Abstract
Long non-coding RNAs (lncRNAs) can play significant regulatory roles in cells that affect the development and acquired drug resistance of lung cancer. Herein, we report that lncRNA linc00665 is significantly upregulated in non-small cell lung cancer (NSCLC) tissues compared with adjacent normal tissues. linc00665 affects the sensitivity of NSCLC cells to the chemotherapy drug cisplatin (DDP), making it a potential target for the treatment of NSCLC. Functional experiments showed that linc00665 enhanced the proliferation and migration of NSCLC cells in vivo and in vitro, and knocking down linc00665 could enhance the drug sensitivity of NSCLC cells to DDP. Further work revealed that linc00665 could recruit enhancer of zeste homolog 2 (EZH2) to the promoter region of cyclin-dependent kinase inhibitor 1C (CDKN1C) to inhibit its transcription and thus carry out its tumorigenic role. In conclusion, our study elucidated the carcinogenic role of the linc00665-EZH2-CDKN1C axis in NSCLC tumors and its ability to influence the sensitivity of these tumors to DDP. These results suggest that linc00665 may be a potential diagnostic marker and therapeutic target in NSCLC, and they also provide a new direction for the development of clinical reversal methods for acquired drug resistance in patients with NSCLC.
Keywords: linc00665, CDKN1C, long non-coding RNA, EZH2, non-small cell lung cancer, cisplatin, drug sensitivity
Graphical Abstract
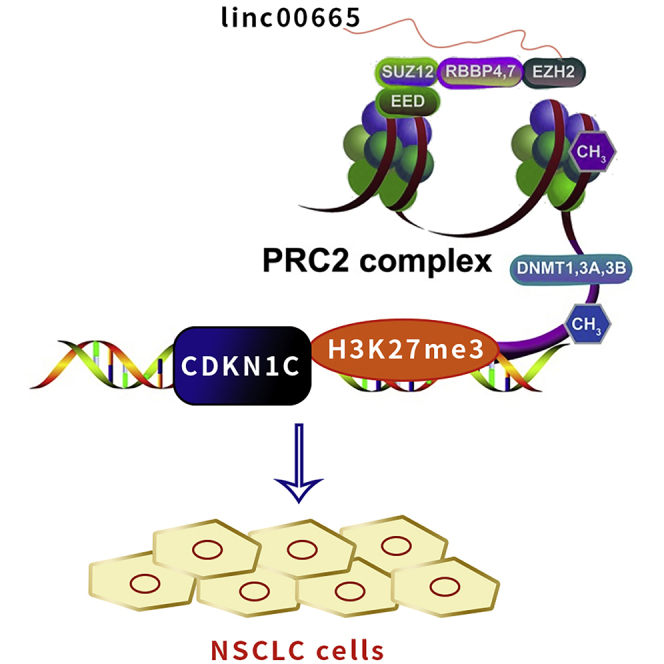
Yang et al. showed that linc00665 could recruit EZH2 enhancer into the promoter region of CDKN1C and inhibit its transcription, thus promoting the development of NSCLC cells and inhibiting their sensitivity to cisplatin. These results provide a new direction for clinical reversal of acquired drug resistance in NSCLC patients.
Introduction
Lung cancer is one of the most common types of cancer, with both the highest incidence and mortality rate in the world. Among the different types of lung cancer, non-small cell lung cancer (NSCLC) is the most common, accounting for 80%–85% of all lung cancer cases, with a 5-year survival rate of less than 15%.1 NSCLC includes lung squamous cell carcinoma (LUSC), lung adenocarcinoma (LUAD), and large cell lung cancer (LCLC). The first-line treatment for NSCLC is cisplatin (DDP), a chemotherapy drug that kills cancer cells by inhibiting their DNA replication process and damaging structures on their cell membranes. However, long-term DDP treatment frequently causes serious side effects, including gastrointestinal effects, fatigue, bone marrow toxicity, and even long-term heart, kidney, and nervous system problems.2 Increasing the sensitivity of tumor cells to DDP would enhance the efficacy and reduce the duration of medication for patients, thereby reducing potential side effects. However, developing strategies that increase NSCLC sensitivity to chemotherapeutic drugs and improve patient treatment and prognosis remains a huge challenge.
Long non-coding RNAs (lncRNAs) are a class of RNA molecules that are generally longer than 200 nt but lack protein-coding potential. They were originally thought to be cloning artifacts or transcriptional noise.3 In recent years, studies have found that lncRNAs are involved in a wide range of biological processes, such as regulating the activity of protein-binding factors,4 directing the localization of chromatin repair complexes,5 and producing RNAs with a 5′ cap structure through post-transcriptional processing.6 An increasing number of research reports also indicate that lncRNAs play a key regulatory role in tumor development,7, 8, 9, 10 including in NSCLC.11, 12, 13 We previously reported that the lncRNA SNHG20 promotes the proliferation and migration of NSCLC cells by interacting with zeste homolog 2 (EZH2) to inhibit the expression of p21, a negative regulator of the cell cycle.14 Recent studies have also demonstrated a relationship between the abnormal expression of lncRNAs and drug resistance in cancer cells.15,16 Our research team found that the lncRNA HOTAIR can upregulate p21 expression and partially reverse the DDP resistance of the NSCLC cell line A549/DDP.17 The literature has increasingly reported that DDP resistance in NSCLC is affected by lncRNAs. For example, knockdown of lncRNA MIAT increased the expression of miR-184 to inhibit DDP resistance in NSCLC cells,18 lncRNA Spry4-IT1 reversed DDP resistance in NSCLC cells by inhibiting the epithelial-mesenchymal transition (EMT) and downregulating myelin protein zero-like 1 (MPZL-1),19 and lncRNA NORAD/miR-202-5p regulated DDP resistance of A549/DDP cells by targeting P-glycoprotein (P-GP).20 These reports revealed that DDP resistance in NSCLC cells can be affected by the actions of various lncRNAs. The regulatory mechanisms of these molecules were complex and diverse, which make them worthy of further study.
One lncRNA of particular interest is linc00665. A previous study reported that linc00665 acts as a microRNA (miRNA) sponge of miR-98 and regulates the expression of aldo-keto reductase family 1, member B10 (AKR1B10) by binding to this miRNA, thus promoting LUAD progression.21 Another study suggested that linc00665 may promote gefitinib resistance in NSCLC cell line PC9 through EZH2, but the mechanism was not fully determined.22 However, no currently published study has examined whether linc00665 plays a role in DDP resistance. In this study, we explored whether linc00665 is involved in the sensitivity of NSCLC to DDP. We confirmed that linc00665 expression is upregulated in NSCLC tissues and cells, and that increased linc00665 expression is associated with poor prognosis in NSCLC patients.
Furthermore, we showed that linc00665 affects the sensitivity of NSCLC cells to DDP treatment. Our results suggest that linc00665 promotes cell proliferation and migration and inhibits NSCLC sensitivity to DDP by interacting with EZH2 and recruiting it to the promoter region of cyclin-dependent kinase inhibitor 1C (CDKN1C), which reduces the expression of CDKN1C.
