Summary
The physical microenvironment of cells plays a fundamental role in regulating cellular behavior and cell fate, especially in the context of cancer metastasis. For example, capillary deformation can destroy arrested circulating tumor cells while the dense extracellular matrix can form a physical barrier for invading cancer cells. Understanding how metastatic cancer cells overcome the challenges brought forth by physical confinement can help in developing better therapeutics that can put a stop to this migratory stage of the metastatic cascade. Numerous in vivo and in vitro assays have been developed to recapitulate the metastatic processes and study cancer cell migration in a confining microenvironment. In this review, we summarize some of the representative techniques and the exciting new findings. We critically review the advantages, as well as challenges associated with these tools and methodologies, and provide a guide on the applications that they are most suited for. We hope future efforts that push forward our current understanding on metastasis under confinement can lead to novel and more effective diagnostic and therapeutic strategies against this dreaded disease.
Subject Areas: Microenvironment, Bioengineering, Tissue Engineering, Cancer
Graphical Abstract
Microenvironment; Bioengineering; Tissue Engineering; Cancer
Introduction
Cancer is the second leading cause of human death worldwide (Bray et al., 2018) in which metastasis is the major cause of cancer-associated death (Welch and Hurst, 2019). Metastasis is a multi-step process involving the dissemination of cancer cells from their primary sites to nearby or distant tissues. For most types of cancer, the 5-year survival rates of patients diagnosed with metastasized tumors are below 30%, and this situation has not improved during the period from 2005-2015 despite advancements made in diagnostic and therapeutic approaches (Jemal et al., 2005; Siegel et al., 2015; Steeg, 2016). Targeting metastasis is thus essential to effectively combat cancer. Understanding how each step in the metastasis cascade progresses is key to developing effective intervention methods to prevent or inhibit metastasis (Mohammadi and Sahai, 2018; Steeg, 2016).
Choosing the right tools before we start investigating the different steps in the metastasis cascade will ensure effective investigation and reliable results. Metastasis does not happen on a flat plastic surface with “free flow” of supplements and unconfined space (Quail and Joyce, 2013; Schindler et al., 2006). Instead, cancer cells live in and interact within a complex three-dimensional (3D) microenvironment involving different types of cells, various types of chemical factors, and diverse extracellular matrix (ECM) (Kapałczyńska et al., 2018; Micek et al., 2020; Riedl et al., 2017; Sarvestani et al., 2020). Among these microenvironmental factors, mechanical confinements which directly or indirectly exert physical forces on cancer cells during their metastatic journey have recently attracted much attention (Chaudhuri et al., 2018; Gensbittel et al., 2020; Paul et al., 2016a; Wirtz et al., 2011).
Mechanical confinements in cancer metastasis
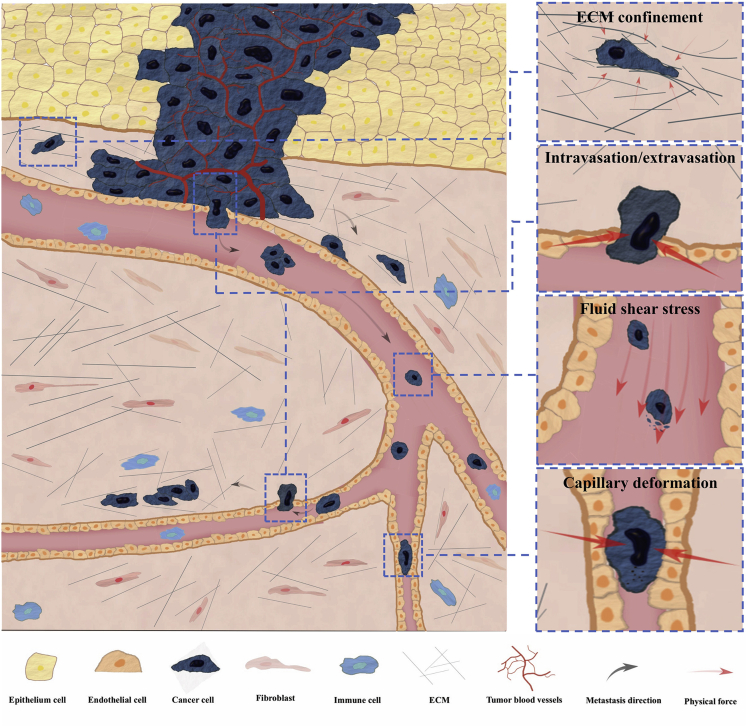
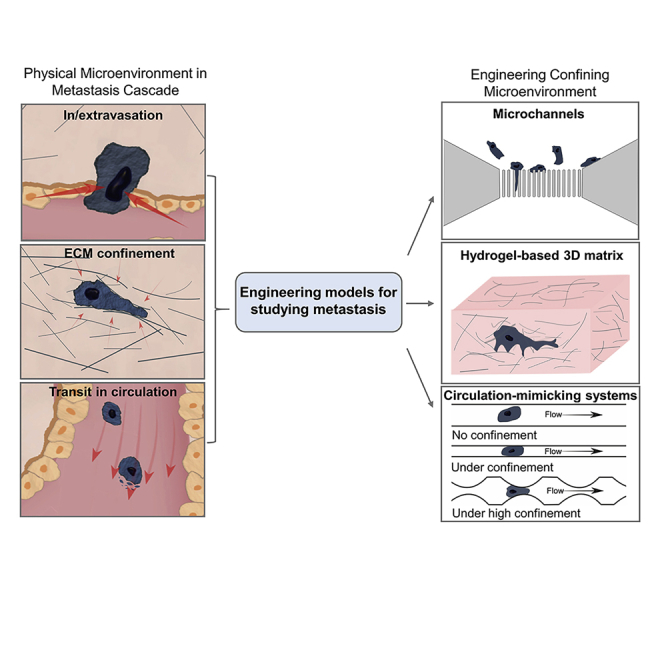
Cancer cells interact with confining microenvironment either actively or passively during metastasis as shown in Figure 1. These mechanical confinements and the associated physical forces have a fundamental impact on the metastatic processes.
Figure 1.
Cancer microenvironment encountered during metastasis
Cancer cells from the primary tumor break the basement membrane and invade into the connective tissues which contain fibrillar ECM. The pore size inside the ECM varies and may cause significant deformation on the cells transmigrating within it. Metastatic cancer cells nearby blood vessels might break the basement membrane and squeeze through the interstitial space to enter the blood circulation. Additionally, primary tumor may stimulate formation of blood vessels through an angiogenesis process. These circulating tumor cells (CTCs) can travel via circulation to reach a distant organ where they can extravasate, invade, and proliferate to form a secondary tumor. During this process, physical forces arising from various sources are exerted on the metastatic cancer cells.
During the metastatic invasion, cancer cells are faced with dense ECM in the connective tissues and tight intercellular spaces within the endothelium layers. The cancer cells typically have diameters from 10 to 20 μm, whereas the microenvironment consists of interstitial space ranging from 3 to 30 μm in width and intercellular space of about only 1 to 2μm (Wolf et al., 2009; Zuela-Sopilniak and Lammerding, 2019). These minute spaces that cancer cells encounter during invasion pose a great challenge to the mobility and deformability of cancer cells (Krause and Wolf, 2015; McGregor et al., 2016; Mohammadi and Sahai, 2018; Paul et al., 2016a). Cancer cells that are not able to generate enough forces or efficiently squeeze their cell body through these small constrictions barely stand a chance to metastasize to a distant site. Apart from playing a role as barriers, the forces exerted on the cancer cells or by the cancer cells in order to overcome the space limitation may rupture their nuclei and cause genome instability (Denais et al., 2016; Irianto et al., 2017), directly alter the genome architecture and transcriptional profile of cancer cells (Uhler and Shivashankar, 2017), or select cancer cells with high metastatic capacity (Rudzka et al., 2019).
