Highlights
-
•
Acute cardiac tamponade is a highly relevant complication in modern cardiology.
-
•
Continuous pericardial drainage is safe and does not increase total drainage volume.
-
•
Continuous drainage associates with lower rates of open-heart surgical interventions.
-
•
Continuous drainage associates with reduced re-tamponades and mortality on day 5.
Keywords: Pericardial effusion, Tamponade, Drainage, Pericardiocentesis, Intervention
Abbreviations: int., intermittent; cont., continiuous
Abstract
Background
Acute cardiac tamponade is a life-threatening pathology in modern cardiology as catheter-based interventions become increasingly relevant. Pericardiocentesis is usually the primary treatment of choice. However, protocols for handling of draining pigtail catheters are very variable due to limit data and require further investigation.
Methods
We retrospectively analyzed 52 patients with acute cardiac tamponade requiring immediate pericardiocentesis from January 2017 to August 2020. Patients were treated with a classical approach of intermittent manual aspiration or continuous pericardial drainage using a redon drainage system.
Results
Mean age of patients was 74 years in both groups. Most common causes for cardiac tamponade were percutaneous coronary interventions in about 50% and transaortic valve implantations in 25% of all cases. 28 patients were treated with classic intermittent drainage from 2017 to 2020. 24 patients were treated with continuous drainage from December 2018–2020. Compared to classical intermittent drainage treatment, continuous drainage was associated with a lower rate of a surgical intervention or cardiac re-tamponade and a lower mortality at 5 days (HR 0.2, 95% CI 0.1–0.9, log-rank p = 0.03). Despite a longer total drainage time under continuous suction, drainage volumes were comparable in both groups.
Conclusion
Acute cardiac tamponade can be efficiently treated by pericardiocentesis with subsequent continuous negative pressure drainage via a pigtail catheter. Our retrospective analysis shows a significantly lower mortality, a decreased rate of interventions and lower rates of cardiac re-tamponade without any relevant side effects when compared to classical intermittent manual drainage. These findings require further investigations in larger, randomized trials.
1. Introduction
The pericardium is a double-walled sac composed of an inner serous visceral and an outer fibrous parietal layer. It surrounds and protects the heart, fixes its position in the mediastinum and prevents excessive cardiac dilatation. Under physiological conditions it contains about 20–60 ml of plasma ultrafiltrate to lubricate the heart [2].
An excessive amount of pericardial fluid is called pericardial effusion. Typical classifications of pericardial effusions refer to the timeframe of development (acute or chronic), hemodynamic relevance, inner composition (blood, serous fluid or pus) as well as size and position in relation to the heart. While low and moderately elevated levels of pericardial pressure lower than 10–15 mmHg have typically only mild hemodynamic impact, severe effusions with more than 15 mmHg of pericardial pressure impair cardiac filling as right atrial and potentially also ventricular pressure is exceeded – a life-threatening condition called cardiac tamponade [1], [3]. However, immediate pericardiocentesis as the favorable primary treatment strategy is also associated with complication rates of 4–10% including arrhythmia, injury of the coronaries, accidental puncture of the right ventricle, hemato- or pneumothorax, pneumopericardium or liver lacerations [4], [5].
The increasing amount of catheter-based interventional strategies to treat cardiac pathologies involve pericardial effusion with potential subsequent tamponade among the most common severe complications. These procedures include ablations for arrhythmias, pacemaker or defibrillator implantations, highly complex percutaneous coronary interventions (PCIs) as well as different valve repair and replacement strategies such as mitral clipping or transcatheter aortic valve implantation (TAVI) [6], [7], [8]. Recent studies reported coronary artery perforations in 0.7% of PCIs and in 4% of chronic total occlusion interventions (CTO) [9], [10]. Any injuries of myocardial tissue or vessels potentially lead to acute cardiac tamponade due to a rapid filling and compression with blood. Therefore, any unexpected hemodynamic changes require immediate echocardiographic or fluoroscopic control to exclude acute pericardial effusion.
