Abstract
Coarse (CF) and Fine (FF) fractions were obtained by dry fractionation (air classification) of raw micronized flour (RM) of kabuli chickpea, green pea, yellow and red lentil. Pea showed the highest phytate content in RM and CF. Stachyose was the main oligosaccharide in lentils, exceeding 50 mg g−1, whereas raffinose (39.9 mg g−1) was abundant in chickpea. Antinutritional factors were significantly enriched in FF, whereas decreased in CF. Total-reflection X-ray fluorescence identified potassium as the main macronutrient in pulses. Ca was highly variable, ranging from 0.92 to 0.28 g kg−1 in pea and yellow lentil, respectively. A significant shift of minerals was observed in FF, but despite the highest phytate content, phytate:Zn ratio of lentils was lower than RM, indicating that Zn was enriched more than phytates. Yellow lentil and pea FF showed a protein content higher than 55 g 100g−1. Dry fractionation significantly affected the physicochemical properties, indicating different potential use of fractions.
Keywords: Dry fractionation, Pulses protein, Legume-based ingredients, Mineral composition, TXRF, Physicochemical properties, Antinutritional factors
Dry fractionation; Pulses protein; Legume-based ingredients; Mineral composition; TXRF; Physicochemical properties; Antinutritional factors.
1. Introduction
Pulses (grain legumes) are the edible seeds of a group of legume crops such as chickpea (Cicer arietinum L.), pea (Pisum sativum L.) and lentil (Lens culinaris Medik.). Pulses demand is rising in developed countries, due to the increased consciousness of health risks associated with the excessive consumption of animal-based protein sources (Daryanto et al., 2015). However, pulses consumption is hampered by their long cooking and preparation time, that does not meet the modern habits of consumers. Several strategies have been proposed to increase the consumption of pulses, such as the incorporation of their flours in daily consumed food such as bakery products (Pasqualone et al., 2019) and pasta (Hooper et al., 2019). Moreover, the development of pulse-based meat analogues (Osen et al., 2014) also satisfies the demand for meat substitutes.
Other approaches to increase the consumption of pulses involve the use of innovative pulse-derived ingredients such as starch, protein (Sozer et al., 2017), exploitable for novel food preparation. Most of the proteins used as ingredients by the food industry are animal-based and derive especially from bovine milk, such as whey protein or casein (Ozturk and McClements, 2016). However, the substitution of these products by more sustainable and healthier vegetable alternatives is widely encouraged (Kristensen et al., 2016).
This scenario explains the growing interest in pulse proteins, which can be used as a functional ingredient to enhance the nutritional and technological value of food. The majority of pulse-derived proteins available on the market are conventionally obtained by a wet extraction (Schutyser et al., 2015), involving flour dispersion in water at pH 9 to solubilize proteins. Proteins are then precipitated reaching the iso-electric point (pH 4.5–4.8). The pH is neutralized, and the protein isolate is dried, providing a protein concentration of 75–90 g 100 g−1. The drawback of this process consists in the use of a considerable quantity of water and chemicals. Moreover, it involves high energy consumption, especially for the drying stage (Schutyser et al., 2015).
A sustainable alternative to wet extraction is the dry fractionation. This technology starts with a fine milling step (micronization), which is a crucial step to obtain the optimal reduction of particle size and the disentanglement of starch granules (20–30 μm) and protein bodies (<10 μm), without damaging starch. It is reported that the fracturing happening in the impact mill are caused mainly by the weaknesses existing in the cell tissue, whereas jet milling is a more intense technology which can easily produce damaged starch or too fine particles (Pelgrom et al., 2013). Moreover, the milling process needs to be calibrated on the basis of the raw material used for air classification (e.g. starch-rich or oil-rich legumes) (Pelgrom et al., 2014; Schutyser et al., 2015). The two components can be then separated on the basis of both density and size by air classification (Schutyser et al., 2015). The flour is fed in a cyclone and separated into two fractions by using a calibrated air flow. The fine fraction is rich in proteins (>50 g 100 g−1) whereas the coarse fraction is composed of more than 70 g 100 g−1 of starch (Li et al., 2019). Dry fractionation has been studied in the past (Tyler et al., 1981), however it received renewed interest in recent years from the scientific community. Despite this, the studies currently present in literature are limited and focused especially on pea (Pelgrom et al., 2013; Rempel et al., 2019; Wang and Maximiuk, 2019), with few studies regarding other pulse species such as chickpea, bean and lentil (Pelgrom et al., 2015). These researches focused primarily on the mechanism of protein separation, with very little information regarding other chemical aspects such as the antinutritional factors and the macro and micronutrients composition. has not been comprehensively investigated, in spite of their well-recognized importance for human nutrition (Ray et al., 2014). Moreover, the physicochemical and functional properties of the air-classified fractions were poorly examined, although they are fundamental to determine the potentiality of each fraction for different purposes in the food industry.
Therefore, in this paper we investigated the hypothesis that the antinutritional factors, macro and micronutrient composition together with the microstructure and the starch morphology, are influenced by dry fractionation and by the type of pulses, showing different characteristics in the coarse and fine fractions.
2. Materials and methods
2.1. Dry fractionation process
Dry fractionated coarse fraction (CF) and fine fraction (FF) from raw micronized (RM) flour of dehulled kabuli chickpea (Cicer arietinum L.) (KC), green pea (Pisum sativum L.) (GP), yellow (YL) and red (RL) lentils (Lens culinaris Medik.) were produced by a plant of Separ Micro System sas (Flero, Brescia, Italy). The micronization process has been performed twice in a KMX-300 micronizer equipped with a rotor operating at a peripheral speed of 157 m s−1. The operating principles of the micronizer have been described by Laudadio et al. (2013). The disentanglement of starch and protein bodies was due to the mechanical impact against stator and rotor serrated surfaces and by turbulent multiple impact between particles. The resulting flours were then air classified in a SX-100 apparatus, consisting in a turbo-separator and in a cyclone. The air flow is driven by an aspirating pump modulated by an inlet restriction-valve set to 270 in order to obtain the CF (collected in the turbo-separator) and FF fraction (collected in the cyclone). The mean percentage of FF recovered were 26.9, 21.5, 22.9 and 18.8 for RL, YL, GP, KC respectively. The mean protein separation efficiency (PSE) was calculated as reported in Eq. (1).
| PSE = PFF × MFF/PRM | (1) |
where PFF is the protein content of the fine fraction, MFF is the mass of the fine fraction, PRM is the protein content of the micronized flours. PSE of the FF was 46.5 for both RL and YL, 53.5 and 34.7 for GP and KC respectively.
The dry fractionation process was performed in duplicate and the samples were stored at -18 °C until the analysis.
2.2. Determination of antinutritional factors
Total phytate content was measured spectrophotometrically according to the method previously described in Summo et al. (2019a). The analysis was carried out in triplicate. Oligosaccharides (verbascose, stachyose, raffinose and sucrose) were determined by high-performance liquid chromatography (HPLC) (Agilent Technologies, Santa Clara, USA), equipped with Refractive Index Detector (RID 1260), as previously reported in Flamminii et al. (2019) with few modifications. Ten mg of flour were dispersed in 5 mL of deionized water, stirred for 5 min and filtered through 0.22 μm cellulose acetate filters. The HPLC separation was carried out isocratically at a 0.8 mL min−1 flow rate through a 300 × 7.8 mm cation exchange column (Rezex RCM column, Ca2+, 8 μm, Torrance, CA) maintained at 80 °C. Deionized water was used as mobile phase. The identification was carried out comparing the retention time with that of the corresponding standard (Merck KGaA, Darmstadt, Germany). A calibration curve for each oligosaccharide was prepared for the quantification. The analysis was carried out in triplicate.