Besides the ECM confinements that metastatic cancer cells need to actively deal with, circulating tumor cells (CTCs) are subjected to confined conditions imposed in the blood flow such as that encountered while traversing capillaries (Follain et al., 2020; Strilic and Offermanns, 2017). In mice, only less than 0.1% of the cancer cells survive in the circulation after tail vein injection (Fidler, 1970). In the 1980s, it was observed that small capillaries can cause fragmentation and apoptosis in CTCs and especially the cells arrested in the pulmonary capillaries where additional stretching and compression can be brought by lung expansion during respiration (Liaw et al., 2016; Weiss and Dimitrov, 1986; Weiss et al., 1985). These mechanical stresses are thought to be the main reason for the inefficiency of blood-borne metastasis (Follain et al., 2020; Honn, 1987; Weiss, 1990). Recent studies also revealed that the shear stress on cancer cells circulating in the blood flow influences cell survival, modulates cellular phenotypes, and determines subsequent extravasation processes (Dart, 2018; Follain et al., 2018, 2020; Gensbittel et al., 2020).
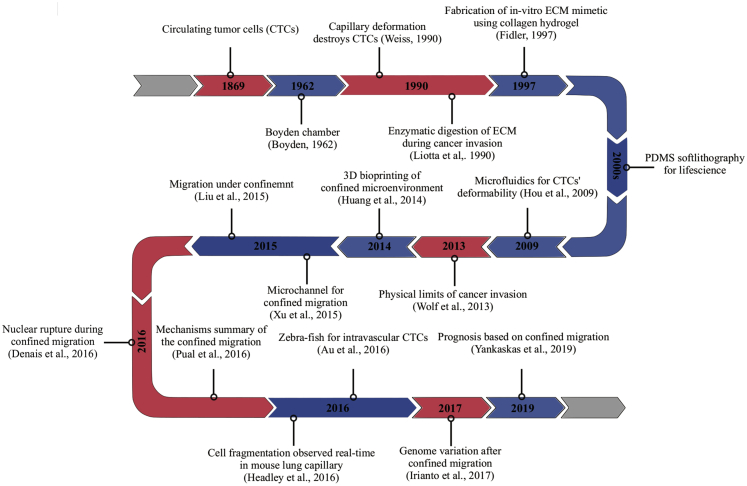
To better understand the interaction between cancer cells and their physical environment, different experimental models have been widely explored in the past two decades. In vivo systems are important but not always feasible, especially when a well-controlled environment is desired. Engineered systems that resemble one or more aspects of the in vivo microenvironment have played a fundamental role in pushing forward such research. In the subsequent sections, we will introduce the techniques developed for studying cancer cells under physical confinement and discuss their advantages and limitations (a technological timeline is summarized in Figure 2, and comparisons of different techniques are presented in Table 1). We will start with a brief introduction of animal models for studying cancer metastasis which have an intimate correlation with the in vitro models developed over the years. Following that, we will focus our discussion on the engineering systems for studying two specific situations where cancer cells encounter confinement during their metastatic journey: (1) The confined migration in the stroma and (2) the shear stress or vessel geometry induced confinement in blood. While summarizing the exciting technological advancements and scientific achievements in studying metastasizing cancer cells under confinement, we hope this review can help guide researchers who are new in this emerging field to work toward a better understanding of metastasis, which to date is still the predominant cause of cancer-associated death.
Figure 2.
Timeline of the key discoveries and the emergence of key technologies
Table 1.
Summary and comparison of different technologies for studying cancer cells under confinement
| Technology | Key principle | Cost | Protocol complexity | Physiological relevance | Profession | Imaging resolution | Representative applications |
|---|---|---|---|---|---|---|---|
| Explant for cancer invasion study | Studying cancer cell interaction with tissues ex vivo | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | Mouse brain slice invasion (Jung et al., 2002); mouse ear ex-plant (Raab et al., 2016), etc. |
| Mouse intravital imaging | Imaging cancer cell real-time in vivo in mammals | ∗∗∗∗ | ∗∗∗∗∗ | ∗∗∗∗∗ | ∗∗∗∗∗ | ∗ | Arresting of CTCs at mouse lung post-injection (Headley et al., 2016) |
| Zebrafish in vivo imaging | Imaging cancer cell real time in vivo in non-mammals | ∗∗∗ | ∗∗∗∗ | ∗∗∗∗ | ∗∗∗∗ | ∗∗ | CTCs traverse capillary collectively (Au et al., 2016) |
| Chick embryo invasion assay | Studying cancer cell invasion in developing embryo | ∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | Chick embryo for cancer cell intravasation study (Koop al., 1995) |
| Microfluidic constricted channels | Studying cancer cell in vitro with well-defined spatial parameters | ∗ | ∗ | ∗ | ∗ | ∗∗∗∗∗ | Deformability measurement of CTCs (Hou et al., 2009); Study of nuclear rupture during constricted migration (Denais et al., 2016), etc. |
| Microfluidic organ-on-a-chip model | Creating in vivo mimicking environment for cancer cell metastasis study | ∗ | ∗∗ | ∗∗ | ∗ | ∗∗∗∗∗ | Blood vessel-on-a-chip for studying cancer cells extravasation (Chen et al., 2017) |
| Cell confiner | Applying confinement on cultured cells through an air pressure-driven PDMS piston | ∗ | ∗ | ∗ | ∗ | ∗∗∗∗∗ | Studying the influence of confinement on the migration modes of cancer cells (Liu et al., 2015) |
| Hydrogel-based 3D matrix | Studying cancer cell in vitro in relatively well-defined 3D matrices | ∗ | ∗∗ | ∗∗ | ∗ | ∗∗∗∗ | Studying protease activity during ECM invasion (Wolf et al., 2009); Studying invasiveness of cancer cells in a 3D matrix (Wisdom et al., 2018), etc. |
Techniques for studying metastasis under confinement
Animal models
In vivo models were used much earlier than most of the in vitro systems for studying cancer metastasis. The aim of using in vitro assays for metastasis study is to reproduce an in vivo mimicking environment with well-controlled conditions. Designing an in vitro system often uses parameters derived from in vivo studies when human samples are not available (Wolf et al., 2009; Zaman, 2013). For example, the arrangement of collagen fibrils in the ECM and the structure of the basement membrane guide the design of synthetic gels for 3D invasion studies (Wolf and Friedl, 2011). Meanwhile, animal models provide the most physiologically relevant microenvironment for supporting and validating a proposed process or mechanism found in in vitro observations. Here, we will briefly mention several successful animal models that were widely used for studying the physical interactions during cancer metastasis to better appreciate the advantages and disadvantages of different experimental models. As animal models are not our focus, we refer readers to the excellent reviews and research articles cited in this section (Entenberg et al., 2018; Follain et al., 2018; Khanna and Hunter, 2005; Noto and Yeshi, 2017; Padmanaban et al., 2020; Wolf et al., 2009).
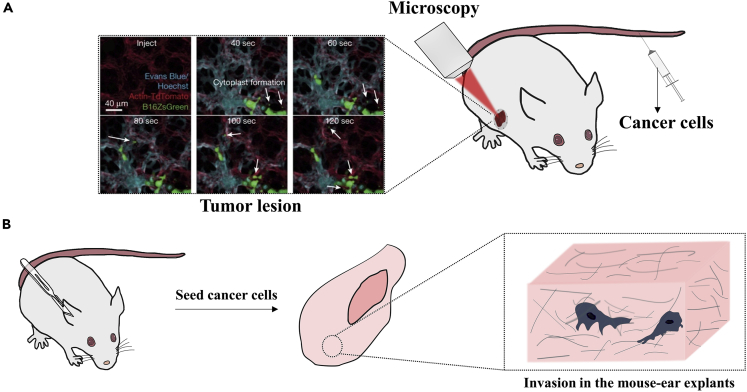
Mice and rats are the most frequently used mammals for studying cancer development in vivo (Gomez-Cuadrado et al., 2017; Noto and Yeshi, 2017). Local invasion and spontaneous metastasis can be investigated through a subcutaneous tumor model, and the development of secondary tumors through blood-borne metastasis can be easily monitored in tumor injection models. By varying the implantation or injection locations, metastasis to different secondary sites can usually be recapitulated in mouse models as well (Gomez-Cuadrado et al., 2017). One challenging task researchers have been pursuing is to visualize the tumor formation or invasion processes in live animals. The advancement of intravital imaging techniques has brought us closer to this goal (Condeelis and Segall, 2003; Entenberg et al., 2018; Paul et al., 2019; Weigelin et al., 2012). Through an imaging window created on mice, immediate arrest at the pulmonary capillary can be observed in real time after tail-vein injection with a lung intravital microscopy (Headley et al., 2016) (Figure 3A). Multiphoton laser scanning microscopy developed by Kienast and co-workers was successfully utilized to visualize the real-time brain metastasis formation in mice as well (Kienast et al., 2010). Despite significantly improved imaging quality, the complexity and cost of intravital imaging make it currently impractical to rely experiments entirely on it. To achieve a higher imaging depth and resolution while maintaining a reasonable cost, tissues can be fixed for imaging after an animal being sacrificed. For example, a mouse lung can be fixed for observing the tumor cells arrested in the lung capillary, and different markers can be immunostained for confocal imaging (Furlow et al., 2015). The limitation is that acquiring the metastasis dynamics of cancer cells will not be possible here. A compromised method is to obtain animal tissues for ex vivo studies, which sacrifices part of the physiological relevance for temporal-spatial resolution. Mouse ear explant (Raab et al., 2016) (Figure 3B) and organotypic brain slices (Jung et al., 2002) are two good examples. Another way of trading physiological relevance for cost reduction and imaging quality is to employ lower vertebrates for the studies. Zebrafish and chick embryos are such systems that have been applied successfully as models to study cancer cell intravasation, transit in blood circulation, and extravasation processes (Au et al., 2016; Busch et al., 2013; Follain et al., 2018; Osmani et al., 2019; Paul et al., 2019).