Results
linc00665 is upregulated in NSCLC tissues and is associated with poor prognosis
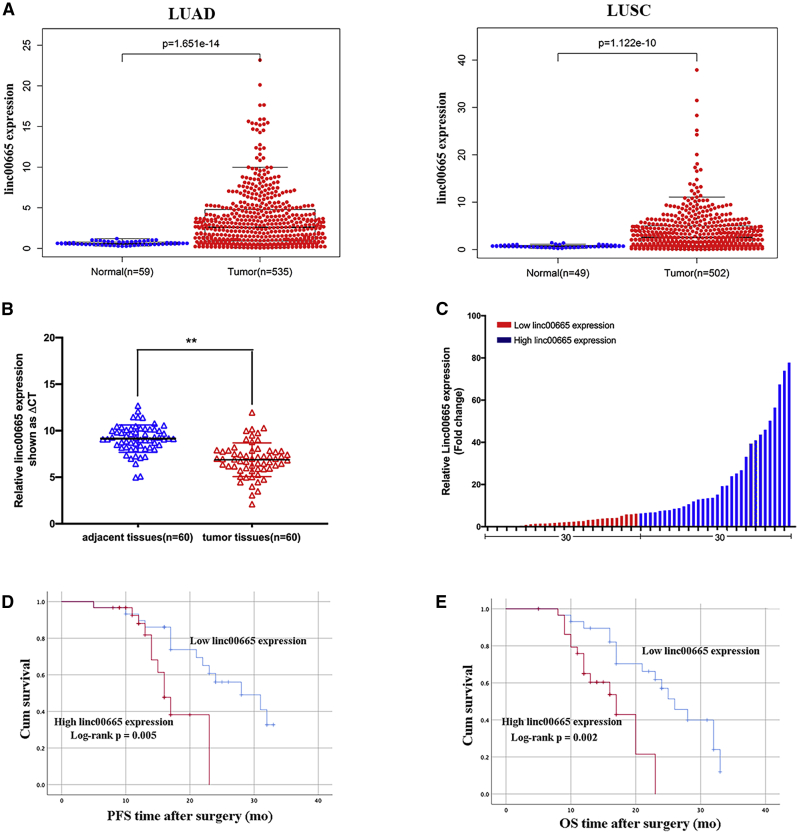
First, we analyzed The Cancer Genome Atlas (TCGA) database and found that linc00665 was highly expressed in both LUAD and LUSC, which is 6.37- and 4.19-fold higher than normal tissue, respectively (Figure 1A). We then analyzed linc00665 expression in 60 paired samples of NSCLC tissues and adjacent non-tumor tissues by qRT-PCR, and we found that this lncRNA was upregulated in 51 of 60 cancerous tissues compared with their respective paracancerous tissues (fold change >1.5; Figure 1B). To examine any potential correlations between linc00665 expression and the clinical characteristics of NSCLC patients, we divided the 31 NSCLC patients into two groups according to the median expression of linc00665 in tumor tissues: the high linc00665 group (n = 30, linc00665 expression ratio ≥6.5532) and low linc00665 group (n = 30, linc00665 expression ratio <6.5532) (Figure 1C). As shown in Table 1, higher linc00665 levels were associated with advanced tumor, lymph node, and metastasis (TNM) stage, lymph node metastasis, and tumor size, but not with age, sex, histological subtype, smoking history, or other factors. Kaplan-Meier survival analysis showed that patients with high linc00665 expression had shorter overall survival (OS) and shorter progression-free survival (PFS) than did patients with low linc00665 expression (Figures 1D and 1E).
Figure 1.
Relative linc00665 expression levels in non-small cell lung cancer (NSCLC) tissues and its clinical relevance
(A) Through data analysis of TCGA database, the expression differences between linc00665 in lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) tissues and normal tissues were compared. (B) Quantitative real-time PCR was used to detect the difference of linc00665 expression in cancer tissues and adjacent tissues of NSCLC patients (n = 60). (C) Patients (n = 60) were divided into two groups based on the amount of linc00665 expressed in the tissues of NSCLC patients. (D and E) The Kaplan-Meier method was used to analyze the expression level of linc00665 and PFS and OS. Patients were grouped according to the median expression of linc00665. Data are presented as the mean ± SEM. ∗∗p < 0.01.
Table 1.
Correlation between the expression of linc00665 and clinicopathological features in patients with non-small cell lung cancer (n = 60)
| Characteristics | linc00665 low no. case (%) | linc00665 high no. case (%) | Chi-square test p value |
|---|---|---|---|
| Age (years) | |||
| ≥55 | 13 (43.3) | 17 (56.7) | 0.302 |
| <55 | 17 (56.7) | 13 (43.3) | |
| Sex | |||
| Male | 16 (53.3) | 21 (70.0) | 0.184 |
| Female | 14 (46.7) | 9 (30.0) | |
| Histological subtype | |||
| Adenocarcinoma | 14 (46.7) | 20 (66.7) | 0.118 |
| Squamous cell carcinoma | 16 (53.3) | 10 (33.3) | |
| TNM stage | |||
| I | 14 (46.7) | 3 (10.0) | 0.007∗∗ |
| II | 10 (33.3) | 16 (53.3) | |
| III–IV | 6 (20.0) | 11 (36.7) | |
| Tumor size | |||
| ≤5 cm | 25 (83.3) | 16 (53.3) | 0.012∗ |
| >5 cm | 5 (16.7) | 14 (46.7) | |
| Lymph node metastasis | |||
| Negative | 18 (60.0) | 7 (23.3) | 0.004∗∗ |
| Positive | 12 (40.0) | 23 (76.7) | |
| Smoking history | |||
| Smokers | 19 (63.3) | 23 (76.7) | 0.269 |
| Never smokers | 11 (36.7) | 7 (23.3) | |
∗p < 0.05.∗∗p < 0.01.
Effect of linc00665 on NSCLC cell proliferation, apoptosis, cell cycle, and migration
To explore the biological function of linc00665 in NSCLC cells, we examined the expression levels of linc00665 in a normal bronchial epithelial cell line (16HBE) and five human NSCLC cell lines (H1975, A549, PC-9, H1299, and SPC-A1). We found that linc00665 was highly expressed in all five lung cancer cell lines, but most significantly in H1975 and H1299 cells (Figure 2A). Next, we designed four small interfering RNAs (siRNAs) to specifically knock down linc00665 expression. The results showed that siRNA (si-)linc00665 #2 and si-linc00665 #4 had knocked down linc00665 levels most efficiently (Figure 2B), and thus these two siRNAs were used for subsequent experiments.
Figure 2.