2.3. Determination of mineral composition
The mineral composition of the pulses fractions was studied using total-reflection X-ray fluorescence (TXRF) spectroscopy, according to Allegretta et al. (2019). For the analysis, 100 mg of sample were transferred in a 12 mL polypropylene tube. Then, 5 mL of a 1% Triton X-100 solution (Merck KGaA, Darmstadt, Germany) were added and the sample was suspended by manual shaking. The suspension was then vortexed for 5 min and finally further treated in an ultrasonic bath for 15 min. Ten μL of a 1000 g L−1 Ga (Gallium) standard solution were added as internal standard and the suspension was vortexed again for 5 min. At the end, 10 μL of suspension were transferred to the center of a quartz sample carrier with a micropipette and left to dry at 50 °C on a heating plate. The analyses were performed using an S2Picofox (Bruker Nano GmbH, Germany) TXRF spectrometer equipped with a Mo source (50 kV, 600 μA) and an SDD of 30 mm2. For the analysis a live time of 1000 s was set. Analyses were carried out in triplicate.
The molar ratios of phytate to iron and zinc were calculated as the moles of phytate divided by the moles of iron or zinc.
2.4. Scanning electron microscopy
The morphology of the grains of each fraction was studied using a field emission gun scanning electron microscope (FEG-SEM) Zeiss Σigma 300 VP (Zeiss Oberkochen, Germany). Micrographs were acquired with a secondary electron detector (SE) using an accelerating voltage of 10–15 kV and a working distance of 3 mm. For the analysis an aluminum stub was covered with a carbon disk. Then, each pulse fraction was deposited over the carbon disk. Before the FEG-SEM analysis, all the samples were carbon coated. Three representative SE micrographs for each sample were further processed by ImageJ software (National Institutes of Health, Bethesda, USA) in order to measure the starch granules diameter. The analysis was carried out in duplicate.
2.5. Chemical composition
Protein (total nitrogen × 6.25), ash and moisture contents were determined according to the AOAC methods 979.09, 923.03, and 925.10 respectively (AOAC, 2006). The analyses were carried out in triplicate.
2.6. Determination of the physicochemical and functional properties
Bulk density (BD), water absorption index (WAI), water solubility index (WSI), water absorption capacity (WAC) and oil absorption capacity (OAC) of flours were determined according to the procedures reported by Summo et al. (2019a).
2.7. Statistical analysis
All the experimental data were subjected to one-way ANOVA followed by the Tukey's HSD test, considering separately the variable type of pulse (in the same fraction) and fraction (in the four types) as independent variables. Significant differences among the data were determined at p ≤ 0.05 by the XLStat software (Addinsoft SARL, New York, NY, USA).
3. Results and discussion
3.1. Antinutritional factors
The contents of total phytates, stachyose, verbascose, raffinose, and sucrose are reported in Table 1. Pea RM had a total phytate content significantly higher than the other pulses, with mean value of 8.7 mg g−1. The same behavior was observed in the CF, whereas for the FF, yellow lentil and pea showed the highest phytates concentration. The lowest phytate content, instead, was displayed by the red lentil FF. A previous study reported no significant differences for phytates among green pea, kabuli chickpea, yellow and red lentils (Shi et al., 2018), although the same authors reported higher values compared to our findings. However, differences in phytate content can be attributed also to different agronomical practices and environmental conditions. Considering the changes occurring during dry fractionation, the statistical analysis highlighted significant differences for total phytate content in CF and FF, with respect to the RM. In particular, a significant decrease of phytates in CF of all the pulses under investigation was observed, whereas the FF were characterized by the highest phytates content. Phytates and phytic acid are the main sources of phosphorous in the seeds and they are strongly associated with protein bodies in the cotyledons (Coulibaly et al., 2011), therefore the protein concentration by dry fractionation inevitably affects the phytates content. Furthermore, a positive and significant correlation between total phytates (Table 1) and P content (Table 2) was observed (R2 = 0.955). Phytates act in the gastrointestinal tract binding Fe, Zn, and Ca, leading to a reduction of their bioavailability (Wang and Daun, 2004). Therefore, the higher phytate content of FF should be carefully considered in view of further uses in food processing.
Table 1.
Mean value, standard deviation and results of statistical analysis one-way ANOVA of the total phytates and sugars contents (expressed as mg g−1 of dry matter) of the four of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
| Total Phytates | Verbascose | Stachyose | Raffinose | Sucrose | ||
|---|---|---|---|---|---|---|
| RM | RL | 6.43 ± 0.51b B | n.d. | 56.51 ± 0.69a B | 21.18 ± 0.00b B | 31.62 ± 0.77a A |
| YL | 7.39 ± 0.04b B | n.d. | 51.02 ± 0.48b B | 15.02 ± 0.99c B | 24.5 ± 1.5b B | |
| GP | 8.7 ± 1.1a B | 36.74 ± 0.61B | 36.5 ± 1.7c B | 8.18 ± 0.41d B | 27.6 ± 1.7ab A | |
| KC | 6.78 ± 0.02b B | n.d. | 31.38 ± 2.62d B | 39.9 ± 2.1a B | 29.6 ± 2.1a B | |
| CF | RL | 5.19 ± 0.02b C | n.d. | 45.74 ± 0.77a C | 18.39 ± 0.47b C | 28.73 ± 0.63a B |
| YL | 5.06 ± 0.18b C | n.d. | 34.9 ± 2.3b C | 11.77 ± 0.58c C | 23.64 ± 0.22b B | |
| GP | 6.86 ± 0.55aC | 28.26 ± 0.46C | 28.1 ± 2.9c C | 7.76 ± 0.01d B | 12.84 ± 0.76d B | |
| KC | 5.32 ± 0.55b C | n.d. | 30.1 ± 2.3bc B | 41.9 ± 1.4a AB | 18.6 ± 1.7c C | |
| FF | RL | 8.72 ± 0.31c A | n.d. | 73.3 ± 3.7ab A | 24.77 ± 0.23b A | 29.8 ± 1.5b B |
| YL | 14.06 ± 0.19a A | n.d. | 87.5 ± 4.9a A | 25.61 ± 0.63b A | 31.7 ± 1.6b A | |
| GP | 13.95 ± 0.02a A | 59.5 ± 2.9A | 55.9 ± 4.0c A | 10.34 ± 0.74c A | 26.36 ± 0.77b A | |
| KC | 12.11 ± 0.06b A | n.d. | 61.0 ± 8.1bc A | 46.5 ± 3.4a A | 47.3 ± 3.6a A |
Lowercase letters mean significant differences among different types of pulse within the same fraction (p < 0.05). Uppercase letters mean significant differences among the fractions within the same type of pulse (p < 0.05).
n.d. = Not detected.
Table 2.