Figure 3.
In vivo and ex vivo models for studying cancer cells in a confined microenvironment
(A) Intravital imaging of cancer cells in vivo. Cancer cells are usually injected through the tail vein, seeded under the dermis or mammalian fat pad. Surgery is usually performed to create a microscopy observation window at the site of interest allowing imaging of tumor development and invasion in vivo. (Reproduced with permission from (Headley et al., 2016)).
(B) Mouse ear explants for ex vivo imaging of cancer cells. Cancer cells are seeded and cultured on dissected ear explants allowing them to invade under physiological conditions. Thereafter, the explant and cancer cells can be fixed and stained for imaging.
While animal models allow us to study metastatic events in a physiologically relevant condition, the contribution of each environmental factor can hardly be decoupled from the rest in such complex systems. Additionally, it is hard to tune the in vivo microenvironment, e.g., the ECM porosity, level of blood shear stress, and matrix stiffness for specific mechanistic studies. Thus, bottom-up engineering systems with well-controlled microenvironmental conditions can provide some help here, and they are playing an increasingly important role in studying cancer metastasis. If physiological relevance becomes a concern for the findings obtained in in vitro models, validation can always be performed in a suitable animal model thereafter. One recent example is the observation of nuclear rupture and repair during cancer cell transmigration through small constrictions and the subsequent validation through intravital imaging of the migrating cancer cells cultured under mouse dermis (Denais et al., 2016).
Engineering systems for studying confined migration of cancer cells
The ECM is a complex 3D environment formed by scaffolding proteins with pore sizes much smaller than the size of a typical cancer cell (Friedl and Wolf, 2003a; Wolf et al., 2009). This confining environment has been regarded as a physical barrier for cancer metastasis, without degradation of which the cancer cells can hardly pass through (Gupta and Massagué, 2006). Indeed, tunnels are found in the ECM with significantly remodeled topographies after the transmigration of cancer cells. Upregulation of genes encoding enzymes that digest ECM structural proteins was shown to correlate with cancer metastasis and poor disease prognosis (Lim et al., 2017). Matrix metalloproteinases (MMPs) that degrade ECM proteins are highly expressed by cancer cells or other cells in the cancer-associated microenvironment (Friedl and Wolf, 2008). Chemically remodeling the microenvironment is widely adopted by cancer cells during metastasis. Targeting the enzymatic activity of cancer cells has thus become a promising candidate for reducing tumor spread. Unfortunately, clinical trials using MMP inhibitors in cancer treatment had resulted in poor performance (Coussens et al., 2002). Accumulating evidence has shown that cancer cells can still transmigrate in a protease-independent manner (Weigelin et al., 2012; Wolf et al., 2003), raising a new question as to how cancer cells can squeeze through densely packed connective tissues without activating ECM cleavage enzymes. Answer to this question will be critical in coming up with new strategies that may inhibit the metastatic spread of cancer cells, and experimental platforms with precisely controlled spatial confinement are essential for such experimental investigation (Morris et al., 2020; Paul et al., 2016b; Um et al., 2017; Zuela-Sopilniak and Lammerding, 2019).
Transwell inserts
One easy-to-use and commercially available assay is a transwell membrane insert (Figure 4D) with custom-designed pore sizes for cell culture developed from the Boyden chamber (Boyden, 1962). The cancer cells are seeded on one side of the membrane allowing those that penetrated the holes to be directly visualized and counted under microscopy. One of the widest applications of a transwell insert in cancer study is to assess the “invasiveness” of cancer cells. Generally, the more efficient a cancer cell transmigrates to the other side of the membrane, the more metastatic it is.
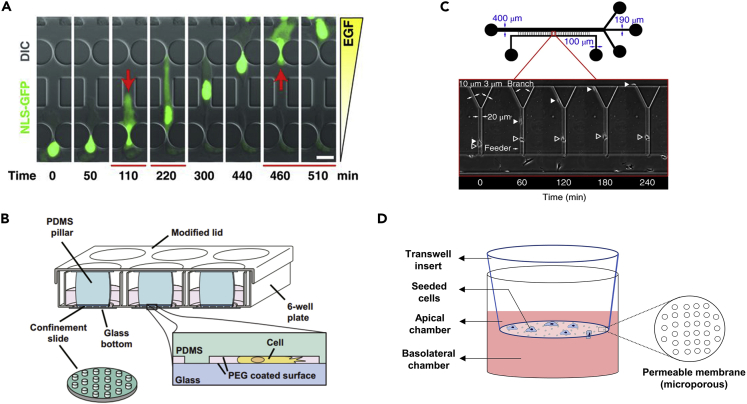
Figure 4.
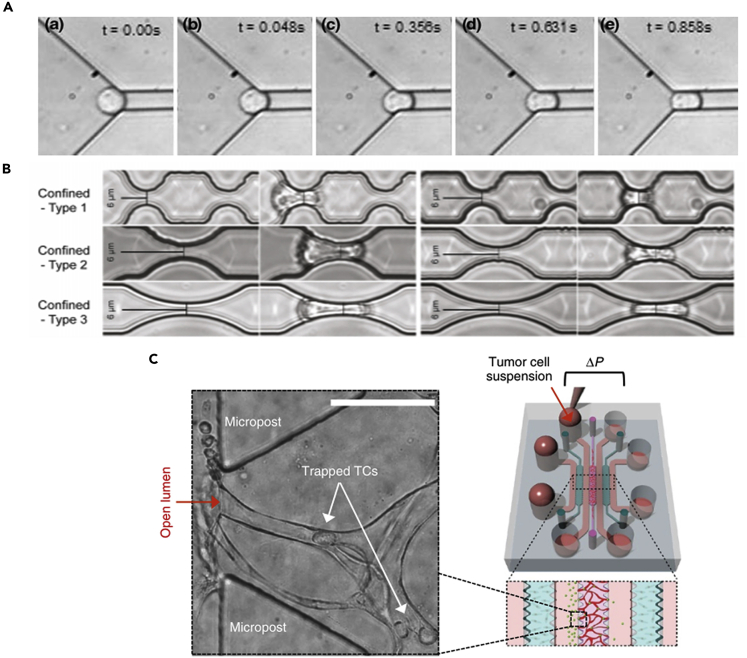
Microchannels for studying cancer cell migration under confinement
(A) Microfluidic channels with different sized constrictions for studying nuclear deformation during confined migration. (Reproduced with permission from (Denais et al., 2016)).
(B) PDMS ceiling with short supporting pillars can create a controllable confined microenvironment for studying the effects of varying degrees of confinement on cancer cells. (Reproduced with permission from (Liu et al., 2015)).
(C) Y-shape microfluidic channel with different sized openings for comparing the metastastic ability of cancer cells. (Reproduced with permission from (Yankaskas et al., 2019)).
(D) Transwell membrane inserts for studying cancer cell transmigration through small pores with defined diameters.
