Abstract
Translesion DNA synthesis (TLS) enables DNA replication through damaging modifications to template DNA and requires monoubiquitination of the proliferating cell nuclear antigen (PCNA) sliding clamp by the Rad6/Rad18 complex. This posttranslational modification is critical to cell survival following exposure to DNA-damaging agents and is tightly regulated to restrict TLS to damaged DNA. Replication protein A (RPA), the major single-strand DNA (ssDNA) binding protein complex, forms filaments on ssDNA exposed at TLS sites and plays critical yet undefined roles in regulating PCNA monoubiquitination. Here, we utilize kinetic assays and single-molecule FRET microscopy to monitor PCNA monoubiquitination and Rad6/Rad18 complex dynamics on RPA filaments, respectively. Results reveal that a Rad6/Rad18 complex is recruited to an RPA filament via Rad18·RPA interactions and randomly translocates along the filament. These translocations promote productive interactions between the Rad6/Rad18 complex and the resident PCNA, significantly enhancing monoubiquitination. These results illuminate critical roles of RPA in the specificity and efficiency of PCNA monoubiquitination and represent, to the best of our knowledge, the first example of ATP-independent translocation of a protein complex along a protein filament.
Graphical Abstract
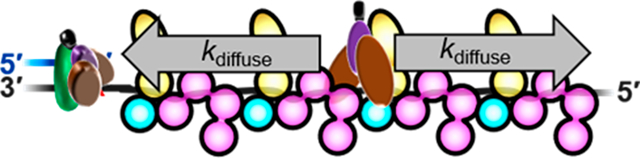
DNA polymerases (pols) ε and δ replicate the majority of the human genome and achieve optimal processivity by anchoring to proliferating cell nuclear antigen (PCNA) sliding clamps encircling primer/template (P/T) junctions.1 These “replicative” pols have very stringent polymerase domains and 3′ to 5′ exonuclease (“proofreading”) domains that collectively ensure accurate replication of native template bases during S phase of the cell cycle. However, DNA is continuously damaged by covalent modifications from reactive metabolites and environmental mutagens, such as ultraviolet radiation (UVR), and the replicative pols cannot accommodate damaged template bases (i.e., lesions). Consequently, primer extension by these pols stalls upon encountering a lesion, leading to persistent exposure of the template strand downstream of the lesion.2,3 Failure to restart primer extension on damaged templates often results in double-strand breaks that may lead to gross chromosomal rearrangements, cell-cycle arrest, and cell death. These stalling events may be overcome by translesion DNA synthesis (TLS), in which specialized TLS pols bind PCNA residing at the stalled P/T junction (i.e., the resident PCNA) and extend the primer across and beyond the DNA lesion, allowing DNA synthesis by a replicative pol to resume downstream of the lesion.4 Characterized by a more “open” DNA polymerase active site and the lack of an associated proofreading activity, a single TLS pol can accommodate multiple DNA lesions, albeit with varying fidelities.5 Hence, tight regulation is required to limit the frequency and extent of TLS.
In humans, TLS requires the covalent attachment of single ubiquitin moieties (i.e., monoubiquitination) to lysine residues K164 of PCNA sliding clamps encircling stalled P/T junctions.6 This critical posttranslational modification (PTM) is fully conserved in eukaryotes and catalyzed by a complex comprised of Rad6 and Rad18 proteins. The former is an E2 ubiquitin conjugating enzyme that covalently attaches a ubiquitin to PCNA, and the latter is a RING E3 ubiquitin ligase that delivers Rad6 to a PCNA target.7 PCNA monoubiquitination contributes to cell survival following exposure to DNA-damaging agents such as UVR and, hence, must be tightly regulated as dysfunction can selectively propagate cells with an increased level of mutagenesis due to aberrant TLS.4,7 Currently, it is unclear how the activity of the Rad6/Rad18 complex is regulated, particularly regarding the roles of replication protein A (RPA), the major single-strand DNA (ssDNA) binding protein complex. RPA has exceptionally high affinity for ssDNA and immediately coats ssDNA templates exposed downstream of stalled P/T junctions (1 RPA/30 ± 2 nucleotides),8−10 forming elongated, directional filaments (Figure 1). These structures protect the underlying ssDNA from degradation, prevent formation of alternative DNA structures,4 and block diffusion of the resident PCNA along the damaged template.11−13 RPA is comprised of three subunits, denoted RPA1–3, that each contain multiple oligonucleotide binding (OB) folds that interact with ssDNA and/or proteins. RPA1 contains four OB folds, three of which (A–C) serve as DNA binding domains (DBD). OB fold F of RPA1 is primarily involved in protein·protein interactions with other cellular factors. RPA2 contains a single OB fold (D) that serves as a DBD and a winged-helix (wh) domain that participates in protein·protein interactions with other cellular factors. RPA3 contains an OB fold (E) that serves as a structural scaffold for individual RPA complexes as well as RPA filaments. In the latter, interactions between OB fold E of one RPA complex and OB fold A of the adjacent RPA complex stabilize an elongated, rigid conformation that allows the engaged ssDNA to pass from one RPA complex to another by a linear path.14−16 Recent studies revealed that Rad6/Rad18 complexes directly interact (via Rad18) with RPA on ssDNA and this nonspecific interaction is required for PCNA monoubiquitination.13,17,18 A pressing issue that has remained unresolved is the functional role(s) of nonspecific Rad18·RPA interactions in PCNA monoubiquitination. To investigate this, we utilized transient-state kinetic studies and single-molecule FRET (smFRET) microscopy to directly monitor PCNA monoubiquitination and the dynamics of Rad6/Rad18 complexes on RPA filaments, respectively. Results from thorough experiments reveal that a Rad6/Rad18 complex is directly recruited to an RPA filament (via Rad18·RPA interactions) and then randomly translocates along the filament by one-dimensional, thermally driven diffusion. These translocations promote productive interactions between the Rad6/Rad18 complex and the resident PCNA at stalled P/T junctions, significantly enhancing monoubiquitination. These results illuminate critical roles of RPA in the specificity and efficiency of PCNA monoubiquitination.
