Abstract
Tissue-engineered vascular grafts (TEVGs) have enormous potential for vascular replacement therapy. However, thrombosis and intimal hyperplasia are important problems associated with TEVGs especially small diameter TEVGs (<6 mm) after transplantation. Endothelialization of TEVGs is a key point to prevent thrombosis. Here, we discuss different types of endothelialization and different seed cells of tissue-engineered vascular grafts. Meanwhile, endothelial heterogeneity is also discussed. Based on it, we provide a new perspective for selecting suitable types of endothelialization and suitable seed cells to improve the long-term patency rate of tissue-engineered vascular grafts with different diameters and lengths.
Keywords: Tissue-engineered vascular grafts, scaffold materials, seed cells, vascular endothelialization, endothelial heterogeneity
Highlights
-
•
The material, diameter and length of tissue-engineered vascular graft are all key factors affecting its long-term patency.
-
•
Endothelialization strategies should consider the different diameters and lengths of tissue-engineered vascular grafts.
-
•
Cell heterogeneity and tissue heterogeneity should be considered in the application of seed cells.
Cardiovascular disease (CVD) is the most common cause of death worldwide, accounting for 31% of all deaths globally and being the cause of 40% of deaths in the Chinese population [1,2]. The number of deaths is expected to grow to more than 23.6 million by 2030 [3]. Autologous or allogeneic vascular transplantation is a common and effective method for the treatment of cardiovascular diseases. For example, the internal thoracic artery and the great saphenous vein are commonly used by surgeons as autologous vascular substitutes [4]. Besides, blood vessels are also needed for hemodialysis that cures diseases such as kidney failure [5]. The data show that more than 500,000 vascular bypass grafts are implanted in patients in the United States every year to replace damaged blood vessels [6]. However, autologous or allogeneic vessels couldn't meet the clinical demands constantly because of vascular availability or donor shortage [7]. There is an increasing demand for vascular grafts that could be used for coronary artery bypass grafting and peripheral artery bypass grafting. Therefore, constructing tissue-engineered vascular grafts (TEVGs) is accepted as a promising and acceptable alternative solution. While autologous veins or arterial grafts with a diameter of 3–5 mm are used for aorta-coronary artery anastomosis constantly [8]. In other words, most of the patients could benefit from constructing small-diameter TEVGs (<6 mm internal diameter; ID).
1. Common types and materials of TEVGs
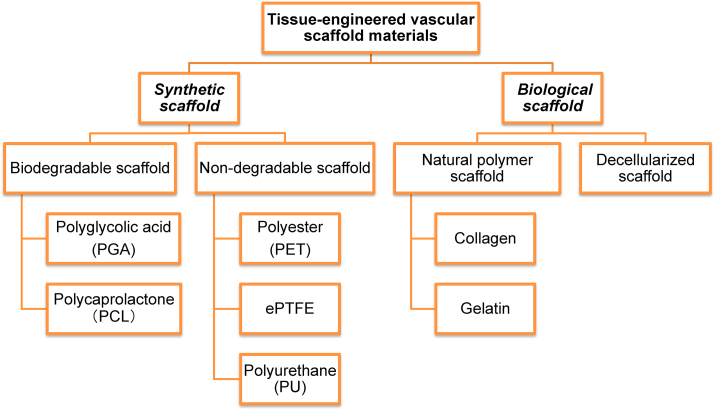
TEVG is a kind of vascular substitute with good biocompatibility and mechanical properties constructed by the tissue engineering methods. It contains three elements: seed cells, scaffold materials and signals [9]. In general, scaffolds are used as supporting structures to make seed cells adhere and proliferate, reaching functional maturity [10]. However, different scaffold materials have variant properties, the common scaffold materials of TEVGs (Fig. 1) will be discussed to select proper materials for producing TEVGs. Among them, suitable vascular scaffold materials should imitate the natural extracellular environment, provide appropriate mechanical and biological properties and possess good biocompatibility simultaneously [11].
Fig. 1.
Common types and materials of TEVGs. At present, a variety of materials have been produced as vascular scaffolds, which are mainly divided into these types: non-degradable synthetic, biodegradable, natural polymer and decellularized scaffolds.
Non-degradable synthetic materials such as expanded polytetrafluoroethylene (ePTFE), polyester (PET), and polyurethane (PU) have been used as substitutes for large blood vessels for decades due to their good mechanical properties, durability and convenient production [12]. In the treatment of superficial femoral artery occlusion diseases and renal diseases requiring hemodialysis, ePTFE stent grafts show satisfactory safety and short-term patency [13]. However, clinical studies found that the long-term patency rate of small-diameter ePTFE grafts were not encouraging [14]. Moreover, these materials are short of cellular communication signals and integrin-binding sites, which might decrease cell attachment and infiltration [15].
Shinoka et al. first to produce tubular scaffolds with regenerative and repair functions by using polyglycolic acid (PGA) [16]. Shum et al. constructed a PGA large-diameter scaffold with a diameter of 7 mm [17]. After the scaffold implanting, the percentages of collagen and DNA contents are close to those in the natural aorta, and the mechanical string-stress curve is close to that of natural blood vessels. PGA is also used to produce small-diameter TEVGs, which remain patent for 24 days [18]. However, PGA grafts degrade rapidly within 2–3 weeks and lose their mechanical integrity [19]. Compared with the PGA, polycaprolactone (PCL) has a slower degradation rate and provides adequate mechanical properties effectively [20]. The porous scaffolds made of PCL have sufficient mechanical strength and porosity to satisfy the demand for clinical vascular transplantation [21]. However, poor regeneration of vascular walls, irregular cell infiltration and partial calcification are still obstacles to limiting PCL applications in the long run [22]. Take cell infiltration for example, pore sizes of PCL scaffolds play an important role in cell processes: the nanopore size membranes are helpful to acquire the collagen fibers and ECM, whereas macropores are significant in cell seeding and neo-vascularization in vivo [23]. Therefore, the macropores PCL grafts could enhance cell infiltration and extracellular matrix (ECM) secretion [24]. However, the irregular cell infiltration restricted the re-construction of vessels’ structure.
Natural polymer materials such as collagen, gelatin, and chitosan are nontoxic and have good biocompatibility, and that promote cell adhesion and retain differentiation function [[25], [26], [27]]. For example, Badhe et al. prepared a double-layer tissue-engineered scaffold with a mixture of chitosan and gelatin, which supports the growth and spreading of cells [28]. Collagen can be used for producing vascular grafts alone [29]. While by combining Hyaluronic acid (HA) and human-like collagen, the vascular scaffolds improve biophysical and mechanical properties that are close to those of the natural ECM [30]. It shows that collagen combined with other materials could demonstrate better prospects for future researches. However, as the activation signals of the coagulation pathway, collagen is easy to form thrombus, which limits collagen to being used in the clinical therapy [[31], [32], [33]]. Hyaluronic acid (HA) can promote adhesion and proliferation of ECs [34]. Moreover, HA is shown to be able to inhibit fibroblast proliferation, which plays a crucial role in vascular thrombosis and anastomotic hyperplasia [35]. When coating a HA hydrogel layer, the decellularized TEVGs have the benefits of inhibition of thrombus formation and promotion of endothelium attachment [36]. These studies indicate that HA has better application prospects for TEVGs.
The decellularized native blood vessels scaffolds retain the structure and properties of ECM. Abundant growth factors and cell adhesion signals are also left behind [37]. So decellularized scaffolds can serve as cell adhesion sites and provide mechanical strength support for TEVGs [38]. Moreover, decellularized scaffolds have many advantages, such as strong affinity, good biocompatibility, and extremely low immunogenicity, etc. However, there are also defects such as low accessibility, fast degradation rate, and inability to alter the content and structure of an ECM [39]. Besides, the implantation of large amounts of complex ECM into the vascular system may promote thrombosis [40].
To solve the problem of intimal hyperplasia and thrombosis of TEVGs, the coating modified on TEVGs is a feasible method. Studies have shown that the use of fluorosurfactant (FSP) or heparin coating can inhibit the intimal hyperplasia and thrombosis of ePTFE vascular grafts effectively [41]. The patency rate of polyurethane (PU) vascular grafts modified by heparin and cell-adhesive peptides was significantly higher than that of unmodified PU vascular grafts after 9 weeks of implantation [42]. Decellularized scaffolds coated with vascular endothelial growth factor (VEGF) and peptide also reduce intima formation and thrombus formation [[43], [44], [45], [46]]. These studies suggest that coating on grafts can reduce intimal hyperplasia and thrombosis, which is important for the application of TEVGs.
Overall, non-degradable synthetic materials are mainly used to produce large-diameter TEVGs, while these materials are not recommended for small-diameter TEVGs. Due to the defects of polymer degradable materials and natural polymer materials, there is a trend of producing composite materials by mixing several materials to give play to complementary advantages. Many studies have shown that composite materials improve the grafts properties and provide positive results [[47], [48], [49], [50]]. From a wide range of sources, decellularized scaffolds have good biological properties and low immunogenicity. Besides, decellularized scaffolds can promote the growth, proliferation, and differentiation of inoculated cells. These good properties make the decellularized scaffolds become a promising material in TEVGs and an active research field. Here, a more complete summary of the advantages and disadvantages of different scaffold materials is shown as follows (Table 1), which helps to provide a reference for choosing proper materials to construct TEVGs.
Table 1.
