Summary
Antarctica is home to an assortment of psychrophilic algae, which have evolved various survival strategies for coping with their frigid environments. Here, we explore Antarctic psychrophily by examining the ∼212 Mb draft nuclear genome of the green alga Chlamydomonas sp. UWO241, which resides within the water column of a perennially ice-covered, hypersaline lake. Like certain other Antarctic algae, UWO241 encodes a large number (≥37) of ice-binding proteins, putatively originating from horizontal gene transfer. Even more striking, UWO241 harbors hundreds of highly similar duplicated genes involved in diverse cellular processes, some of which we argue are aiding its survival in the Antarctic via gene dosage. Gene and partial gene duplication appear to be an ongoing phenomenon within UWO241, one which might be mediated by retrotransposons. Ultimately, we consider how such a process could be associated with adaptation to extreme environments but explore potential non-adaptive hypotheses as well.
Subject areas: biological sciences, genomic analysis, sequence analysis, genomics
Graphical abstract
Highlights
-
•
Chlamydomonas sp. UWO241 is a green alga originating from Lake Bonney, Antarctica
-
•
We present a draft nuclear genome sequence of UWO241 (∼212 Mb).
-
•
The UWO genome contains hundreds of highly similar duplicated genes
-
•
These duplicates, we argue, might be involved in cold adaptation
Biological sciences; genomic analysis; sequence analysis; genomics
Introduction
“Antarctica is a very alien environment, and you can't survive here more than minutes if you're not equipped properly and doing the right thing all the time” — Jon Krakauer
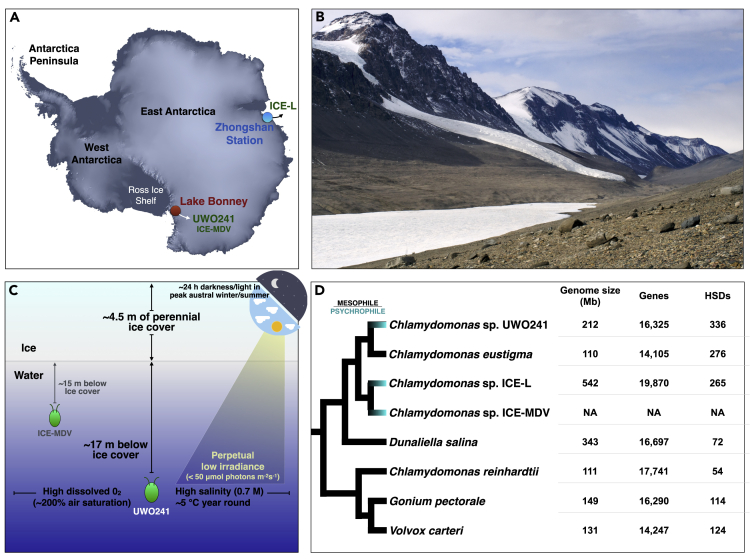
Life persists in the harshest places. Take Chlamydomonas sp. UWO241, for instance. This unicellular chlorophycean green alga—formerly called Chlamydomonas raudensis (Possmayer et al., 2016)—resides in the permanently ice-covered Lake Bonney (McMurdo Dry Valleys, Antarctica) (Pocock et al., 2004) (Figures 1A and 1B) and is so attuned to cold water (∼5°C year-round) that it cannot grow above 18°C, making it a true psychrophile (Morita, 1975). Moreover, its position within the water column (∼17 m below surface) brings other challenges: high salinity (0.7 M), low levels of phosphorus (N:P ∼1000), seasonal extremes in photoperiod, unusually high oxygen concentrations (200% air saturation), and perpetually low irradiance (Morgan-Kiss et al., 2006; Dore and Priscu, 2001) (Figure 1C). How has UWO241 adapted to this extreme habitat?
Figure 1.
Chlamydomonas sp. UWO241
(A) Origins of isolation of UWO241 and Chlamydomonas sp. ICE-MDV (Lake Bonney), as well as Chlamydomonas sp. ICE-L (sea ice off of Zhongshan Station); image from NASA Earth Observatory.
(B) Photograph of Lake Bonney (Wikimedia-Commons, 2020).
(C) Simplified diagram showing environmental conditions in Lake Bonney.
(D) Tree of various chlamydomonadalean algae and their nuclear genome statistics; branching order based on previous phylogenetic analyses (Nakada et al., 2008; Possmayer et al., 2016; Zhang et al., 2020).
Recently, it was shown that UWO241, unlike other surveyed eukaryotic algae, produces two near-identical copies of photosynthetic ferredoxin (PETF), resulting from a duplication of the nuclear petf gene (Cvetkovska et al., 2018). The retention and expression of this duplicate gene is hypothesized to be an adaptation to the cold, leading to higher protein accumulation (i.e., gene dosage); indeed, UWO241 accumulates greater amounts of PETF than its mesophilic relative Chlamydomonas reinhardtii (Merchant et al., 2007; Cvetkovska et al., 2018). Similarly, UWO241 expresses three isoforms of an unusual bidomain enzyme, allowing it to produce high levels of osmoprotectant glycerol (>400 mM) (Kalra et al., 2020). If gene dosage is contributing to psychrophily in UWO241, one might expect other genes to be duplicated. Here, we show that this expectation is true.