The increasing relevance of cardiac tamponade as a rare but common and potentially life-threatening complication of modern interventional cardiology in combination with very limited data on post-pericardiocentesis patient management drove us to establish this retrospective analysis. To this end, we compared our standard of care procedure of intermittent pericardial aspiration via an intrapericardial pigtail catheter (intermittent suction: int.) with a continuous negative pressure pericardial drainage system (continuous group: cont.) to optimize patient management for an increasingly important clinical condition of acute cardiac tamponade [11].
2. Methods
2.1. Patients
We retrospectively analyzed 52 consecutive patients with acute cardiac tamponade requiring emergent pericardiocentesis and pigtail catheter insertion for repetitive drainage between January 2017 to August 2020. In December 2018 we established a new approach to drain pericardial tamponade after pericardiocentesis via continuous negative pericardial pressure using a redon drainage system (Redovac®, B. Braun, Germany) as previously published by our group [11]. Patients, who died immediately in the catheter laboratory or those, who received a pericardial drainage as a bridge for optimized transportation to direct surgery, were excluded from our analysis. We also excluded patients, in which a drainage could not be placed and those with missing data about the modality of drainage (continuous or intermittent). In compliance with the Declaration of Helsinki and German data protection laws, all patients in this analysis suffered from cardiogenic shock and were treated in cardiac intensive care unit (ICU) of Ludwig-Maximilians-University (LMU) hospital and were included in a registry (LMUshock). The latter is registered at the WHO International Clinical Trials Registry Platform (DRKS00015860) and was approved by the local ethics committee (IRB number: 18-001) [12], [13].
2.2. Study definition and endpoints
Acute cardiac tamponade was defined as a sudden onset of hemodynamic instability, mostly directly associated with invasive catheter procedures, in combination with an echocardiographic (or fluoroscopic) confirmed pericardial effusion. Study endpoints were overall survival, drained blood volume, drainage time, drainage clotting, re-tamponade and the requirement of open-heart surgery.
2.3. Procedure
While rare cases of severe emergency require immediate blind decompression by pericardiocentesis, standard of care techniques include echocardiographic and/or fluoroscopic guidance [1], [14].
After preparation with local anesthesia, a needle is protruded in a 15 degrees angle from subxiphoid position towards the left shoulder under gentle aspiration until pericardial fluid is obtained [3], [15]. In cases of uncertainty, we use echocardiographic or fluoroscopic visualization by injection of agitated fluid or contrast medium respectively, to confirm proper needle position. Afterwards a standard guide wire (Angiocard, Germany) is inserted and an injection sheath is placed (usually 7 French). Subsequently, a pigtail catheter (usually 6 French) is placed to immediately drain pericardial effusion. This catheter is used for intermittent manual aspiration as our conventional treatment method. Aspiration intervals vary based on clinical presentation and hemodynamics. In our new approach, the pigtail catheter is directly connected to a redon drainage system (Redovac®, B. Braun, Germany) which ensures continuous drainage by applying negative pericardial pressure [11]. The catheter was usually withdrawn when a drainage volume of <50 ml/ 24 h was reached [3], [15].
2.4. Data collection
Demographic, procedural and outcome data were obtained from review of our LMUshock registry [12], [13]. Clinical follow-up data were collected upon discharge, from rehabilitation clinic reports and by telephone follow-up.
2.5. Statistical analysis
Statistical analysis was performed using R (version 4.0.1, The R foundation). Normally distributed continuous variables were reported as mean with standard deviation and non-normally distributed continuous variables as median with interquartile ranges (25th and 75th percentile). To compare groups, t-test for normally distributed continuous variables and Mann-Whitney-U test for non-normally distributed continuous variables were used. Categorical variables were reported as absolute numbers and percentages and Chi-square test was utilized for comparison. All tests were 2-tailed, and p-values < 0.05 were considered as significant. Mortality was calculated using the Kaplan–Meier method and comparisons were made by using log-rank tests.