Figure 1.
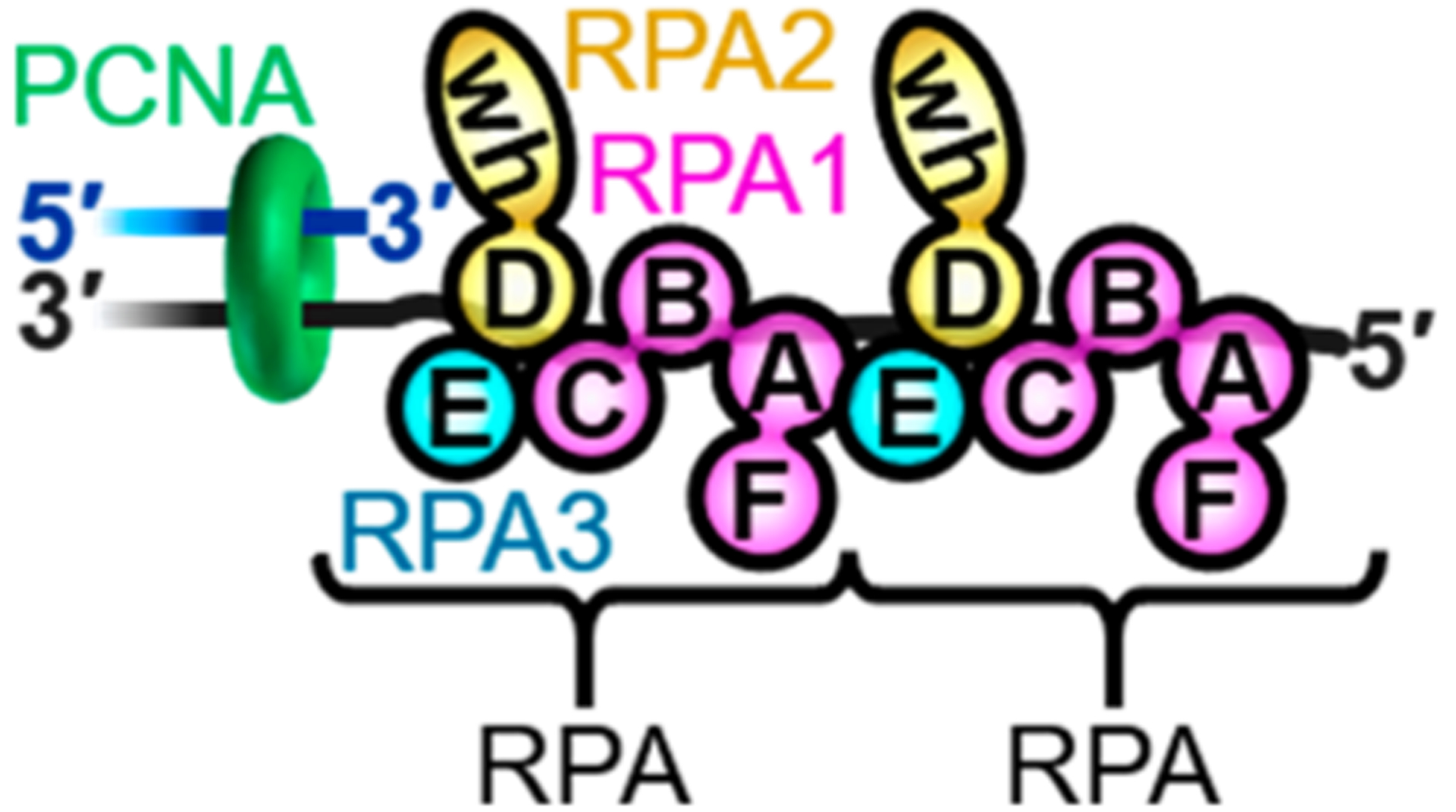
RPA interactions at stalled P/T junctions. The primer and template DNA strands are colored blue and gray, respectively. PCNA (green) encircles the P/T junction. The ssDNA downstream of the P/T junction is coated by two RPAs. Each RPA is colored by subunit (RPA1 in pink, RPA2 in yellow, and RPA3 in blue) and depicted to illustrate the OB folds (A–E) and winged-helix (wh) domain. Each RPA interacts with 30 ± 2 nucleotides in an orientation-specific manner such that the RPA2 subunit is oriented toward the P/T junction. The RPAs can interact with each other via interaction between OB folds A and E.