Genome sequencing of UWO241 exposed hundreds of gene duplicates for crucial cellular pathways and dozens of genes encoding ice-binding proteins (IBPs). These findings for UWO241 (isolated from a constantly cold but non-freezing environment) mirror many of those from the recent genomic analysis of Chlamydomonas sp. ICE-L (Zhang et al., 2020), which originates from a cold but fluctuating Antarctic sea ice environment (Figure 1A), and enhance our understanding of photopsychrophily and the evolutionary dynamics within ice-covered Antarctic lakes.
Results and discussion
Draft nuclear genome sequence of an alga from an Antarctic lake
The haploid nuclear genome of UWO241 was assembled de novo using a combination of long-read PacBio (∼16.5 Gb) and short-read Illumina (∼40 Gb) data, resulting in 2,458 scaffolds (N50 = 375.9 kb) with an accumulative length of 211.6 Mb (%GC = 60.6) (Figures 1D and S1). This length is consistent with flow cytometry and k-mer spectral analysis of UWO241, which predicted an overall genome size of ∼230 Mb (Figure S1). In total, 16,325 protein-coding genes were annotated (all supported by transcriptomic data), capturing ∼85% of the Chlorophyte Benchmarking Universal Single-Copy Orthologs data sets (Figure S1), indicating a high level of gene region completeness. The UWO241 genome is rich in functional RNAs (630 tRNAs and 480 rRNAs) as well as noncoding DNA (∼87%), having the highest average intron density yet observed from a green alga (∼10 introns/gene; avg. intron length 0.9 kb). The intergenic regions abound with repeats, accounting for ∼104 Mb (∼49%) of the total assembly length, ∼70 Mb of which are represented by transposable elements (TEs) (discussed below).
The past decade has brought draft nuclear genomes for >25 different green algal species, with especially strong sampling from the order Chlamydomonadales (Chlorophyceae) (Figure 1D). The UWO241 genome is the second to be sequenced from the Moewusinia clade of the Chlamydomonadales (Nakada et al., 2008), the other coming from the acidophilic species Chlamydomonas eustigma (Hirooka et al., 2017). The Moewusinia is closely affiliated with the Monadinia, the clade to which the Antarctic psychrophiles ICE-L and Chlamydomonas ICE-MDV belong (Figures 1A and 1D) (Demchenko et al., 2012). Keep in mind that the Chlamydomonas genus is polyphyletic and that UWO241, C. eustigma, and ICE-L branch closer to Dunaliella salina (Figure 1D), for example, than they do to C. reinhardtii, which hails from the Reinhardtinia clade (Possmayer et al., 2016; Zhang et al., 2020). What immediately stands out for the UWO241 genome as compared to other available green algal nuclear DNAs (nucDNAs) is its relatively large size (approximately twice that of C. reinhardtii), record-setting intron density, and high repeat content, outdone only by that of ICE-L (∼64% repeats) (Zhang et al., 2020). However, close inspection of the UWO241 coding regions uncovered something much more unique: widespread gene duplication to a degree unmatched in any chlorophyte studied to date.
Hundreds of gene duplicates
Functional annotation of the 16,325 RNA-supported gene models revealed the standard cohort of proteins typically encoded in green algal nuclear genomes (Data S1), as well as many hypothetical proteins (21.8%), paralleling the trends from other available chlamydomonadalean nuclear gene sets, which are generally 20-30% hypothetical. There were no obvious signs of contamination in the assembly or annotations and, with one conspicuous exception (discussed below), little evidence of horizontal gene transfer (HGT). When examining the annotations in detail, it became obvious that many genes were represented two or more times within the assembly. To explore the validity of these multi-copy genes, we performed a series of BLAST (Basic Local Alignment Search Tool) -based analyses with strict downstream filtering.