The abilities of cancer cells to migrate through different sized pores are varied. For example, in larger pores, the migratory ability is the major determinant of the transmigration efficiency while nuclear deformability dominates in smaller sized pores (Harada et al., 2014; McGregor et al., 2016). It should also be noted here that transwell membranes may be selecting a subpopulation of cancer cells from mixed genomic backgrounds, and the transmigration process can generate additional genomic alternations in single clonal cancer cells. A work by Irianto et al. showed that by selecting single clonal cancer cells that migrated through 3μm diameter pores for multiple rounds, a significant amount of chromosome gain and loss could be observed in the transwell-selected cancer cells (Irianto et al., 2017). A study involving a similar experimental design also found that cancer cells that passed through the transwell assays exhibited an upregulated mitogen-activated protein kinase (MAPK) pathway which modulated the populational migratory behavior and cell stiffness (Rudzka et al., 2019). These studies highlighted that the mechanical environment of metastasizing cancer cells can apply a selection pressure on them. Expanding the mechanical selection experiments to different cell types or in a microenvironment mimicking different steps in the metastatic cascade can likely provide us a profound insight of the advantageous traits required for successfully circumventing the mechanical barriers, which can lead to novel druggable targets.
Artificial ceiling
Confinement on cancer cells can be induced on demand by introducing a ceiling and spacing pillar with a desired height (Figure 4B). Utilizing the deformable nature of polydimethylsiloxane (PDMS), the degree of confinement can be made adjustable through an externally applied pressure. This method is employed in determining the height threshold that induces nuclear rupture on cancer cell nuclei (Le Berre et al., 2012). It is also found that under different degrees of confinement between low adhesion surfaces, cancer cells can transit from a mesenchymal migration mode to an ameboid movement, which significantly increases their migration speed (Liu et al., 2015).
Microfluidic channel
Microfluidic-based systems are convenient tools for studying the confined migration of cancer cells. Channels and pillars with different geometries and dimensional parameters can be customized and created easily on a piece of glass through PDMS soft lithography. One of the classic examples is to create an array of pillars with different-sized gaps to study how cancer cells squeeze through small constrictions (Figure 4A). A chemogradient is usually created across the two sides of the migration channel for stimulating the directional movement of cancer cells. The behavior of cancer cells squeezing inside the channels can be recorded easily via time-lapse microscopies. Using this kind of microfluidic system, the ability to maintain nuclear integrity is found to be a key determinant of the transmigration survival of cancer cells, as nuclear ruptures can occur when squeezed through small gaps (Davidson et al., 2014; Denais et al., 2016; Raab et al., 2016) . Such detailed molecular dynamics will not be easily captured in in vivo models or other in vitro systems, and these findings identified potential vulnerabilities of the metastasizing cancer cells, e.g., the nuclear repair process after squeezing through small gaps.
Apart from creating holes in microchannels for studying cellular squeezing events, a long straight microchannel confining cancer cells can offer insights on how cancer cells migrate along linear tracks. MDA-MB-231 breast cancer cells were found to undergo mesenchymal to ameboid transition to facilitate their invasion in the confining matrix (Holle et al., 2019). Another emerging aspect of tumor invasion is the decision-making ability of cancer cells when they are faced with branching tracks. The ECM is like a maze for cancer cells migrating inside, and a recent study demonstrated a surprising ability of pancreatic cancer cells to navigate efficiently inside a microfluidic-based maze (Tweedy et al., 2020). Another recent study on immune cells also unveiled that the cell nucleus and microtubule-organizing center can act as a gauge to efficiently navigate neutrophil transmigration in matrices with differently sized pores (Renkawitz et al., 2019). It is unclear how cancer cells behave in a maze under confinement or differently sized branches. In our opinion, these are important factors determining the metastatic efficiency of cancer cells.
Hydrogel 3D matrix
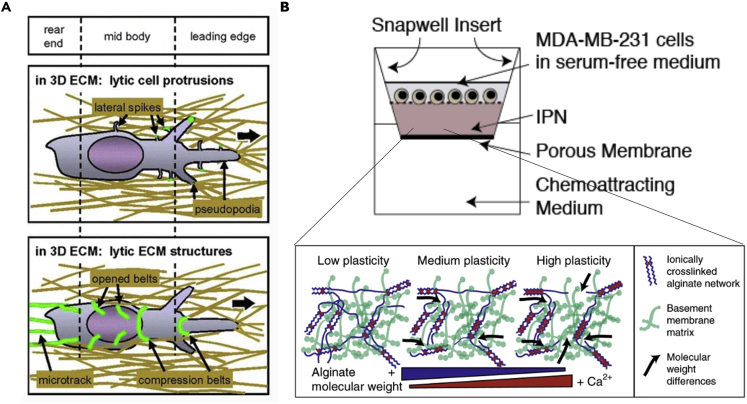
Self-assembling collagen hydrogel has made in vitro ECM with tunable stiffness, different pore sizes, and fibrillar structures possible (Geiger et al., 2019; Gillette et al., 2008; Riching et al., 2015; Wolf et al., 2009). Briefly, the degree of cross-linking and the matrix mechanical property can be controlled by additives and the concentration of collagen stock solution used. The hydrogel solidifies at room temperature naturally and can be cast or injected easily into pre-designed molds or microchannels. The resulting devices are thin and transparent, making them suitable for imaging. There is a relatively long history of using natural collagen derived from animals to study 3D cancer cell invasion (Friedl et al., 1997). Protease digestion is a prevailing mechanism found to assist cancer cell penetration through the dense collagen matrix (Figure 5A), providing early evidence for an active remodeling of the ECM by metastatic cancer cells. Subsequent development identified a change of migration modes when protease activity gets inhibited (Friedl and Wolf, 2003b; Wolf et al., 2003). Recently, a jamming transition is well characterized in invading cancer cells confined in a collagen matrix (Haeger et al., 2014; Ilina et al., 2020), which explained the plasticity of single-cell and collective cell invasion under physical confinement.
Figure 5.
Hydrogel-based 3D matrix for studying cancer cell invasion
(A) The invasion of cancer cells into collagen gel elicits proteolytic activity of cancer cells. (Reproduced with permission from (Wolf and Friedl, 2009)).
(B) An ECM mimicking system with tunable plasticity formed by basement membrane proteins and alginate cross-linking network. (Reproduced with permission from (Wisdom et al., 2018))
Reproducibility is a challenging aspect of animal-derived collagens (rat tail, porcine skin, etc.). Heterogeneity and batch difference of the collagen may affect the reproducibility of results (Shinsato et al., 2020). These limitations can likely be overcome by changing the formula, chemical modification of the gels, high precision 3D bioprinting, and advanced imaging techniques that can visualize cell-ECM interaction at the subcellular level (Huang et al., 2014; Li and Kumacheva, 2018; Trujillo-de Santiago et al., 2019; Vasudevan et al., 2020). Synthetic hydrogels such as polyethylene glycol (PEG) (Raeber et al., 2005), hyaluronic acid (Xu et al., 2012), and alginate (Xu et al., 2013) can offer better quality control, as well as enable tunability of the mechanical properties of the fabricated 3D matrices. A recent study shows that tuning the plasticity of the ECM is possible by adjusting the molecular weight and degree of cross-linking of interpenetrating networks formed by reconstituted basement membrane and alginate (Figure 5B). Cancer-cell-generated forces are found to be sufficient for the cells to create metastatic tracks through negotiating the plasticity of the local matrix and deforming the ECM that allows them to translocate the seemly impenetrable small pores (Wisdom et al., 2018). In another study, breast tumor spheroids growing inside PEG-heparin 3D hydrogel matrix were found responsive to the matrix stiffness in a p21- and ROCK-dependent manner, and the force exerted by cancer cells can be calculated from the movement of beads embedded inside the gel (Taubenberger et al., 2019).
Perfusion systems for studying cancer cells in transit
Studying cancer cells in an in vitro system that mimics blood circulation will allow us to better investigate the fate of CTCs, especially the properties that are necessary for their intravascular survival and efficient transmigration in blood vessels (Castro-Giner and Aceto, 2020; Furlow et al., 2015; Massagué and Obenauf, 2016; Zheng et al., 2017). CTCs in transit experience two kinds of confinement: (1) confinement induced by shear stress in blood flow and (2) intravascular deformation exerted by the geometry of blood vessels. The mechanical properties of cancer cells determined by the cytoskeletal arrangement and nuclear lamina are crucial factors influencing the metastatic efficiency in these two scenarios (Follain et al., 2020; Gensbittel et al., 2020). Perfusion-based systems recapitulating the mechanical stress experienced by cancer cells in these situations have played an important role in the research conducted in this field.