MATERIALS AND METHODS
Oligonucleotides.
DNA constructs were synthesized by Integrated DNA Technologies (Coralville, IA) and purified on denaturing polyacrylamide gels. Concentrations of unlabeled DNAs were determined from the absorbance at 260 nm using the calculated extinction coefficients. For DNA labeled with a cyanine dye, concentrations were determined from the absorbance at 550 nm (for Cy3) or 650 nm (for Cy5) using the extinction coefficient of the respective dye. Primer/Template (P/T) DNAs were annealed by mixing the primer and equimolar amounts of complementary template strands in 1× annealing buffer [10 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 1 mM EDTA], heated to 95 °C for 5 min, and allowed to cool slowly to room temperature. All DNAs utilized in this study are depicted in Figure S1.
Recombinant Human Proteins.
Wild type PCNA, a site-specifically labeled Cy5-PCNA, Rad6, the Rad6/Rad18 complex, RFC, RPA, Uba1, and fluorescein-labeled ubiquitin (Fl-Ub) were expressed and purified as previously described.1−5 The concentration of the purified Rad6/Rad18 complex was determined from the extinction coefficient (ε280 =68570 M−1 cm−1) assuming a Rad6·(Rad18)2 stoichiometry,19 and the concentration of Rad6 within the complex was confirmed by a Bradford assay using BSA (VWR) as a standard. The concentration of active RPA was confirmed as previously described (Figure S2).15 The plasmid (pET28-NHis-SUMO-Rad18) for the expression of Rad18 was a generous gift from J. Huang (Life Sciences Institute, Zhejiang University, Hangzhou, China).20 Rad18 was expressed in Escherichia coli and purified by via slight modifications of published protocols20,21 (see the Supporting Information). The Rad18 concentration was determined via a Bradford assay using BSA as a standard.
Protein Labeling for smFRET Measurements.
The N-terminus of Rad6 was labeled with Cy5 (GE Healthcare). Briefly, the solution of NHS-ester-functionalized Cy5 in DMSO was added dropwise under stirring conditions to a solution of Rad6 in 10 mM HEPES (pH 7.5) containing 468 mM NaCl, 2 μM ZnCl2, and 1 mM TCEP. The final protein:dye ratio was 1:1.1, and the labeling reaction mixture was incubated overnight at 4 °C. Labeled Rad6 was separated from free Cy5 dye by being dialyzed twice against 10 mM HEPES buffer (pH 7.5). Finally, the solution was concentrated, washed twice with the storage buffer [10 mM HEPES (pH 7.5), 468 mM NaCl, 2 μM ZnCl2, and 1 mM TCEP] via centrifugal filtration (Amicon, 3 kDa molecular weight cutoff), and stored at −80 °C. The labeling efficiency was calculated by dividing the concentration of Cy5 by the concentration of Rad6. The former was determined from the absorbance at 650 nm using the extinction coefficient for Cy5. The latter was determined by Bradford assay using unlabeled Rad6 as the standard and correcting for the absorbance of Cy5 at 595 nm (ε595 = 140000 ± 4010 M−1 cm−1). On average, each Rad6 contained one Cy5 dye (labeling efficiency of 1.10 ± 0.08 Cy5/Rad6). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of Cy5-Rad6 indicated a single labeled species (Figure S3). Together, this indicated that Rad6 was uniformly labeled with a single Cy5 dye/protein.
Ubiquitination Assays.