Advantages and disadvantages of different scaffold materials for TEVGs.
| Scaffold type | Materials | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Non-degradable | ePTFE, PET, PU, etc. | *Have successfully been employed for decades to bypass and reconstruct medium to large diameter vessels *Good mechanical properties, durability, and convenient production |
*Short of cellular communication signals and integrin-binding sites *Mechanical properties incompatible with soft tissue regeneration (may be in composite scaffolds) |
[7,12,15,51] |
| Biodegradable | PGA, PCL, etc. | *Can be tailored with specific physical properties to suit particular applications *Convenient production *Appropriate mechanical properties |
*Toxic degradation products and loss of mechanical properties during degradation *Can't accurately mimic the in vivo microenvironment of cells *Poor regeneration of vascular wall and partial calcification |
[19,20,[52], [53], [54]] |
| Natural polymer scaffold | Collagen, gelatin, chitosan, etc. | *Promote adhesion and proliferation of ECs *Excellent biodegradable and biocompatible properties *Stimulate the colonization of recruited cells Inhibit thrombosis and promote endothelium attachment |
*May degrade rapidly and poor mechanical strength *Material sourced from an animal could lead to potential disease transmission *Variable quality assurance |
[35,[55], [56], [57]] |
| Decellularized scaffold | Umbilical artery, umbilical vein, animal artery, etc. | *Keep the structure and properties of ECM *Extractable from specific tissue of interest *Extremely low immunogenicity *Strong affinity, good biocompatibility |
*Fast degradation rate of scaffolds *Inability to alter the content and structure of an ECM *Risk of viral transmission from animal tissues *Graft-related thrombosis, infection, and aneurysm *Variable in composition/quality from batch to batch |
[9,39,58,59] |
2. Selection of endothelialization forms for TEVGs with different lengths and diameters
Due to the environment, pressure and blood flow of TEVGs with different diameters and lengths are different in vivo, the patency of vascular grafts is significantly affected by cell composition, length, diameter, internal surface modification and pretreatment of TEVGs [60]. For example, with the same scaffold and diameter, the vascular patency time will decrease when the length of TEVGs increases (Table 2). Similarly, with the same scaffold and length, TEVGs of smaller diameters will shorten the vascular patency time (Table 3). That is, the patency time of TEVGs is positively correlated with the diameter and negatively correlated with length. Classifying the TEVGs and giving different solutions respectively might be a possible method to obtain a satisfactory long-term patency rate. In other words, TEVGs of different lengths and diameters should have different ways to achieve endothelialization.
Table 2.
Comparison of patency of TEVGs with different length.
| Number | Scaffold | Inner diameter (ID: mm) | Length (cm) | Graft patency time | Date and References |
|---|---|---|---|---|---|
| 1 | Decellularized vascular scaffold | <4 | 1 | 100% at 8 weeks | 2014 [61] |
| <4 | 3 | 100% at 2 weeks | 2015 [62] | ||
| 2 | 2 | 4 | 50% at 3 months | 2013 [63] | |
| 2 | 7 | 100% at 4 weeks | 2019 [64] | ||
| 3 | 4 | 12 | 100% at 30 days | 2011 [65] | |
| 4 | 6 | 80% at 6 months | 2017 [66] |
Table 3.
Comparison of patency of TEVGs with different inner diameter.
2.1. Vascular grafts larger than 6 mm in diameter
With stable biological properties, PET and ePTFE are commonly used as substitutes for large-diameter (ID ≥ 6 mm) vascular grafts. The 5-year patency rate of large-diameter PET graft (7–9 mm) used in aortic bypass grafts is 93% [71,72]. These large vascular grafts usually do not need endothelialization actively to obtain satisfactory results. However, when these materials are applied to smaller-diameter vessels (ID < 6 mm), it is commonly difficult to reach expected targets due to occlusive thrombosis [73]. Besides, biomaterials are prone to chronic foreign body reactions, and fibrosis capsules are formed around the grafts, which is not favorable to the long-term patency of TEVGs [74,75].
2.2. Vascular grafts with a diameter of 4–6 mm and a length less than 5 cm
Small-diameter vascular grafts are prone to inducing obstruction because of Thrombosis, so these vascular grafts should possess some properties to avoid thrombosis [76]. Under normal conditions, ECs regulate blood coagulation and platelet functions, which reduces platelet aggregation and inhibits thrombosis. Once vascular injury (EC injury or detachment) occurs, thrombi will form at the site of the injury to prevent excessive blood loss (Fig. 2). So endothelialization is viewed as one of the most important factors in inhibiting thrombosis [77]. Therefore, vascular grafts with endothelium are considered as a key to inhibiting intimal hyperplasia and thrombosis [78,79].
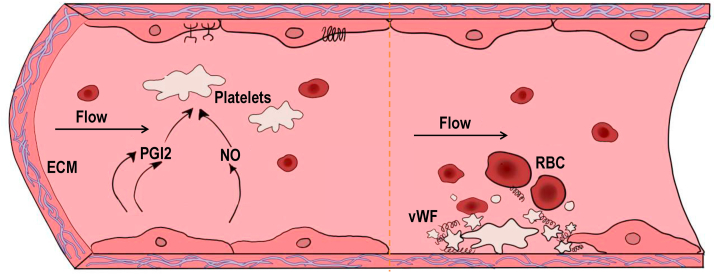
Fig. 2.
The role of endothelial cells in regulating thrombosis. Under normal physiological conditions, ECs secrete Prostacyclin (PGI2) and nitric oxide (NO), which are important for the regulation of blood coagulation and platelet functions by synergistically increasing cAMP content in platelets. Ecto-nucleotidase derived from ECs hydrolyze ATP and ADP to AMP and adenosine, which also reduces platelet aggregation. In addition to the factors above, ECs also inhibit thrombosis by inactivating coagulation factors and inhibiting thrombin activity [80]. When endothelium is damaged, negatively charged extracellular matrix(ECM)such collagen exposed to blood, result in accumulation of platelets, von Willebrand factor (vWF) and red blood cells (RBCs), eventually lead to the formation of thrombus [15].
Compared with decellularized vascular grafts of non-endothelialization, re-endothelialized decellularized vascular grafts have an excellent anti-thrombus property and ability to inhibit the abnormal proliferation of smooth muscle cells (SMCs). That avoids the occurrence of intimal hyperplasia and maintains patency for 6 weeks after implantation [67]. In an endothelialization study of porcine coronary arteries using adipose stem cells, there is integration between host tissue and implanted tissue, and the graft patency rate reaches 100% [81]. One study found that ECs on implanted vascular grafts originated entirely from the host, while seed cells were lost after vascular transplantation [82]. Therefore, the in situ endothelialization of TEVGs with a diameter of 4–6 mm is a feasible option.
2.3. Vascular grafts less than 4 mm in diameter and less than 5 cm in length
With an inner diameter of less than 4 mm, the vascular grafts usually form thrombus after implantation in the early stage, resulting in vascular occlusion and failure to maintain long-term patency. In one study about the decellularized vascular grafts (length: 1 cm; ID: ~1–1.5 mm), which was constructed by the static seeding method in 5 days. After the 14-day follow-up, only one of six rats was alive in the non-endothelialization group, the vascular grafts were short of ECs and there was endothelial damage and thrombotic plaque formation on the luminal side. In contrast, four of six rats were alive in the re-endothelialization groups, ECs are observed in the implanted vascular grafts and there were fewer thrombotic plaques or fibrin clots that were noted in the lumen [83]. Besides, compared with the control group, the decellularized vessels of re-endothelialization in vitro retained >95% monolayer EC coverage in 1 h after implantation, endothelial coverage increased. 9 weeks later, intimal hyperplasia decreased, and growth of the basement membrane was observed [84]. Two patients were treated by the grafts with endothelialization, there was no myocardial infarction occurred, and the grafted vessels remained patent in the six-month follow-up [85]. At six months after the implantation of the reendothelialized decellularized porcine aorta, the vascular graft had acquired a complete layer of ECs, and the fibroblasts had grown uniformly into the medial membrane, showing a structure similar to that of normal arteries, with good patency and no apparent thrombosis [66].
In a word, TEVGs (ID:>6 mm, length>5 cm) do not need to facilitate endothelialization since the faster blood flow speed does not easily form thrombus; TEVGs (ID:4–6 mm, length<5 cm) are prone to thrombosis and graft occlusion due to the decreased blood flow velocity and smaller blood vessel diameter, so this type of TEVGs requires vascular endothelialization. Compared with endothelialization in vitro, relying on seed cells to realize endothelialization in vivo is a safer and more convenient option. For TEVGs (ID < 4 mm, length<5 cm), acute thrombus occlusion may occur after implantation in the early stage, leading to a major cause of graft failure. Therefore, it is necessary to implant TEVGs after endothelialization in vitro.
3. Selection of different seed cells for endothelialization of TEVGs
The vascular endothelial layer has antithrombotic functions and prevents platelet adhesion, which is very important to increase the patency of vessels and reduce the incidence of vascular stenosis or occlusion [93]. studies (Table 4) showed that even though the inner diameter and length of TEVGs are different, re-endothelialized TEVGs get better results in increasing the patency rates of grafts than TEVGs without endothelialization. Therefore, a complete endothelial layer is crucial to the TEVGs, and selecting the ideal seed cell source of the vascular endothelial layer is considered a key factor. The sources of common endothelial seed cells in studies are shown in Fig. 3. To meet clinical needs, seed cells should have the following characteristics: (1) sufficient number of seed cells to meet the requirements, and the sources of acquisition should be diverse; (2) the processes of isolating and acquiring seed cells should be relatively easy; and (3) trauma for both patients and donors should be minimized.