A protein BLAST of the UWO241 gene models against themselves (E-value cut-off 10−5) detected 901 putative duplicates (encompassing 2,012 gene copies) all with pairwise amino acid identities ≥80%. We filtered this gene set to only those with near-identical protein lengths (within 10 amino acids) and ≥90% pairwise identities, giving a pared-down list of 336 highly similar duplicates (HSDs), totaling 1,339 gene copies (Table 1; Data S2). By setting such a strict cutoff, we have undoubtedly removed some genuine duplicates from this list, but we would rather be conservative in our approach, ensuring that the gene pairs in question are bona fide duplicates rather than spurious ones. The protein sequences of the HSDs were searched against the KEGG (Kyoto Encyclopedia of Genes and Genomes) and Pfam databases, providing a functional breakdown (Table 1; Data S2). HSDs in UWO241 are involved in various cellular pathways, including gene expression, cell growth, membrane transport, and energy metabolism, but also include hypothetical proteins (∼37%) and reverse transcriptases (11%) (Table 1; Data S2). HSDs for protein translation, DNA packaging, and photosynthesis were particularly prevalent, with 19 duplications of genes for ribosomal proteins, 10 for histones, and at least 4 for proteins of the chlorophyll a/b binding light-harvesting complex (Table 1; Figures 2A–2C). As with the previously described petf duplication (Cvetkovska et al., 2018), many of these HSDs are virtually indistinguishable from each other at the amino acid level and 65 are identical across their nucleotide coding regions (Data S2).
Table 1.
Summary statistics of highly similar duplicate genes (HSDs) in UWO241.
| Database | Example identifiersa | Number of HSDs (%)b | Number of gene copies (%)b |
|---|---|---|---|
| Pfam | |||
| Chlorophyll A-B binding protein | PF00504 | 4 (1%) | 25 (2%) |
| Ribosomal protein | PF01015; PF01775; PF00828 | 19 (5%) | 42 (3%) |
| Core histone H2A/H2B/H3/H4 | PF00125 | 5 (1%) | 99 (7%) |
| Ice-binding protein (DUF3494) | PF11999 | 8 (2%) | 21 (2%) |
| Reverse transcriptases | PF00078 | 38 (11%) | 151 (11%) |
| KEGG | |||
| 09,101 Carbohydrate metabolism | K13979 (alcohol dehydrogenase) | 12 (4%) | 89 (7%) |
| 09,102 Energy metabolism | K02639 (ferredoxin); K08913 (light-harvesting complex II chlorophyll a/b binding protein 2) | 10 (3%) | 51 (4%) |
| 09,103 Lipid metabolism | K01054 (acylglycerol lipase) | 3 (1%) | 15 (1%) |
| 09,122 Translation | K02868 (large subunit ribosomal protein L11e) | 27 (8%) | 47 (4%) |
| Hypothetical proteins | NA | 125 (37%) | 357 (27%) |
Not all identifiers are listed.
A total of 336 HSDs were identified within the UWO241 genome, encompassing 1,339 gene copies. HSDs share ≥90% pairwise amino acid identity and have lengths within 10 amino acids of each other.
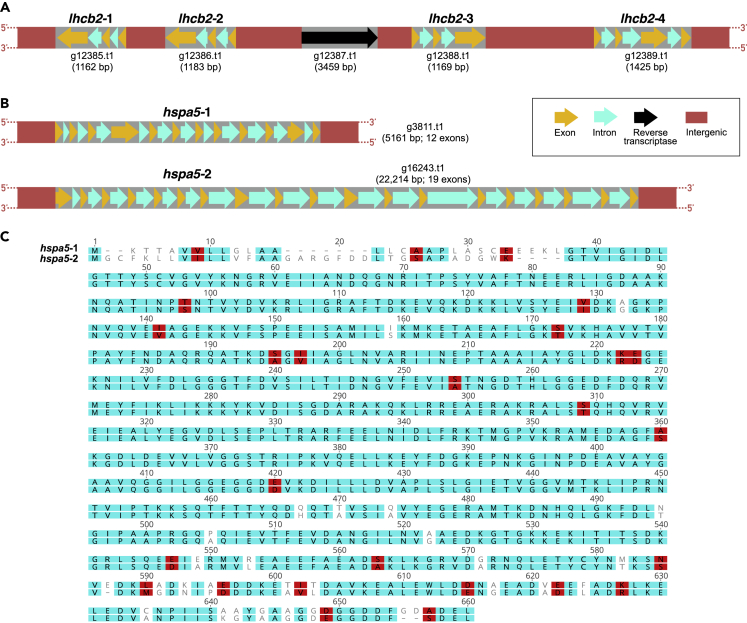
Figure 2.
Examples of duplicate genes in Chlamydomonas sp. UWO241
(A) Four distinct copies of lhcb2, all located on scaffold scf7180000014917.
(B) Two distinct copies of hspa5, located on scaffolds scf7180000011611 (hspa5-1) and scf7180000015050 (hspa5-2).
(C) Pairwise alignment of the deduced amino acid sequences of hspa5-1 and hspa5-2.