All ubiquitination assays were performed at room temperature (23 ± 2 °C) in 1× ubiquitination assay buffer [25 mM HEPES (pH 7.5), 10 mM Mg(OAc)2, and 125 mM KOAc] supplemented with 1 mM TCEP, and the ionic strength was adjusted to a physiological concentration (200 mM) by the addition of appropriate amounts of KOAc. All concentrations indicated below are final (i.e., after mixing). Unless indicated otherwise, experiments were performed as described previously with minor changes.13,22 In solution A, PCNA (700 nM homotrimer) was preloaded by RFC (700 nM with 0.5 mM ATP) onto a P/T DNA (700 nM with 2.8 μM NeutrAvidin) in the presence of excess RPA such that all ssDNA is saturated with RPA and the concentration of free RPA is 1.0 μM. Both RPA and the biotin/neutravidin complexes serve to prevent loaded PCNA from sliding off of the DNA. Under these conditions, all PCNA was loaded onto DNA and stabilized,11,12 and hence, the concentration of loaded PCNA was 700 nM. In solution B, Rad6/Rad18 complexes (7 nM heterotrimer) were preincubated with Uba1 (14 nM with 0.5 mM ATP) and fluorescein-labeled ubiquitin (Fl-Ub, 4.55 μM) for 10 min. Under these conditions, all Rad6/Rad18 complexes were precharged with ubiquitin22 and, hence, the concentration of active Rad6/Rad18 complexes was 7 nM. Ubiquitination of target proteins was initiated by mixing equal volumes of solutions A and B. Aliquots were removed at the indicated time points and quenched 1.33-fold into 1× reducing loading buffer [5 mM Tris (pH 6.8), 7.5% (v/v) glycerol, 0.375% SDS, 0.51 M β-mercaptoethanol, and bromophenol blue]. Under these reducing and denaturing conditions, only proteins containing covalent isopeptide bonds with ubiquitin were observed. After all time points had been completed, samples were analyzed by fluorescence scanning as described previously to yield the concentration of monoubiquitinated PCNA clamps ([P]T = [E·P] + [P]).13 The extent of each reaction was determined by dividing the concentration of monoubiquitinated PCNA clamps observed at the last time point by the initial concentration of PCNA clamps {([P]T/[S]0) × 100% = [[P]T/(700 nM)] × 100%}. For all reactions, ≤5.53 ± 1.05% of PCNA clamps were monoubiquitinated over time. Data points were divided by the initial concentration of active Rad6/Rad18 complexes ([E]0 = 7 nM) and plotted as a function of time. For each condition, the steady-state phase was fit to the equation23 where vss = kmu,obskcat/(kmu,obs + kcat) and A0 = [kmu,obs/(kmu,obs + kcat)]2.Values for A0 and vss are reported in Table 1 and were utilized to calculate values for kmu,obs and kcat.
Table 1.
Kinetics Obtained from Transient-State Kinetic Assays Reveal a Critical Role for RPA in Monoubiquitination of PCNA by the Rad6/Rad18 Complex
| substrate | BioP/T-33ssa | BioP/T-171ss | BioP/T-33ss+138ss | |
|---|---|---|---|---|
| experimental | no. of RPAs bound to ssDNA | 1 | 6 | 6 |
| condition | no. of RPAs next to PCNA | 1 | 6 | 1 |
| kinetic | A0 (unitless) | 0.0967 ± 0.0228 | 0.616 ± 0.033 | 0.0548 ± 0.0081 |
| variables | vss (min−1) | 0.0243 ± 0.0015 | 0.0498 ± 0.0019 | 0.0169 ± 0.00056 |
Reference conditions.
Ensemble FRET Measurements.
All experiments were performed at room temperature (23 ± 2 °C) in 1× ubiquitination buffer [25 mM HEPES, 125 mM KOAc, and 10 mM Mg(OAc)2] supplemented with 1 mM TCEP. The ionic strength was adjusted to 200 mM by the addition of appropriate amounts of KOAc. First, a solution containing 110 nM Cy3-labeled P/T DNA substrate (Figure S1), NeutrAvidin (440 nM, Thermo Scientific), and ATP (1 mM) was preincubated with excess RPA such that the concentration of free RPA was 550 nM. Cy5-PCNA (100 nM homotrimer) and RFC (100 nM) were sequentially added, and retention of PCNA on DNA at equilibrium was monitored via FRET as described previously.6,7
smFRET Measurements.
Quartz microscope slides (Finkenbeiner) were thoroughly cleaned as previously described,24 and slide surfaces were coated with polyethylene glycol (PEG) and PEG-biotin at a 99:1 ratio. First, the BioCy3P/T-70ss DNA substrate (Figure S1) was immobilized on a microscope slide surface via biotin/streptavidin conjugation and then preincubated with 0.5 μM RPA for 10 min followed by a wash. Next, a solution containing 10 nM Cy5-Rad6, 20 nM Rad18, 1.6 mM protocatechuic acid (PCA, HWI Pherma Services), 0.16 unit/mL protocatechuate 3,4-dioxygenase (PCD, Sigma), and 1 mM trolox (Sigma, St. Louis, MO) was injected. After a 10 min incubation to deplete oxygen, two-color smFRET measurements were performed using a prism-coupled total internal reflection fluorescence (TIRF) microscope system that was based on a Nikon TE2000 microscope (Nikon) as previously described.24 Briefly, the slide surface was illuminated with a 532 nm laser through a prism mounted on top of the slide. Fluorescence emission was collected through a water immersion objective lens (Nikon, Plan Apo, 60×, 1.2 NA) and bifurcated into two different paths to separately image donor (Cy3) and acceptor (Cy5) signals on an EMCCD camera (Cascade-II, Photometrics). A time-series stack of fluorescence images with 150 ms signal integration was recorded until ~70% Cy3 spots are photo-bleached. Several stacks of images were recorded focusing on different regions of the slide surface. The intensities of Cy3 emission and corresponding Cy5 emission were obtained from the stacks of images. The background fluorescence signal after photobleaching was taken as the zero-fluorescence level and subtracted from the fluorescence signal. From the relative intensities of Cy3 and Cy5, the FRET efficiencies were estimated with the term ICy5/(ICy3 + ICy5), where I is the fluorescence intensity. From the dynamics of the FRET efficiency levels, the time windows of the FRET-on states were defined. We first identified FRET-on events with a four-frame average for FRET efficiency of ≥0.1, which was also verified by visual inspection. The first and last points of each event that show anticorrelated Cy3 and Cy5 intensities were defined as the starting and ending points of a τon window. We observed a total of 88 τon events over 1100 s of the 362250 s total observation time. Under these conditions, the probability of observing a double binding event in which two Cy5-Rad6/Rad18 complexes were engaged with a single BioCy3P/T-70ss DNA substrate was negligible (~0.0009%).