3. Results
3.1. Baseline characteristics according to study group
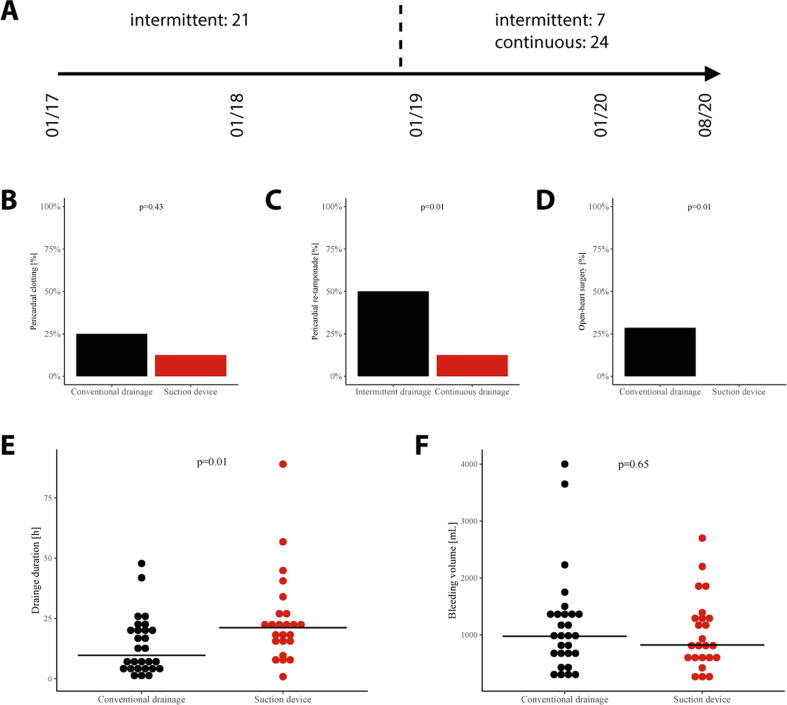
28 patient in the intermittent suction group were enrolled between January 2017 and August 2020 and 24 patients in the continuous suction group between December 2018 and August 2020. The predominant treatment strategy between December 2018 and August 2020 was the continuous approach in 77% of patients with pericardial tamponade. Fig. 1 provides an overview about the two treatment strategies according to both treatment periods (Fig. 1).
Fig. 1.
Procedural success: (A) Time-dependent choice of intermittent or continuous drainage therapy. Procedural success parameters including pericardial clotting (B), re-tamponade (C), conversion to open-heart surgery (D), drainage duration (E) and total bleeding volume (F).
The median age and body mass index (BMI) was 74.5 years and 27.3 kg/m2 in the classic intermittent and 74.0 years and 25.8 kg/m2 in the continuous drainage group, respectively. In both groups, most patients were male (int.: 60.7%/ cont.: 66.7%), had hypertension (int.: 75.0%, cont.: 79.2%) and about 60% suffered from hyperlipidemia (int.: 60.7%/ cont.: 58.3%). The rate of active smoking (int.: 3.6%/ cont.: 20.8%) and diabetes (int.: 10.7%/ cont.: 20.8%) was slightly – albeit not significant - higher in our experimental group. Coronary artery disease was known in about three quarters of our patients (int.: 71.4%/ cont.: 75.0%). Yet only a minority had a previous myocardial infarction (int.: 17.9%/ cont.: 25.0%). A previous PCI was performed in 21.4% of intermittent and 41.7% of continuous suction cohort patients. Atrial fibrillation was present in about half of all cases (int.: 39.3%/ cont.: 54.2%) and a minority suffered from chronic kidney disease (int.: 17.9%/ cont.: 12.5%). All baseline characteristics are displayed in Table 1.