Effect of linc00665 expression on the development of NSCLC cells in vitro
(A) Quantitative real-time PCR was used to detect the difference in the expression of linc00665 in NSCLC cell lines (H1975, H1299, SPCA1, A549, and PC9) and normal human bronchial epithelial cells (16HBE). (B) Expression levels of linc00665 in H1299 and H1975 cells transfected with si-linc00665 #1, si-linc00665 #2, si-linc00665 #3, si-linc00665 #4, and scrambled siRNA were analyzed by quantitative real-time PCR technology. (C) The proliferation of H1299 and H1975 cells transfected with si-linc00665 was tested by the CCK-8 method. (D and E) A colony formation test and EdU staining test were used to detect the proliferation ability of H1299 and H1975 cells transfected with si-linc00665. Representative images and data are based on three independent experiments. (F) Flow cytometry was used to detect NSCLC cells transfected with si-linc00665 and cultured for 48 h. The LR quadrant represents early apoptotic cells; the UR quadrant represents terminal apoptotic cells. (G) Flow cytometry was used to detect the percentage of NSCLC cells transfected with si-linc00665 in the G0/G1, S, or G2/M phase. (H) Differences in expression of caspase-3, cleaved caspase-3, and Bcl-2 after knockdown of linc00665. (I) Transwell test was used to detect the migration ability of NSCLC cells transfected with si-linc00665. Data are presented as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
Data from a Cell Counting Kit-8 (CCK-8) assay suggested that the survival rates of H1975 and H1299 cells transfected with si-linc00665 were significantly reduced compared with the blank control group (Figure 2C). Furthermore, NSCLC cells transfected with si-linc00665 showed decreased proliferation rates compared with the controls (Figure 2D). 5-Ethynyl-2′-deoxyuridine (EdU) immunostaining assays also confirmed that interference with linc00665 could reduce the proliferation of NSCLC cells (Figure 2E).
To determine whether cell cycle progression or apoptosis rates were affected by linc0065 knockdown in NSCLC cells, we performed flow cytometry experiments. Both H1975 and H1299 cells transfected with si-linc00665 #2 or #4 had higher apoptosis rates and showed cell cycle arrest in the G0/G1 phase compared with control cells (Figures 2F and 2G). We then found that following linc00665 knockdown, caspase-3 and Bcl-2 expression were significantly reduced, while cleaved caspase-3 levels increased (Figure 2H). We also performed transwell migration assays and found that linc00665 knockdown significantly inhibited H1975 and H1299 cell migration (Figure 2I).
Taken together, these results indicate that linc00665 promotes NSCLC cell proliferation by inhibiting apoptosis and promoting cell cycle progression, and that linc00665 also positively regulates NSCLC cell migration.
Knockdown of linc00665 enhances the sensitivity of NSCLC cells to DDP in vitro
Numerous studies have reported that changes in lncRNA expression levels are related to drug resistance in human tumors.23,24 To determine whether linc00665 expression affects NSCLC cell sensitivity to DDP, we treated H1975 and H1299 cells transfected with si-linc00665 with various concentrations of DDP (0, 5, 10, 15, 20, and 25 μg/mL) for 12 h or 5 μg/mL DDP for 0, 12, 24, 36, and 48 h. CCK-8 assays suggested that knocking down linc00665 significantly reduced the viability of NSCLC cells at the same DDP concentration or at the same reaction time (Figures 3A and 3B). We also performed colony formation experiments on cells treated with 5 μg/mL DDP for 12 h and found that the proliferative abilities of H1975 and H1299 cells treated with si-linc00665 and DDP were significantly reduced (p < 0.05) (Figure 3C). Flow cytometry results further revealed that knocking down linc00665 significantly enhanced the pro-apoptotic effect of DDP on H1299 and H1975 cells compared with the control group (Figure 3D). In summary, inhibition of linc00665 expression may improve the sensitivity of NSCLC cells to DDP.
Figure 3.
Effects of NSCLC cells transfected with si-linc00665 on DDP drug sensitivity in vitro
(A) Effects of various concentrations (0, 5, 10, 15, 20, and 25 μg/mL) of DDP on cells (H1299 and H1975 cells/scrambled, H1299 and H1975 cells/si-linc00665 #2, or H1299 and H1975 cells/si-linc00665 #4) for 12 h as assessed by a CCK-8 assay. (B) Effects of 5 μg/mL DDP on cells (H1299 and H1975 cells/scrambled, H1299 and H1975 cells/si-linc00665 #2, or H1299 and H1975 cells/si-linc00665 #4) for varied lengths of time (0, 12, 24, 36, and 48 h) evaluated by CCK-8 assays. (C) Effect of downregulated linc00665 combined with DDP (5 μg/mL) on proliferation of H1299 and H1975 cells. (D) Effect of combined linc00665 downregulation with DDP (5 μg/mL) on apoptosis of H1299 and H1975 cells. Data are presented as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
linc00665 knockdown increases the sensitivity of H1299 cells to DDP in vivo
We next examined whether linc00665 is involved in NSCLC cell sensitivity to DDP in vivo. Nude mice were inoculated with H1299 cells stably transfected with short hairpin RNA (sh-)linc00665 or empty vector, and then injected with DDP or phosphate-buffered saline (PBS). The growth of tumors derived from H1299 cells transfected with sh-linc00665 was significantly slower than that of tumors derived from H1299 cells transfected with the empty vector. In addition, the sensitivity to DDP of tumors derived from H1299 cells transfected with sh-linc00665 was significantly increased compared with tumors derived from empty vector-transfected H1299 cells. Furthermore, the size and weight of these tumors were significantly lower than those in the control group (Figures 4A–4C).
Figure 4.
Effect of interfering with the expression of linc00665 on tumor development in vivo
(A) Nude mouse model of NSCLC induced with H1299 cells stably transfected with sh-linc00665/sh-linc00665+cisplatin or empty vector/empty vector+cisplatin was established to observe the effects of linc00665 and linc00665+cisplatin on the transplanted carcinoma. (B and C) Average weight and volume of transplanted tumors from respective groups. Tumor volume was measured every 3 days. (D) Expression levels of proteins (Ki-67 and H&E stains) in tumor tissues of the interference group and the control group were analyzed by immunohistochemistry. Data are presented as the mean ± SEM. ∗∗p < 0.01.
Immunohistochemical analysis revealed that the Ki-67-positive rate in linc00665 knockdown tumor samples was lower than that of the control group. DDP treatment further reduced the Ki-67-positive rate of the linc00665 knockdown tumor samples (Figure 4D). Overall, these data suggest that linc00665 may promote the proliferation of NSCLC cells and affects the sensitivity of these cells to DDP in vivo.