RESULTS
Monoubiquitination of PCNA Encircling a P/T Junction Is Promoted by the Adjacent RPA Filament.
To investigate the effects of nonspecific Rad18·RPA interactions on PCNA monoubiquitination, we designed a fluorescence assay to analyze the transient-state kinetics of the reaction pathway for PCNA monoubiquitination (Figure 2A). PCNA was loaded onto a P/T DNA that was saturated with RPA (Figure 2B). The duplex regions of all P/T DNAs were identical, and the total lengths of the poly(dT) ssDNA regions were either 33 or 171 nucleotides, which accommodated at least 1 and 6 RPA molecules, respectively.8−10 All experiments were carried out such that the concentrations of excess RPA were identical to account for any effects of “free” RPA in solution on PCNA monoubiquitination.13 Rad6/Rad18 complexes were precharged with fluorescein-labeled ubiquitin by Uba1, the ubiquitin-activating enzyme (E1).7 Reactions were initiated by mixing a limiting concentration of ubiquitin-charged Rad6/Rad18 complexes with a large excess of a loaded PCNA assemblage. Upon mixing, a ubiquitin-charged Rad6/Rad18 complex must first locate and engage with a PCNA target (i.e., substrate, S) in a productive complex. Under the conditions of the assay, the likelihood that a loaded PCNA assemblage is engaged by more than one ubiquitin-charged Rad6/Rad18 complex at a time is negligible (<0.15%). In the subsequent chemical step, the ubiquitin-charged Rad6/Rad18 complex covalently transfers the associated ubiquitin from itself to the engaged PCNA target, forming monoubiquitinated PCNA (i.e., the product). The apo Rad6/Rad18 complex devoid of ubiquitin then releases the monoubiquitinated PCNA product. For all experiments, <5.53 ± 1.05% of PCNA is monoubiquitinated over time. Thus, product release is irreversible due to the relatively low abundance of monoubiquitinated PCNA. Once free in solution, the apo Rad6/Rad18 complex is recharged with fluorescein-labeled ubiquitin by Uba1, completing the catalytic reaction cycle. The product release and ubiquitin recharging steps together regenerate the active Rad6/Rad18 complex that is charged with a ubiquitin and, hence, allow catalytic turnovers after the initial chemical steps. In this setup, the first turnover of Rad6/Rad18 complexes encompasses only formation of the productive complex (kon[S]) and the chemical step (kchem), whereas subsequent, catalytic turnovers encompass all steps of the reaction cycle (Figure 2A). As observed in Figure 3A, products accumulate during an initial burst of Rad6/Rad18 activity (i.e., burst phase) and then the level of products increases at a slower, constant rate (i.e., steady-state phase). This biphasic behavior indicates that all steps up to and including monoubiquitination of PCNA (kon[S] and kchem) are comparable to any subsequent steps (krelease and krecharge). Fitting the steady-state phases to linear regressions yields the amplitudes (A0) for the burst phases and the steady-state rates (vss) for catalytic turnover (reported in Table 1). The observed rate constants for PCNA monoubiquitination (kmu,obs) and catalytic turnover (kcat) are calculated from the values for vss and A0 (Figure 3B).23 kmu,obs reflects formation of the productive complex (kon[S]), the chemical step (kchem), or a combination of both steps. kcat reflects the product release step (krelease), the ubiquitin recharging step (krecharge), or a combination of both steps (Figure 2A). The products (monoubiquitinated PCNA) and apo Rad6/Rad18 complexes are identical for all conditions. Thus, it is expected that kcat remains constant.
Figure 2.