Table 4.
Comparison of patency rate of reendothelialized or non-reendothelialized small-diameter TEVGs.
| Number | Inner diameter(mm) | Length(cm) | Experimental group | Control group | Observing time | Date and References |
|---|---|---|---|---|---|---|
| 1 | 4 | 5–6 | 66.7% | 37.5% | 24 weeks | 2013 [86] |
| 2 | 3 | 5 | 83.3% (5/6) | 16.7% (1/6) | 3 months | 2014 [87] |
| 3 | 1 | 0.6 | 89% | 29% | 4 weeks | 2005 [88] |
| 4 | 4 | 4–5 | 100% (4/4) | 0 (0/9) | 6 months | 2008 [89] |
| 5 | 3–4 | 4 | 100% (2/2) | 0 (0/3) | 5 months | 2010 [90] |
| 6 | 1 | 0.5 | 90% (9/10) | 10% (1/10) | 6 months | 2018 [91] |
| 7 | 4 | 12 | 100% (5/5) | 37.5% (3/8) | 30 days | 2011 [65] |
| 8 | 3 | 4–5 | 95% (19/20) | 60% (12/20) | 3 months | 2012 [92] |
Note: Experimental group: reendothelialized small-diameter TEVGs; Control group: non-reendothelialized small-diameter TEVGs.
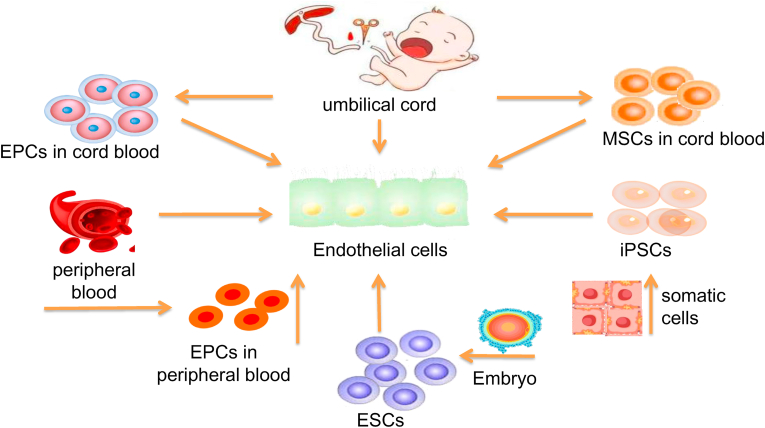
Fig. 3.
Sources of common endothelial seed cells. The most commonly used cells in. tissue engineering blood vessels (TEBV) include human umbilical vein endothelial cells (HUVECs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), embryonic stem cells (ESCs), and pluripotent stem cells (iPSCs).
3.1. Umbilical vein endothelial cells (HUVECs)
To date, HUVEC is still the preferred seed cell for TEVGs. An analysis of all studies from 2013 to 2018 found that 59% of studies still used HUVECs as a source of human ECs [94]. The use of umbilical cord cells has the advantage of harvesting the patient's complete vascular structure without invasive procedure and obtaining approximately 20–30 cm of vascular tissue. Meanwhile, the umbilical cord can separate a large number of immature and fast-growing cells, so a sufficient number of cells can be obtained in a short time for scaffold seeding in a short time. Experimental studies have shown that the human umbilical cord artery (UCA) and umbilical cord vein (UCV) have similar cell growth characteristics to the saphenous vein, and no difference has been found in the mechanical properties of tissue engineering constructs [95]. Schechner et al. demonstrated that TEVGs with HUVECs could connect with the host circulatory system and be remodeled into complex capillaries after surgical implantation into severe combined immunodeficiency (SCID) mice [96]. However, it is difficult to purify umbilical cord-derived endothelial cells, which are easily mixed with a large number of fibroblasts and SMCs without antithrombotic ability. Moreover, HUVECs are unable to adhere to the scaffold materials firmly and easily washed away by blood flow, resulting in the formation of thrombus.
3.2. Mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs) exist in a variety of tissues and can be isolated, grown and differentiated into many cell lineages in vitro. After MSCs are implanted into the transplant recipient, there is no obvious immune rejection response [97]. Under natural conditions, some kinds of MSCs have biological properties that promote angiogenesis [98]. Among them, umbilical cord mesenchymal stem cells (UC-MSCs) are easy to obtain and not limited by ethical concerns. UC-MSCs also have a high proliferation rate and amplification potential in vitro, as well as the ability to downregulate the immune response and promote tissue repair. Moreover, UC-MSCs could reduce the risk of contamination and damage during amplification in vitro [99]. In recent years, MSC products derived from UCs have been increasingly introduced into clinical research, accounting for approximately 27% of current clinical trial products [100]. With the support of UC-MSC and cytokine supplementation, fibrin scaffolds can achieve efficient expansion of umbilical cord blood endothelial cells [101]. That provides a solution to the problem of limited and insufficient expansion in vitro. A recent report records cases of forearm venous thrombosis after getting UC-MSC treatment [102]. These cases may be associated with a higher level of the pro-coagulant factor TF/CD142 expressed by UC-MSCs and a stronger inflammatory response [103]. This puts forward new requirements for the potential safety issues of UC-MSCs and indicates the clinical application of UC-MSCs would be cautious. However, a retrospective study indicated that 93% of published studies had safe or positive results, and an additional 18% of published studies indicated that the applications of UC-MSC were safe and that any patient's death in these studies was usually attributed to their underlying disease [104].
3.3. Endothelial progenitor cells (EPCs)
Endothelial progenitor cells (EPCs) are usually derived from bone marrow or peripheral blood, and EPCs have the potential to be induced to differentiate into ECs of various tissues [104,105]. EPCs can form vascular structures both in vitro and in vivo, which have permeability that is similar to the vascular endothelium, and EPCs may be well superior to vascular-derived endothelium in the formation of vascular networks [105]. There is a good prospect to obtain ECs from EPCs of peripheral blood with the noninvasive operation, which is beneficial to clinical promotion and application [106]. Moreover, endothelial progenitor cell-induced endothelial cells that are from porcine peripheral blood were similar to the normal aortic ECs of pigs in growth structures, phenotypes and functions [107]. Kaushal et al. use EPCs obtained from peripheral blood to construct TEVGs, which maintain patency for 130 days and show similar contractility and NO-mediated vasodilation to those of the natural carotid artery [108]. In addition, EPCs can be rapidly covered on implanted artificial vascular scaffolds [109], which provides important support for the in situ induction of vascular endothelialization.
Compared with EPCs derived from peripheral blood, cord blood endothelial progenitor cells (CB-EPCs) are more stable and have better physiological functions in TEVGs [110]. CB-EPCs proliferated in vitro and cultured on a three-dimensional PGA-PLLA scaffold, which retained an endothelial phenotype [111]. Martin et al. demonstrated the feasibility that cord blood and adult peripheral blood are used as sources of EPCs [112]. However, the TEVGs formed by EPCs in adult peripheral blood were unstable and gradually disappeared within 3 weeks. In contrast, the vascular function formed by CB-EPCs is normal and can be maintained for more than 4 months [110]. Therefore, CB-EPCs may be more suitable as a source of seed cells for TEVGs.
At present, the major limitations to the widespread of EPCs are isolating difficulty and few quantities in peripheral blood or bone marrow. In addition, the characteristics and mechanism of EPCs differentiation are also unclear, possibly due to the lack of standardized methods for isolation and identification [113]. Moreover, EPCs in different periods and subsets of EPCs are heterogeneous in angiogenic potential and tissue specificity [113,114]. CB-EPCs also have limitations in autologous cell therapy because most patients are unable to obtain their own cord blood. Establishing a cell bank is a good strategy to solve this problem [94].
3.4. Embryonic stem cells (ESCs)
Embryonic stem cells (ESCs) are unique cells population isolated from early embryos (before the gastrula stage) or primary gonads, which have the ability of unlimited proliferation, self-renewal and multipotent differentiation [115]. Moreover, the differentiation of ESCs into ECs is feasible in theory [116,117]. The differentiation of ESCs depends on their microenvironment, including biomechanical stress, cytokines or growth factors, ECM, and communication with neighboring cells [118]. Therefore, regulation of the extracellular environment can promote the differentiation of ESCs into ECs. The addition of exogenous ETV2 can promote the directional differentiation of primitive endothelial cells derived from ESCs, and the differentiated endothelial cells show no differences from arteriovenous endothelial cells [119]. Leptin also regulates the differentiation of ESCs into ECs [120]. VEGF plays an important role in improving survival and migration of ECs induced by ESCs and promoting vascular remodeling [121,122]. In addition, regulating signaling pathways is an effective mechanism to promote directional differentiation of ESCs. Upregulating the Notch1 signaling pathway can promote the differentiation of ESC into ECs [123], while the downregulating TGF-β signaling pathway also has similar effects [124]. However, TGF-β signaling also plays a role in ESCs differentiating into SMCs [125], and the release of TGF-β stimulates extracellular matrix production by SMCs [126], which are the primary cell type in the pre-atherosclerotic intima [127]. Therefore, the specific mechanisms of between TGF-β and embryonic stem cell differentiation need to be further clarified. To accomplish efficient differentiation of ECs from ESCs, it is necessary to regulate the MAPK and PI3K signaling pathways, and this approach does not require cell sorting or magnetic purification to acquire a very pure endothelial cell population [128].