Mean value, standard deviation and results of statistical analysis one-way ANOVA of macronutrients (expressed as g kg−1 of dry matter) of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
| P | S | Cl | K | Ca | ||
|---|---|---|---|---|---|---|
| RM | RL | 3.53 ± 0.10b B | 2.01 ± 0.08ab B | 0.60 ± 0.04cB | 9.23 ± 0.55c B | 0.53 ± 0.17bc AB |
| YL | 3.85 ± 0.61b B | 1.79 ± 0.20b B | 0.65 ± 0.04cB | 10.7 ± 1.6bc B | 0.28 ± 0.06c B | |
| GP | 6.01 ± 0.28a B | 2.62 ± 0.03a B | 0.89 ± 0.04bA | 15.67 ± 0.49a B | 0.92 ± 0.12a B | |
| KC | 3.58 ± 0.21b B | 2.08 ± 0.42ab B | 1.18 ± 0.13aA | 11.93 ± 0.53b B | 0.64 ± 0.05ab B | |
| CF | RL | 3.02 ± 0.24ab C | 1.79 ± 0.06b C | 0.78 ± 0.04b A | 9.31 ± 0.46a B | 0.34 ± 0.01c B |
| YL | 3.19 ± 0.06ab B | 1.54 ± 0.02c B | 0.69 ± 0.04b B | 9.33 ± 0.46a B | 0.22 ± 0.00d B | |
| GP | 3.41 ± 0.05a C | 1.44 ± 0.01c C | 0.79 ± 0.04b B | 9.77 ± 0.40a C | 0.55 ± 0.05b C | |
| KC | 2.81 ± 0.20b C | 2.07 ± 0.13a B | 1.14 ± 0.10a A | 9.60 ± 0.64a C | 0.70 ± 0.04a B | |
| FF | RL | 6.94 ± 0.22c A | 3.88 ± 0.09c A | 0.56 ± 0.01b B | 12.53 ± 0.13c A | 0.76 ± 0.01c A |
| YL | 9.52 ± 0.60a A | 4.92 ± 0.28b A | 1.15 ± 0.19a A | 19.93 ± 0.92b A | 0.74 ± 0.02c A | |
| GP | 10.33 ± 0.22a A | 4.81 ± 0.01b A | 0.52 ± 0.01b C | 22.42 ± 0.29a A | 1.20 ± 0.03a A | |
| KC | 8.39 ± 0.27b A | 5.42 ± 0.08a A | 1.21 ± 0.05a A | 20.62 ± 0.43b A | 1.01 ± 0.01b A |
Lowercase letters mean significant differences among the different types of pulse within the same fraction (p < 0.05). Uppercase letters mean significant differences among the fractions within the same type of pulse (p < 0.05).
Verbascose was only detected in green pea, and it accounted for 36.74 mg g−1 in the raw material. Stachyose was the main oligosaccharide in lentils, with a content exceeding 50 mg g−1, whereas the main oligosaccharide detected in chickpea was raffinose (39.88 mg g−1), which was as low as 8.18 mg g−1 in pea. Small but significant differences were found for sucrose, which ranged between 24.5 mg g−1 in yellow lentil and 31.62 mg g−1 in red lentil. The sugar composition of pulses is strongly related to genotype (Martinez-Villaluenga et al., 2008), therefore significantly varied among the species. Stachyose is one of the most abundant α-galactosides in chickpea, lentil (Pedrosa et al., 2012) and pea (Wang and Daun, 2004). Similarly to phytates, also sugars were affected by dry fractionation, showing a significantly lower content of verbascose, stachyose, raffinose and sucrose in CF compared to RM, with only few exceptions. Chickpea CF, indeed, showed stachyose and raffinose contents similar to the RM flour. A similar behavior was observed for raffinose in green pea and sucrose in yellow lentil. Furthermore, FF was always characterized by the highest contents of sucrose and α-galactosides. The same trend was previously reported by other authors (Wang and Maximiuk, 2019) and these results should be considered in relation to further utilization of dry-fractionated ingredients in the food industry, paying particular attention to the protein concentrates (FF).
A controversy exists regarding the α-galactosides, also known as ‘raffinose family oligosaccharides’. They are usually considered as antinutrients, or non-nutritive factors, owing to their ability to cause flatulence (Wang and Daun, 2004), which contributes to lower the acceptance of pulses. However, the flatulence-causing activity is actually due to fermentation by the colon microbiota (Martinez-Villaluenga et al., 2008). Therefore, α-galactosides have a prebiotic activity, being able to promote the growth of Lactobacillus and Bifidobacterium (Martinez-Villaluenga et al., 2008). The optimal conditions for dry fractionation of different pulses can be identified by using an approach of experimental design. In this respect, Wang and Maximiuk (2019) reported optimal processing conditions in terms of air flow and speed of the classifier wheel to obtain the best compromise between yield, protein/starch content and antinutritional factors for field peas. Moreover, these issues raise the need of further processing the protein concentrates produced by dry fractionation – e.g. by fermentation (Xing et al., 2020) or thermal treatments such as extrusion cooking or microwave heating (Pasqualone et al., 2020), to reduce phytates, α-galactosides and the other antinutrients usually contained in legumes (i.e lectins, tannins, trypsin inhibitor) before being used in food. Lectins of legumes are considered as antinutritional factors, but recently some potential health benefits (Lagarda-Diaz et al., 2017) have led to a re-evaluation of these compounds.
3.2. Mineral composition
To investigate the mineral nutrients present in the different fractions, the elemental concentrations were determined by TXRF. Several macronutrients were detected, in particular P, S, Cl, K and Ca (Table 2), together with micronutrients, i.e. Mn, Fe, Ni, Cu, Ze, and Se (Table 3). Moreover, three non-essential elements (Br, Rb, Sr) were identified. Both macro and micronutrient composition significantly varied among the pulses with some exceptions. Potassium was the most abundant element in pulse flours, with the highest content in green pea, which also showed the highest P and S contents. Ca was highly variable among pulses of different types, ranging from 0.92 g kg−1 in green pea to 0.28 g kg−1 in yellow lentil. Previous studies confirm that pea contains more Ca than other pulses, such as chickpea or lentil (Ray et al., 2014). Chlorine showed the highest content in chickpea.
Table 3.