Fluorescence assay to analyze the transient-state kinetics of PCNA monoubiquitination. (A) Schematic representation of the fluorescence assay. RPA is colored by subunit as in Figure 1. Denotations of the OB folds and the winged-helix domain of RPA have been removed for the sake of clarity. PCNA is preloaded onto a BioP/T DNA as depicted in panel B. The BioP/T-33ss DNA is shown in panel A as an example. Rad6/Rad18 complexes are precharged with fluorescein-labeled ubiquitin by Uba1. Reactions are initiated by mixing limiting concentrations of ubiquitin-charged Rad6/Rad18 complexes with a large excess of a loaded PCNA assemblage (depicted in panel B), and PCNA monoubiquitination is monitored over time. Under these conditions, the first turnover of enzymatic activity starts from ubiquitin-charged Rad6/Rad18 complexes in solution. Subsequent turnovers of enzymatic activity start from apo Rad6/Rad18 complexes that are engaged with monoubiquitinated PCNA. (B) Schematic representations of PCNA assembled onto the BioP/T DNA substrates.
Figure 3.
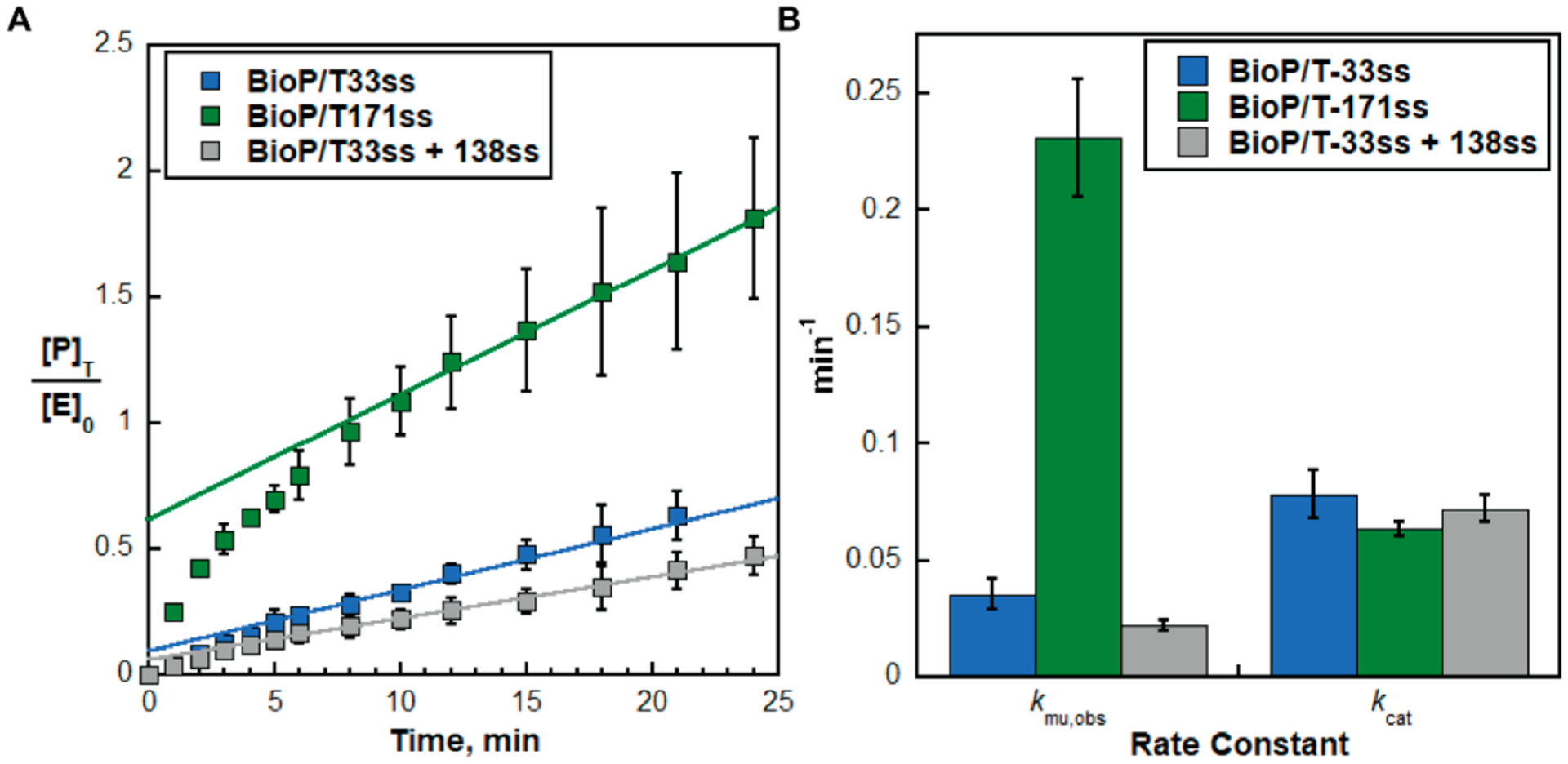
RPA filament adjacent to a P/T junction stimulates monoubiquitination of the resident PCNA. (A) Data from transient-state kinetic assays of PCNA monoubiquitination (Figure 2A). Extents of PCNA monoubiquitination. The concentrations of monoubiquitinated PCNA clamps are divided by the initial concentration of charged Rad6/Rad18 and plotted as a function of time. Data for each PCNA assemblage (Figure 2B) represent the average ± the standard deviation of three independent experiments. For each PCNA assemblage, the linear phase is fit to a linear regression that is extrapolated back to the y-axis. The y-intercept and the slope of the fit represent the amplitude (A0) and the steady-state rate (vss), respectively. (B) Kinetics of PCNA monoubiquitination. Values for rate constants kmu,obs and kcat are calculated from the values for A0 and vss determined from panel A (and reported in Table 1) and plotted for each PCNA assemblage. kmu,obs is dependent on the length of RPA molecules adjacent to the target PCNA. kcat remains constant.