Although ESCs have good prospects for the future studies, there are still some problems to be solved. First, there is still no conclusion on the ethical controversy about ESCs [129]. Second, the serious risk of teratoma formation hinders the use of ESCs in TEVGs [130]. Therefore, how to isolate and identify ESCs that can't form teratomas but can differentiate into ECs is a great challenge for researchers. In fact, solving the immune rejection of transplantation is also a huge challenge for the application of ESCs [131,132]. While establishing a cell bank through immune matching is a good solution strategy [133].
3.5. Induced pluripotent stem cells (iPSCs)
Since Shinya Yamanaka et al. succeeded in inducing somatic cells to pluripotent stem cells [134], induced pluripotent stem cells (iPSCs) have become the focus of research. The vascular grafts constructed by iPSC-induced ECs, morphology and inflammatory response are similar to normal blood vessels [135]. The scaffold material or the decellularized vascular scaffold is similar to the basement membrane of normal blood vessels, which can provide good adhesion to ECs [136,137]. This promotes TEVGs to achieve endothelialization in vitro. Besides, the smooth muscle layer planted in vitro can provide ECs with microenvironment and biomechanical support close to normal blood vessels, which can better help TEGVs endothelialization in vitro [138]. ECs from iPSCs have the same angiogenic effect as those derived from ESCs [139], and the endothelialization potential is very similar [140]. Besides, iPSCs provide an unlimited cell bank that can be used to obtain a large number of SMCs and ECs with good biological characteristics [141].
Margariti et al. found that the PiPS-ECs displayed good attachment, stabilization, patency, and typical vascular structure when seeded on decellularized vessel scaffolds [142]. Samuel et al. successfully obtained ECs and multipotent progenitor cells (MPCs) from type I diabetic iPSC lines and used them to construct functional and durable blood vessels in vivo [143]. It shows that iPSCs from patients can also construct normal, patient-specific TEVGs. Li et al. constructed TEVGs by using vascular SMCs induced by iPSCs and found that the new type of TEVG can withstand surgical operation and arterial pressure. But there appeared to be dilatation in the vascular grafts [144]. That is because the hiPSC-derived TEVGs have low mechanical strength that leads to significant radial dilation after implantation. Luo et al. produced the hiPSC-derived TEVGs, which have good patency without luminal dilation, effectively maintaining mechanical and contractile function [145]. This is a beneficial attempt at exploration for the clinical study of TEVGs seeded with iPSCs. In addition, iPSCs derived from peripheral blood are successfully used to construct TEVGs, providing a new basis to obtain somatic cells as seed cells conveniently and rapidly [146]. Some studies suggest that some kinds of iPSC-derived cells show immunogenicity [147], but iPSC-derived ECs could tolerate the immune response and survive for a long time [148]. These low-immunogenicity iPSCs still retain their pluripotent stem cell potential and differentiation ability [149]. Li et al. achieved the allotransplantation of TEVGs based on iPSCs [144], which provides experimental evidence for the application of iPSCs in allograft transplantation.
However, pluripotent stem cells may cause malformations or tumors [150], and the cell differentiation of iPSCs is a complex and not fully defined process. Besides, the heterogeneity of cell subsets produced by iPSCs is also a major problem that researchers need to solve [151]. What's more, the long time and high cost of reprogramming and differentiation process also limit the application of iPSCs [152].
In various types of seed cells, the most commonly used seed cells are HUVECs, while iPSCs are considered to be a better seed cell source. iPSCs could come from the patients, and the iPSCs are differentiated to the same type of ECs to get the function similar to the endothelium of the transplanted site, which makes the TEVGs adapt to the nearby vascular endothelium and reduces the occurrence of complications. In addition, for patients with vascular abnormalities caused by gene mutations, the patient-specific iPSCs can be genetically edited to differentiate into normal vascular cells and regain vascular function. Based on the above conclusions, we proposed a tissue-engineered vascular therapy method based on patient-specific iPSC (Fig. 4). A more complete summary of these common seed cells is as follows (Table 5), which helps to know the advantages and disadvantages of the seed cells and to select proper seed cells for TEVGs.
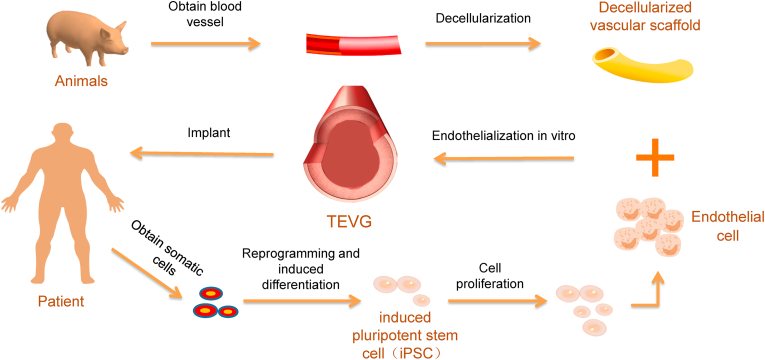
Fig. 4.
A tissue-engineered vascular therapy strategy based on the patient's iPSCs. Blood vessels from animals were decellularised preserving microarchitecture, and extracellular matrix. Then we acquired the decellularized scaffolds, which provide good adhesion to ECs. Meanwhile, somatic cells from the patient are reprogrammed to iPSCs. Next, the iPSCs differentiated to ECs and seeded on the decellularized scaffolds to achieve endothelialization of scaffold in vitro, finally implanted into patients.
Table 5.
Advantages and disadvantages of different seed cells.
| Seed cell | Advantages | Disadvantages | References |
|---|---|---|---|
| HUVEC | *Non-invasive harvesting method from “medical waste” *Can be easily isolated in high numbers *A large number of published studies = comparable results |
*Lack of organ-specificity *Heterogeneity of ECs *Limited replicative capacity in culture |
[94,153] |
| MSC | *Can be isolated from virtually all tissues *Multilineage differentiation potential *Paracrine and immunomodulatory properties *Antithrombogenic property |
*Heterogeneity of the MSC population *Harvested from elderly or diabetic patients have diminished regenerative potential in vascular tissue engineering *Difficulties in identifying and isolating native MSCs |
[[154], [155], [156], [157], [158], [159], [160]] |
| EPC | *Non-invasive means *Robust proliferative and blood vessel-forming abilities *Can create a non-thrombogenic surface |
*Standardization of detection and cultivation procedures is essential *Heterogeneity of EPC populations from different origins *Few quantity and isolating difficulty |
[[161], [162], [163], [164], [165], [166]] |
| ESC | *Potential to differentiate into every cell type of the body *Consistency with physiological development |
*Ethical controversy *Risk of teratocarcinoma formation *Inefficiency of Induction differentiation |
[[167], [168], [169], [170]] |
| iPSC | *Obtain large numbers of clinically relevant cells *A robust source of patient-specific cells *Avoid allogenic immune rejection |
*Potential genetic and epigenetic alternations *Tumorigenicity *Lack of robust and highly reproducible *Differentiation protocols *High costs |
[167,[171], [172], [173], [174], [175], [176]] |
Currently, research on the endothelialization of vascular grafts by using seed cells is in full swing. While some studies have found that seeded cells are not incorporated into the developing TEVGs, they are lost in the early stages after the implant, and TEVGs are composed of recruited host cells which mainly come from the adjacent vessel walls [82,177,178]. This may be a departure from the traditional concept that seed cells are viewed as part of neovessels. But with seed cells, vascular grafts have more satisfactory results in patency rate, intimal hyperplasia, and tissue regeneration [65,179]. Therefore, seed cells may only play a temporary role in the development of blood vessels at the early stages, and likely act in a paracrine manner to inhibit thrombosis and maintain long-term patency [157].
Besides, there is a problem with how the density of seed cells affects the endothelialization of TEVGs. In the process of planting in TEVGs, the density of ECs varies from 5 × 105 (decellularized scaffold) to 1 × 108/ml (PGA scaffold) [142,144]. When using bone marrow as a cell source, higher seeding densities may mitigate graft stenosis [180]. And a cell density of more than 400 cells per cm3 of the scaffold material is needed because the cell density is able to prevent acute thrombosis and bring beneficial scaffold remodeling after implantation [181]. Therefore, for vascular scaffolds of different materials and diameters, appropriate cell density plays a very important role in achieving endothelialization in vitro.
4. Heterogeneity of vascular endothelium
4.1. Morphological and functional differences of arteriovenous endothelium
There are significant differences in morphology between arterial and venous endothelium [182,183]. Arterial ECs are long and narrow or oval, while venous ECs are short and wide, which is related to the fact that the velocity of blood flow in the venous circulation is significantly lower than that in the arterial circulation. There are also differences in intercellular junctions between the two endothelia, as well as in mediated intercellular adhesion and communication. For example, the intercellular junctions between arteries of all diameters were tighter than those in veins, especially postcapillary venules, which showed loose tight junctions [184].
Besides, the function between arterial endothelium and venous endothelium is also obviously different, which may be because of the different types of blood vessels and the sites within the vasculature [185]. Arteries have greater vascular tension and vasoconstriction to maintain blood pressure and maintain blood perfusion. However, veins, especially postcapillary venules, are the main sites of leukocyte chemotaxis during inflammation [184,186]. In the oxidative stress response, the venous endothelium showed a significant increase in inflammation and a decrease in proliferative phenotype [187].