The arrangements of the HSDs are informative. Approximately, 20% contain gene copies that are situated close to one another (often in a head-to-head or head-to-tail orientation) and have very similar intron numbers and intronic sequences (based on pairwise alignments), implying that they result from recent tandem duplication events (Figure 2A; Data S2). A clear example of this is the duplication of the lhcb2 gene (Figure 2A). The remaining HSDs are generally far apart (most on distinct scaffolds) and, despite their matching coding regions, many (∼50%) have un-alignable intronic sequences and differing numbers of introns, suggesting that they derive from more ancient duplication events (Figures 2B and 2C; Data S2). This is the case for petf (Cvetkovska et al., 2018), as well as for hspa5 (encoding heat shock 70-kDa protein 5), the two copies of which are found in the middle of distinct scaffolds and share 93% coding sequence identity but <25% nucleotide similarity across their introns (Figures 2B and 2C). Whatever their arrangement, the exonic sequences of more than half of the HSDs (∼190) are under strong purifying selection as evidenced by low (<1) nonsynonymous to synonymous substitution rates (dN/dS), ranging from 0 to 0.5 (avg. = 0.2) (Figure S2). It is possible that the strong coding sequence preservation of these duplicates could be aiding the survival of UWO241 in Lake Bonney, perhaps due to increased gene dosage (Innan and Kondrashov, 2010; Kondrashov, 2012), as previously suggested (Cvetkovska et al., 2018). But various non-adaptive explanations are also plausible. The HSDs, however, represent only a fraction of duplicated regions within the genome.
A genome in upheaval
The UWO241 nucDNA contains thousands of partial duplicates, characterized by gene fragments and pseudogenes, as well as duplicated segments of intergenic and intronic DNA (Figure S3; Data S3). These incomplete duplicates, which range in size from ∼100-12,000 bp, can exist in high copy numbers (>6) and, like the HSDs, can be found in tandem or on different scaffolds (Figure S3; Data S3). But unlike the HSDs, they are in various states of decay, possibly reflecting an ongoing birth-death process, which is supported by the fact that many of the complete and partial duplicates are directly associated with or occur near to retrotransposons (RTs) (Figure S3; Data S3), as outlined for the duplication of lhcb2 in Figure 2A.
RT-mediated gene duplication is a recurring theme within nuclear genomes (Qian and Zhang, 2014; Panchy et al., 2016; Casola and Betrán, 2017; Kubiak and Makałowska, 2017), including those of green algae (Jąkalski et al., 2016), and the UWO241 genome contains the standard hallmarks of such a phenomenon, such as poly-(A) tail insertions and target site duplications (Figure S3). But this certainly does not rule out the possibility that other processes, such as unequal crossing-over (Zhang, 2003), are contributing to gene duplication within UWO241. Do note that 83% of the HSDs contain introns, a characteristic not generally associated with RT-mediated duplications, but not unprecedented (Casola and Betrán, 2017; Kubiak and Makałowska, 2017). Retrocopies often inherit introns from parental genes, flanking genomic DNA, or the fusion of transcripts (Catania and Lynch, 2008; Zhu et al., 2009; Szcześniak et al., 2011; Kang et al., 2012; Zhang et al., 2014). Altogether, we identified 401 putatively functional RTs in the nucDNA, including 77 long terminal repeat (LTR) and 324 non-LTR RTs. These numbers are likely underestimates of the true total as they do not include retropseudogenes, partial retroelements, or identified RTs with no RNA-seq support, which together account for >10% of the assembly. What’s more, there are >480 duplicated regions containing a reverse-transcriptase domain, including ones in noncoding DNA. UWO241 has more retroelements than all other surveyed chlorophytes (4 times that of C. reinhardtii) with the exception of ICE-L, for which non-LTR RTs account for a staggering ∼23% of the genome (Zhang et al., 2020). In addition to RTs, the UWO241 and ICE-L genomes share another atypical feature—genes for IBPs.