Mechanophenotyping of cancer cells in circulation
The mechanical properties of CTCs, in a way, reflect the resilience of cancer cells to the mechanical stresses, as well as their metastatic efficiency in circulation (Alibert et al., 2017; Wirtz et al., 2011). The mechanical characterization of cancer cells has led to an emerging field of mechanophenotyping that aims to elucidate the differences of mechanical properties between cancer and healthy cells for diagnostic and prognostic purposes (Urbanska et al., 2020; Wu et al., 2018). Traditionally, methods developed for the mechanical characterization of cells (Wu et al., 2018) include atomic force microscopy (Li et al., 2008), optical tweezers (Dao et al., 2003), micropipe aspiration (Mak and Erickson, 2013), etc. While these techniques can reveal a specific aspect of the mechanical properties of cells, the tests are not exerting a physiological relevant deformation on cancer cells and they generally suffer from low throughput. In this context, microfluidic systems with channel dimensions mimicking that of capillaries have been developed to probe the deformability of CTCs (Hou et al., 2009). Cancer cells can flow through a microfluidic channel with small openings, and the entry time and transit time can be recorded to quantify how easily a cell can traverse these constrictions (Figure 6A). With this microfluidic assay, breast cancer cells were shown to be more deformable than the non-cancerous breast epithelial cells.
Figure 6.
In vitro perfusion assays mimicking blood circulation for metastasis studies
(A) Microfluidic constricted channels for quantifying cancer cell deformability in a microcirculation mimicking condition. (Reproduced with permission from (Hou et al., 2009)).
(B) Microfluidic constricted channels with different geometries for studying the influence of intravascular deformation on CTCs. (Reproduced with permission from (Cognart et al., 2020)).
(C) In vitro microcirculation-on-a-chip microfluidic device for studying cancer cell behavior in capillaries under flow condition. (Reproduced with permission from (Chen et al., 2017))
Recently, microfluidic systems with a similar concept were developed for more precise, efficient, and reproducible quantifications of cellular mechanics (Davidson et al., 2019; Li et al., 2013; Mak and Erickson, 2013). Additionally, there are concerns that the interaction between cancer cells and microchannel walls may influence the accuracy of measured deformability, so non-contact methods such as optical stretching (real-time deformability cytometry) (Otto et al., 2015) and hydrodynamic stretching (Tse et al., 2013) on flowing cancer cells have been developed. As examining the heterogeneity of cancer cells for discovering metastatic subpopulations requires high throughput phenotyping of cancer cells, the advantages of microfluidic systems are highly valued in this emerging field (Che et al., 2018; Davidson et al., 2019).
Studying shear stress on circulating tumor cells
Shear stresses acting on CTCs can be deleterious (Fan et al., 2016; Regmi et al., 2017). On the other hand, these mechanical forces can also modulate the cancer cells in circulation (Follain et al., 2020). Simulating physiological shear stress on cancer cells is achievable by flowing suspended cells through a syringe tip with appropriate size at a defined flow rate. The shear stress on cells can be controlled by manipulating the infusion rate of a syringe pump. Using this method, malignant cancer cells are found to be generally more resistant to fluid shear stress than less malignant cancer cells (Barnes et al., 2012). Nuclear lamina protein lamin A and C are found to regulate the survival of cancer cells under shear stress (Mitchell et al., 2015). Using a peristaltic pump to drive the flow will allow the cell suspension to continuously flow from several hours to days. Long-term exposure of cancer cells to shear stress reduces their viability significantly, and the survival is regulated in an actomyosin-dependent way (Xin et al., 2019). Interestingly, recent evidence also shows that the epithelial-to-mesenchymal transition of CTCs and their enhanced chemotherapy resistance may also be mediated by the shear stress in the blood (Gong et al., 2015; Xin et al., 2019, 2020).
Studying vessel geometry-induced deformation
Intravascular deformation is thought to cause cellular fragmentation and apoptosis in cancer cells (Albertsson et al., 1995; Weiss et al., 1985). Recent studies have shown that a similar level of deformation on the cell nucleus can cause nuclear ruptures, DNA damage, and genome instability in cancer cells squeezing through constrictions as well (Denais et al., 2016; Irianto et al., 2017; Raab et al., 2016). Studies on circulating white blood cells have also shown that intravascular deformation can influence immune cell function (Ekpenyong et al., 2017; Worthen et al., 1989). However, studies on CTCs that involved both shear flow and vessel geometry are limited despite successful investigation involving just shear stress. Flowing cancer cells through constricting microfluidic channels can be used to evaluate the performance of surviving cancer cells after traversing capillary-mimicking structures (Nath et al., 2018). This can subsequently allow us to mechanistically investigate the factors mediating cell survival and the potential alternations on capillary-arrested CTCs. In a recent study, a minimal model of microcirculation was developed to study the effects of shear stress and geometry-induced deformation on CTCs (Figure 6B). The researchers flowed epithelial or mesenchymal origin breast cancer cells through a microfluidic channel with small constrictions and studied their cellular phenotype changes after deformation (Cognart et al., 2020). Significant cell plasticity was observed after such deformation, and an increased DNA damage level was found. Subsequent gene expression study revealed increased expression of epithelial-mesenchymal transition-associated genes under the circulatory conditions.
Blood vessel-on-a-chip
A human microcirculation-mimicking microfluidic device with a vascular network formed by endothelium cells can provide a platform to dynamically study the arrest, transendothelial migration, and formation of an early metastatic lesion with the interaction of different cell types (Chen et al., 2017) (Figure 6C). A recent study exploring the seeding of endothelial cells on PDMS tubes also showed promise in replicating the vascular structure with the cellular environment in vitro for imaging CTCs transiting in capillaries (Xi et al., 2017). Extensive on-going efforts are trying to build an in vivo relevant blood vessel-on-chip model for cancer studies (Coughlin and Kamm, 2020). Pioneering investigations have demonstrated their potential in studying cancer cell-blood vessel interaction or the extravasation processes from multiple aspects, for example, with the interstitial fluid flow (Hajal et al., 2021) or considering the mechanics of endothelium (Escribano et al., 2019).
Summary and perspectives
How cancer cells interact with their physical environment during metastasis is an essential question to address with the growing evidence highlighting significant mechanical modulations on metastasizing cancer cells (Follain et al., 2020; Gensbittel et al., 2020; Paul et al., 2016a; Wirtz et al., 2011). Mechanical stresses can cause DNA damage and genome instability (Denais et al., 2016; Irianto et al., 2017), actively select a more invasive subpopulation (Rudzka et al., 2019; Xin et al., 2019), and promote the malignancy of metastasizing cancer cells (Gong et al., 2015; Xin et al., 2020). These aspects are not only closely related to cancer progression but can also offer critical insights for developing novel therapeutics to inhibit such progression. To further study this, multiple in vitro models at different levels of complexity and with controllable parameters have been developed to explore such interactions. Microfluidics-based systems and hydrogel-based 3D matrices are two major categories of in vitro techniques for exploring confined migration, 3D invasion, and capillary deformation of cancer cells. These techniques have apparent advantages for their simplicity, low cost, and precise control of experimental conditions. Such technological advancements have led to fruitful results and a profound understanding of how physical confinement influences the metastatic process of cancer cells, marking good progress of our explorations in this field.
While the advantages of in vitro systems are evident, several associated issues need to be addressed: (1) Can we make the system more integrative in a controllable manner? For example, alter the surface that cancer cells interact with or include several additional cell types and mechanical cues from the tumor microenvironment (Kami, 2015) in one microfluidic device. This is in line with the pursuit for the “controlled complexity” of in vitro systems that will allow us to better recapitulate the actual tumor environment in vitro (Zaman, 2013). (2) Different types of cancer cells or even different subpopulations in the same cancer type behave differently under confinement. Can we separate the cells into different subpopulations efficiently and study their phenotypic differences? Preliminary studies have shown that the metastatic propensity and patient prognosis can be tested directly using such microfluidic platforms (Yankaskas et al., 2019) (Figure 4C). Can these findings be further extended and help design and test drugs that target the relevant migratory or navigating pathways?