Mean value, standard deviation and results of statistical analysis one-way ANOVA of micronutrients (expressed as mg kg−1 of dry matter), the phytate:Fe and phytate:Zn ratios of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
| Mn | Fe | Ni | Cu | Zn | Br | Rb | Sr | Se |
Phytate:Fe Ratio |
Phytate:Zn Ratio |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RM | RL | 11.30 ± 0.95bc B | 54.9 ± 6.7a B | 3.44 ± 0.06a B | 8.97 ± 0.31b B | 36.9 ± 1.1b B | 3.31 ± 0.28c B | 4.24 ± 0.76c A | 0.31 ± 0.02d C | n.d. | 10.0 ± 1.2b AB | 17.3 ± 0.8b A |
| YL | 9.1 ± 1.5c B | 46.7 ± 4.1a B | 2.85 ± 0.91a B | 6.73 ± 0.95b B | 28.5 ± 4.5b B | 5.27 ± 0.29b B | 8.5 ± 1.4b B | 1.48 ± 0.06b A | 0.39 ± 0.12B | 13.4 ± 1.3a A | 26.1 ± 4.5a A | |
| GP | 13.15 ± 0.31b B | 56.1 ± 2.3a B | 2.32 ± 0.04a B | 11.45 ± 0.18a B | 47.22 ± 0.90a B | 4.29 ± 0.02bc A | 18.61 ± 0.42a B | 0.97 ± 0.17c B | n.d. | 13.1 ± 1.0a B | 18.2 ± 1.5ab B | |
| KC | 19.07 ± 0.34a B | 47.4 ± 4.1a B | 0.88 ± 0.26b B | 8.7 ± 1.5b B | 32.0 ± 6.0b B | 11.98 ± 0.77a A | 6.08 ± 0.71c B | 2.44 ± 0.31a B | n.d. | 12.2 ± 1.1ab B | 21.5 ± 4.3ab A | |
| CF | RL | 8.80 ± 0.53b C | 45 ± 11ab B | 2.89 ± 0.27a C | 7.24 ± 0.71a C | 29.2 ± 2.0a C | 3.92 ± 0.08c A | 4.75 ± 0.19c A | 1.28 ± 0.05b A | n.d. | 10.7 ± 2.6b A | 18.3 ± 1.7c A |
| YL | 6.83 ± 0.24c B | 49.2 ± 2.9a B | 2.06 ± 0.09b B | 5.32 ± 0.17b B | 22.39 ± 0.24b B | 5.44 ± 0.39b B | 7.60 ± 0.52b B | 1.48 ± 0.07b A | 0.24 ± 0.02C | 8.7 ± 0.8b B | 22.4 ± 0.4b AB | |
| GP | 5.86 ± 0.38c C | 29.3 ± 3.8b C | 1.25 ± 0.22c C | 5.83 ± 0.29b C | 23.91 ± 0.40b C | 4.65 ± 0.39bc A | 11.62 ± 0.27a C | 0.37 ± 0.11c B | n.d. | 20.0 ± 1.5a A | 28.4 ± 1.2a A | |
| KC | 14.74 ± 0.69a C | 52.0 ± 3.2a B | 0.56 ± 0.14d B | 7.98 ± 0.22a B | 31.92 ± 0.94a B | 11.71 ± 0.55a A | 4.50 ± 0.37c C | 3.14 ± 0.26a C | n.d. | 8.7 ± 0.7b C | 16.5 ± 1.3c A | |
| FF | RL | 21.99 ± 0.46c A | 82.6 ± 5.2a A | 5.46 ± 0.03a A | 17.04 ± 0.49b A | 67.6 ± 1.7b A | 2.80 ± 0.12c C | 5.03 ± 0.12c A | 0.67 ± 0.10c B | n.d. | 6.3 ± 0.6b B | 8.9 ± 1.0c B |
| YL | 28.33 ± 0.96b A | 82.5 ± 5.0a A | 5.56 ± 0.21a A | 18.58 ± 0.62a A | 79.2 ± 1.8a A | 6.85 ± 0.73b A | 13.44 ± 0.86b A | 1.73 ± 0.73bc A | 1.00 ± 0.09A | 14.5 ± 0.9a A | 17.6 ± 0.4b B | |
| GP | 23.00 ± 0.12c A | 83.1 ± 2.3a A | 3.89 ± 0.26b A | 18.90 ± 0.22a A | 68.2 ± 2.4b A | 2.54 ± 0.08c B | 24.03 ± 0.21a A | 2.96 ± 0.99ab A | n.d. | 14.2 ± 0.4a B | 20.3 ± 0.7a B | |
| KC | 35.63 ± 0.46a A | 69.5 ± 1.2b A | 2.20 ± 0.11c A | 18.01 ± 0.25ab A | 66.23 ± 0.60b A | 10.60 ± 0.37a A | 12.36 ± 0.47b A | 3.91 ± 0.13a A | n.d. | 14.8 ± 0.2a A | 18.1 ± 0.2b A |
Lowercase letters mean significant differences among the different types of pulse within the same fraction (p < 0.05). Uppercase letters mean significant differences among the fractions within the same type of pulse (p < 0.05).
Considering micronutrients (Table 3), pea showed the highest content of Cu and Zn, whereas no significant differences were observed among the other types of pulses. Iron did not show significant differences among pulses. Finally, Se was detected only in yellow lentil. Regarding FF, chickpea showed the lowest content of Fe but the highest content of Mn, whereas yellow lentil showed the highest Zn content. Furthermore, a noticeable high content of Se in yellow lentil FF was observed, which make it suitable for supplementation in Se-deficient individuals (dos Reis et al., 2017). Dry fractionation by air classification caused consistent changes in the mineral elements’ concentrations, leading to a significant shif of both macro and micronutrients in the fine fraction. Simultaneously, we observed a general lower content of macro and micronutrients in CF. To the best of our knowledge, the effect of air classification on the macro and micronutrients composition has been poorly investigated. Tecklenburg et al. (1984) studied just a few elements, reporting an increase of minerals in the fine fraction of dry-fractionated bean flour, which were significantly and positively correlated with phytates, suggesting the presence of metal-phytates complexes. Both macro and micro elements have several biological functions in humans and their absorption from vegetable sources is strongly related to the phytate content (Wang and Daun, 2004). In order to estimate the dietary bioavailability of minerals, the molar ratio between phytates and Zn or Fe should be considered. When this ratio is above 15 for zinc and above 1 for iron, the bioavailability of these micronutrients is inhibited (Ma et al., 2007), however the amount of protein can improve their absorption (Lonnerdal, 2000). In our case, the molar ratios phytate:Fe and phytate:Zn were little influenced by both pulse type and dry fractionation process. In RM we found small but significant differences, with the lowest phytate:Fe and phytate:Zn ratios in red lentil. Phytate:Fe ratio was highly variable in the CF, ranging from 8.7 (yellow lentil and chickpea) to 20.0 in green pea, which was also characterized by the highest phytate:Zn. In FF, despite the highest phytate content, the ratios remained nearly constant with no significant differences compared to the RM. Phytate:Zn ratios of yellow and red lentils were significantly lower than those of RM, indicating that Zn was enriched in FF more than phytates. However, despite these values, the highest protein content of the FF could lead to an improvement of the zinc absorption (Lonnerdal, 2000). These findings should be considered in case of preparation of food supplements using FF.