Horizontally acquired and duplicated ice-binding proteins
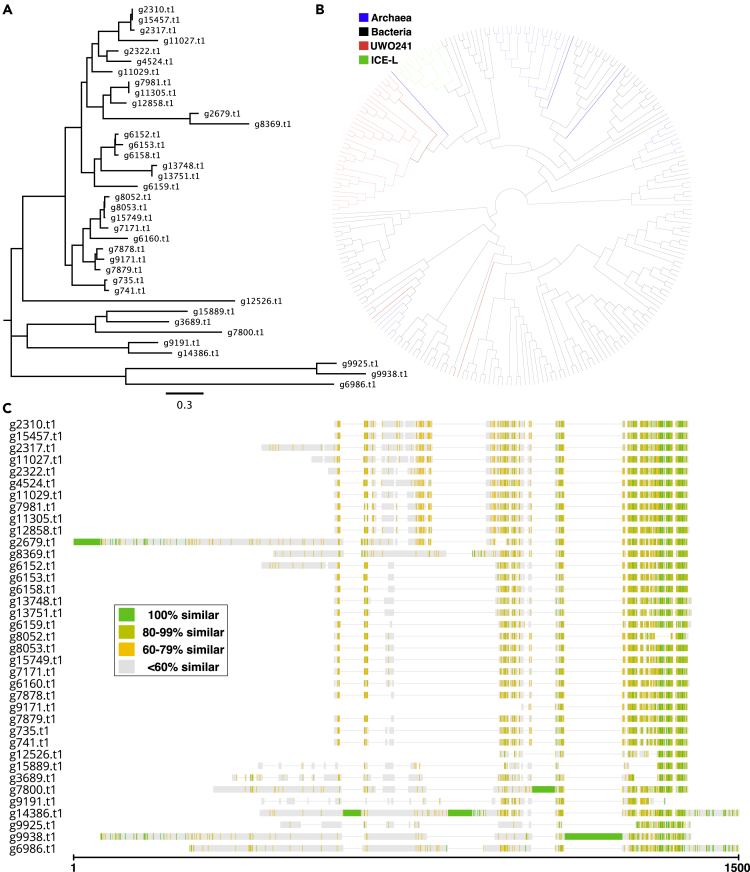
The UWO241 genome encodes no fewer than 37 proteins with an ice-binding domain (DUF3494) (Figure 3A), which is among the largest number of IBPs ever recorded in a photosynthetic protist. This wealth of IBPs appears to be the consequence of HGT events in combination with gene duplication. Phylogenetic analyses of the IBP genes, which range in size from 483-37,549 bp, show their similarity to type I bacterial and archaeal IBPs (Figure 3B), which is consistent with previous work (Raymond and Morgan-Kiss, 2013). Nuclear genes acquired via recent HGT events from bacteria usually lack introns (Keeling and Palmer, 2008), as do 14 of the IBP genes from UWO241; the remaining genes, with 4 exceptions, all have a single, short intron at their 3’ ends. The largest IBP gene, however, contains 29 introns. The IBP genes show varying degrees of similarity with each other (Figure 3C), including 8 groupings of almost identical genes, suggesting a complicated history of IBP gene acquisition and duplication within UWO241. The presence of pseudogenes and gene fragments with similarity to IBPs (Data S3) indicates that some previously functional IBP coding regions might have been lost.
Figure 3.
Ice-binding proteins from UWO241
(A) Maximum likelihood (ML) phylogenetic tree based on the amino acid alignments of 37 IBPs in UWO241.
(B) ML phylogenetic relationships of IBPs in UWO241 (red), ICE-L (green), Archaea (blue), and bacteria (black).
(C) Amino acid alignment of 37 IBPs in UWO241 via Clustal Omega, version 1.2.4, using default parameters.
These findings add to the growing list of psychrophilic and psychrotolerant algae encoding IBPs (Blanc et al., 2012; Raymond and Morgan-Kiss, 2013, 2017; Mock et al., 2017), mirroring the pattern of ice-associated bacteria and fungi (Margesin et al., 2008). Genome sequencing of the polar diatom Fragilariopsis cylindrus identified 11 IBPs (Mock et al., 2017), almost as many as found in ICE-L (12) (Zhang et al., 2020). The IBPs of UWO241 and ICE-L show a surprising degree of similarity with each other as evidenced in the phylogenetic analysis (Figure 3B), especially given that these two algae were isolated from locations that are more than 2500 km apart (Figure 1A). Chlamydomonas sp. ICE-MDV, a close relative of ICE-L and a resident of Lake Bonney (Figures 1A, 1C, and 1D), currently holds the record for the greatest number of IBP isoforms (50) in a green alga (Raymond and Morgan-Kiss, 2017). In all these examples, the IBPs are believed to have been acquired from bacteria via HGT, and their existence is thought to be an adaptation to polar environments (Raymond and Kim, 2012). It might seem obvious why a species that lives in the Antarctic would acquire IBPs, which can have ice recrystallization inhibition activities and, thus, protect cells from freezing damage (Davies, 2014). However, the potential benefits bestowed upon UWO241 and ICE-MDV by having these genes is not immediately clear. Unlike ICE-L, UWO241 does not live on ice or snow (Morgan-Kiss et al., 2006) but deep within lake water, which remains at ∼5°C year-round (this is also true for ICE-MDV). We do not know the evolutionary history of UWO241 or how long it has been isolated in Lake Bonney, meaning the presence of IBPs could be a remnant of an ancestral lifestyle involving close association with ice and snow.
Genome evolution in a permanently ice-covered Antarctic lake
One must be mindful not to instantly invoke positive selection when trying to explain the evolution of genomic architecture (Lynch, 2007; Brunet and Doolittle, 2018). It is tempting to propose that pervasive gene duplication within the UWO241 genome is an adaptation to life in Lake Bonney. But one could also reason that these features are neutral (or slightly deleterious) outcomes of random genetic events, such as the whims of selfish elements. As with many aspects of molecular evolution, the truth likely falls somewhere in-between these two extremes.