One goal of studying the behavior of metastasizing cancer cells in a confining microenvironment is to find out how cancer cells overcome the mechanophysical challenges. Targetting these dedicated mechanisms is promising in limiting the metastatic invasion of cancer cells. Earlier studies have shown that the MMP activity of invading cancer cells allows them to digest ECM proteins for penetration after which MMP inhibitors immediately became novel drug candidates. However, further research showed that activated compensation mechanisms might have resulted in the inefficiency of the MMP inhibiting therapies (Coussens et al., 2002; Wolf et al., 2003). From research done in the past two decades, additional mechanisms utilized by metastasizing cancer cells to cope with the confining microenvironment are emerging. These include nuclear rupture repair mechanisms (Denais et al., 2016), shear-induced cytoskeleton alternations (Moose et al., 2020; Xin et al., 2019), and change in migration modes (Liu et al., 2015; Paul et al., 2016a; Wolf et al., 2003), etc. Our understanding of these processes is growing, and their importance has been widely appreciated. We believe subsequent work attempting to target the essential mechanisms for cancer cells to successfully metastasize in confining microenvironments can perturb and disrupt cancer cells in the metastasis cascade and greatly improve the overall survival rate of patients with cancer.
Acknowledgments
K.J. and L.L. thank the Mechanobiology Institute at the National University of Singapore for the Ph.D. Fellowship.
Authors contribution
Conceptual development: C.T.L. and K.J.; Drafting the manuscript: C.T.L., K.J., and L.L.; Revision and editing: C.T.L., K.J., and L.L.; Illustrating figures: L.L.
Declaration of interests
The authors declare no competing interests.
References
- Albertsson P.A., Nannmark U., Johansson B.R. Melanoma cell destruction in the microvasculature of perfused hearts is reduced by pretreatment with vitamin E. Clin. Exp. Metastasis. 1995;13:269–276. doi: 10.1007/BF00133482. [DOI] [PubMed] [Google Scholar]
- Alibert C., Goud B., Manneville J.B. Are cancer cells really softer than normal cells? Biol. Cell. 2017;109:167–189. doi: 10.1111/boc.201600078. [DOI] [PubMed] [Google Scholar]
- Au S.H., Storey B.D., Moore J.C., Tang Q., Chen Y.-L., Javaid S., Sarioglu A.F., Sullivan R., Madden M.W., O’Keefe R. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J.M., Nauseef J.T., Henry M.D. Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS One. 2012;7:e50973. doi: 10.1371/journal.pone.0050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre M., Aubertin J., Piel M. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr. Biol. (United Kingdom) 2012;4:1406–1414. doi: 10.1039/c2ib20056b. [DOI] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Busch C., Krochmann J., Drews U. The chick embryo as an experimental system for melanoma cell invasion. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0053970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Giner F., Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:1–12. doi: 10.1186/s13073-020-00728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P.K., Low B.C., Lim C.T. Mechanobiology of tumor growth. Chem. Rev. 2018;118 doi: 10.1021/acs.chemrev.8b00042. acs.chemrev.8b00042. [DOI] [PubMed] [Google Scholar]
- Che J., Yu V., Garon E.B., Goldman J., Carlo D. Di, Angeles L., Hematology S.M., Angeles L. Biophysical isolation and identification of circulating tumor cells. 2018;17:1452–1461. doi: 10.1039/c7lc00038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.B., Whisler J.A., Fröse J., Yu C., Shin Y., Kamm R.D. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc. 2017;12:865–880. doi: 10.1038/nprot.2017.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognart H.A., Viovy J.L., Villard C. Fluid shear stress coupled with narrow constrictions induce cell type-dependent morphological and molecular changes in SK-BR-3 and MDA-MB-231 cells. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-63316-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Segall J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- Coughlin M.F., Kamm R.D. The use of microfluidic platforms to probe the mechanism of cancer cell extravasation. Adv. Healthc. Mater. 2020;9:e1901410. doi: 10.1002/adhm.201901410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Dao M., Lim C.T., Suresh S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Sol. 2003;51:2259–2280. [Google Scholar]
- Dart A. Metastasis: go with the flow. Nat. Rev. Cancer. 2018;18:207. doi: 10.1038/nrc.2018.25. [DOI] [PubMed] [Google Scholar]
- Davidson P.M., Denais C., Bakshi M.C., Lammerding J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng. 2014;7:293–306. doi: 10.1007/s12195-014-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.M., Fedorchak G.R., Mondésert-Deveraux S., Bell E.S., Isermann P., Aubry D., Allena R., Lammerding J. High-throughput microfluidic micropipette aspiration device to probe time-scale dependent nuclear mechanics in intact cells. Lab Chip. 2019;19:3652–3663. doi: 10.1039/c9lc00444k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denais C.M., Gilbert R.M., Isermann P., Mcgregor A.L., Weigelin B., Davidson P.M., Friedl P., Wolf K. Nuclear envelope rupture and repair during cancer cell migration Log in to view PDF Science. Science. 2016;6:1–4. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekpenyong A., Töpfner N., Fiddler C., Herbig M., Li W., Summers C., Guck J., Chilvers E.R. Mechanical deformation induces depriming of neutrophils. Sci. Adv. Under Rev. 2017:1–12. doi: 10.1126/sciadv.1602536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D., Voiculescu S., Guo P., Borriello L., Wang Y., Karagiannis G.S., Jones J., Baccay F., Oktay M., Condeelis J. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods. 2018;15:73–80. doi: 10.1038/nmeth.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano J., Chen M.B., Moeendarbary E., Cao X., Shenoy V., Garcia-Aznar J.M., Kamm R.D., Spill F. Balance of mechanical forces drives endothelial gap formation and may facilitate cancer and immune-cell extravasation. Plos Comput. Biol. 2019;15:1–21. doi: 10.1371/journal.pcbi.1006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R., Emery T., Zhang Y., Xia Y., Sun J., Wan J. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I.J. Metastasis : quantitative analysis of Dis- tribution and fate of tumor emboli labeled. J. Natl. Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- Follain G., Osmani N., Azevedo A.S., Allio G., Mercier L., Karreman M.A., Solecki G., Garcia Leòn M.J., Lefebvre O., Fekonja N. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell. 2018;45:33–52.e12. doi: 10.1016/j.devcel.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Follain G., Herrmann D., Harlepp S., Hyenne V., Osmani N., Warren S.C., Timpson P., Goetz J.G. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer. 2020;20:107–124. doi: 10.1038/s41568-019-0221-x. [DOI] [PubMed] [Google Scholar]
- Friedl P., Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Friedl P., Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem. Soc. Symp. 2003:277–285. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- Friedl P., Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- Friedl P., Maaser K., Klein C.E., Niggemann B., Krohne G., Zänker K.S. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of α2 and β1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- Furlow P.W., Zhang S., Soong T.D., Halberg N., Goodarzi H., Mangrum C., Wu Y.G., Elemento O., Tavazoie S.F. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat. Cell Biol. 2015;17:943–952. doi: 10.1038/ncb3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger F., Rüdiger D., Zahler S., Engelke H. Fiber stiffness, pore size and adhesion control migratory phenotype of MDA-MB-231 cells in collagen gels. PLoS One. 2019;14:1–14. doi: 10.1371/journal.pone.0225215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensbittel V., Kräter M., Harlepp S., Busnelli I., Guck J., Goetz J.G. Mechanical adaptability of tumor cells in metastasis. Dev. Cell. 2020:1–16. doi: 10.1016/j.devcel.2020.10.011. S1534-5807(20)30801-30807. [DOI] [PubMed] [Google Scholar]