3.3. Starch morphology, protein and ash contents
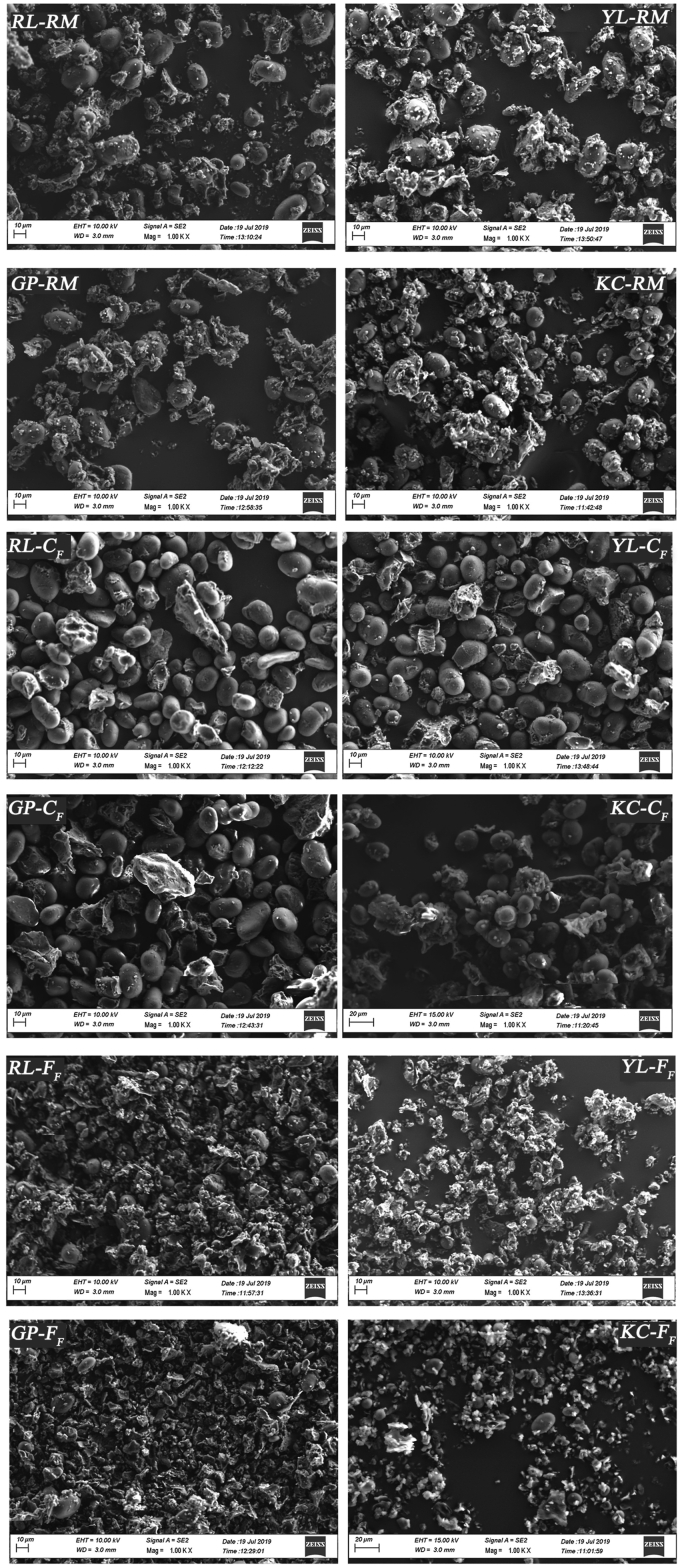
Scanning electron (SE) micrographs (Figure 1) show the microstructure of the raw materials (RM), the coarse (CF) and fine (FF) fractions. In the RM, it is possible to observe a homogeneous mix of starch granules and protein fragments, generated by the milling process which caused a consistent disentanglement of the protein bodies from the starch granules. In particular, the protein bodies consist of small bright fragments adhering to the surface of starch granules (Rempel et al., 2019). CF are all characterized by the predominant presence of large and spherical starch granules, together with few macro-complexes of starch embedded in the protein matrix. Dry fractionation caused the accumulation of these complexes in the CF, being of similar dimension and weight of the starch granules. Differently from other pulses, the chickpea CF was characterized also by the presence of starch aggregates and this could be explained by the higher lipid content of chickpea, compared to other pulses (Pelgrom et al., 2015). FF consisted largely of protein bodies with rare residual starch granules, which likely migrated in the fraction during dry fractionation.
Figure 1.
SE Micrographs of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
In order to better understand the separation process during dry fractionation, the mean diameter of the starch granules was measured by analyzing the SE micrographs (Table 4). The starch granules diameter varied significantly among the different pulse types as previously reported by Chung et al. (2008). In particular, among the RM, pea flour showed the largest starch granules, with a mean diameter of 25.4 μm. The dimension of starch granules, the physical and chemical characteristics of the raw materials (i.e. seed hardness, particle density, lipid content), and the intensity of the milling step are significant factors affecting the air classification process in terms of yield and protein shifting (Pelgrom et al., 2015), because the key factor of the process consists in the efficiency of the disentanglement of starch from proteins. In particular, the larger starch granules are more easily separated from the protein bodies compared to the smaller ones (Schutyser et al., 2015).
Table 4.
Mean value, standard deviation and results of statistical analysis one-way ANOVA of the mean starch granules diameter (μm), protein and ash contents (expressed as g 100g−1 of dry matter) of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
| Starch granules diameter (μm) | Protein (g 100g-1 d.m.) | Ash (g 100g-1 d.m.) | ||
|---|---|---|---|---|
| RM | RL | 19.5 ± 4.1b B (n = 28)∗ | 28.65 ± 0.24a B | 2.57 ± 0.12b B |
| YL | 20.7 ± 3.9b B (n = 23) | 26.42 ± 0.28ab B | 2.70 ± 0.01ab B | |
| GP | 25.4 ± 4.0a A (n = 28) | 24.00 ± 0.84c B | 2.96 ± 0.01a B | |
| KC | 16.6 ± 2.8c B (n = 23) | 25.0 ± 1.5bc B | 2.65 ± 0.27ab B | |
| CF | RL | 23.9 ± 4.2a A (n = 30) | 21.53 ± 0.10a C | 2.02 ± 0.13a C |
| YL | 24.3 ± 4.2a A (n = 33) | 19.45 ± 0.62bc C | 2.25 ± 0.18a C | |
| GP | 23.6 ± 4.4a A (n = 36) | 18.2 ± 1.2c C | 2.28 ± 0.05a C | |
| KC | 19.8 ± 2.0b A (n = 25) | 20.46 ± 0.20ab C | 2.11 ± 0.03a C | |
| FF | RL | 15.7 ± 3.5a C (n = 26) | 49.41 ± 0.20b A | 4.20 ± 0.11c A |
| YL | 10.9 ± 2.3b C (n = 12) | 57.2 ± 1.2a A | 5.59 ± 0.18ab A | |
| GP | 15.9 ± 6.5a B (n = 9) | 56.08 ± 0.01a A | 5.91 ± 0.05a A | |
| KC | 11.0 ± 3.1b C (n = 18) | 46.5 ± 1.4c A | 5.35 ± 0.13b A |
Lowercase letters mean significant differences among different types of pulse within the same fraction (p < 0.05). Uppercase letters mean significant differences among different fractions within the same type of pulse (p < 0.05).
In brackets the number of starch granules used for the length measurement.
The differences found in the RM are less evident in CF. Indeed, only the chickpea CF showed significantly smaller starch granules compared to the other pulses. Finally, in FF significantly smaller starch granules were found for yellow lentil and chickpea compared to pea and red lentil. In general, larger starch granules were found in the CF compared to RM, in all the pulses investigated. On the contrary, in FF a smaller starch granule size was observed. Overall, dry fractionation allowed separation of larger starch granules of homogeneous size in the CF.
As shown in FF micrographs (Figure 1), some small and/or damaged starch granules of various sizes were identified together with protein bodies. This is because dry fractionation does not allow a complete separation of starch and protein in CF and FF respectively (Van der Goot et al., 2016).
The starch morphology and dimension can help to explain the data regarding the protein content (Table 4). The RM showed a protein content with slight but still significant differences among the considered pulses. In particular, the protein content was significantly higher in red lentil than in chickpea and pea, but it was statistically similar to yellow lentil.
As shown in Table 4, CF contained a protein residue ranging from 18.2 g 100g−1 in pea, to 21.53 g 100g−1 in red lentil, showing the same trend observed for the RM. As for the FF, the highest protein content was in yellow lentil and pea, with mean values higher than 55 g 100g−1, whereas chickpea displayed the lowest protein content. As reported by previous studies, compared to other pulses such as pea, chickpea flour has not the optimal characteristics for protein separation due to the presence of small starch granules (Pelgrom et al., 2015). Therefore, chickpea needs a more intense milling process to increase the protein separation (Pelgrom et al., 2015). The authors used an impact mill, based on a similar technology of the equipment used in this study, obtaining therefore similar protein separation in the chickpea fine fraction (Pelgrom et al., 2015). In our study, we used the same set-up of milling and air classification for all pulse types, in order to understand how different pulses behave under the same processing conditions. Despite the lower performances of chickpea during dry fractionation, it is important to study and work on this species because it is the second most produced pulse worldwide (FAO, 2018), receiving large attention from the scientific community (Summo et al., 2019b). Furthermore, for some food applications, such as a protein fortification or amino acid compensation, also a protein content lower than 50 g 100g−1 as found in chickpea could be sufficient (Van der Goot et al., 2016). Moreover, also the CF obtained by dry fractionation can be a valuable ingredient due to both starch and protein contents, which make it suitable for some food application such as pasta and other cereal-based products (Giuberti and Gallo, 2018).