It is our belief that the underlying mechanisms behind the duplications within the UWO241 nucDNA, be it retrotransposition and/or other processes, are neutral or even maladaptive. Likewise, we contend that most of the observed duplicates in the genome, such as those encoding reverse transcriptases, were fixed through random genetic drift, perhaps exacerbated by the hermetic environment of Lake Bonney. (Unfortunately, there are no data on the effective population size of UWO241 and how it compares to that of other green algae, but chlorophytes appear to be relatively rare in Antarctic lake autotrophic communities (Dolhi et al., 2015)). But if enough duplicates are generated, it stands to reason that eventually one will arise resulting in an increase in fitness. For instance, if an increase in dosage of a particular gene is beneficial, then the duplication of this gene could be fixed (or at least maintained after the fact) by positive selection (Innan and Kondrashov, 2010; Kondrashov, 2012). This is arguably the best explanation for the existence of the petf duplicates (Cvetkovska et al., 2018), as well as some of the other HSDs in UWO241, including the IBP genes. However, more work is needed, including additional genome sequences from Moewusinia algae, especially close relatives of UWO241, before one can definitively say if adaptation to an extreme environment is contributing to the retention of HSDs in UWO241. It is noteworthy in this context that neither the UWO241 mitochondrial or chloroplast genomes (Cvetkovska et al., 2019) contain duplicated genes or retroelement-like sequences.
Gene duplication is increasingly being identified as a means of adaptation to extreme environments (Kondrashov, 2012; Qian and Zhang, 2014). Moreover, duplication events resulting in increased gene dosage are known to play a key role in the initial retention of duplicate genes (Innan and Kondrashov, 2010). The data presented here add to this theme. But, again, it is not necessarily true that the infrastructure responsible for generating putatively beneficial duplications is adaptive. Rather, something neutral can sometimes give rise to something useful, which we think is the case for UWO241. The question remains: what precise molecular mechanism(s) are causing genetic duplications in this alga? We favor an RT-based model because of the close association between RTs and duplicates in the genome, as well as the preponderance of reverse transcriptases. But other models are possible. If RTs are contributing to gene duplications in UWO241, then this could help explain the general upheaval we observed throughout the genome but also raises questions about how the HSDs acquired functional regulatory regions—retro-duplication does not typically include regulatory elements but they can be acquire by RTs via other means (Kubiak and Makałowska, 2017). Moreover, RT insertions can also alter the function of nearby genes, which, in turn, can have a cascade of effects (Carelli et al., 2016; Conrad and Antonarakis, 2007).
Remarkably, similar evolutionary processes appear to be operating in the ICE-L genome, in which gene duplication, potentially driven by RTs, has led to large expansions in various gene families, including IBP genes (Zhang et al., 2020), as well as HSDs (265 duplicates covering 717 gene copies) (Figure 1D; Data S4). Many of the HSDs in ICE-L have similar functions to those in UWO241 (Data S4). C. eustigma, the closest relative of UWO241 for which a draft genome sequence is available, also has a considerable number of gene duplicates (276), which could be contributing to its survival in an extremely acidic environment (Hirooka et al., 2017). The UWO241, ICE-L, and C. eustigma genomes stand in contrast to other explored green algal nucDNAs, which do not have large numbers of HSDs. Indeed, when the same bioinformatics procedures used to identify and classify HSDs in UWO241 were carried out on available chlamydomonadalean genomes, small to moderate numbers of gene duplications were identified (Figures 1D and S4), which is consistent with previous analyses of these genomes. It will be especially interesting to see if ICE-MDV—which like UWO241 lives deep within the water column of Lake Bonney—also harbors expanded gene families and HSDs. Whatever the result, Antarctic lakes have a lot to teach us about genome evolution at the extremes of life.
Limitations of the study
A large number of RTs and rampant gene duplication can cause errors during genome assembly (Zimin et al., 2017). We performed multiple iterations of the UWO241 assembly, using different protocols and algorithms, and are confident that the available draft genome sequence in GenBank is of good quality. The HSDs, in particular, are supported by RNA-seq, meaning there exists a specific transcript corresponding to each duplicate gene. But given the massive extent of duplications in the UWO241 genome, it is likely that some regions were misassembled, especially segments of duplicated noncoding DNA, and will need to be resolved through subsequent sequencing projects. That said, the overall conclusions presented here should not be affected.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, David R. Smith (dsmit242@uwo.ca).
Materials availability
This study did not generate new reagents or other materials.
Data and code availability
The assembled genome sequences and the raw sequencing data of UWO241 are deposited at the US National Center for Biotechnology Information (NCBI) database under BioProject accession PRJNA547753, nucleotide accession VFSX00000000, and BioSample accessions SAMN11975472 and SAMN11975511. This study did not generate new code.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
MC, NPAH, and DRS are supported by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada (NSERC). We thank Bojian Zhong and Jinlai Miao for sharing the ICE-L genome sequences, as well as Rory Craig for useful discussion on TE curation. We also thank Yining Hu for her assistance with computer programming.