- Gillette B.M., Jensen J.A., Tang B., Yang G.J., Bazargan-Lari A., Zhong M., Sia S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- Gomez-Cuadrado L., Tracey N., Ma R., Qian B., Brunton V.G. Mouse models of metastasis: progress and prospects. DMM Dis. Model. Mech. 2017;10:1061–1074. doi: 10.1242/dmm.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C., Liu B., Yao Y., Qu S., Luo W., Tan W., Liu Q., Yao H., Zou L., Su F. Potentiated DNA damage response in circulating breast tumor cells confers resistance to chemotherapy. J. Biol. Chem. 2015;290:14811–14825. doi: 10.1074/jbc.M115.652628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G.P., Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Haeger A., Krause M., Wolf K., Friedl P. Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim. Biophys. Acta. 2014;1840:2386–2395. doi: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Hajal C., Ibrahim L., Serrano J.C., Offeddu G.S., Kamm R.D. The effects of luminal and trans-endothelial fluid flows on the extravasation and tissue invasion of tumor cells in a 3D in vitro microvascular platform. Biomaterials. 2021;265:120470. doi: 10.1016/j.biomaterials.2020.120470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Swift J., Irianto J., Shin J.W., Spinler K.R., Athirasala A., Diegmiller R., Dingal P.C.D.P., Ivanovska I.L., Discher D.E. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley M.B., Bins A., Nip A., Roberts E.W., Looney M.R., Gerard A., Krummel M.F. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531:513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holle A.W., Govindan Kutty Devi N., Clar K., Fan A., Saif T., Kemkemer R., Spatz J.P. Cancer cells invade confined microchannels via a self-directed mesenchymal-to-amoeboid transition. Nano Lett. 2019;19:2280–2290. doi: 10.1021/acs.nanolett.8b04720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn V. Interactions of cancer cells with the microvasculature during metastasis. FASEB J. 1987;2:12–21. doi: 10.1096/fasebj.2.1.3275560. [DOI] [PubMed] [Google Scholar]
- Hou H.W., Li Q.S., Lee G.Y.H., Kumar A.P., Ong C.N., Lim C.T. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices. 2009;11:557–564. doi: 10.1007/s10544-008-9262-8. [DOI] [PubMed] [Google Scholar]
- Huang T.Q., Qu X., Liu J., Chen S. 3D printing of biomimetic microstructures for cancer cell migration. Biomed. Microdevices. 2014;16:127–132. doi: 10.1007/s10544-013-9812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina O., Gritsenko P.G., Syga S., Lippoldt J., La Porta C.A.M., Chepizhko O., Grosser S., Vullings M., Bakker G.J., Starruß J. Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 2020;22:1103–1115. doi: 10.1038/s41556-020-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irianto J., Xia Y., Pfeifer C.R., Athirasala A., Ji J., Alvey C., Tewari M., Bennett R.R., Harding S.M., Liu A.J. DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 2017;27:210–223. doi: 10.1016/j.cub.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Murray T., Ward E., Samuels A., Tiwari R.C., Ghafoor A., Feuer E.J., Thun M.J. Cancer statistics, 2005. Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jung S., Kim H.W., Lee J.H., Kang S.S., Rhu H.H., Jeong Y. Il, Yang S.Y., Chung H.Y., Bae C.S., Choi C. Brain tumor invasion model system using organotypic brain-slice culture as an alternative to in vivo model. J. Cancer Res. Clin. Oncol. 2002;128:469–476. doi: 10.1007/s00432-002-0366-x. [DOI] [PubMed] [Google Scholar]
- Kami K. The role of the cell – cell interactions in cancer progression Cancer cell – fibroblast interaction in cancer progression. 2015;19:283–296. doi: 10.1111/jcmm.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł., Lamperska K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C., Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26:513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- Kienast Y., Von Baumgarten L., Fuhrmann M., Klinkert W.E.F., Goldbrunner R., Herms J., Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- Koop S., MacDonald I.C., Luzzi K., Schmidt E.E., Morris V.L., Grattan M., Khokha R., Chambers A.F., Groom A.C. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995;55:2520–2523. [PubMed] [Google Scholar]
- Krause M., Wolf K. Cancer cell migration in 3d tissue: negotiating space by proteolysis and nuclear deformability. Cell Adhes. Migr. 2015;9:357–366. doi: 10.1080/19336918.2015.1061173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kumacheva E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018;4:1–11. doi: 10.1126/sciadv.aas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Stratton Z.S., Dao M., Ritz J., Huang T.J. Probing circulating tumor cells in microfluidics. Lab Chip. 2013;13:602–609. doi: 10.1039/c2lc90148j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.S., Lee G.Y.H., Ong C.N., Lim C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008;374:609–613. doi: 10.1016/j.bbrc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Liaw C.-C., Chang H., Liao T.-Y., Wen M.-S., Yu C.-T., Juan Y.-H. The role of pulmonary veins in cancer progression from a computed tomography viewpoint. J. Oncol. 2016;2016:1872627. doi: 10.1155/2016/1872627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. Bin, Tan S.J., Lim W.T., Lim C.T. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat. Commun. 2017;8:1–10. doi: 10.1038/s41467-017-01430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Le Berre M., Lautenschlaeger F., Maiuri P., Callan-Jones A., Heuzé M., Takaki T., Voituriez R., Piel M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160:659–672. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Mak M., Erickson D. A serial micropipette microfluidic device with applications to cancer cell repeated deformation studies. Integr. Biol. 2013;5:1374–1384. doi: 10.1039/c3ib40128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Obenauf A.C. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A.L., Hsia C.R., Lammerding J. Squish and squeeze - the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol. 2016;40:32–40. doi: 10.1016/j.ceb.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micek H.M., Visetsouk M.R., Masters K.S., Kreeger P.K. Engineering the extracellular matrix to model the evolving tumor microenvironment. IScience. 2020;23:101742. doi: 10.1016/j.isci.2020.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.J., Denais C., Chan M., Wang Z., Lammerding J., King M.R. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am. J. Physiol. Cell Physiol. 2015;309 doi: 10.1152/ajpcell.00050.2015. C736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi H., Sahai E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 2018;20:766–774. doi: 10.1038/s41556-018-0131-2. [DOI] [PubMed] [Google Scholar]
- Moose D.L., Krog B.L., Kim T.H., Zhao L., Williams-Perez S., Burke G., Rhodes L., Vanneste M., Breheny P., Milhem M. Cancer cells resist mechanical destruction in circulation via RhoA/actomyosin-dependent mechano-adaptation. Cell Rep. 2020;30:3864–3874.e6. doi: 10.1016/j.celrep.2020.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.H., Orbach S.M., Bushnell G.G., Oakes R.S., Jeruss J.S., Shea L.D. Engineered niches to analyze mechanisms of metastasis and guide precision medicine. Cancer Res. 2020;80:3786–3794. doi: 10.1158/0008-5472.CAN-20-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath B., Raza A., Sethi V., Dalal A., Ghosh S.S., Biswas G. Understanding flow dynamics, viability and metastatic potency of cervical cancer (HeLa) cells through constricted microchannel. Sci. Rep. 2018;8:17357. doi: 10.1038/s41598-018-35646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto F.K., Yeshi T. Humanized mouse and rat PDX cancer models. In: Wang Y., Lin D., Gout P.W., editors. Patient-Derived Xenograft Models of Human Cancer. Springer International Publishing); Cham: 2017. pp. 43–57. [Google Scholar]
- Osmani N., Follain G., García León M.J., Lefebvre O., Busnelli I., Larnicol A., Harlepp S., Goetz J.G. Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Rep. 2019;28:2491–2500.e5. doi: 10.1016/j.celrep.2019.07.102. [DOI] [PubMed] [Google Scholar]
- Otto O., Rosendahl P., Mietke A., Golfier S., Herold C., Klaue D., Girardo S., Pagliara S., Ekpenyong A., Jacobi A. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods. 2015;12:199–202. doi: 10.1038/nmeth.3281. [DOI] [PubMed] [Google Scholar]