In the RM, we found significantly different ash content between pea and red lentils, the latter showing the lowest level. No significant differences were observed among the ash content of the four types of pulses in the CF, whereas pea showed higher ash content than chickpea and red lentil in FF. According to the macro and micronutrients analysis, the effect of dry fractionation on ash content (total mineral compounds) was significant, and in CF we observed the lowest ash content, whereas all the pulses showed significantly higher ash content in FF than both the RM and the CF. More specifically, FF was characterized by an ash content approximately double that of RM, with mean values reaching 5.91 g 100 g−1 in the case of pea. Considering the yield of the fractions, the data are consistent with the composition of the raw materials and are further confirmed by the elemental analysis. The shift of ash content in the fine fraction was previously reported by other authors (Rempel et al., 2019).
3.4. Physicochemical and functional properties
Table 5 reports the physicochemical and functional properties of RM and the respective fractions obtained by dry fractionation. Bulk density (BD) is the mass per occupied volume and it is expressed as g mL−1. As for RM, red lentil displayed the highest BD, indicating a denser flour, whereas a significantly lower BD was found in chickpea. We observed a significant variation of BD moving to CF and FF. In particular, CF was characterized by a higher BD compared to RM for all types of pulses, whereas FF showed the lowest values of BD. This was mainly due to the different particle size of the fractions, as described in a previous study (Drakos et al., 2017). As stated above, indeed FF was composed of smaller particles, i.e. protein bodies and small starch granules, which make this fraction less dense than the CF, mainly composed of large starch granules. BD plays an important role for food formulation, especially in weaning foods (Summo et al., 2019b), with low values required to obtain an adequate texture. Hence, in view of highlighting the best end-use of the fractions, FF could help obtaining adequate texture while improving the protein content of food products.
Table 5.
Mean value, standard deviation and results of statistical analysis one-way ANOVA of the physicochemical and functional properties of the pulses flours before (RM) and after separation by dry fractionation in coarse (CF) and fine (FF) fractions. Red Lentil (RL), Yellow Lentil (YL), Green Pea (GP), Kabuli Chickpea (KC).
| Bulk Density (g ml−1) | Water Absorption Index | Water Solubility Index (%) | Water Absorption Capacity (g water g−1 flour) | Oil Absorption Capacity (g oil g−1 flour) | ||
|---|---|---|---|---|---|---|
| RM | RL | 0.73 ± 0.01a B | 4.61 ± 0.48a A | 14.23 ± 2.03c B | 0.93 ± 0.01b A | 0.32 ± 0.02b B |
| YL | 0.69 ± 0.02b B | 3.72 ± 0.02b B | 21.06 ± 0.37a B | 0.91 ± 0.04b A | 0.31 ± 0.03b B | |
| GP | 0.70 ± 0.02b B | 3.89 ± 0.12b B | 18.58 ± 0.05b B | 0.99 ± 0.04a A | 0.35 ± 0.00ab AB | |
| KC | 0.63 ± 0.01c B | 4.44 ± 0.05a B | 14.28 ± 0.55c B | 0.88 ± 0.01b B | 0.38 ± 0.03a B | |
| CF | RL | 0.95 ± 0.03c A | 4.48 ± 0.02 b A | 13.31 ± 0.17b B | 0.90 ± 0.02c AB | 0.16 ± 0.00c C |
| YL | 0.88 ± 0.02a A | 3.94 ± 0.17c A | 14.70 ± 0.47a C | 0.91 ± 0.01c A | 0.18 ± 0.02b C | |
| GP | 0.84 ± 0.01b A | 4.70 ± 0.09a A | 12.71 ± 0.31c C | 1.07 ± 0.03a A | 0.20 ± 0.01b B | |
| KC | 0.65 ± 0.00d A | 4.65 ± 0.01ab A | 11.19 ± 0.09d C | 1.01 ± 0.03b A | 0.35 ± 0.01a C | |
| FF | RL | 0.54 ± 0.01a C | 3.83 ± 0.05b B | 21.38 ± 0.23c A | 0.89 ± 0.03a B | 0.45 ± 0.02a A |
| YL | 0.48 ± 0.01b C | 3.98 ± 0.05a A | 27.30 ± 0.72a A | 0.75 ± 0.03b B | 0.50 ± 0.01a A | |
| GP | 0.47 ± 0.01bc C | 3.98 ± 0.02a B | 25.01 ± 0.28b A | 0.76 ± 0.06b B | 0.51 ± 0.28a A | |
| KC | 0.46 ± 0.02c C | 3.48 ± 0.01c C | 27.11 ± 0.15a A | 0.42 ± 0.04c C | 0.53 ± 0.01a A |
Lowercase letters mean significant differences among the different types of pulse within the same fraction (p < 0.05). Uppercase letters mean significant differences among the fractions within the same type of pulse (p < 0.05).
Water absorption index (WAI) concerns the physical state of starch granules and its swelling capacity, indicating the volume occupied by the starch after being put in hot water (Du et al., 2014). Water solubility index (WSI) is highly related to WAI and quantifies the percentage of soluble solids that persist in the aqueous phase after the heating process. Generally, the higher WAI, the lower WSI. Regarding RM, red lentil and chickpea showed WAI values significantly higher than yellow lentil and green pea. Therefore, the same pulses showed also the lowest WSI, with values lower than 15 %, whereas green pea and particularly yellow lentil showed significantly higher WSI values. WAI and WSI of the chickpea RM were in accordance with our previous work, in which we attributed these values to a low content of damaged starch (Summo et al., 2019b). Other studies carried out on different pulse species reported slightly higher WAI and WSI for whole lentil flour (Du et al., 2014), and comparable WAI and WSI for pea flour (Maninder et al., 2007). Owing to their close relationship with the starch characteristics, WAI and WSI are interesting parameters specially to explain the quality of the CF. In our study, chickpea CF was characterized by the lowest WSI and a high WAI, highlighting that this fraction had good gelation properties. Slight but significant differences were found for FF, with yellow lentil and pea having the highest WAI, whereas red lentil showed the lowest WSI, suggesting a lower presence of damaged starch.
Analyzing the changes induced by dry fractionation, it possible to highlight an increase of WAI in CF, and a reduction of this index in FF. At the same time, WSI showed the lowest value in CF and the highest in FF, compared to RM. These results point out that less damaged starch granules, with better gelling capacity, are more concentrated in CF compared to FF. In the latter, indeed, the presence of small fragments of starch was observed in SE micrographs. Li et al. (2019) evaluated the damaged starch level of starch-rich fractions obtained by dry fractionation, reporting a content of 1.9–3.4 %. Moreover, Pelgrom et al. (2013) observed a content of damaged starch in the fine fraction higher than 2.3%, which was strongly dependent on the milling technology.
The water absorption capacity (WAC) indicates the amount of water that can be bound by a gram of flour. Considering RM, green pea showed the highest WAC, whereas no significant differences were found among the other types of pulses. As for CF, red and yellow lentils showed a WAC of 0.90 and 0.91 g water g−1 respectively, significantly lower than chickpea and pea CF (the latter showing the highest WAC). Chickpea FF, instead, showed very low WAC compared to the other species.