4.2. Difference of gene expression of the vascular endothelium in different parts
In the process of angiogenesis, progenitor cells gradually acquire markers of ECs phenotype and then are directionally differentiated into arterial, venous and capillary ECs [188,189]. ECs in different areas are heterogeneous cell populations [190,191], and each of them has unique molecular markers [192], but this molecular marker can be altered [193]. While tissue microenvironment may play an important role in regulating endothelial heterogeneity [194,195].
Mike et al. found six genes encoding brain endothelium–specific transcription factors [196]. Among them, Foxf2, Foxl2, Foxq1 and Lef1 play a role in the differentiation of the central nervous system ECs in early development; Ppard and Zfp551 may contribute to the phases of late development or maturity. Besides, genes such as Ptgds and Atp1a2, which are involved in classical neurological expression processes such as neurotransmitter transport, axonal development and regulation of ion transmembrane transport, are significantly enriched in the brain ECs [197].
Renomedullary ECs upregulate OXPHOS and other genes involved in hypoxia reaction, glycolysis and oxidative phosphorylation to cope with hypertonic dehydration response [198]. Genes such as Myl2, Myl3 and Aqp7 that are specifically upregulated by cardiac ECs, and these genes are involved in processes such as myocardial tissue development, myofibril assembly and cardiac contraction [197]. Although the aorta is in the neighborhood of the heart, genes such as Ehd3 and Fam167b are specifically expressed in aortic ECs and are not expressed in cardiomyocytes [199].
Compared with lymphatic ECs, pulmonary vascular ECs were highly enriched for the genes Epas1, Klf4, Gata2, Klf2 and Sox17 [200]. Moreover, genes involved in immune function were significantly upregulated [197]. In newborn alveoli (AT1/AT2), the tissue factor of regulating UPR genes and endoplasmic reticulum pressure sensors are selectively enriched to regulate the gene expression of surfactant protein and lipid biosynthesis and metabolism [200].
Although all vascular ECs originate in the embryonic mesoderm, the ECs located in different tissues and organs show functional, transcriptomic and metabolomic heterogeneity. That may match with the functions of relative tissues and organs. Besides, the differentiation of ECs relies on both epigenetic mechanisms and the interaction with the microenvironment [201]. Therefore, if we do not pay sufficient attention to endothelial cell heterogeneity at the transplantation site, the seed cells on TEVGs may not match the host endothelium in function.
4.3. Seed cells should be homogeneous with host endothelium
Due to the microenvironmental differences between arteries and veins, saphenous veins often used in coronary artery bypass surgery confront a problem of graft adaptation [202]. If there is no adaptability, the probability of transplant failure increased 13 times [203]. Kudo et al. found that ECs from veins are not matched with the artery when implanted in the arteries, which means that the heterogenous cell population is restricted in clinical application [204,205]. However, there is heterogeneity in seed cells from different sources. For example, ESC differentiates into the venous EC by default [206], and iPSC-derived arterial ECs exhibit functional differences from iPSC-derived venous ECs [207]. Due to the existence of endothelial heterogeneity, attention should be focused on the heterogeneity between seed cells and host endothelium, which may be a significant factor influencing vascular endothelialization. To maintain the long-term patency of TEVGs after implantation and make seed cells easy to adapt to the transplantation environment, reducing the heterogeneity between seed cells and host endothelium may be a solution. Besides, identifying endothelial heterogeneity is helpful for the construction of organ-specific TEVGs.
5. Summary and future
Compared with autologous or allogeneic vessels, TEVG is a good choice for the treatment of cardiovascular diseases because of decreased trauma, low immunogenicity, easy to preserve and so on. However, the scaffold materials, inner diameter, and length of TEVGs affect the application of TEVGs. So different endothelialization strategies should adapt to the TEVGs with different inner diameters and lengths. Further, 1) TEVGs (ID > 6 mm) do not require endothelialization; 2) achieving endothelialization in vivo is a safer and more convenient option for TEVGs (ID:4–6 mm, length<5 cm); 3) for the TEVGs (ID < 4 mm, length<5 cm), it is necessary to achieve endothelialization in vitro before implantation. Besides, cell heterogeneity and tissue heterogeneity should be considered in the selection of seed cells. Suitable seed cells may need to remain homogenous with the ECs of the host at the implantation site.
In future studies, the surface coating of the grafts and the preparation of composite materials can both improve the material properties, which are feasible and promising research directions. To reduce thrombosis and maintain long-term vascular patency, suitable seed cells should be selected in different environments for vascular endothelialization. However, which kind of seed cells are used in a special environment remains unknown. After vascular graft implantation, defining the function of seed cells is critical to promote further research. Besides, it is also meaningful and necessary for TEVGs to study the effects of between the density of seed cells and vascular endothelialization. From the perspective of clinical needs, TEVGs should be prepared faster, more efficiently and safely. At the same time, TEVGs should be inexpensive and convenient to store. In conclusion, the results of scientific research should be combined with clinical practice so that the research results benefit patients and society.
Declaration of competing interest
Authors do not have any conflicts of interest to declare.
Acknowledgements
This work was supported by The National Science Fund for Outstanding Young Scholars (No:31822021); The Key Research and Development Plan Young Scientists Program (No:2017YFA0106000); The National Key Research and Development Plan (No:2016YFC1101100).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Qingjin Cai, Email: 1623675523@qq.com.
Wanshan Liao, Email: 317208975@qq.com.
Fangchao Xue, Email: 501157875@qq.com.
Xiaochen Wang, Email: wangxiaochenchen@163.com.
Weiming Zhou, Email: 610952881@qq.com.
Yanzhao Li, Email: liyanzhao@tmmu.edu.cn.
Wen Zeng, Email: zengw0105@163.com.
References
- 1.Evans M.A. Cardiovascular disease, aging, and clonal hematopoiesis. J. Annu Rev Pathol. 2020;15:419–438. doi: 10.1146/annurev-pathmechdis-012419-032544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao D. Epidemiology of cardiovascular disease in China: current features and implications. J. Nat Rev Cardiol. 2019;16:203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 3.Smith S.C., Jr. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke) J. Glob Heart. 2012;7:297–305. doi: 10.1016/j.gheart.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Canver C.C. Conduit options in coronary artery bypass surgery. J. Chest. 1995;108:1150–1155. doi: 10.1378/chest.108.4.1150. [DOI] [PubMed] [Google Scholar]
- 5.Radakovic D. A multilayered electrospun graft as vascular access for hemodialysis. J. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D. Executive summary: heart disease and stroke statistics--2016 update: a report from the American heart association. J. Circulation. 2016;133:447–454. doi: 10.1161/cir.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 7.Rocco K.A. In vivo applications of electrospun tissue-engineered vascular grafts: a review. J. Tissue Eng. Part B Rev. 2014;20:628–640. doi: 10.1089/ten.TEB.2014.0123. [DOI] [PubMed] [Google Scholar]
- 8.Kurobe H. Development of small diameter nanofiber tissue engineered arterial grafts. J. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuzaki Y. The evolution of tissue engineered vascular graft technologies: from preclinical trials to advancing patient care. J. Appl. Sci. 2019;9 doi: 10.3390/app9071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karkan S.F. Electrospun nanofibers for the fabrication of engineered vascular grafts. J. J Biol Eng. 2019;13:83. doi: 10.1186/s13036-019-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan H. Strategies in cell-free tissue-engineered vascular grafts. J. J Biomed Mater Res A. 2020;108:426–445. doi: 10.1002/jbm.a.36825. [DOI] [PubMed] [Google Scholar]
- 12.Hsia K. Scaffolds and cell-based tissue engineering for blood vessel therapy. J. Cells Tissues Organs. 2016;202:281–295. doi: 10.1159/000448169. [DOI] [PubMed] [Google Scholar]
- 13.Mcquade K. Randomized comparison of ePTFE/nitinol self-expanding stent graft vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J. J Vasc Surg. 2009;49:109–115. doi: 10.1016/j.jvs.2008.08.041. 116.e101-109; discussion 116. [DOI] [PubMed] [Google Scholar]
- 14.Mclarty A.J. Aortocoronary bypass grafting with expanded polytetrafluoroethylene: 12-year patency. Jpn. Ann. Thorac. Surg. 1998;65:1442–1444. doi: 10.1016/s0003-4975(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 15.Radke D. Tissue engineering at the blood-contacting surface: a review of challenges and strategies in vascular graft development. J. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinoka T. Creation of viable pulmonary artery autografts through tissue engineering. Jpn. J. Thorac. Cardiovasc. Surg. 1998;115:536–545. doi: 10.1016/s0022-5223(98)70315-0. ; discussion 545-536. [DOI] [PubMed] [Google Scholar]
- 17.Shum-Tim D. Tissue engineering of autologous aorta using a new biodegradable polymer. Jpn. Ann. Thorac. Surg. 1999;68:2298–2304. doi: 10.1016/s0003-4975(99)01055-3. ; discussion 2305. [DOI] [PubMed] [Google Scholar]
- 18.Niklason L.E. Functional arteries grown in vitro. J. Sci. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 19.Tara S. Vessel bioengineering. Jpn. Circ. J. 2014;78:12–19. doi: 10.1253/circj.cj-13-1440. [DOI] [PubMed] [Google Scholar]
- 20.Fukunishi T. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. J. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q. Experimental studies on preparation of the porous and small-diameter poly(ε-caprolactone) external vascular scaffold and its degradability and biocompatibility. J. Regen Med Res. 2018;6:2. doi: 10.1051/rmr/180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Valence S. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. J. Biomaterials. 2012;33:38–47. doi: 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Bružauskaitė I. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. J. Cytotechnology. 2016;68:355–369. doi: 10.1007/s10616-015-9895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. J. Biomaterials. 2014;35:5700–5710. doi: 10.1016/j.biomaterials.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 25.Sipilä K.H. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. J Biol Chem. 2018;293:7645–7658. doi: 10.1074/jbc.RA118.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simionescu D.T. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. J. Biomaterials. 2006;27:702–713. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Fukayama T. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. J. Ann Vasc Surg. 2015;29:341–352. doi: 10.1016/j.avsg.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Badhe R.V. A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. J. Carbohydr Polym. 2017;157:1215–1225. doi: 10.1016/j.carbpol.2016.09.095. [DOI] [PubMed] [Google Scholar]