CDKN1C is a target of linc00665
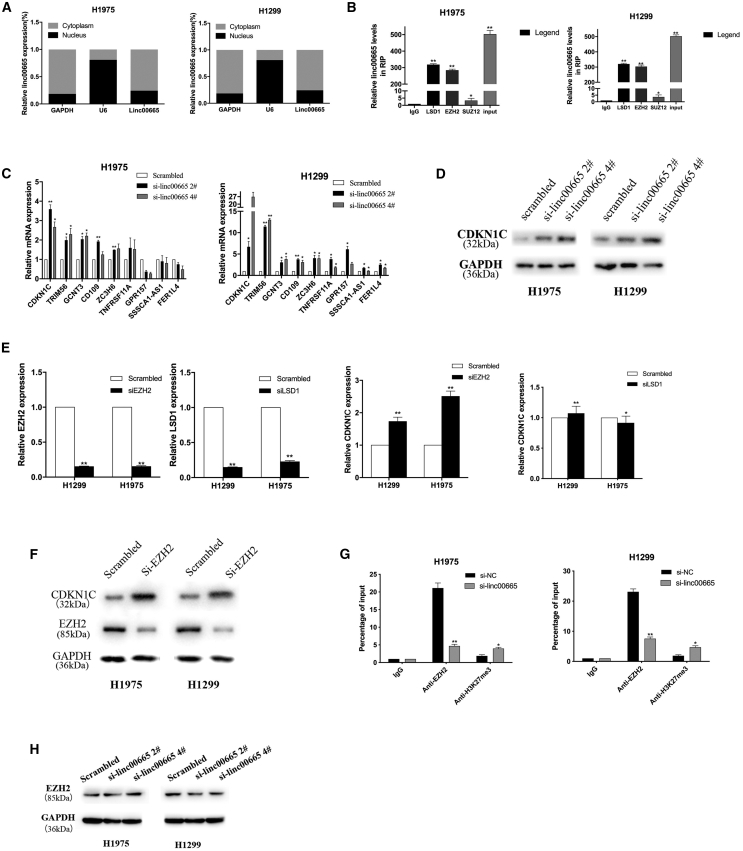
Upon further investigation of the molecular mechanism by which linc00665 affects NSCLC cells, we found through a nucleoplasm separation experiment that linc00665 is mainly distributed in the cytoplasm (Figure 5A). Our previous studies demonstrated that lncRNAs can inhibit the transcription of downstream genes by interacting with histone modification enzymes or other RNA-binding proteins.25 Therefore, we performed RNA immunoprecipitation (RIP) experiments to examine the potential interaction of linc00665 with several RNA binding proteins, such as EZH2, lysine-specific demethylase 1 (LSD1), and recombinant suppressor of zeste 12 homolog (SUZ12). The results showed that linc00665 bound to LSD1 and EZH2, but not to SUZ12, in H1975 and H1299 cells (Figure 5B).
Figure 5.
linc00665 interacts with EZH2 to regulate CDKN1C in NSCLC cells
(A) Quantitative real-time PCR was used to detect the relative expression of linc00665 in the cytoplasm and nucleus of H1299 and H1975 cell lines. GAPDH was used as a cytoplasmic marker; U6 was used as a nuclear marker. (B) Using RNA immunoprecipitation analysis and qRT-PCR to detect the expression level of linc00665 RNA, we compared the immunoprecipitation of LSD1, EZH2, and SUZ12 with that of IgG. (C) qRT-PCR was used to detect the expression level of tumor suppressor in NSCLC cells transfected with si-linc00665. (D) CDKN1C protein levels in linc00665 knockdown H1299 and H1975 cells transfected with si-linc00665 #2 or si-linc00665 #4. (E and F) mRNA and protein levels of EZH2/LSD1 and CDKN1C in H1975 and H1299 cells transfected with si-EZH2 and si-LSD1 were detected by qRT-PCR and western blot analysis. (G) The binding ratio of H3K27me3 to EZH2 in the CDKN1C initiating region in H1299 and H1975 cells transfected with si-linc00665 was determined by a ChIP assay. (H) The expression of EZH2 was detected after linc00665 was knocked down. ∗p < 0.05, ∗∗p < 0.01.
We next used RNA transcriptome sequencing (RNA-seq) to identify genes and transcripts that were differentially expressed in linc00665 knockdown H1299 cells compared with control cells. The results identified 1,447 differentially expressed transcripts (fold change ≥2, q ≤ 0.05), including 865 upregulated and 582 downregulated transcripts. We selected several upregulated genes for further verification (Table S1). We evaluated the expression levels of CDKN1C and other genes in H1975 and H1299 cells with linc00665 knocked down. The results showed that CDKN1C mRNA was significantly upregulated in these cells after linc00665 was knocked down (Figure 5C). Western blot analysis further showed that CDKN1C protein levels were elevated in si-linc00665-transfected NSCLC cells (Figure 5C).
CDKN1C is a cyclin-dependent kinase inhibitor of the Cip/Kip family, which is involved in many processes in human cancer. Studies have shown that melanoma cells can use CDKN1C/P57 to regulate cell cycle arrest.26,27 To determine whether linc00665 silenced the expression of CDKN1C by interacting with LSD1 or EZH2 in H1975 and H1299 cells, we knocked down the expression of EZH2 and LSD1 in both cell lines. We found that knocking down EZH2 increased the expression of CDKN1C. However, CDKN1C expression did not change significantly after interference with LSD1 expression (Figures 5E and 5F). To determine whether EZH2 binds to the promoter region of the CDKN1C gene, we designed primers that span a 2,000-bp portion of the promoter. A chromatin immunoprecipitation (ChIP) assay in NSCLC cells demonstrated that EZH2 bound to the CDKN1C promoter region and mediated H3K27 demethylation, while linc00665 knockdown reduced EZH2 binding ability to the CDKN1C promoter region (Figure 5G). EZH2 levels did not change following interference with linc00665 expression (Figure 5H). Taken together, these results suggest that linc00665 inhibits transcription of the CDKN1C gene by interacting with EZH2 in NSCLC cells.
Overexpression of CDKN1C inhibits the proliferation and migration of NSCLC cells
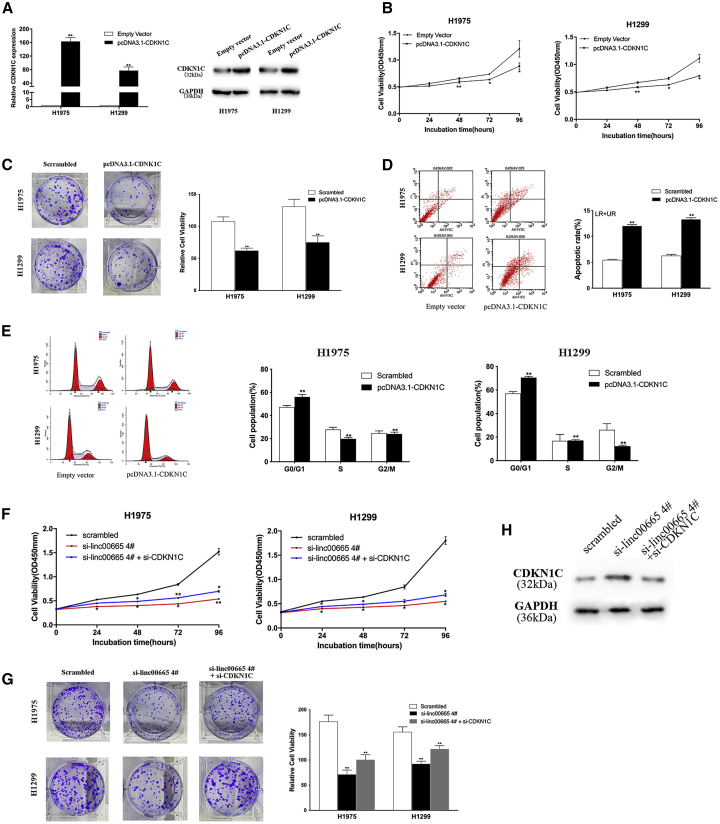
To investigate the function of CDKN1C in NSCLC cells, we conducted a series of functional experiments. qRT-PCR and western blot analyses confirmed upregulation of CDKN1C mRNA and protein in H1975 and H1299 cells transfected with the pcDNA3.1-CDKN1C vector (Figure 6A). CCK-8 and colony formation experiments showed that overexpression of CDKN1C reduced the proliferation rates of NSCLC cells compared with controls (Figures 6B and 6C). Flow cytometry analysis suggested that overexpression of CDKN1C led to the arrest of H1975 and H1299 cells in the G1/G0 phase and enhanced the rate of apoptosis (Figures 6D and 6E).