Table 1.
Baseline characteristics.
|
Drainage type |
|||
|---|---|---|---|
| Intermittent | Continuous | p value | |
| n | 28 | 24 | |
| Basic information | |||
| Age (mean (SD)) | 74.5 (12.5) | 74.0 (11.8) | 0.87 |
| Gender (mean (SD)) | 17 (60.7) | 16 (66.7) | 0.88 |
| Height (mean (SD)) | 170.7 (8.4) | 170.5 (10.6) | 0.95 |
| Weight (mean (SD)) | 79.5 (16.3) | 75.2 (15.4) | 0.33 |
| BMI (mean (SD)) | 27.3 (5.3) | 25.8 (4.2) | 0.27 |
| Cardiovascular risk profile | |||
| Smoking (%) | 0.07 | ||
| active-smoker | 1 (3.6) | 5 (20.8) | |
| ex-smoker | 12 (42.9) | 5 (20.8) | |
| never | 15 (53.6) | 14 (58.3) | |
| Hypertension (%) | 21 (75.0) | 19 (79.2) | 0.98 |
| Hyperlipidemia (%) | 17 (60.7) | 14 (58.3) | 1.00 |
| Diabetes mellitus | 3 (10.7) | 5 (20.8) | 0.53 |
| Family history CV (%) | 5 (17.9) | 4 (16.7) | 1.00 |
| Medical history | |||
| Previous MI (%) | 5 (17.9) | 6 (25.0) | 0.77 |
| Previous PCI (%) | 6 (21.4) | 10 (41.7) | 0.20 |
| PCI on admission (%) | 14 (50.0) | 14 (58.3) | 0.75 |
| CAD by ICD (%) | 20 (71.4) | 18 (75.0) | 1.00 |
| Atrial fibrillation by ICD (%) | 11 (39.3) | 13 (54.2) | 0.43 |
| CKD by ICD (%) | 5 (17.9) | 3 (12.5) | 0.88 |
All values are presented as mean and standard deviation (SD) or percent of total, respectively. BMI, body mass index; MI, myocardial infarction; PCI, percutaneous coronary intervention; CAD, coronary artery disease; ICD, international classification of diseases; CKD, chronic kidney disease.
3.2. Etiology of acute cardiac tamponade
The most common cause of acute cardiac tamponade were complex PCIs, including CTO, (int.: 46.4%, cont.: 50.0%) and TAVI-associated complications, namely annulus rupture (int.: 10.7%, cont.: 4.2%), a ventricular damage (int.: 3.6%, cont.: 4.2%) or temporal pacemaker lead perforation (int.: 10.7%, cont.: 16.7%). Less common causes were electrophysiological (EP) studies, lead perforation during pacemaker/ ICD implantation and rupture of the free ventricular wall after myocardial infarction. PCI-associated bleeding sources were mainly located in the left anterior descending artery, whereas ventricular bleedings were mainly associated with TAVI or pacemaker/ICD implantations (Table 2).
Table 2.
Bleeding causes and sources.