A single RPA molecule resides next to the resident PCNA encircling a BioP/T-33ss DNA (Figure 2A). Thus, the target (PCNA) and nonspecific binding sites (RPA) are equally abundant on a BioP/T-33ss DNA, and a Rad6/Rad18 complex has an equal probability of binding either from solution. In this context, kmu,obs = 0.0353 ± 0.00654 and kcat = 0.0782 ± 0.0104 min−1 (Table 1 and Figure 3B). On a BioP/T-171ss DNA (Figure 2A), the number of RPA molecules residing next to the resident PCNA is increased to at least 6. Here, a Rad6/Rad18 complex is at least 6 times more likely to initially engage a nonspecific site along the RPA filament rather than the resident PCNA target. In other words, a Rad6/Rad18 complex is first localized and/or recruited to the RPA filament before engaging the resident PCNA and catalyzing monoubiquitination.17 As observed in Figure 3B, kmu,obs is stimulated by a factor of 6.55 ± 0.03 relative to the reference condition but kcat is unaffected. The latter supports the validity of the approach.
kmu,obs is dependent on the formation of the productive complex (kon[S]) and the chemical step (kchem) (Figure 2A). The concentration of the resident PCNA targets ([S]) and kchem are identical for both the BioP/T-33ss and BioP/T-171ss DNAs as the number of RPA molecules residing next to the P/T junctions has no effect on the retention of the resident PCNA or its orientation on DNA (Figure 4).13 This suggests that kmu,obs is rate-limited by kon, and hence, overabundant nonspecific sites (i.e., an RPA filament) adjacent to a P/T junction stimulate monoubiquitination of the resident PCNA target (kmu,obs) by increasing kon. A near stoichiometric increase in kmu,obs (6.55-fold) with the 6-fold overabundance of RPA molecules also supports this conclusion. To confirm this further, we repeated the experiments by separating the RPA filament from the P/T junction [BioP/T-33ss+138ss (Figure 2B)]. Here, the loaded PCNA assemblage (on the BioP/T-33ss) and the detached RPA filament (on the 138ss) are stoichiometric. Hence, the number of RPA molecules bound to ssDNA is increased from 1 to 6 compared to the reference condition but still only a single RPA resides next to the P/T junction and resident PCNA. As observed in Figure 3B, the significant stimulation of kmu,obs on the BioP/T-171ss DNA disappears when the RPA filament is not physically connected to the P/T junction encircled by PCNA. Again, kcat remains constant, confirming the validity of the experimental approach. Altogether, the results presented thus far indicate that recruitment of a Rad6/Rad18 complex to an RPA filament adjacent to a P/T junction stimulates monoubiquitination of the resident PCNA (kmu,obs) by promoting formation of a productive complex (kon).
Figure 4.

Retention of PCNA on a P/T junction is independent of the length of the adjacent RPA filament. (A) Schematic representation of the FRET experiment. Cy5-labeled PCNA is loaded onto a Cy3-labeled BioP/T DNA by the human clamp loader, RFC, in the presence of RPA, and FRET is monitored at equilibrium. The PCNA assemblage on the BioP/T-33ss DNA (Figure 2B) is shown as an example. (B) FRET data for PCNA assemblages on the BioP/T-33ss and BioP/T-171ss DNAs. For each, FRET is observed only when RFC is included. FRET values measured in the presence of RFC are within experimental error for the BioCy3P/T-33ss (0.487 ± 0.0194) and BioCy3P/T-171ss (0.456 ± 0.0246) DNAs that accommodate at least 1 and 6 RPA molecules, respectively. This indicates that the same amount of PCNA is loaded onto and stabilized at a P/T junction in the same FRET state (i.e., orientation), regardless of the length of the adjacent RPA filament.
Rad6/Rad18 Complexes Diffuse Randomly along RPA Filaments.