Figure 6.
Effect of overexpression of CDKN1C on proliferation ability, apoptosis, and cell cycle of H1299 and H1975 cells in vitro
(A) qRT-PCR and western blot were used to detect the expression of CDKN1C in H1299 and H1975 cells transfected with pcDNA3.1-CDKN1C. (B and C) CCK-8 and colony formation test to detect the proliferation ability of NSCLC cells transfected with pcDNA3.1-CDKN1C. (D and E) Flow cytometry showed that the apoptosis rate of NSCLC cells transfected with pcDNA3.1-CDKN1C was increased, and the cell cycle was blocked. (F and G) CCK-8 and colony formation experiments verified that co-transfection of si-linc00665 and si-CDKN1C can partially reverse the effect of knocking down linc00665 on cell proliferation. (H) Western blot analysis of CDKN1C protein expression levels after co-transfection with si-linc00665 and si-CDKN1C. Data are presented as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
We further examined whether CDKN1C is involved in the tumorigenic function of linc00665 in NSCLC cells. We co-transfected si-linc00665 and si-CDKN1C in H1975 and H1299 cells, and we performed CCK-8 and colony formation experiments. The observed decrease in NSCLC cell proliferation rates with linc00665 knockdown was partially restored with si-linc00665 and si-CDKN1C co-transfection (Figure 6F and 6G). Western blot analysis showed that CDKN1C upregulation resulting from linc00665 knockdown was reversed in cells co-transfected with si-linc00665 and si-CDKN1C (Figure 6H). These data suggest that linc00665 may play a role in promoting NSCLC by inhibiting the expression of CDKN1C.
Overexpression of CDKN1C enhances the sensitivity of NSCLC cells to DDP
We next examined whether CDKN1C is involved in the sensitivity of NSCLC cells to DDP. We treated H1975 and H1299 cells transfected with pcDNA3.1-CDKN1C with various concentrations of DDP (0, 5, 10, 15, 20, and 25 μg/mL) for 12 h or 5 μg/mL DDP for 0, 12, 24, 36, and 48 h. Overexpression of CDKN1C significantly reduced the proliferation of NSCLC cells after DDP treatment (Figures 7A and 7B). Colony formation experiments yielded similar results (Figure 7C). Flow cytometry analysis revealed that the apoptosis rates after DDP treatment of NSCLC cells overexpressing CDKN1C were significantly higher than those of the control cells (Figure 7D). These results suggest that linc00665 may influence the sensitivity of NSCLC cells to DDP by regulating CDKN1C.
Figure 7.
In vitro, overexpression of CDKN1C H1299 and A549 cells affected DDP sensitivity
(A) Using a CCK-8 assay, we evaluated the various concentrations (0, 5, 10, 15, 20, and 25 μg/mL) of DDP for 12 h on cells (H1299 and H1975 cells/scrambled, H1299 and H1975 cells/pcDNA3.1-CDKN1C). (B) Using a CCK-8 assay, cells (H1299 and H1975 cells/scrambled, H1299 and H1975 cells/pcDNA3.1-CDKN1C) treated with 5 μg/mL DDP for different lengths of time (0, 12, 24, 36, and 48 h) were detected. (C) Cell cycle changes of NSCLC cells (H1975 and H1299) treated with 5 μg/mL DDP and pcDNA3.1-CDKN1C were detected. (D) The apoptosis rate of H1299 and H1975 cells overregulated by CDKN1C combined with DDP (5 μg/mL) was determined by flow cytometry. Data are presented as the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
Discussion
In recent years, an increasing number of studies have revealed that lncRNAs are involved in tumor development and chemotherapy drug resistance. HOTAIR, TUG1, PVT1, MEG3, and other lncRNAs play an important role in the development of NSCLC through transcriptional or post-transcriptional regulation of specific target genes.28, 29, 30, 31 Our previous work showed that the lncRNA AFAP1-AS1 is upregulated in NSCLC tissues and promotes NSCLC cell proliferation through LSD1-mediated silencing of HMG-box transcription factor 1 (HBP1).32 In this study, we found another lncRNA, linc00665, to be upregulated in NSCLC tissues and cells. High expression of linc00665 may be a marker of poor prognosis in NSCLC patients. In addition, downregulation of linc00665 inhibited the proliferation of NSCLC cells in vitro and tumor growth in vivo, and it also enhanced the sensitivity of NSCLC cells to DDP. These findings suggest that linc00665 may play a carcinogenic role in the development of NSCLC and impact the efficacy of DDP.
Many lncRNAs regulate the expression of local or distal genes and contribute to malignant biological behaviors of cancer cells.33,34 In this study, we conducted RIP experiments and found that linc00665 can bind to EZH2 in NSCLC cells. RNA-seq analysis of H1299 cells with linc00665 expression knocked down suggested that the tumor suppressor gene CDKN1C is a downstream regulatory gene of linc00665 in NSCLC cells. We found that CDKN1C expression increased after transfection of si-EZH2 in NSCLC cells. Furthermore, ChIP assays showed that EZH2 binds to the promoter region of CDKN1C and inhibits its transcription by mediating the demethylation of H3K27me3. These results indicate that linc00665 plays a key role in NSCLC cells by inhibiting EZH2-mediated CDKN1C expression.
In this study, we found that overexpression of CDKN1C inhibited the proliferation of NSCLC cells, induced cell cycle arrest and apoptosis, and enhanced the sensitivity of NSCLC cells to DDP. Additionally, knocking down CDKN1C partially reversed the inhibitory effect of knocking down linc00665 on NSCLC cell growth, suggesting that CDKN1C may also be involved in linc00665 effects on the malignant phenotype of NSCLC cells and the inhibition of DDP sensitivity.
It has been previously reported that linc00665 plays a cancer-promoting role in LUAD and is associated with the development of gefitinib resistance. However, we demonstrated for the first time that the linc00665/EZH2/CDKN1C axis mediates the development of NSCLC cells and their drug sensitivity to DDP. In conclusion, our study found that linc00665 was upregulated in NSCLC tissues and cells, and its high expression was associated with poor prognosis in NSCLC patients. We showed that linc00665 promotes the proliferation of NSCLC cells and inhibits the sensitivity of these cells to DDP. We revealed that linc00665 can recruit EZH2 to the CDKN1C promoter region to induce the demethylation of histone H3K27 and inhibit CDKN1C transcription. Although our results have helped elucidate the downstream pathways of linc00665 in NSCLC, other potential target genes and their mechanisms are still worth exploring. Our findings enrich the understanding of the pathogenesis of NSCLC and suggest the potential of linc00665 as a diagnostic marker or therapeutic target for NSCLC. Our results also indicate a role for linc0065 in mediating the efficacy of DDP treatment, which provides potential targets for improving this treatment in NSCLC.