- 29.Wu H.C. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. J. Biomaterials. 2007;28:1385–1392. doi: 10.1016/j.biomaterials.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C. Human-like collagen/hyaluronic acid 3D scaffolds for vascular tissue engineering. J. Mater Sci Eng C Mater Biol Appl. 2014;34:393–401. doi: 10.1016/j.msec.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Meijden P.E. Dual role of collagen in factor XII-dependent thrombus formation. J. Blood. 2009;114:881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 32.Gambaryan S. Thrombin and collagen induce a feedback inhibitory signaling pathway in platelets involving dissociation of the catalytic subunit of protein kinase A from an NFkappaB-IkappaB complex. J. J Biol Chem. 2010;285:18352–18363. doi: 10.1074/jbc.M109.077602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kee M.F. Platelet mechanosensing of collagen matrices. J. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slevin M. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. J. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz A. Differential support of cell adhesion and growth by copolymers of polyurethane with hyaluronic acid. J. 2013;101:2870–2882. doi: 10.1002/jbm.a.34597. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrievska S. Glycocalyx-like hydrogel coatings for small diameter vascular grafts. J. Mater. Chem. B. 2020;30 doi: 10.1002/adfm.201908963. [DOI] [Google Scholar]
- 37.Lopera Higuita M., Griffiths L.G. Small diameter xenogeneic extracellular matrix scaffolds for vascular applications. J. Tissue Eng. Part B Rev. 2020;26:26–45. doi: 10.1089/ten.TEB.2019.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing Q. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. J. Tissue Eng. Part C Methods. 2015;21:77–87. doi: 10.1089/ten.tec.2013.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez C.E. Biological and engineering design considerations for vascular tissue engineered blood vessels (TEBVs) J. Curr Opin Chem Eng. 2014;3:83–90. doi: 10.1016/j.coche.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y. Extracellular matrix proteins in the regulation of thrombus formation. J. Curr Opin Hematol. 2016;23:280–287. doi: 10.1097/moh.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 41.Bastijanic J.M. Dual biofunctional polymer modifications to address endothelialization and smooth muscle cell integration of ePTFE vascular grafts. J. J Biomed Mater Res A. 2016;104:71–81. doi: 10.1002/jbm.a.35541. [DOI] [PubMed] [Google Scholar]
- 42.Choi W.S. Enhanced patency and endothelialization of small-caliber vascular grafts fabricated by coimmobilization of heparin and cell-adhesive peptides. J. ACS Appl Mater Interfaces. 2016;8:4336–4346. doi: 10.1021/acsami.5b12052. [DOI] [PubMed] [Google Scholar]
- 43.Tsai T.N. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. J. Am J Pathol. 2012;181:362–373. doi: 10.1016/j.ajpath.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Mahara A. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. J. Biomaterials. 2015;58:54–62. doi: 10.1016/j.biomaterials.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Mahara A. Accelerated endothelialization and suppressed thrombus formation of acellular vascular grafts by modifying with neointima-inducing peptide: a time-dependent analysis of graft patency in rat-abdominal transplantation model. J. Colloids Surf B Biointerfaces. 2019;181:806–813. doi: 10.1016/j.colsurfb.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka H. Tissue-engineered submillimeter-diameter vascular grafts for free flap survival in rat model. J. Biomaterials. 2018;179:156–163. doi: 10.1016/j.biomaterials.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Haghjooy Javanmard S. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. J Biomater Appl. 2016;31:438–449. doi: 10.1177/0885328216652068. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y.C. Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. J. Mater Sci Eng C Mater Biol Appl. 2017;71:901–908. doi: 10.1016/j.msec.2016.10.083. [DOI] [PubMed] [Google Scholar]
- 49.Bertram U. Vascular tissue engineering: effects of integrating collagen into a PCL based nanofiber material. J. Biomed Res Int. 2017;2017:9616939. doi: 10.1155/2017/9616939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wise S.G. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. J. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 51.Mcbane J.E. Tissue engineering a small diameter vessel substitute: engineering constructs with select biomaterials and cells. J. Curr Vasc Pharmacol. 2012;10:347–360. doi: 10.2174/157016112799959378. [DOI] [PubMed] [Google Scholar]
- 52.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. J. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 53.Natasha G. Tissue engineering vascular grafts a fortiori: looking back and going forward. J. Expert Opin Biol Ther. 2015;15:231–244. doi: 10.1517/14712598.2015.980234. [DOI] [PubMed] [Google Scholar]
- 54.Richbourg N.R. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. J Tissue Eng Regen Med. 2019;13:1275–1293. doi: 10.1002/term.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdulghani S., Mitchell G.R. Biomaterials for in situ tissue regeneration: a review. J. Biomolecules. 2019;9 doi: 10.3390/biom9110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obiweluozor F.O. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: a review. J. Cardiovasc Eng Technol. 2020;11:495–521. doi: 10.1007/s13239-020-00482-y. [DOI] [PubMed] [Google Scholar]
- 57.Carrabba M., Madeddu P. Current strategies for the manufacture of small size tissue engineering vascular grafts. J. Front Bioeng Biotechnol. 2018;6:41. doi: 10.3389/fbioe.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin C.H. In vivo performance of decellularized vascular grafts: a review article. J. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bittner S.M. Three-dimensional printing of multilayered tissue engineering scaffolds. J. Mater Today (Kidlington). 2018;21:861–874. doi: 10.1016/j.mattod.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skovrind I. Concise review: patency of small-diameter tissue-engineered vascular grafts: a meta-analysis of preclinical trials. J. Stem Cells Transl Med. 2019;8:671–680. doi: 10.1002/sctm.18-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dall'olmo L. Blood vessel-derived acellular matrix for vascular graft application. J. Biomed Res Int. 2014;2014:685426. doi: 10.1155/2014/685426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Negishi J. Porcine radial artery decellularization by high hydrostatic pressure. J. J Tissue Eng Regen Med. 2015;9:E144–E151. doi: 10.1002/term.1662. [DOI] [PubMed] [Google Scholar]
- 63.Xiong Y. Decellularized porcine saphenous artery for small-diameter tissue-engineered conduit graft. J. Artif. Organs. 2013;37:E74–E87. doi: 10.1111/aor.12014. [DOI] [PubMed] [Google Scholar]
- 64.Yamanami M. Development of xenogeneic decellularized biotubes for off-the-shelf applications. J. Artif. Organs. 2019;43:773–779. doi: 10.1111/aor.13432. [DOI] [PubMed] [Google Scholar]
- 65.Quint C. Decellularized tissue-engineered blood vessel as an arterial conduit. J. Proc Natl Acad Sci U S A. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma X. Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J. J Cardiothorac Surg. 2017;12:101. doi: 10.1186/s13019-017-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dahan N. Dynamic autologous reendothelialization of small-caliber arterial extracellular matrix: a preclinical large animal study. J. Tissue Eng. Part A. 2017;23:69–79. doi: 10.1089/ten.TEA.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Syedain Z.H. A completely biological "off-the-shelf" arteriovenous graft that recellularizes in baboons. J. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan4209. [DOI] [PubMed] [Google Scholar]
- 69.Nagaoka Y. Reconstruction of small diameter arteries using decellularized vascular scaffolds. J. J Med Dent Sci. 2014;61:33–40. PMID: 24658963. [PubMed] [Google Scholar]
- 70.Bai H. A rat arteriovenous graft model using decellularized vein. J. Vascular. 2020 doi: 10.1177/1708538120923191. 1708538120923191. [DOI] [PubMed] [Google Scholar]
- 71.Abbott W.M. Evaluation and performance standards for arterial prostheses. J. J Vasc Surg. 1993;17:746–756. doi: 10.1067/mva.1993.45222. [DOI] [PubMed] [Google Scholar]
- 72.Bennion R.S. Patency of autogenous saphenous vein versus polytetrafluoroethylene grafts in femoropopliteal bypass for advanced ischemia of the extremity. J. Surg Gynecol Obstet. 1985;160:239–242. PMID: 3975795. [PubMed] [Google Scholar]
- 73.Chlupác J. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. J. Physiol Res. 2009;58(Suppl 2):S119–S139. doi: 10.33549/physiolres.931918. PMID: 20131930. [DOI] [PubMed] [Google Scholar]
- 74.Walpoth B.H., Bowlin G.L. The daunting quest for a small diameter vascular graft. J. Expert Rev Med Devices. 2005;2:647–651. doi: 10.1586/17434440.2.6.647. [DOI] [PubMed] [Google Scholar]
- 75.Van Damme H. Intrinsic structural failure of polyester (Dacron) vascular grafts. A general review. J. Acta Chir Belg. 2005;105:249–255. doi: 10.1080/00015458.2005.11679712. [DOI] [PubMed] [Google Scholar]
- 76.Scharn D.M. Biological mechanisms influencing prosthetic bypass graft patency: possible targets for modern graft design. J. Eur J Vasc Endovasc Surg. 2012;43:66–72. doi: 10.1016/j.ejvs.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Pennel T. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. J. Biomaterials. 2014;35:6311–6322. doi: 10.1016/j.biomaterials.2014.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubanyi G.M. The role of endothelium in cardiovascular homeostasis and diseases. J. J Cardiovasc Pharmacol. 1993;22(Suppl 4):S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 79.Post A. A review of integrin-mediated endothelial cell phenotype in the design of cardiovascular devices. J. Ann Biomed Eng. 2019;47:366–380. doi: 10.1007/s10439-018-02171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krüger-Genge A. Vascular endothelial cell biology: an update. J. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin C.H. Decellularized porcine coronary artery with adipose stem cells for vascular tissue engineering. J. Biomed Mater. 2019;14 doi: 10.1088/1748-605X/ab2329. [DOI] [PubMed] [Google Scholar]
- 82.Pellegata A.F. Arterial decellularized scaffolds produced using an innovative automatic system. J. Cells Tissues Organs. 2015;200:363–373. doi: 10.1159/000439082. [DOI] [PubMed] [Google Scholar]