Author contributions
The study was conceptualized by MC, NPAH, and DRS. The data were analyzed by MC and XZ. DRS and XZ drafted the manuscript and all authors commented to produce the manuscript for peer review.
Declaration of interests
The authors declare no competing interests.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102084.
Contributor Information
Norman P.A. Hüner, Email: nhuner@uwo.ca.
David Roy Smith, Email: dsmit242@uwo.ca.
Supplemental information
References
- Blanc G., Agarkova I., Grimwood J., Kuo A., Brueggeman A., Dunigan D.D., Gurnon J., Ladunga I., Lindquist E., Lucas S. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012;13:1–12. doi: 10.1186/gb-2012-13-5-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet T., Doolittle W.F. The generality of constructive neutral evolution. Biol. Philos. 2018;33:2. [Google Scholar]
- Carelli F.N., Hayakawa T., Go Y., Imai H., Warnefors M., Kaessmann H. The life history of retrocopies illuminates the evolution of new mammalian genes. Genome Res. 2016;26:301–314. doi: 10.1101/gr.198473.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola C., Betrán E. The genomic impact of gene retrocopies: what have we learned from comparative genomics, population genomics, and transcriptomic analyses? Genome Biol. Evol. 2017;9:1351–1373. doi: 10.1093/gbe/evx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania F., Lynch M. Where do introns come from? PLoS Biol. 2008;6:e283. doi: 10.1371/journal.pbio.0060283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Antonarakis S.E. Gene duplication: a drive for phenotypic diversity and cause of human disease. Annu. Rev. Genomics Hum. Genet. 2007;8:17–35. doi: 10.1146/annurev.genom.8.021307.110233. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M., Orgnero S., Hüner N.P., Smith D.R. The enigmatic loss of light-independent chlorophyll biosynthesis from an Antarctic green alga in a light-limited environment. New Phytol. 2019;222:651–656. doi: 10.1111/nph.15623. [DOI] [PubMed] [Google Scholar]
- Cvetkovska M., Szyszka-Mroz B., Possmayer M., Pittock P., Lajoie G., Smith D.R., Hüner N.P. Characterization of photosynthetic ferredoxin from the Antarctic alga Chlamydomonas sp. UWO241 reveals novel features of cold adaptation. New Phytol. 2018;219:588–604. doi: 10.1111/nph.15194. [DOI] [PubMed] [Google Scholar]
- Davies P.L. Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2014;39:548–555. doi: 10.1016/j.tibs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Demchenko E., Mikhailyuk T., Coleman A.W., Pröschold T. Generic and species concepts in Microglena (previously the Chlamydomonas monadina group) revised using an integrative approach. Eur. J. Phycol. 2012;47:264–290. [Google Scholar]
- Dolhi J.M., Teufel A.G., Kong W., Morgan-Kiss R.M. Diversity and spatial distribution of autotrophic communities within and between ice-covered Antarctic lakes (McMurdo Dry Valleys) Limnol. Oceanogr. 2015;60:977–991. [Google Scholar]
- Dore J.E., Priscu J.C. Phytoplankton phosphorus deficiency and alkaline phosphatase activity in the McMurdo Dry Valley lakes, Antarctica. Limnol. Oceanogr. 2001;46:1331–1346. [Google Scholar]
- Hirooka S., Hirose Y., Kanesaki Y., Higuchi S., Fujiwara T., Onuma R., Era A., Ohbayashi R., Uzuka A., Nozaki H. Acidophilic green algal genome provides insights into adaptation to an acidic environment. Proc. Natl. Acad. Sci. U S A. 2017;114:8304–8313. doi: 10.1073/pnas.1707072114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan H., Kondrashov F. The evolution of gene duplications: classifying and distinguishing between models. Nat. Rev. Genet. 2010;11:97–108. doi: 10.1038/nrg2689. [DOI] [PubMed] [Google Scholar]
- Jąkalski M., Takeshita K., Deblieck M., Koyanagi K.O., Makałowska I., Watanabe H., Makałowski W. Comparative genomic analysis of retrogene repertoire in two green algae Volvox carteri and Chlamydomonas reinhardtii. Biol. Direct. 2016;11:1–12. doi: 10.1186/s13062-016-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra I., Wang X., Cvetkovska M., Jeong J., Mchargue W., Zhang R., Hüner N., Yuan J.S., Morgan-Kiss R. Chlamydomonas sp. UWO 241 exhibits high cyclic electron flow and rewired metabolism under high salinity. Plant Physiol. 2020;183:588–601. doi: 10.1104/pp.19.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L.-F., Zhu Z.-L., Zhao Q., Chen L.-Y., Zhang Z. Newly evolved introns in human retrogenes provide novel insights into their evolutionary roles. BMC Evol. Biol. 2012;12:128. doi: 10.1186/1471-2148-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P.J., Palmer J.D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008;9:605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- Kondrashov F.A. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. R. Soc. B. 2012;279:5048–5057. doi: 10.1098/rspb.2012.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak M.R., Makałowska I. Protein-coding genes’ retrocopies and their functions. Viruses. 2017;9:80. doi: 10.3390/v9040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. The frailty of adaptive hypotheses for the origins of organismal complexity. Proc. Natl. Acad. Sci. U S A. 2007;104:8597–8604. doi: 10.1073/pnas.0702207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margesin R., Schinner F., Marx J.-C., Gerday C. Springer Verlag; 2008. Psychrophiles: From Biodiversity to Biotechnology. [Google Scholar]
- Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Maréchal-Drouard L. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock T., Otillar R.P., Strauss J., Mcmullan M., Paajanen P., Schmutz J., Salamov A., Sanges R., Toseland A., Ward B.J. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature. 2017;541:536–540. doi: 10.1038/nature20803. [DOI] [PubMed] [Google Scholar]
- Morgan-Kiss R.M., Priscu J.C., Pocock T., Gudynaite-Savitch L., Huner N.P. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006;70:222–252. doi: 10.1128/MMBR.70.1.222-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975;39:144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada T., Misawa K., Nozaki H. Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Mol. Phylogenet. Evol. 2008;48:281–291. doi: 10.1016/j.ympev.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Panchy N., Lehti-Shiu M., Shiu S.-H. Evolution of gene duplication in plants. Plant Physiol. 2016;171:2294–2316. doi: 10.1104/pp.16.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock T., Lachance M.A., Pröschold T., Priscu J.C., Kim S.S., Huner N.P. Identification of a psychrophilic green alga from Lake Bonney Antarctica: Chlamydomonas raudensis ETTL. (UWO241) chlorophyceae. J. Phycol. 2004;40:1138–1148. [Google Scholar]
- Possmayer M., Gupta R.K., Szyszka-Mroz B., Maxwell D.P., Lachance M.A., Hüner N.P., Smith D.R. Resolving the phylogenetic relationship between Chlamydomonas sp. UWO 241 and Chlamydomonas raudensis SAG 49.72 (Chlorophyceae) with nuclear and plastid DNA sequences. J. Phycol. 2016;52:305–310. doi: 10.1111/jpy.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W., Zhang J. Genomic evidence for adaptation by gene duplication. Genome Res. 2014;24:1356–1362. doi: 10.1101/gr.172098.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.A., Kim H.J. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS One. 2012;7:e35968. doi: 10.1371/journal.pone.0035968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.A., Morgan-Kiss R. Separate origins of ice-binding proteins in Antarctic Chlamydomonas species. PLoS One. 2013;8:e59186. doi: 10.1371/journal.pone.0059186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.A., Morgan-Kiss R. Multiple ice-binding proteins of probable prokaryotic origin in an Antarctic lake alga, Chlamydomonas sp. ICE-MDV (Chlorophyceae) J. Phycol. 2017;53:848–854. doi: 10.1111/jpy.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szcześniak M.W., Ciomborowska J., Nowak W., Rogozin I.B., Makałowska I. Primate and rodent specific intron gains and the origin of retrogenes with splice variants. Mol. Biol. Evol. 2011;28:33–37. doi: 10.1093/molbev/msq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikimedia-Commons Wikimedia commons, the free media repository. 2020. https://commons.wikimedia.org/wiki/Main_Page
- Zhang C., Gschwend A.R., Ouyang Y., Long M. Evolution of gene structural complexity: an alternative-splicing-based model accounts for intron-containing retrogenes. Plant Physiol. 2014;165:412–423. doi: 10.1104/pp.113.231696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Evolution by gene duplication: an update. Trends Ecol. Evol. 2003;18:292–298. [Google Scholar]
- Zhang Z., Qu C., Zhang K., He Y., Zhao X., Yang L., Zheng Z., Ma X., Wang X., Wang W. Adaptation to extreme Antarctic environments revealed by the genome of a sea ice green alga. Curr. Biol. 2020;30:1–12. doi: 10.1016/j.cub.2020.06.029. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Zhang Y., Long M. Extensive structural renovation of retrogenes in the evolution of the Populus genome. Plant Physiol. 2009;151:1943–1951. doi: 10.1104/pp.109.142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimin A.V., Puiu D., Luo M.-C., Zhu T., Koren S., Marçais G., Yorke J.A., Dvořák J., Salzberg S.L. Hybrid assembly of the large and highly repetitive genome of Aegilops tauschii, a progenitor of bread wheat, with the MaSuRCA mega-reads algorithm. Genome Res. 2017;27:787–792. doi: 10.1101/gr.213405.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The assembled genome sequences and the raw sequencing data of UWO241 are deposited at the US National Center for Biotechnology Information (NCBI) database under BioProject accession PRJNA547753, nucleotide accession VFSX00000000, and BioSample accessions SAMN11975472 and SAMN11975511. This study did not generate new code.