Materials and methods
Tissue samples and clinical data collection
We acquired 60 paired NSCLC and adjacent non-tumor lung tissues from patients who did not receive chemotherapy or radiotherapy before surgery and underwent surgery at Jiangsu Province Hospital between 2016 and 2018. In the light of clinicopathological diagnosis from the Pathology Department, all patients were diagnosed with NSCLC and detailed follow-up data on clinicopathological features. We gathered and summarized the correlation between specific pseudogene expression and clinicopathological characteristics of NSCLC patients, including advanced TNM staging (Table 1). We obtained written informed consent from all patients as well as the Research Ethics Committee of Nanjing Medical University approval of our study protocol and methods. Moreover, all collected postoperative tissue samples were promptly snap-frozen in liquid nitrogen and stored at − 80°C until required to extract total RNAs and proteins. OS was defined as the interval between the dates of surgery and death caused by any causes; PFS was defined as the interval between the date of surgery and the date of recurrence. Patients who were lost to follow-up before death usually ended up with the time of the last recorded contact with the patient.
Cell lines
A normal human bronchial epithelial cell line (16HBE) serving as a control cell line and five NSCLC cell lines (PC9, SPC-A1, H1975, H1299, and A549) were acquired from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). A549, H1975, and H1299 cells were cultivated in RPMI 1640 (Invitrogen, Shanghai, China); 16HBE, PC9, and SPC-A1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Shanghai, China). The RPMI 1640 and DMEM medium were supplemented with 10% fetal bovine serum (FBS; Gibco), 100 IU/mL penicillin, and 100 μg/mL streptomycin (according to Invitrogen instructions). All cells were cultured in large plates or cell culture bottles, added with suitable medium, and cell growth was observed every other day. Almost all adherent cells were overgrowth, we were passaged these cells and culturing these cells choose to continue or cryopreserved. The cell incubator was set at humidified air at 37°C and CO2 content of 5%, which was sterilized regularly.
Cell transfection
Plasmid vectors and siRNA were transfected in NSCLC cell lines H1975 and H1299 using Lipofectamine 2000 for 48 h, and then the total RNA and proteins were extracted for qRT-PCR and western blot experiments. There are four individual linc00665 siRNAs (si-linc00665 #1, #2, #3, and #4) and a scrambled negative control siRNA (si-NC) purchased from Invitrogen. siRNA sequences are provided in Table S1.
RNA extraction and qPCR assays
Total RNA of tissues or cultured cells was extracted using TRIzol reagent (Invitrogen, Grand Island, NY, USA) based on the manufacturer’s instructions. Under standard conditions for the PrimeScript RT reagent kit (TaKaRa, Dalian, China), total RNA (500 ng) was reverse transcribed in cDNA (10 μL) using random primers. SYBR Premix Ex Taq (TaKaRa, Dalian, China) was used to monitor linc00665 expression levels, following the manufacturer’s instructions. Results were standardized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Specific primers used are shown in Table S1. We performed quantitative PCR (qPCR) and data collection to analyze the relative expression level of the gene adopting the comparative cycle threshold (CT) (2−ΔΔCT) method on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The CT value was obtained according to the amplification curve.
Construction of plasmid
The CDKN1C sequences designed on the NCBI database were synthesized by Realgene (Nanjing, China) and subcloned into the pcDNA3.1(+) vector (Invitrogen) (Genechem, Shanghai, China), generating the pcDNA3.1-CDKN1C vector for ectopic expression in cells. Plasmid vectors (pcDNA3.1-CDKN1C and empty vector) were transfected into NSCLC cells cultured in six-well plates using the X-tremeGENE HP DNA transfection reagent (Roche, Basel, Switzerland). Western blot and qPCR were conducted to assess CDKN1C expressions of NSCLC cells harvested after 48 h of transfection.
Cell viability assays
A cell proliferation assay was executed with CCK-8 (Roche Applied Science, Basel, Switzerland) according to the manufacturer’s instructions. H1299 or H1975 cells transfected with si-linc00665 (1,500 cells/well) were grown in 96-well plates. Cell viability was tested every 24 h following the manufacturer’s method. The cell survival rate was assessed by the proliferation curve of different treatment groups of 5 days. For the colony formation assay, a total of 1,100 H1299 or H1975 cells transfected with si-linc00665 were placed in each well of a six-well plate and maintained in RPMI 1640 containing 10% FBS, changing the medium every 4 days. After 14 days, the colonies were immobilized with methanol and stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA), and the numbers of stained visible colonies were counted to describe colony formation. For each treatment group, we repeated the experiments three times and assessed the wells in triplicate.
Flow cytometric analysis
H1299 and H1975 cells transfected with si-linc00665 for 48 h were harvested by trypsinization and doubly stained with fluorescein isothiocyanate (FITC) annexin V and propidium iodide (PI) using a FITC annexin V apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. There are viable cells, dead cells, early apoptotic cells, and apoptotic cells by the classification. Then, we compared the relative ratio of early apoptotic cells with control cells for each experiment. The cells were then stained with PI using the Cycletest Plus DNA reagent kit (BD Biosciences) following the manufacturer’s method and analyzed by FACScan flow cytometry for cell-cycle analysis counting and comparing the percentages of cells in the G0/G1, S, and G2/M phases.
Cell migration assays
For the Transwell migration assays, the upper chamber of an insert (8-μm pore size; Millipore) was placed in 5 × 10 4 cells incubated with the serum-free medium as well as the lower chamber added in medium containing 10% FBS at 48 h post-transfection. Twenty-four hours later, cotton wool was used to softly remove the cells remaining on the upper membrane. Cells were stained with methanol and 0.1% crystal violet and imaged, and cells were counted that had migrated or invaded through the membrane using an IX71 inverted microscope (Olympus, Tokyo, Japan). The experiment was performed in triplicate and repeated three times.
Western blot
Cell protein lysates were isolated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to 0.22-μm polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), and incubated with specific antibodies. Then, the quantization of the enhanced chemiluminescence (ECL) chromogenic substrate was measured by densitometry (Quantity One software; Bio-Rad, Hercules, CA, USA), with GAPDH antibody as a control. Anti-CDKN1C and anti-EZH2 were purchased from Cell Signaling Technology (Boston, MA, USA).
EdU assay
We used an EdU labeling/detection kit (Ribobio, Guangzhou, China) to assess proliferating cells per the manufacturer’s protocol. In short, H1299 or H1975 cells transfected with si-linc00665 were cultured in 12-well plates at 5 × 104 cells/well for 2 days. Next, cells were added to 50 μM EdU labeling medium and incubated for 2 h in a standard incubator. The cultured cells were treated with 4% paraformaldehyde (pH 7.4) for 30 min and then 0.5% Triton X-100 for 20 min at room temperature. After washing with PBS, the samples were stained with an anti-EdU working solution for 30 min at room temperature without light. DAPI (4′,6-diamidino-2-phenylindole) was used as a nuclear/chromosomal counterstain. Subsequently, the cells were observed under a fluorescent microscope. The percentage of EdU-positive cells was calculated from five random fields in three wells.