|
Drainage type |
|||
|---|---|---|---|
| Intermittent | Continuous | p value | |
| n | 28 | 24 | |
| Tamponade cause (%) | 0.37 | ||
| EP study | 1 (3.6) | 4 (16.7) | |
| Lead perforation | 1 (3.6) | 2 (8.4) | |
| Ventricile rupture after MI | 2 (7.1) | 0 (0.0) | |
| Pacemaker/ ICD implantation | 2 (7.1) | 0 (0.0) | |
| PCI | 13 (46.4) | 12 (50.0) | |
| TAVI | 7 (25.0) | 6 (25.0) | |
| Annulus rupture | 3 (10.7) | 1 (4.2) | |
| Lead perforation | 3 (10.7) | 4 (16.7) | |
| Ventricle perforation/ rupture | 1 (3.6) | 1 (4.2) | |
| Unkown | 2 (7.1) | 0 (0.0) | |
| Tamponade bleeding source (%) | 0.06 | ||
| Main stem | 0 (0.0) | 1 (4.2) | |
| LAD | 4 (14.3) | 7 (29.2) | |
| LCX | 2 (7.1) | 3 (12.5) | |
| RCA | 3 (10.7) | 1 (4.2) | |
| Any unkown coronary | 4 (14.3) | 1 (4.2) | |
| Right atrium | 2 (7.1) | 0 (0.0) | |
| Right ventricule | 1 (3.6) | 5 (20.8) | |
| Left atrium | 0 (0.0) | 3 (12.5) | |
| Left ventricle | 6 (21.4) | 1 (4.2) | |
| Annulus rupture | 2 (7.1) | 1 (4.2) | |
| Unkown | 4 (14.3) | 1 (4.2) | |
All values are presented as percent of total. EP, electrophysiology; MI, myocardial infarction; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
3.3. Intensive care parameters
Median duration of stay on intensive care unit was 46.1 vs 49.2 h with a SAPS2 of slightly over 60 (int.: 63.5 / cont.: 62.0). 39.3% of intermittent and 29.2% of continuous suction cohort patients had mechanical ventilation (not significant, p = 0.64). Coagulation-relevant blood values such as INR, PTT and platelet counts did not differ significantly between groups, while 32.1% of intermittent drainage and 54.2% of continuous drainage patients received full therapeutic dosage unfractionated heparin (p = 0.19). About half of all patients had a double anti-platelet therapy consistent of acetylsalicylic acid (ASA) (int.: 53.6%/ cont.: 58.3%) and clopidogrel (int.: 46.4%/ cont.: 58.3%) (Table 3).
Table 3.
Intensive care parameters.
|
Drainage type |
|||
|---|---|---|---|
| Intermittent | Continuous | p value | |
| n | 28 | 24 | |
| ICU parameters | |||
| Cardiac arrest (%) | 10 (35.7) | 11 (45.8) | 0.65 |
| Mechanical ventilation (%) | 11 (39.3) | 7 (29.2) | 0.64 |
| ICU stay duration (h) (median [IQR]) | 46.1 [15.8, 82.8] | 49.2 [29.4, 116.8] | 0.19 |
| SAPS2 (median [IQR]) | 63.5 [59.2, 76.8] | 62.0 [51.0, 72.0] | 0.28 |
| Catecholamines* (median [IQR]) | 4.3 [0.0, 20.1] | 3.7 [0.0, 9.2] | 0.57 |
| Dialysis on ICU (%) | 0 (0.0) | 1 (4.2) | 0.94 |
| vaECMO (%) | 2 (7.1) | 2 (8.3) | 1.00 |
| Laboratory values | |||
| PTT (median [IQR]) | 26.8 [24.7, 36.6] | 31.5 [25.8, 38.9] | 0.51 |
| INR (median [IQR]) | 1.1 [1.0, 1.2] | 1.1 [1.0, 1.3] | 0.44 |
| Platelets (median [IQR]) | 164.0 [133.5, 209.0] | 166.8 [132.7, 195.2] | 0.76 |
| Medication | |||
| ASA (%) | 15 (53.6) | 14 (58.3) | 0.95 |
| Clopidogrel (%) | 13 (46.4) | 14 (58.3) | 0.56 |
| Prasugrel (%) | 1 (3.6) | 2 (8.3) | 0.89 |
| Heparin (%) | 9 (32.1) | 13 (54.2) | 0.19 |
All values are presented as median and interquartile range or percent of total, respectively. For catecholamines a cumulative dosage equivalent was calculated as follows: dobutamine (mg/h) + 100* epinephrine (mg/h) + 100* norepinephrine (mg/h). ICU, intensive care unit; SAPS2, simplified acute physiology score 2; vaECMO, venoarterial extracorporeal membrane oxygenation; PTT, partial thromboplastin time; INR, international normalized ratio; ASA, acetylsalicylic acid.
