity, or if there was no response to the medication use question.
In an effort to better understand what drives individuals to utilize laxative medications, an individual’s likelihood of treating on a particular day (used laxatives) given self-reported daily bowel movement pattern as well as reported daily symptom severity was modeled. Using a fixed-effects panel regression, the following linear probability model was estimated:
| 2 |
where:
medicationi,t is a binary variable that is 1 for individual i on day t if a laxative is reported taken, else 0,
symptomj,i,t is an ordinal symptom severity indicator for individual i on day t with levels ranging from 0 to 4 for each of the j symptom types (e.g., gas, difficulty passing, etc.) with 0 indicating no symptoms, and 4 indicating most severe symptoms,
BMpatternk,i,t is a dummy variable indicating if individual i reported being constipated, irregular, or “None of the above” as opposed to regular on day t, with regular BM pattern being the reference state.
We looked at daily responses to symptom severity across treatment approaches categories (general management and medication frequency) as determined from the monthly surveys. Comparison of means across different groups was conducted with Kruskal–Wallis (non-parametric) ANOVA tests or the Wilcoxon rank sum test when appropriate. For all the analyses, we adjusted p-values (q-value ≤0.05 significant) for multiple comparisons with the Benjamini–Hochberg procedure for false discovery rate (FDR). All statistical tests were two-sided.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Supplementary information
Acknowledgements
We would like to thank the study participants. We also would like to thank Sophie Maurel and Heike Siedler from Sanofi who supported protocol development. The study was sponsored by Sanofi-Aventis. Medical writing support was provided by Evidation Health and funded by Sanofi-Aventis in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author contributions
Benjamin Bradshaw, Sabine Landes, and Petra Kammann designed the study and participated in data acquisition and analyzed and interpreted the data. They contributed to critically revising the drafts for important intellectual content. Allison Shapiro participated in designing the analysis plan, contributed to analyzing and the interpretation of the data and critically revised each manuscript draft for important intellectual content. Wei-Nchih Lee wrote the manuscript and contributed to analyzing and the interpretation of the data. He critically revised the drafts for important intellectual content. Beatrice Bois De Fer and Robert Lange participated in the analysis and interpretation of the data and critically revised each draft for intellectual content. All authors approved the final version and its submission to npj Digital Medicine. All authors are accountable for the accuracy and integrity of the publication.
Data availability
Qualified researchers may request access to the data and related study documents including the study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Code availability
The code that supports the findings of this study are available from the corresponding author upon reasonable request. Analysis to process and analyze the data was generated with Python 3 and the R programming language.
Competing interests
Sabine Landes, Petra Kammann, Beatrice Bois De Fer, and Robert Lange are employees of Sanofi-Aventis. Wei-Nchih Lee, Allison Shapiro, and Benjamin Bradshaw are employees of Evidation Health. Evidation Health designed the study in collaboration with Sanofi-Aventis and performed the study. The authors have not signed any agreement with the funding sponsor that will bias the results of this research in any way.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/24/2021
A Correction to this paper has been published: 10.1038/s41746-021-00434-3
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-021-00391-x.
References
- 1.Koloski NA, et al. Impact of persistent constipation on health-related quality of life and mortality in older community-dwelling women. Am. J. Gastroenterol. 2013;108:1152–1158. doi: 10.1038/ajg.2013.137. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am. J. Gastroenterol. 1999;94:3530–3540. doi: 10.1111/j.1572-0241.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Dennison C, et al. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sun SX, et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig. Dis. Sci. 2011;56:2688–2695. doi: 10.1007/s10620-011-1639-5. [DOI] [PubMed] [Google Scholar]
- 5.Kamm MA, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol. 2011;9:577–583. doi: 10.1016/j.cgh.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharm. Ther. 1995;9:639–647. doi: 10.1111/j.1365-2036.1995.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration: Framework for FDA’s Real-World Evidence Program. https://www.fda.gov/media/120060/download. Accessed 20 March 2020 (2018).
- 8.Berger, M. et al. A framework for regulatory use of real-world evidence. Duke Margolis Center for Health Policy. https://healthpolicy.duke.edu/publications/framework-regulatory-use-real-world-evidence. Accessed 20 March 2020 (2019).