Subcellular fractionation
The separation of nuclear and cytosolic fractions was implemented using the Paris kit (Life Technologies) following the manufacturer’s instructions.
RIP
By performing RIP, we investigated whether si-linc00665 could interact or bind with potential binding proteins (EZH2 or LSD1) in H1299 and H1975 cells using a Magna RIP RNA-binding protein immunoprecipitation kit (Millipore) following the manufacturer’s protocol. H1299 and H1975 cells at 80%–90% confluency were scraped off from the 15-cm plate. Then, 100 μL of whole-cell lysates lysed in complete RIP lysis buffer was incubated with protein A Sepharose beads conjugated with recognized antibodies to EZH2, LSD1, or negative control immunoglobulin G (IgG) (Millipore, USA) for 30 min at 4°C. After that, the supernatants of whole-cell extracts were incubated with treated beads for 6 h at 4°C. Furthermore, the beads were washed with wash buffer six times and incubated with 0.1% SDS/0.5 mg/mL Proteinase K for 30 min at 55°C in a water bath to digest proteins. Ultimately, immunoprecipitated RNA was subjected to qPCR analysis using specific primers to demonstrate the presence of si-linc00665.
ChIP assays
ChIP assays were conducted using a Magna ChIP kit (Millipore, Billerica, MA, USA) following the manufacturer’s protocol. H1299 and H1975 cells at 80%–90% confluency were treated with paraformaldehyde and incubated for 10 min to prepare DNA-protein crosslinking. Then, the cells were fully lysed by cell lysis and nuclear cracking liquid. Next, the cell debris was sonicated to generate 200- to 300-bp chromatin pieces and then compounded with anti-EZH2, anti-LSD1, anti-H3K27, anti-H3K4 (as positive reference), and IgG (as negative control) antibody-magnetic beads for immunoprecipitation. Finally, after digestion with Proteinase K, precipitated chromatin DNA was extracted and purified for qRT-PCR analysis.
Tumor formation assay in vivo
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Nanjing Medical University. The suspended H1299 cells (0.1 mL) stably transfected with sh-linc00665 or empty vector were subcutaneously injected into either side of the armpit regions of male BALB/c nude mice (4 or 5 weeks old). All of the cells were collected at a concentration of 2 × 107 cells/mL. Next, half of the mice were treated with a total of 0.1 mL of DDP, and the other half were treated with an equal amount of PBS. Tumor volumes and weights were measured every 3 days, and tumor volumes were calculated using the following equation: (length × width2) × 0.5. At 21 days after injection, mice were killed, and the subcutaneous growth of each tumor was examined. Primary tumors were excised, and tumor tissues were used for immunostaining of protein(Ki-67 and H&E stains).
Immunohistochemistry
Primary tumors were immunostained (Ki-67 and H&E), as previously described.
Statistical analysis
All statistical analyses were performed using SPASS 20.0 software (IBM). A Student’s t test and Wilcoxon test or the χ2 test estimated the significance of differences between groups. The Kaplan-Meier method was used to determine PFS and OS and used the log-rank test for comparison. Survival data were evaluated via univariate and multivariate Cox proportional hazards models. Variables with a value of p <0.05 in univariate analysis were used in subsequent multivariate analysis on the basis of Cox regression analyses. Pearson correlation analyses were conducted to investigate the correlation of si-linc00665 expression. p values <0.05 were viewed as statistically significant.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 81871871); the Key Research and Development Plan (Social Development) of the Science and Technology Department of Jiangsu Province (no. BE2019760); the Jiangsu Graduate Practice Innovation Program (no. SJCX19-0325); the Medical Innovation Team Foundation of the Jiangsu Provincial Enhancement Health Project (no. CXTDA2017021); and the “333 High Class Talented Man Project” (no. 2016-II-426).
Author contributions
Z.W., Y. Zhu, and W.W. designed experiments and supervised their completion. D.Y. participated in flow cytometry analysis and animal experiments and wrote the paper. W.F. participated in qRT-PCR. W.W. and Y. Zhuang collected and classified human tissue samples. J.L. and T.X. analyzed the experimental data. Z.W. and Z.F. provided technical and administrative support for a platform for scientific research. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2021.01.013.
Contributor Information
Wei Wang, Email: wangwei15261883958@163.com.
Yefei Zhu, Email: zhuyf@njmu.edu.cn.
Zhaoxia Wang, Email: zhaoxiawang66@126.com.
Supplemental information
References
- 1.Chheang S., Brown K. Lung cancer staging: clinical and radiologic perspectives. Semin. Intervent. Radiol. 2013;30:99–113. doi: 10.1055/s-0033-1342950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostova I. Platinum complexes as anticancer agents. Recent Patents Anticancer Drug Discov. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 3.Perkel J.M. Visiting “noncodarnia”. Biotechniques. 2013;54(301):303–304. doi: 10.2144/000114037. [DOI] [PubMed] [Google Scholar]
- 4.Mariner P.D., Walters R.D., Espinoza C.A., Drullinger L.F., Wagner S.D., Kugel J.F., Goodrich J.A. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol. Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Mattick J.S., Amaral P.P., Dinger M.E., Mercer T.R., Mehler M.F. RNA regulation of epigenetic processes. BioEssays. 2009;31:51–59. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- 6.Mercer T.R., Dinger M.E., Bracken C.P., Kolle G., Szubert J.M., Korbie D.J., Askarian-Amiri M.E., Gardiner B.B., Goodall G.J., Grimmond S.M., Mattick J.S. Regulated post-transcriptional RNA cleavage diversifies the eukaryotic transcriptome. Genome Res. 2010;20:1639–1650. doi: 10.1101/gr.112128.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Chen Z., Yu S., Nie F., Yan S., Ma P., Chen Q., Wei C., Fu H., Xu T. Long noncoding RNA LINC01234 functions as a competing endogenous RNA to regulate CBFB expression by sponging miR-204-5p in gastric cancer. Clin. Cancer Res. 2018;24:2002–2014. doi: 10.1158/1078-0432.CCR-17-2376. [DOI] [PubMed] [Google Scholar]