3.4. Procedural success rates and clinical outcome of intermittent versus continuous drainage
Pericardiocentesis led to a comparable median drainage volume of 1000 ml for the intermittent and 822.5 ml for the continuous suction group, although total drainage time was longer with continuous suction (int.: 9.8 h/ cont.: 21.2 h, p = 0.01). Clotting rate was higher – albeit not significant – in the intermittent suction group (int.: 21.4%/ cont.: 12.5%, p = 0.43). Significantly higher rates were observed for cardiac re-tamponade in patients with intermittent drainage compared to continuous drainage patients (int.: 50.0%/ cont.: 12.5%, p = 0.01). A higher rate of conversion to open-heart surgery was observed in the intermittent drainage group vs. continuous drainage (int.: 28.6%/ cont.: 0.0%, p = 0.01) (Fig. 1). Survival on day 5 was higher when continuous pericardial drainage was applied (int.: 21.4%, cont.: 4.2%, HR 0.2, 95% CI 0.1–0.9, log-rank p = 0.03). 30 day mortality was still higher in the intermittent group but without statistical significance (int.: 21.4%, cont.: 12.5%, HR 0.4, 95% CI 0.1–1.6, log-rank p = 0.19). (Fig. 2)
Fig. 2.
Survival rates at 5 and 30 days: Depicted are Kaplan-Meier curves for survival on day 5 (A) and 30 (B) after acute cardiac tamponade and subsequent drainage with either intermittent or continuous suction.
4. Discussion
This is the first study which compares classic intermittent pericardial drainage versus continuous drainage using a redon drainage system (Redovac®, B. Braun, Germany). The results of our study are as follows: (a) continuous negative pericardial pressure drainage is safe without increasing total drainage volume, (b) it reduces rates of re-tamponade as well as conversion rates to open-heart surgery and (c) is associated with a significantly lower mortality on day 5 but not on day 30.
Modern cardiology critically relies on an increasing amount of catheter-based interventional approaches including multiple PCI techniques, valve interventions and ablations of different forms of arrhythmias as well as pacemaker or defibrillator implantations. All these procedures go along with a rare but common risk for pericardial effusion and potentially lethal acute cardiac tamponades. Continuous hemodynamic monitoring and rapid on-demand diagnostic tools such as echocardiography facilitate immediate detection of these conditions.
Due to relevant comorbidities and the need for patient transportation under unstable conditions surgical treatments of pericardial effusions are often secondary and limited to special conditions such as a clotted hemopericardium or the impossibility to reach the pericardial space by needle insertion. Therefore, the treatment of choice according to 2015 ESC guidelines is usually on-site pericardiocentesis including sheath and pig tail insertion for potential repetitive drainage [4], [5]. Volume expansion can be used as a temporal therapy to overcome time to definite pericardiocentesis and is especially helpful in patients with a systolic blood pressure < 100 mmHg [16], [17].
While conventional treatment strategies require repetitive manual suction, our newer approach applies a continuous negative pressure to the pigtail catheter as it is connected to a redon drainage (Redovac®, B. Braun, Germany). This reduces clotting of the draining catheter – a severe complication potentially leading to re-tamponade which requires emergent sheath and/ or pigtail catheter exchange, re-puncture or even a switch to open-heart surgery. These latter conditions are associated with a relevant increase in mortality due to an uncontrollable hemodynamic insufficiency. Furthermore, the intermittent drainage system comes with the disadvantage that continuous vigilance by physician is necessary and if not performed bears the risk of unrecognized re-tamponade. Additionally, the repetitive withdrawal of lagged blood and subsequent saline flushing may lead to an increased risk of infections for the conventional open drainage system as opposed to the closed continuous drainage system. Some people argue that continuous suction impedes spontaneous healing of potential bleeding sources such as coronary arteries or the ventricular myocardium. However, total bleeding volume did not differ significantly between both groups and of note was lower in the continuous drainage group [3], [18].