- 9.Solem CT, et al. Treatment patterns, symptom reduction, quality of life, and resource use associated with lubiprostone in irritable bowel syndrome constipation subtype. Curr. Med. Res. Opin. 2016;32:899–905. doi: 10.1185/03007995.2016.1150262. [DOI] [PubMed] [Google Scholar]
- 10.Trinkley KE, Porter K, Nahata MC. Prescribing patterns for the outpatient treatment of constipation in the United States. Dig. Dis. Sci. 2010;55:3514–3520. doi: 10.1007/s10620-010-1196-3. [DOI] [PubMed] [Google Scholar]
- 11.Wanner M, et al. Validation of the long international physical activity questionnaire: influence of age and language region. Prev. Med. Rep. 2016;3:250–256. doi: 10.1016/j.pmedr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanner M, et al. Validation of the global physical activity questionnaire for self-administration in a European context. BMJ Open Sport Exerc. Med. 2017;3:e000206. doi: 10.1136/bmjsem-2016-000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176:1216–1217. doi: 10.1001/jamainternmed.2016.2775. [DOI] [PubMed] [Google Scholar]
- 14.Wright SP, Hall Brown TS, Collier SR, Sandberg K. How consumer physical activity monitors could transform human physiologic research. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312:R358–R367. doi: 10.1152/ajpregu.00349.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomersall SR, et al. Estimating physical activity and sedentary behavior in a free-living context: a pragmatic comparison of consumer-based activity trackers and ActiGraph accelerometry. J. Med. Internet Res. 2016;18:e239. doi: 10.2196/jmir.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR. Accuracy of PurePulse photoplethysmography technology of fitbit charge 2 for assessment of heart rate during sleep. Chronobiol. Int. 2019;36:927–933. doi: 10.1080/07420528.2019.1596947. [DOI] [PubMed] [Google Scholar]
- 17.Block VJ, et al. Validation of a consumer-grade activity monitor for continuous daily activity monitoring in individuals with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019;5:2055217319888660. doi: 10.1177/2055217319888660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, R. et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining (KDD ‘19) 2145–2155 (Association for Computing Machinery, 2019).
- 19.Samson, S. I. et al. Quantifying the impact of influenza among persons with type 2 diabetes mellitus: a new approach to determine medical and physical activity impact. J Diabetes Sci Technol. 15, 1932296819883340 (2019). [DOI] [PMC free article] [PubMed]
- 20.Migueles JH, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–1845. doi: 10.1007/s40279-017-0716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi W, et al. Epidemiology and risk factors of functional constipation in pregnant women. PLoS ONE. 2015;10:e0133521. doi: 10.1371/journal.pone.0133521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emmanuel A, Johnson M, McSkimming P, Dickerson S. Laxatives do not improve symptoms of opioid-induced constipation: results of a patient survey. Pain Med. 2016;18:1932–1940. doi: 10.1093/pm/pnw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am. J. Gastroenterol. 2005;100:124–129. doi: 10.1111/j.1572-0241.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 24.Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am. J. Gastroenterol. 2003;98:1790–1796. doi: 10.1111/j.1572-0241.2003.07591.x. [DOI] [PubMed] [Google Scholar]
- 25.Brown WJ, Mishra G, Lee C, Bauman A. Leisure time physical activity in Australian women: relationship with well being and symptoms. Res. Q. Exerc. Sport. 2000;71:206–216. doi: 10.1080/02701367.2000.10608901. [DOI] [PubMed] [Google Scholar]
- 26.Meshkinpour H, et al. Effects of regular exercise in management of chronic idiopathic constipation. Dig. Dis. Sci. 1998;43:2379–2383. doi: 10.1023/A:1026609610466. [DOI] [PubMed] [Google Scholar]
- 27.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer determined steps per day in US adults. Med. Sci. Sports Exerc. 2009;41:1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- 28.Baril JF, et al. Use of free-living step count monitoring for heart failure functional classification: validation study. JMIR Cardio. 2019;3:e12122. doi: 10.2196/12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tudor-Locke C, Hart TL, Washington TL. Expected values for pedometer determined physical activity in older populations. Int. J. Behav. Nutr. Phys. Act. 2009;6:59. doi: 10.1186/1479-5868-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donald IP, Smith RG, Cruikshank JG, Elton RA, Stoddart ME. A study of constipation in the elderly living at home. Gerontology. 1985;31:112–118. doi: 10.1159/000212689. [DOI] [PubMed] [Google Scholar]
- 31.Feehan LM, et al. Accuracy of fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6:e10527. doi: 10.2196/10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton E, et al. Reliability and validity of two fitness tracker devices in the laboratory and home environment for older community-dwelling people. BMC Geriatr. 2018;18:103. doi: 10.1186/s12877-018-0793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Pino F, Porras-Segovia A, López-Esteban P, Artés A, Baca-García E. Validation of fitbit charge 2 and fitbit Alta HR against polysomnography for assessing sleep in adults with obstructive sleep apnea. J. Clin. Sleep Med. 2019;15:1645–1653. doi: 10.5664/jcsm.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clevenger KA, Molesky MJ, Vusich J, Montoye AH. Free-living comparison of physical activity and sleep data from fitbit activity trackers worn on the dominant and nondominant wrists. Meas. Phys. Educ. Exerc. Sci. 2019;23:194–204. doi: 10.1080/1091367X.2019.1577737. [DOI] [Google Scholar]
- 35.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am. J. Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 36.Bakker JP, et al. A systematic review of feasibility studies promoting the use of mobile technologies in clinical research. NPJ Digit. Med. 2019;2:47. doi: 10.1038/s41746-019-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, et al. Design, recruitment, and baseline characteristics of a virtual 1-year mental health study on behavioral data and health outcomes: observational study. JMIR Ment. Health. 2020;7:e17075. doi: 10.2196/17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quisel T, Foschini L, Zbikowski SM, Juusola JL. The association between medication adherence for chronic conditions and digital health activity tracking: retrospective analysis. J. Med. Internet Res. 2019;21:e11486. doi: 10.2196/11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev. Chronic Dis. 2012;9:E113. doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to the data and related study documents including the study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
The code that supports the findings of this study are available from the corresponding author upon reasonable request. Analysis to process and analyze the data was generated with Python 3 and the R programming language.