- Padmanaban V., Grasset E.M., Neumann N.M., Fraser A.K., Henriet E., Matsui W., Tran P.T., Cheung K.J., Georgess D., Ewald A.J. Organotypic culture assays for murine and human primary and metastatic-site tumors. Nat. Protoc. 2020;15:2413–2442. doi: 10.1038/s41596-020-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C.D., Mistriotis P., Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer. 2016;17:131–140. doi: 10.1038/nrc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C.D., Hung W.-C., Wirtz D., Konstantopoulos K. Engineered models of confined cell migration. Annu. Rev. Biomed. Eng. 2016;18:159–180. doi: 10.1146/annurev-bioeng-071114-040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C.D., Bishop K., Devine A., Paine E.L., Staunton J.R., Thomas S.M., Thomas J.R., Doyle A.D., Miller Jenkins L.M., Morgan N.Y. Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 2019;9:187–206.e16. doi: 10.1016/j.cels.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M., Gentili M., de Belly H., Thiam H.-R., Vargas P., Jimenez A.J., Lautenschlaeger F., Voituriez R., Lennon-Dumenil A.-M., Manel N. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- Raeber G.P., Lutolf M.P., Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi S., Fu A., Luo K.Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz J., Kopf A., Stopp J., de Vries I., Driscoll M.K., Merrin J., Hauschild R., Welf E.S., Danuser G., Fiolka R. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature. 2019;568:546–550. doi: 10.1038/s41586-019-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riching K.M., Cox B.L., Salick M.R., Pehlke C., Riching A.S., Ponik S.M., Bass B.R., Crone W.C., Jiang Y., Weaver A.M. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2015;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl A., Schlederer M., Pudelko K., Stadler M., Walter S., Unterleuthner D., Unger C., Kramer N., Hengstschläger M., Kenner L. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017;130:203–218. doi: 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- Rudzka D.A., Spennati G., McGarry D.J., Chim Y.-H., Neilson M., Ptak A., Munro J., Kalna G., Hedley A., Moralli D. Migration through physical constraints is enabled by MAPK-induced cell softening via actin cytoskeleton re-organization. J. Cell Sci. 2019;132:jcs224071. doi: 10.1242/jcs.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvestani S.K., DeHaan R.K., Miller P.G., Bose S., Shen X., Shuler M.L., Huang E.H. A tissue engineering approach to metastatic colon cancer. IScience. 2020;23:101719. doi: 10.1016/j.isci.2020.101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Nur-E-Kamal A., Ahmed I., Kamal J., Liu H.Y., Amor N., Ponery A.S., Crockett D.P., Grafe T.H., Chung H.Y. Living in three dimensions: 3D nanostructured environments for cell culture and regenerative medicine. Cell Biochem. Biophys. 2006;45:215–227. doi: 10.1385/CBB:45:2:215. [DOI] [PubMed] [Google Scholar]
- Shinsato Y., Doyle A.D., Li W., Yamada K.M. Direct comparison of five different 3D extracellular matrix model systems for characterization of cancer cell migration. Cancer Rep. 2020;3:e1257. doi: 10.1002/cnr2.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA. Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Steeg P.S. Targeting metastasis. Nat. Rev. Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B., Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32:282–293. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Taubenberger A.V., Girardo S., Träber N., Fischer-Friedrich E., Kräter M., Wagner K., Kurth T., Richter I., Haller B., Binner M. 3D microenvironment stiffness regulates tumor spheroid growth and mechanics via p21 and ROCK. Adv. Biosyst. 2019;3:1–16. doi: 10.1002/adbi.201900128. [DOI] [PubMed] [Google Scholar]
- Trujillo-de Santiago G., Flores-Garza B.G., Tavares-Negrete J.A., Lara-Mayorga I.M., González-Gamboa I., Zhang Y.S., Rojas-Martínez A., Ortiz-López R., Álvarez M.M. The tumor-on-chip: recent advances in the development of microfluidic systems to recapitulate the physiology of solid tumors. Materials (Basel) 2019;12:2945. doi: 10.3390/ma12182945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H.T.K., Gossett D.R., Moon Y.S., Masaeli M., Sohsman M., Ying Y., Mislick K., Adams R.P., Rao J., Carlo D. Di. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. 2013;5:212ra163. doi: 10.1126/scitranslmed.3006559. [DOI] [PubMed] [Google Scholar]
- Tweedy L., Thomason P.A., Paschke P.I., Martin K., Machesky L.M., Zagnoni M., Insall R.H. Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science. 2020;369:eaay9792. doi: 10.1126/science.aay9792. [DOI] [PubMed] [Google Scholar]
- Uhler C., Shivashankar G.V. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017;18:717–727. doi: 10.1038/nrm.2017.101. [DOI] [PubMed] [Google Scholar]
- Um E., Oh J.M., Granick S., Cho Y.K. Cell migration in microengineered tumor environments. Lab Chip. 2017;17:4171–4185. doi: 10.1039/c7lc00555e. [DOI] [PubMed] [Google Scholar]
- Urbanska M., Muñoz H.E., Shaw Bagnall J., Otto O., Manalis S.R., Di Carlo D., Guck J. A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat. Methods. 2020;17:587–593. doi: 10.1038/s41592-020-0818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan J., Lim C.T., Fernandez J.G. Cell migration and breast cancer metastasis in biomimetic extracellular matrices with independently tunable stiffness. Adv. Funct. Mater. 2020;2005383:1–10. [Google Scholar]
- Weigelin B., Bakker G.-J., Friedl P. Intravital third harmonic generation microscopy of collective melanoma cell invasion. IntraVital. 2012;1:32–43. doi: 10.4161/intv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. Metastatic inefficiency. Adv. Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- Weiss L., Dimitrov D.S. Mechanical aspects of the lungs as cancer cell-killing organs during hematogenous metastasis. J. Theor. Biol. 1986;121:307–321. doi: 10.1016/s0022-5193(86)80110-2. [DOI] [PubMed] [Google Scholar]
- Weiss L., Dimitrov D.S., Angelova M. The hemodynamic destruction of intravascular cancer cells in relation to myocardial metastasis. Proc. Natl. Acad. Sci. U S A. 1985;82:5737–5741. doi: 10.1073/pnas.82.17.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D.R., Hurst D.R. Defining the hallmarks of metastasis. Cancer Res. 2019;79:3011–3027. doi: 10.1158/0008-5472.CAN-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz D., Konstantopoulos K., Searson P.C.P.P.C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom K.M., Adebowale K., Chang J., Lee J.Y., Nam S., Desai R., Rossen N.S., Rafat M., West R.B., Hodgson L. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018;9:4144. doi: 10.1038/s41467-018-06641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin. Exp. Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- Wolf K., Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011;21:736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., Von Andrian U.H., Deryugina E.I., Strongin A.Y., Bröcker E.B., Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., Deryugina E., Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen G.S., Schwab B.I., Elson E.L., Downey G.P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- Wu P.-H., Aroush D.R.-B., Asnacios A., Chen W.-C., Dokukin M.E., Doss B.L., Durand-Smet P., Ekpenyong A., Guck J., Guz N.V. A comparison of methods to assess cell mechanical properties. Nat. Methods. 2018;15:491–498. doi: 10.1038/s41592-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W., Sonam S., Beng Saw T., Ladoux B., Teck Lim C. Emergent patterns of collective cell migration under tubular confinement. Nat. Commun. 2017;8:1517. doi: 10.1038/s41467-017-01390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y., Chen X., Tang X., Li K., Yang M., Tai W.C.S., Liu Y., Tan Y. Mechanics and actomyosin-dependent survival/chemoresistance of suspended tumor cells in shear flow. Biophys. J. 2019;116:1803–1814. doi: 10.1016/j.bpj.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y., Li K., Yang M., Tan Y. Fluid shear stress induces emt of circulating tumor cells via jnk signaling in favor of their survival during hematogenous dissemination. Int. J. Mol. Sci. 2020;21:1–16. doi: 10.3390/ijms21218115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Gurski L.A., Zhang C., Harrington D.A., Farach-Carson M.C., Jia X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33:9049–9060. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.xi, Liu C., Liu Y., Li N., Guo X., Wang S. jun, Sun G. wei, Wang W., Ma X. jun. Encapsulated human hepatocellular carcinoma cells by alginate gel beads as an in vitro metastasis model. Exp. Cell Res. 2013;319:2135–2144. doi: 10.1016/j.yexcr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Yankaskas C.L., Thompson K.N., Paul C.D., Vitolo M.I., Mistriotis P., Mahendra A., Bajpai V.K., Shea D.J., Manto K.M., Chai A.C. A microfluidic assay for the quantification of the metastatic propensity of breast cancer specimens. Nat. Biomed. Eng. 2019 doi: 10.1038/s41551-019-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M.H. The role of engineering approaches in analysing cancer invasion and metastasis. Nat. Rev. Cancer. 2013;13:596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Miyamoto D.T., Wittner B.S., Sullivan J.P., Aceto N., Jordan N.V., Yu M., Karabacak N.M., Comaills V., Morris R. Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat. Commun. 2017;8:1–12. doi: 10.1038/ncomms14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuela-Sopilniak N., Lammerding J. Engineering approaches to studying cancer cell migration in three-dimensional environments. Philos. Trans. R. Soc. B Biol. Sci. 2019;374:20180219. doi: 10.1098/rstb.2018.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]