In general, the pulses investigated showed WAC values lower than those reported in previous studies (Du et al., 2014; Maninder et al., 2007; Summo et al., 2019b). This can be explained by the lower content of total dietary fiber of these flours, due to dehulling carried out before milling and air classification. The effect of dry fractionation on WAC was not significant moving from RM to CF, with the only exception of chickpea CF, which showed a higher WAC than RM. At the same time, a significant decrease of WAC in the FF was observed, compared to both RM and CF, probably related to the lower presence of starch. Other authors, indeed, correlated WAC to the presence of polysaccharides and other hydrophilic constituents (Maninder et al., 2007; Du et al., 2014). Overall, the trend observed for WAC is confirmed by a previous study carried out by do Carmo et al. (2020). Moreover, the dry fractionation process can be adapted to produce fractions with properties suited to particular applications. For example, do Carmo et al. (2020) reported an increase of water holding capacity when the raw materials were not dehulled, probably because of the higher content of dietary fibers in the resulting fractions. WAC is an important index to be considered for food formulation, due to its technological interest. WAC indeed plays a relevant role in bakery products, helping to improve the texture and preserving the shelf life of products (Foschia et al., 2017). In this perspective, CF could be used in these foods, whereas FF could be used for protein complementation and/or fortification. In particular, Gómez et al. (2012) reported the use of dry fractionated pea flours in sponge cakes and highlighted the potentially of the starch-rich fraction to be used to substitute up to 50% of wheat flour. By contrast, the use of a rice-derived fine fraction in gluten-free bread was responsible of low specific volume, probably related to an excessively fine particle size of the fraction (Park et al., 2014). Moreover, Xing et al. (2020) prepared a sourdough from chickpea air-classified fractions and suggested a possible utilization as fortifying ingredient in bread and other fermented foods, also because lactic acid bacteria grow well in both starch and protein rich fractions, due to a higher availability of nutrients (Coda et al., 2015). With this regard, fermented bakery products can be a very promising application for the air-classified fractions, but more research need to be carried out in order to identify the better strategies to exploit such ingredients.
Oil absorption capacity (OAC) indicates the weight of oil retained by a gram of flour. In RM we observed an OAC comprised between 0.31 and 0.38 g oil g−1 flour for yellow lentil and chickpea, respectively. Also in CF, chickpea showed the highest OAC, whereas we found the lowest OAC in red lentil. No significant differences were recognized within the FF. Observing the influence of dry fractionation, CF showed a significant decrease of OAC, which instead was significantly higher in FF, with mean values near 0.50 g oil g−1. This trend was previously reported by other authors (do Carmo et al., 2020). OAC is principally associated to capillary interactions which help the absorption and retention of oil in the matrix (Du et al., 2014). Moreover, in a previous study a positive and significant correlation between protein content and OAC was reported (Summo et al., 2019b). This observation could steadily explain the higher values found for FF. The obtained results point out a possible utilization of FF in different types of foods, such as bakery goods (Foschia et al., 2017) or meat systems, where is required a good incorporation of oil in order to support flavor and texture (Summo et al., 2019b).
4. Conclusions
This research highlights that dry fractionation of micronized pulse flours (RM) had a significant effect on the chemical, nutritional and physicochemical properties of the resulting coarse (CF) and fine (FF) fractions. These properties are also influenced by the type of pulses. RM and CF of pea showed the highest phytate content, whereas FF of red lentil displayed the lowest content. Stachyose was the main oligosaccharide in lentils, exceeding 50 mg g−1, whereas chickpea showed the highest raffinose content. Both phytates and oligosaccharides were significantly enriched in the FF produced by dry fractionation, while they decreased in CF.
TXRF analysis identified potassium as the main macronutrient in pulses. Furthermore, we found a high Ca variability among the types of pulses, ranging from 0.92 to 0.28 g kg−1 in pea and yellow lentil, respectively. Dry fractionation caused a significant shifting of minerals in FF. However, despite the highest phytate content of FF, phytate:Zn ratios of yellow and red lentils were lower than RM, indicating that Zn was enriched in FF more than phytates. Yellow lentil and pea showed good attitude to dry fractionation as demonstrated by the high protein content (higher than 55 g 100g−1) found in FF.
Dry fractionation significantly affected also the physicochemical and functional properties, highlighting a lower bulk density in FF together with values of WAI and WSI which suggest the higher presence of damaged starch in this fraction. CF showed high WAC, particularly relevant in pea, whereas FF was characterized by the highest OAC, without significant differences among the pulses considered.
In summary, these results represent a step forward for the exploitation of dry fractionated flours of pulses, providing information on their nutritional and functional properties.
Declarations
Author contribution statement
Davide De Angelis, Ignazio Allegretta, Carlo Porfido, Giacomo Squeo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Antonella Pasqualone: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Roberto Terzano: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Carmine Summo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Agropolis Fondation (ANR-10-LABX-0001-01), Fondazione Cariplo (ANR-10-LABX-0001-01), and Ministero dell’Istruzione, dell’Università e della Ricerca (2017SFTX3Y) and (AIM1809249).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledges
The authors gratefully thanks Dr. Luigi Manfredi, CEO of InnovaProt srl and Mr. Ernesto Bastoni, of Separ Micro System SAS, for the support in the air classification process, and Andriani SPA for providing the raw materials used in the experimental trials.
TXRF and FEG-SEM analyses were carried out at the joint “Micro X-ray Lab” of the University of Bari and Polytechnic University of Bari.
Contributor Information
Antonella Pasqualone, Email: Antonella.pasqualone@uniba.it.
Carmine Summo, Email: carmine.summo@uniba.it.