Overall, continuous drainage was associated with reduced rate of re-tamponade and conversion to open-heart surgery in our analysis – two conditions that come along with severe hemodynamic instability and death. Continuous drainage did not have any negative side-effects when compared to conventional standard of care. We observed a significant reduction of mortality on day 5 when continuous suction was applied (int.: 21.4%, cont.: 4.2%, HR 0.2, 95% CI 0.1–0.9, log-rank p = 0.03) although this did not translate in a significant difference at 30 days (int.: 21.4%, cont.: 12.5%, HR 0.4, 95% CI 0.1–1.6, log-rank p = 0.19). One possible explanation for missing significance of this single treatment method in the longer-term observation, might be an overlapping effect of common complications of intensive care medicine like ventilator associated pneumonia. Yet, this small retrospective trial provides a clear hint that continuous suction might be beneficial, and a large randomized trial would be needed to proof a potential superiority of this new approach.
4.1. Limitations
This pioneering trial to treat acute cardiac tamponade is limited by its small single center patient cohort, its retrospective design and the lack of randomization. Complications during transaortic valve implantations were among the predominant etiologies with 25% of all cases in both groups. As this procedure itself is associated with a high risk for mortality, it might have influenced our mortality evaluation for pericardial drainage strategies. Although treatment was at the discretion of the attending physician and not randomized, continuous suction was predominantly used between December 2018 and August 2020 (77% of all drainages during this time period) and intermittent suction between January 2017 and December 2018 (100% of this time period).
4.2. Conclusions
Acute cardiac tamponade can be efficiently treated by pericardiocentesis with subsequent continuous drainage via a pigtail catheter using a redon drainage. The finding of a lower rate of re-tamponade, open-heart surgical intervention and mortality requires further investigations in larger, randomized trials. Our retrospective analysis shows a significantly lower mortality on day 5, a decreased rate of interventions and lower rates of cardiac re-tamponade without any relevant side effects when compared to intermittent manual drainage.
Funding
There was no funding for this study.
Ethical standards
All ethical standards were met in writing and submitting this correspondence.
Author contributions
Christopher Stremmel, Clemens Scherer and Martin Orban designed the study, interpreted data and wrote the manuscript. Enzo Lüsebrink, Danny Kupka, Thomas Stocker, Konstantin Stark, Mathias Orban, Tobias Petzold, Antonia Kellnar, Jan Kleeberger, Moritz F. Sinner, Julinda Mehilli, Teresa Schmid, Daniel Braun, collected and analysed data and critically revised the manuscript. Jörg Hausleiter and Steffen Massberg interpreted data and critically revised the manuscript.
Declaration of Competing Interest
Mathias Orban and Daniel Braun received speaker honoraria from Abbott Vascular, outside the submitted work. Jörg Hausleiter received speaker honoraria and research support from Abbott Vascular and Edwards Lifesciences, outside the submitted work. Martin Orban received speaker honoraria from Abbott Medical, AstraZeneca, Abiomed, Bayer vital, BIOTRONIK, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences Services, Sedana Medical, outside the submitted work. The other authors declare no conflict of interests.
Acknowledgements
None.