- 83.Hsia K. Sphingosine-1-phosphate in endothelial cell recellularization improves patency and endothelialization of decellularized vascular grafts in vivo. J. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meier L.A. Blood outgrowth endothelial cells alter remodeling of completely biological engineered grafts implanted into the sheep femoral artery. J. J Cardiovasc Transl Res. 2014;7:242–249. doi: 10.1007/s12265-013-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrmann F.E.M. Autologous endothelialized vein allografts in coronary artery bypass surgery - long term results. J. Biomaterials. 2019;212:87–97. doi: 10.1016/j.biomaterials.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 86.Wang S. Fabrication of small-diameter vascular scaffolds by heparin-bonded P(LLA-CL) composite nanofibers to improve graft patency. J. Int J Nanomedicine. 2013;8:2131–2139. doi: 10.2147/ijn.S44956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou M. Development and in vivo evaluation of small-diameter vascular grafts engineered by outgrowth endothelial cells and electrospun chitosan/poly(ε-caprolactone) nanofibrous scaffolds. J. Tissue Eng. Part A. 2014;20:79–91. doi: 10.1089/ten.TEA.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borschel G.H. Tissue engineering of recellularized small-diameter vascular grafts. J. Tissue Eng. 2005;11:778–786. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 89.Narita Y. Decellularized ureter for tissue-engineered small-caliber vascular graft. Jpn. J. Artif. Organs. 2008;11:91–99. doi: 10.1007/s10047-008-0407-6. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. J. Biomaterials. 2010;31:296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 91.Fukunishi T. Role of bone marrow mononuclear cell seeding for nanofiber vascular grafts. J. Tissue Eng. Part A. 2018;24:135–144. doi: 10.1089/ten.TEA.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou M. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J. J Biomed Mater Res B Appl Biomater. 2012;100:111–120. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 93.Gimbrone M.A., Jr., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. J. Circ Res. 2016;118:620–636. doi: 10.1161/circresaha.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang K. Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. J. Cell Mol Life Sci. 2019;76:421–439. doi: 10.1007/s00018-018-2939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kadner A. Human umbilical cord cells for cardiovascular tissue engineering: a comparative study. J. Eur J Cardiothorac Surg. 2004;25:635–641. doi: 10.1016/j.ejcts.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 96.Schechner J.S. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. J. Proc Natl Acad Sci U S A. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lynch K., Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. J. Organogenesis. 2014;10:289–298. doi: 10.4161/15476278.2014.970089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Warrier S. Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: vascularization from an avascular tissue. J. Placenta. 2012;33:850–858. doi: 10.1016/j.placenta.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Lindenmair A. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. J. Cell. 2012;1:1061–1088. doi: 10.3390/cells1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendicino M. MSC-based product characterization for clinical trials: an FDA perspective. J. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira M.S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. J. Biomaterials. 2012;33:6987–6997. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 102.Wu Z. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. J. Transplant Proc. 2017;49:1656–1658. doi: 10.1016/j.transproceed.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 103.Moll G. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. J. Trends Mol Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Hristov M., Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters E.B. Endothelial progenitor cells for the vascularization of engineered tissues. J. Tissue Eng. Part B Rev. 2018;24:1–24. doi: 10.1089/ten.TEB.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asahara T. Isolation of putative progenitor endothelial cells for angiogenesis. J. Sci. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 107.Allen J. Characterization of porcine circulating progenitor cells: toward a functional endothelium. J. Tissue Eng. Part A. 2008;14:183–194. doi: 10.1089/ten.a.2007.0265. [DOI] [PubMed] [Google Scholar]
- 108.Kaushal S. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. J. Nat. Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rafii S. Characterization of hematopoietic cells arising on the textured surface of left ventricular assist devices. Jpn. Ann. Thorac. Surg. 1995;60:1627–1632. doi: 10.1016/0003-4975(95)00807-1. [DOI] [PubMed] [Google Scholar]
- 110.Au P. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. J. Blood. 2008;111:1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu X. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. J. Am J Physiol Heart Circ Physiol. 2004;287:H480–H487. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 112.Melero-Martin J.M. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. J. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 113.Richardson M.R., Yoder M.C. Endothelial progenitor cells: quo vadis? J. J Mol Cell Cardiol. 2011;50:266–272. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mukai N. A comparison of the tube forming potentials of early and late endothelial progenitor cells. J. Exp Cell Res. 2008;314:430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 115.Young R.A. Control of the embryonic stem cell state. J. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang L. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. J. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 117.Hill K.L. Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. J. Exp. Hematol. 2010;38:246–257. doi: 10.1016/j.exphem.2010.01.001. e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Margariti A. Stem cells, vascular smooth muscle cells and atherosclerosis. J. Histol. Histopathol. 2006;21:979–985. doi: 10.14670/hh-21.979. [DOI] [PubMed] [Google Scholar]
- 119.Lindgren A.G. ETV2 expression increases the efficiency of primitive endothelial cell derivation from human embryonic stem cells. J. Cell Regen. 2015;4:1. doi: 10.1186/s13619-014-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurtovic S. Leptin enhances endothelial cell differentiation and angiogenesis in murine embryonic stem cells. J. Microvasc Res. 2015;97:65–74. doi: 10.1016/j.mvr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 121.Lee S. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. J. Biomaterials. 2015;63:158–167. doi: 10.1016/j.biomaterials.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiao Q. Sca-1+ progenitors derived from embryonic stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. J. Arterioscler Thromb Vasc Biol. 2006;26:2244–2251. doi: 10.1161/01.Atv.0000240251.50215.50. [DOI] [PubMed] [Google Scholar]
- 123.Park J.K. Role of Notch1 in the arterial specification and angiogenic potential of mouse embryonic stem cell-derived endothelial cells. J. Stem Cell Res. Ther. 2018;9:197. doi: 10.1186/s13287-018-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong H. PAR1 scaffolds TGFβRII to downregulate TGF-β signaling and activate ESC differentiation to endothelial cells. J. Stem Cell Reports. 2016;7:1050–1058. doi: 10.1016/j.stemcr.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinha S. Transforming growth factor-β1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am. J. Physiol. Cell Physiol. 2004;287:C1560–C1568. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 126.Vergallo R., Crea F. Atherosclerotic plaque healing. J. N Engl J Med. 2020;383:846–857. doi: 10.1056/NEJMra2000317. [DOI] [PubMed] [Google Scholar]
- 127.Allahverdian S. Smooth muscle cell fate and plasticity in atherosclerosis. J. Cardiovascular Research. 2018;114:540–550. doi: 10.1093/cvr/cvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harding A. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. J. Stem Cell. 2017;35:909–919. doi: 10.1002/stem.2577. [DOI] [PubMed] [Google Scholar]
- 129.Kimmelman J. Policy: global standards for stem-cell research. J. Nature. 2016;533:311–313. doi: 10.1038/533311a. [DOI] [PubMed] [Google Scholar]
- 130.Hentze H. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. J. Stem Cell Res. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 131.Charron D. Immunogenicity and allogenicity: a challenge of stem cell therapy. J. J Cardiovasc Transl Res. 2009;2:130–138. doi: 10.1007/s12265-008-9062-9. [DOI] [PubMed] [Google Scholar]
- 132.Thompson H.L., Manilay J.O. Embryonic stem cell-derived hematopoietic stem cells: challenges in development, differentiation, and immunogenicity. J. Curr Top Med Chem. 2011;11:1621–1637. doi: 10.2174/156802611796117702. [DOI] [PubMed] [Google Scholar]
- 133.De Almeida P.E. Immunogenicity of pluripotent stem cells and their derivatives. J. Circ Res. 2013;112:549–561. doi: 10.1161/circresaha.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. J. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 135.Nakayama K.H. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. J. Regen Med. 2015;10:745–755. doi: 10.2217/rme.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pashneh-Tala S. The tissue-engineered vascular graft-past, present, and future. J. Tissue Eng. Part B Rev. 2016;22:68–100. doi: 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou J. Dual layer collagen-GAG conduit that mimic vascular scaffold and promote blood vessel cells adhesion, proliferation and elongation. J. Mater. Sci. Eng. C. 2018;92:447–452. doi: 10.1016/j.msec.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 138.Jin Q. Biomimetic human small muscular pulmonary arteries. J. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kohler E.E. Flk1+ and VE-cadherin+ endothelial cells derived from iPSCs recapitulates vascular development during differentiation and display similar angiogenic potential as ESC-derived cells. J. PLoS One. 2013;8 doi: 10.1371/journal.pone.0085549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takebe T. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. J. Nat Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 141.Patsch C. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. J. Nat Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Margariti A. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. J. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Samuel R. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. J. Proc Natl Acad Sci U S A. 2013;110:12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gui L. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. J. Biomaterials. 2016;102:120–129. doi: 10.1016/j.biomaterials.