- 8.Cui C., Zhai D., Cai L., Duan Q., Xie L., Yu J. Long noncoding RNA HEIH promotes colorectal cancer tumorigenesis via counteracting miR-939-mediated transcriptional repression of Bcl-xL. Cancer Res. Treat. 2018;50:992–1008. doi: 10.4143/crt.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou J., Wang L., Wu Q., Zheng G., Long H., Wu H., Zhou C., Guo T., Zhong T., Wang L. Long noncoding RNA H19 upregulates vascular endothelial growth factor A to enhance mesenchymal stem cells survival and angiogenic capacity by inhibiting miR-199a-5p. Stem Cell Res. Ther. 2018;9:109. doi: 10.1186/s13287-018-0861-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing H., Qu X., Liu L., Xia H. A novel long noncoding RNA (lncRNA), LL22NC03-N64E9.1, promotes the proliferation of lung cancer cells and is a potential prognostic molecular biomarker for lung cancer. Med. Sci. Monit. 2018;24:4317–4323. doi: 10.12659/MSM.908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia X., Wang Z., Qiu L., Yang Y., Wang Y., Chen Z., Liu Z., Yu L. Upregulation of lncRNA-HIT promotes migration and invasion of non-small cell lung cancer cells by association with ZEB1. Cancer Med. 2016;5:3555–3563. doi: 10.1002/cam4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Lyu H., Liu H., Shi X., Song Y., Liu B. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci. Rep. 2016;6:31093. doi: 10.1038/srep31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai N., Xia Y., Yin R., Liu J., Gao F. A negative regulation loop of long noncoding RNA HOTAIR and p53 in non-small-cell lung cancer. OncoTargets Ther. 2016;9:5713–5720. doi: 10.2147/OTT.S110219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z., Chen X., Chen P., Yu S., Nie F., Lu B., Zhang T., Zhou Y., Chen Q., Wei C. Long non-coding RNA SNHG20 promotes non-small cell lung cancer cell proliferation and migration by epigenetically silencing of P21 expression. Cell Death Dis. 2017;8:e3092. doi: 10.1038/cddis.2017.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Wan L., Lu K., Sun M., Pan X., Zhang P., Lu B., Liu G., Wang Z. The long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinoma. PLoS ONE. 2015;10:e0114586. doi: 10.1371/journal.pone.0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S., Ma H., Zhang D., Xie S., Wang W., Li Q., Lin Z., Wang Y. lncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9:742. doi: 10.1038/s41419-018-0793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., Sun M., Lu K., Liu J., Zhang M., Wu W., De W., Wang Z., Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21WAF1/CIP1 expression. PLoS ONE. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L., Liu C., Zhang Z. Knockdown of lncRNA MIAT inhibits proliferation and cisplatin resistance in non-small cell lung cancer cells by increasing miR-184 expression. Oncol. Lett. 2020;19:533–541. doi: 10.3892/ol.2019.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y., Gu J., Liu P., Wang H., Jiang L., Lei T., Yu S., Han G., Wang Z. Long non-coding RNA SPRY4-IT1 reverses cisplatin resistance by downregulating MPZL-1 via suppressing EMT in NSCLC. OncoTargets Ther. 2020;13:2783–2793. doi: 10.2147/OTT.S232769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J.G., Xu S.N., Yin L.G. lncRNA NORAD/miR-202-5p regulates the drug resistance of A549/DDP to cisplatin by targeting P-gp. Gen. Physiol. Biophys. 2020;39:481–489. doi: 10.4149/gpb_2020027. [DOI] [PubMed] [Google Scholar]
- 21.Cong Z., Diao Y., Xu Y., Li X., Jiang Z., Shao C., Ji S., Shen Y., De W., Qiang Y. Long non-coding RNA linc00665 promotes lung adenocarcinoma progression and functions as ceRNA to regulate AKR1B10-ERK signaling by sponging miR-98. Cell Death Dis. 2019;10:84. doi: 10.1038/s41419-019-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Lu X., Zhen F., Jin S., Yu T., Zhu Q., Wang W., Xu K., Yao J., Guo R. LINC00665 induces acquired resistance to gefitinib through recruiting EZH2 and activating PI3K/AKT pathway in NSCLC. Mol. Ther. Nucleic Acids. 2019;16:155–161. doi: 10.1016/j.omtn.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian L.J., Wu Y.P., Wang D., Zhou Z.H., Xue S.B., Zhang D.Y., Wei Y.G., Liu W. Upregulation of long noncoding RNA (lncRNA) X-inactive specific transcript (XIST) is associated with cisplatin resistance in non-small cell lung cancer (NSCLC) by downregulating microRNA-144-3p. Med. Sci. Monit. 2019;25:8095–8104. doi: 10.12659/MSM.916075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan Y., Abuduwaili K., Wang X., Shen Y., Nuerlan S., Liu C. Knockdown of long non-coding RNA HOTAIR suppresses cisplatin resistance, cell proliferation, migration and invasion of DDP-resistant NSCLC cells by targeting miR-149-5p/doublecortin-like kinase 1 axis. Cancer Manag. Res. 2020;12:7725–7737. doi: 10.2147/CMAR.S246299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q.N., Chen X., Chen Z.Y., Nie F.Q., Wei C.C., Ma H.W., Wan L., Yan S., Ren S.N., Wang Z.X. Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression. Mol. Cancer. 2017;16:17. doi: 10.1186/s12943-017-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howlin J., Cirenajwis H., Lettiero B., Staaf J., Lauss M., Saal L., Borg Å., Gruvberger-Saal S., Jönsson G. Loss of CITED1, an MITF regulator, drives a phenotype switch in vitro and can predict clinical outcome in primary melanoma tumours. PeerJ. 2015;3:e788. doi: 10.7717/peerj.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanagh E., Joseph B. The hallmarks of CDKN1C (p57, KIP2) in cancer. Biochim. Biophys. Acta. 2011;1816:50–56. doi: 10.1016/j.bbcan.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.S., Peng M., Zhou G.Z., Pu Y.C., Yi M.C., Zhu Y., Jiang B. Long non-coding RNA HOTAIR regulates the development of non-small cell lung cancer through miR-217/DACH1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:670–678. doi: 10.26355/eurrev_201901_16905. [DOI] [PubMed] [Google Scholar]
- 29.Wan L., Sun M., Liu G.J., Wei C.C., Zhang E.B., Kong R., Xu T.P., Huang M.D., Wang Z.X. Long noncoding RNA PVT1 promotes non-small cell lung cancer cell proliferation through epigenetically regulating LATS2 expression. Mol. Cancer Ther. 2016;15:1082–1094. doi: 10.1158/1535-7163.MCT-15-0707. [DOI] [PubMed] [Google Scholar]
- 30.Xu J., Su C., Zhao F., Tao J., Hu D., Shi A., Pan J., Zhang Y. Paclitaxel promotes lung cancer cell apoptosis via MEG3-P53 pathway activation. Biochem. Biophys. Res. Commun. 2018;504:123–128. doi: 10.1016/j.bbrc.2018.08.142. [DOI] [PubMed] [Google Scholar]
- 31.Zhang E.B., Yin D.D., Sun M., Kong R., Liu X.H., You L.H., Han L., Xia R., Wang K.M., Yang J.S. p53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S., Yang D., Ye Y., Liu P., Chen Z., Lei T., Pu J., Liu L., Wang Z. Long noncoding RNA actin filament-associated protein 1 antisense RNA 1 promotes malignant phenotype through binding with lysine-specific demethylase 1 and repressing HMG box-containing protein 1 in non-small-cell lung cancer. Cancer Sci. 2019;110:2211–2225. doi: 10.1111/cas.14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q., Liu L., Zhang S., Ming Y., Liu S., Cheng K., Zhao Y. Long noncoding RNA NEAT1 suppresses hepatocyte proliferation in fulminant hepatic failure through increased recruitment of EZH2 to the LATS2 promoter region and promotion of H3K27me3 methylation. Exp. Mol. Med. 2020;52:461–472. doi: 10.1038/s12276-020-0387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W., Yan Z., Hu F., Wei W., Yang C., Sun Z. Long non-coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2-mediated CDKN1C downregulation. Cancer Cell Int. 2020;20:116. doi: 10.1186/s12935-020-01167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.