References
- Allegretta I., Gattullo C.E., Renna M., Paradiso V.M., Terzano R. Rapid multi-element characterization of microgreens via total-reflection X-ray fluorescence (TXRF) spectrometry. Food Chem. 2019;296:86–93. doi: 10.1016/j.foodchem.2019.05.187. [DOI] [PubMed] [Google Scholar]
- Chung H.J., Liu Q., Donner E., Hoover R., Warkentin T.D., Vandenberg B. Composition, molecular structure, properties, and in vitro digestibility of starches from newly released Canadian pulse cultivars. Cereal Chem. 2008;85(4):471–479. [Google Scholar]
- Coda R., Melama L., Rizzello C.G., Curiel J.A., Sibakov J., Holopainen U.…Sozer N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015;193:34–42. doi: 10.1016/j.ijfoodmicro.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Coulibaly A., Kouakou B., Chen J. Phytic acid in cereal grains: structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am. J. Plant Nutr. Fertiliz. Tech. 2011;1(1):1–22. [Google Scholar]
- Daryanto S., Wang L., Jacinthe P.A. Global synthesis of drought effects on food legume production. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0127401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo C.S., Silventoinen P., Nordgård C.T., Poudroux C., Dessev T., Zobel H., Holtekjølen A.,K., Draget K.,I., Holopainen-Mantila U., Knutsen S.,H., Sahlstrøm S. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein-and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020:109937. [Google Scholar]
- dos Reis A.R., El-Ramady H., Santos E.F., Gratão P.L., Schomburg L. Selenium in Plants. Springer; Cham: 2017. Overview of selenium deficiency and toxicity worldwide: affected areas, selenium-related health issues, and case studies; pp. 209–230. [Google Scholar]
- Drakos A., Kyriakakis G., Evageliou V., Protonotariou S., Mandala I., Ritzoulis C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017;215:326–332. doi: 10.1016/j.foodchem.2016.07.169. [DOI] [PubMed] [Google Scholar]
- Du S.K., Jiang H., Yu X., Jane J.L. Physicochemical and functional properties of whole legume flour. LWT - Food Sci. Technol. 2014;55(1):308–313. [Google Scholar]
- Flamminii F., Di Mattia C.D., Difonzo G., Neri L., Faieta M., Caponio F., Pittia P. From by-product to food ingredient: evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019;99(14):6620–6627. doi: 10.1002/jsfa.9949. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2018. Data of Crops Production.http://www.fao.org/faostat/en/#data/QC (accessed on March 2020) [Google Scholar]
- Foschia M., Horstmann S.W., Arendt E.K., Zannini E. Legumes as functional ingredients in gluten-free bakery and pasta products. Ann. Rev. Food Sci. Tech. 2017;8:75–96. doi: 10.1146/annurev-food-030216-030045. [DOI] [PubMed] [Google Scholar]
- Giuberti G., Gallo A. Reducing the glycaemic index and increasing the slowly digestible starch content in gluten-free cereal-based foods: a review. Int. J. Food Sci. Technol. 2018;53(1):50–60. [Google Scholar]
- Gómez M., Doyagüe M.J., de la Hera E. Addition of pin-milled pea flour and air-classified fractions in layer and sponge cakes. LWT - Food Sci. Technol. 2012;46(1):142–147. [Google Scholar]
- Hooper S.D., Glahn R.P., Cichy K.A. Single varietal dry bean (Phaseolus vulgaris L.) pastas: nutritional profile and consumer acceptability. Plant Foods Hum. Nutr. 2019:1–8. doi: 10.1007/s11130-019-00732-y. [DOI] [PubMed] [Google Scholar]
- Kristensen M.D., Bendsen N.T., Christensen S.M., Astrup A., Raben A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork)–a randomized cross-over meal test study. Food Nutr. Res. 2016;60(1):32634. doi: 10.3402/fnr.v60.32634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarda-Diaz I., Guzman-Partida A.M., Vazquez-Moreno L. Legume lectins: proteins with diverse applications. Int. J. Mol. Sci. 2017;18(6):1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudadio V., Bastoni E., Introna M., Tufarelli V. Production of low-fiber sunflower (Helianthus annuus L.) meal by micronization and air classification processes. CyTA - J. Food. 2013;11(4):398–403. [Google Scholar]
- Li L., Yuan T.Z., Setia R., Raja R.B., Zhang B., Ai Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019;276:599–607. doi: 10.1016/j.foodchem.2018.10.064. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130(5):1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- Ma G., Li Y., Jin Y., Zhai F., Kok F.J., Yang X. Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. Eur. J. Clin. Nutr. 2007;61(3):368–374. doi: 10.1038/sj.ejcn.1602513. [DOI] [PubMed] [Google Scholar]
- Maninder K., Sandhu K.S., Singh N. Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem. 2007;104(1):259–267. [Google Scholar]
- Martinez-Villaluenga C., Frias J., Vidal-Valverde C. Alpha-galactosides: antinutritional factors or functional ingredients? Crit. Rev. Food Sci. Nutr. 2008;48(4):301–316. doi: 10.1080/10408390701326243. [DOI] [PubMed] [Google Scholar]
- Osen R., Toelstede S., Wild F., Eisner P., Schweiggert-Weisz U. High moisture extrusion cooking of pea protein isolates: raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014;127:67–74. [Google Scholar]
- Ozturk B., McClements D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016;7:1–6. [Google Scholar]
- Park J.H., Kim D.C., Lee S.E., Kim O.W., Kim H., Lim S.T., Kim S.S. Effects of rice flour size fractions on gluten free rice bread. Food Sci. Biotechnol. 2014;23(6):1875–1883. [Google Scholar]
- Pasqualone A., De Angelis D., Squeo G., Difonzo G., Caponio F., Summo C. The effect of the addition of Apulian black chickpea flour on the nutritional and qualitative properties of durum wheat-based bakery products. Foods. 2019;8(10):504. doi: 10.3390/foods8100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualone A., Costantini M., Coldea T.E., Summo C. Use of legumes in extrusion cooking: a review. Foods. 2020;9(7):958. doi: 10.3390/foods9070958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa M.M., Cuadrado C., Burbano C., Allaf K., Haddad J., Gelencsér E., Takàcs K., Gullamon E., Muzquiz M. Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 2012;131(3):862–868. [Google Scholar]
- Pelgrom P.J., Berghout J.A., van der Goot A.J., Boom R.M., Schutyser M.A. Preparation of functional lupine protein fractions by dry separation. LWT - Food Sci. Technol. 2014;59(2):680–688. [Google Scholar]
- Pelgrom P.J., Boom R.M., Schutyser M.A. Method development to increase protein enrichment during dry fractionation of starch-rich legumes. Food Bioprocess Technol. 2015;8(7):1495–1502. [Google Scholar]
- Pelgrom P.J., Vissers A.M., Boom R.M., Schutyser M.A. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013;53(1):232–239. [Google Scholar]
- Ray H., Bett K., Tar’an B., Vandenberg A., Thavarajah D., Warkentin T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2014;54(4):1698–1708. [Google Scholar]
- Rempel C., Geng X., Zhang Y. Industrial scale preparation of pea flour fractions with enhanced nutritive composition by dry fractionation. Food Chem. 2019;276:119–128. doi: 10.1016/j.foodchem.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Schutyser M.A.I., Pelgrom P.J.M., Van der Goot A.J., Boom R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015;45(2):327–335. [Google Scholar]
- Shi L., Arntfield S.D., Nickerson M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018;107:660–668. doi: 10.1016/j.foodres.2018.02.056. [DOI] [PubMed] [Google Scholar]
- Sozer N., Holopainen-Mantila U., Poutanen K. Traditional and new food uses of pulses. Cereal Chem. 2017;94:66–73. [Google Scholar]
- Summo C., De Angelis D., Ricciardi L., Caponio F., Lotti C., Pavan S., Pasqualone A. Data on the chemical composition, bioactive compounds, fatty acid composition, physico-chemical and functional properties of a global chickpea collection. Data Brief. 2019;27:104612. doi: 10.1016/j.dib.2019.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summo C., De Angelis D., Ricciardi L., Caponio F., Lotti C., Pavan S., Pasqualone A. Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compos. Anal. 2019;84:103306. doi: 10.1016/j.dib.2019.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecklenburg E., Zabik M.E., Uebersax M.A., Dietz J.C., Lusas E.W. Mineral and phytic acid partitioning among air-classified bean flour fractions. J. Food Sci. 1984;49(2):569–572. [Google Scholar]
- Tyler R.T., Youngs C.G., Sosulski F.W. Air classification of legumes [beans, lentils, peas]. I. Separation efficiency, yield, and composition of the starch and protein fractions. Cereal Chem. 1981;58(2):144–148. [Google Scholar]
- Van der Goot A.J., Pelgrom P.J., Berghout J.A., Geerts M.E., Jankowiak L., Hardt N.A., Keijer J., Schutyser M., Nikiforidis V.,C., Boom R.M. Concepts for further sustainable production of foods. J. Food Eng. 2016;168:42–51. [Google Scholar]
- Wang N., Daun J.K. Effect of variety and crude protein content on nutrients and certain antinutrients in field peas (Pisum sativum) J. Sci. Food Agric. 2004;84(9):1021–1029. [Google Scholar]
- Wang N., Maximiuk L. Effect of air classification processing variables on yield, composition, and certain antinutrients of air-classified fractions from field peas by response surface methodology. J. Food Process. Preserv. 2019 [Google Scholar]
- Xing Q., Dekker S., Kyriakopoulou K., Boom R.M., Smid E.J., Schutyser M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innovat. Food Sci. Emerg. Technol. 2020;59:102269. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.