References
- 1.Hoit B.D. Pericardial Effusion and Cardiac Tamponade in the New Millennium. Curr. Cardiol. Rep. 2017;19:57. doi: 10.1007/s11886-017-0867-5. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez E.R., Tan C.D. Structure and Anatomy of the Human Pericardium. Prog. Cardiovasc. Dis. 2017;59:327–340. doi: 10.1016/j.pcad.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Spodick D.H. Acute cardiac tamponade. N. Engl. J. Med. 2003;349:684–690. doi: 10.1056/NEJMra022643. [DOI] [PubMed] [Google Scholar]
- 4.Adler Y., Charron P., Imazio M., Badano L., Baron-Esquivias G., Bogaert J. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumrukcuoglu H.A., Odabasi D., Akdag S., Ekim H. Management of Cardiac Tamponade: A Comperative Study between Echo-Guided Pericardiocentesis and Surgery-A Report of 100 Patients. Cardiol. Res. Pract. 2011;2011 doi: 10.4061/2011/197838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglis R., King A.J., Gleave M., Bradlow W., Adlam D. Pericardiocentesis in contemporary practice. J. Invasive Cardiol. 2011;23:234–239. [PubMed] [Google Scholar]
- 7.Imazio M., Hoit B.D. Post-cardiac injury syndromes. An emerging cause of pericardial diseases. Int. J. Cardiol. 2013;168:648–652. doi: 10.1016/j.ijcard.2012.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Lusebrink E., Massberg S., Orban M. Ten things ICU specialists need to know about new valvular procedures in interventional cardiology. Intensive Care Med. 2020;46:102–106. doi: 10.1007/s00134-019-05824-6. [DOI] [PubMed] [Google Scholar]
- 9.Lemmert M.E., van Bommel R.J., Diletti R., Wilschut J.M., de Jaegere P.P., Zijlstra F. Clinical Characteristics and Management of Coronary Artery Perforations: A Single-Center 11-Year Experience and Practical Overview. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danek B.A., Karatasakis A., Tajti P., Sandoval Y., Karmpaliotis D., Alaswad K. Incidence, Treatment, and Outcomes of Coronary Perforation During Chronic Total Occlusion Percutaneous Coronary Intervention. Am. J. Cardiol. 2017;120:1285–1292. doi: 10.1016/j.amjcard.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Stremmel C., Lusebrink E., Massberg S., Orban M. Treatment of acute pericardial tamponade with a high-vacuum drainage system. Clin. Res. Cardiol. 2020;109:263–265. doi: 10.1007/s00392-019-01527-6. [DOI] [PubMed] [Google Scholar]
- 12.Scherer C., Kupka D., Stocker T.J., Joskowiak D., Scheuplein H., Schonegger C.M. Isoflurane Sedation in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation Treatment for Cardiogenic Shock-An Observational Propensity-Matched Study. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherer C., Lusebrink E., Kupka D., Stocker T.J., Stark K., Stremmel C. Long-Term Clinical Outcome of Cardiogenic Shock Patients Undergoing Impella CP Treatment vs. Standard of Care. J. Clin. Med. 2020;9 doi: 10.3390/jcm9123803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vakamudi S., Ho N., Cremer P.C. Pericardial Effusions: Causes, Diagnosis, and Management. Prog. Cardiovasc. Dis. 2017;59:380–388. doi: 10.1016/j.pcad.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Sinnaeve P.R., Adriaenssens T. A contemporary look at pericardiocentesis. Trends Cardiovasc. Med. 2019;29:375–383. doi: 10.1016/j.tcm.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Kerber R.E., Gascho J.A., Litchfield R., Wolfson P., Ott D., Pandian N.G. Hemodynamic effects of volume expansion and nitroprusside compared with pericardiocentesis in patients with acute cardiac tamponade. N. Engl. J. Med. 1982;307:929–931. doi: 10.1056/NEJM198210073071506. [DOI] [PubMed] [Google Scholar]
- 17.Sagrista-Sauleda J., Angel J., Sambola A., Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation. 2008;117:1545–1549. doi: 10.1161/CIRCULATIONAHA.107.737841. [DOI] [PubMed] [Google Scholar]
- 18.Imazio M., De Ferrari G.M. Editorial commentary: Pericardiocentesis: No more a subspecialty technique! Trends Cardiovasc. Med. 2019;29:384–385. doi: 10.1016/j.tcm.2019.01.010. [DOI] [PubMed] [Google Scholar]