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo J. Tissue-engineered vascular grafts with advanced mechanical strength from human iPSCs. J. Cell Stem Cell. 2020;26:251–261. doi: 10.1016/j.stem.2019.12.012. e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Generali M. Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells. J. Acta Biomater. 2019;97:333–343. doi: 10.1016/j.actbio.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 147.Mehler V.J. Human iPSC-derived neural crest stem cells exhibit low immunogenicity. J. Mol Ther Methods Clin Dev. 2020;16:161–171. doi: 10.1016/j.omtm.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Almeida P.E. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. J. Nat Commun. 2014;5:3903. doi: 10.1038/ncomms4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deuse T. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. J. Nat Biotechnol. 2019;37:252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ben-David U., Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. J. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 151.Kane N.M. Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation) J. Pharmacol. Ther. 2011;129:29–49. doi: 10.1016/j.pharmthera.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 152.Grath A., Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J. J Biol Eng. 2019;13:14. doi: 10.1186/s13036-019-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kocherova I. Human umbilical vein endothelial cells (HUVECs) Co-culture with osteogenic cells: from molecular communication to engineering prevascularised bone grafts. Jpn. J. Clin. Med. 2019;8 doi: 10.3390/jcm8101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bagno L. Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. J. Mol Ther. 2018;26:1610–1623. doi: 10.1016/j.ymthe.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. J. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Samsonraj R.M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. J. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hashi C.K. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. J. Proc Natl Acad Sci U S A. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee R.H. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. J. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cunnane E.M. Future perspectives on the role of stem cells and extracellular vesicles in vascular tissue regeneration. J. Front Cardiovasc Med. 2018;5:86. doi: 10.3389/fcvm.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. J. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 161.Tasev D. Therapeutic potential of human-derived endothelial colony-forming cells in animal models. J. Tissue Eng. Part B Rev. 2016;22:371–382. doi: 10.1089/ten.TEB.2016.0050. [DOI] [PubMed] [Google Scholar]
- 162.Krawiec J.T., Vorp D.A. Adult stem cell-based tissue engineered blood vessels: a review. J. Biomaterials. 2012;33:3388–3400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 163.Munisso M.C., Yamaoka T. Circulating endothelial progenitor cells in small-diameter artificial blood vessel. Jpn. J. Artif. Organs. 2020;23:6–13. doi: 10.1007/s10047-019-01114-6. [DOI] [PubMed] [Google Scholar]
- 164.Wang Y. The combination of stem cells and tissue engineering: an advanced strategy for blood vessels regeneration and vascular disease treatment. J. Stem Cell Res. Ther. 2017;8:194. doi: 10.1186/s13287-017-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ingram D.A. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. J. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 166.Mund J.A. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. J. Arterioscler Thromb Vasc Biol. 2012;32:1045–1053. doi: 10.1161/atvbaha.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mora C. Clinical potentials of human pluripotent stem cells. J. Cell Biol Toxicol. 2017;33:351–360. doi: 10.1007/s10565-017-9384-y. [DOI] [PubMed] [Google Scholar]
- 168.Robertson J.A. Embryo stem cell research: ten years of controversy. J. J Law Med Ethics. 2010;38:191–203. doi: 10.1111/j.1748-720X.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 169.Bulic-Jakus F. Teratoma: from spontaneous tumors to the pluripotency/malignancy assay. J. Wiley Interdiscip Rev Dev Biol. 2016;5:186–209. doi: 10.1002/wdev.219. [DOI] [PubMed] [Google Scholar]
- 170.Cossu G. Lancet Commission: stem cells and regenerative medicine. J. Lancet. 2018;391:883–910. doi: 10.1016/s0140-6736(17)31366-1. [DOI] [PubMed] [Google Scholar]
- 171.Li K. Differentiation of pluripotent stem cells for regenerative medicine. J. Biochem Biophys Res Commun. 2016;471:1–4. doi: 10.1016/j.bbrc.2016.01.182. [DOI] [PubMed] [Google Scholar]
- 172.Cochrane A. Advanced in vitro models of vascular biology: human induced pluripotent stem cells and organ-on-chip technology. J. Adv Drug Deliv Rev. 2019;140:68–77. doi: 10.1016/j.addr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 173.Wu S.M., Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. J. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barrilleaux B., Knoepfler P.S. Inducing iPSCs to escape the dish. J. Cell Stem Cell. 2011;9:103–111. doi: 10.1016/j.stem.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stevens K.R., Murry C.E. Human pluripotent stem cell-derived engineered tissues: clinical considerations. J. Cell Stem Cell. 2018;22:294–297. doi: 10.1016/j.stem.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ortuño-Costela M.D.C. The challenge of bringing iPSCs to the patient. J. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20246305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roh J.D. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. J. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hibino N. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. J. Faseb j. 2011;25:2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mirensky T.L. Tissue-engineered vascular grafts: does cell seeding matter? J. J Pediatr Surg. 2010;45:1299–1305. doi: 10.1016/j.jpedsurg.2010.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zilla P. Progressive reinvention or destination lost? Half a century of cardiovascular tissue engineering. J. Front Cardiovasc Med. 2020;7:159. doi: 10.3389/fcvm.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cunnane E.M. Development of a semi-automated, bulk seeding device for large animal model implantation of tissue engineered vascular grafts. J. Front Bioeng Biotechnol. 2020;8:597847. doi: 10.3389/fbioe.2020.597847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dela Paz N.G., D'amore P.A. Arterial versus venous endothelial cells. J. Cell Tissue Res. 2009;335:5–16. doi: 10.1007/s00441-008-0706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arora S. Environmental specification of pluripotent stem cell derived endothelial cells toward arterial and venous subtypes. J. Front Bioeng Biotechnol. 2019;7:143. doi: 10.3389/fbioe.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geenen I.L. Endothelial cells (ECs) for vascular tissue engineering: venous ECs are less thrombogenic than arterial ECs. J. J Tissue Eng Regen Med. 2015;9:564–576. doi: 10.1002/term.1642. [DOI] [PubMed] [Google Scholar]
- 185.Aird W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. J. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 186.Cahill P.A., Redmond E.M. Vascular endothelium – gatekeeper of vessel health. J. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shrestha B. Differential arterial and venous endothelial redox responses to oxidative stress. J. Microcirculation. 2018;25 doi: 10.1111/micc.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fang J., Hirschi K. Molecular regulation of arteriovenous endothelial cell specification. J. F1000Res. 2019;8 doi: 10.12688/f1000research.16701.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Red-Horse K., Siekmann A.F. Veins and arteries build hierarchical branching patterns differently: bottom-up versus top-down. J. Bioessays. 2019;41 doi: 10.1002/bies.201800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaur H. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. J. Nat Commun. 2017;8:15700. doi: 10.1038/ncomms15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chavkin N.W., Hirschi K.K. Single cell analysis in vascular biology. J. Front Cardiovasc Med. 2020;7:42. doi: 10.3389/fcvm.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marcelo K.L. Regulation of endothelial cell differentiation and specification. J. Circ Res. 2013;112:1272–1287. doi: 10.1161/circresaha.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wolf K. Molecular identity of arteries, veins, and lymphatics. J. J Vasc Surg. 2019;69:253–262. doi: 10.1016/j.jvs.2018.06.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Potente M., Mäkinen T. Vascular heterogeneity and specialization in development and disease. J. Nat Rev Mol Cell Biol. 2017;18:477–494. doi: 10.1038/nrm.2017.36. [DOI] [PubMed] [Google Scholar]
- 195.Le Noble F. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. J. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 196.Hupe M. Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. J. Sci Signal. 2017;10 doi: 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]
- 197.Jambusaria A. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. J. Elife. 2020;9 doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dumas S.J. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. J Am Soc Nephrol. 2020;31:118–138. doi: 10.1681/asn.2019080832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Feng W. Single cell analysis of endothelial cells identified organ-specific molecular signatures and heart-specific cell populations and molecular features. J. Front Cardiovasc Med. 2019;6:165. doi: 10.3389/fcvm.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guo M. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. J. Nat Commun. 2019;10:37. doi: 10.1038/s41467-018-07770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mastrullo V. Angiogenesis in tissue engineering: as nature intended? J. Front Bioeng Biotechnol. 2020;8:188. doi: 10.3389/fbioe.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Muto A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Jpn. Circ. J. 2010;74:1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Owens C.D. Vein graft failure. J. J Vasc Surg. 2015;61:203–216. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kudo F.A. Venous identity is lost but arterial identity is not gained during vein graft adaptation. J. Arterioscler Thromb Vasc Biol. 2007;27:1562–1571. doi: 10.1161/atvbaha.107.143032. [DOI] [PubMed] [Google Scholar]
- 205.Muto A. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. Jpn. J. Exp. Med. 2011;208:561–575. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Glaser D.E. Functional characterization of embryonic stem cell-derived endothelial cells. J. J Vasc Res. 2011;48:415–428. doi: 10.1159/000324752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosa S. Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. J. Sci Rep. 2019;9:3826. doi: 10.1038/s41598-019-40417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]