In assays carried out with the BioP/T-171ss DNA, a Rad6/Rad18 complex in solution most likely engages a nonspecific site along the RPA filament that is separated from the resident PCNA target by one or more intervening RPA molecules. This recruitment significantly enhances PCNA monoubiquitination (kmu,obs) by promoting formation of the productive complex (kon) (Figure 3). For catalysis to occur after recruitment, the engaged Rad6/Rad18 complex must transfer from a distal, nonspecific site along the RPA filament to the resident PCNA target and, hence, the RPA filament must provide a pathway for transfer. A possible mechanism is direct transfer via ssDNA looping. However, RPA filaments linearize the underlying ssDNA in a rigid rod type structure by engaging the ssDNA in an elongated manner that extends the bound sequence and increases its bending rigidity 2–3-fold.15,25 Alternatively, a Rad6/Rad18 complex may translocate along the RPA filament by random, thermally driven diffusion. In other words, a Rad6/Rad18 complex diffuses toward and away from the resident PCNA during each engagement with the adjacent RPA filament. Such movements would promote formation of the productive complex (kon) by decreasing the time to locate a PCNA target and/or permitting multiple encounters with a resident PCNA target during each interaction with a DNA. To directly observe diffusion of Rad6/Rad18 complexes, we investigated the dynamics of Rad6/Rad18 complexes on RPA filaments by smFRET microscopy (Figure 5A). The P/T DNA [BioCy3P/T-70ss (Figure S1)] contains a biotin tag at the blunt duplex end and a Cy3 dye at the P/T junction and accommodates two RPA molecules on the ssDNA region of the template DNA strand.8−10 The ssDNA is saturated with RPA and extended into an elongated, rigid filament.26 Rad6 is first labeled with a single, N-terminal Cy5 dye (Figure S3) and then reconstituted with Rad18 to form the Rad6/Rad18 complex.21 In this setup, smFRET occurs when Cy5-Rad6 (FRET acceptor) is in the proximity of the Cy3-P/T junction (FRET donor). smFRET is observed (i.e., FRETon) only when both Rad18 and RPA are included (Figure 5B); smFRET events are not detected during ~5520 min of total observation when either Rad18 or RPA is omitted. This confirms that (1) Rad18 functionally interacts with Cy5-Rad6 in a manner that directs Cy5-Rad6 to the vicinity of the P/T junction7 and (2) the Rad6/Rad18 complex has immeasurably weak affinity for naked ssDNA at physiological ionic strength and, hence, must be recruited to DNA by RPA filaments.13,17,18 Furthermore, when Rad18 and RPA are included, the observed smFRET efficiencies fluctuate during FRETon, lacking defined, stable conformational states. It is possible that a Cy5-Rad6/Rad18 complex remains bound to a random position on the RPA filament after recruitment and the observed fluctuations arise due to the conformational dynamics of either the P/T junction or the engaged RPA molecules that enable contact between the FRET dyes. However, the conformational dynamics of DNA junctions are very fast and average out during the measurements with our signal integration time of 150 ms.27,28 Furthermore, the average dwell times (ton) of RPA DBDs that undergo microscopic dissociation and/or reassociation on ssDNA range from 300 ms to 1 s and such events would be clearly visible with the time resolution of the current experiments.14 Although the possibility that Rad6/Rad18 complexes affect these microscopic dissociation/reassociation events cannot be ruled out, defined conformational states are not observed in the smFRET time trajectory depicted in Figure 5B. Alternatively, the observed fluctuations in the smFRET efficiencies may arise from random translocation of a Cy5-Rad6/Rad18 complex toward and away from the Cy3-P/T junction. To confirm this, we investigated the directionality of Rad6/Rad18 translocation (Figure 5C).
Figure 5.

smFRET analysis of the dynamics of Rad6/Rad18 complexes on RPA filaments reveals random diffusion of Rad6/Rad18 complexes on RPA filaments. (A) Schematic representation of the experimental setup. The BioCy3P/T-70ss DNA substrate is immobilized on a microscope slide surface via biotin/streptavidin conjugation, and the ssDNA region is saturated with two RPA molecules. Cy5-Rad6/Rad18 is injected, and smFRET is monitored over time. (B) Example of a time trajectory showing the fluctuating smFRET efficiency during FRETon. (C) Collective smFRET efficiency time trajectories (n = 88) synchronized at the starting point of smFRET events (i.e., starting points of the FRETon windows shown in panel B) were overlaid (left). The average smFRET efficiency (indicated) is calculated from the histogram of the smFRET efficiencies observed during the FRETon windows (right). The FRET efficiencies fluctuate randomly about the average FRET efficiency (0.30) without discrete FRET states.
The 88 detectable smFRET events from the collective time trajectories were synchronized at the starting points of the FRETon windows and overlaid (Figure 5C, left). Histograms of the smFRET efficiencies observed during the FRETon windows were constructed to show the distribution of the FRET efficiency (Figure 5C, right). The average FRET efficiency calculated from the distribution is 0.30, and the FRET efficiency fluctuates randomly about this value with no sign of discrete FRET states. This suggests that the fluctuations of smFRET efficiencies during FRETon reflect the translocation of a Rad6/Rad18 complex toward and away from the P/T junction via random diffusion along the RPA filament. Such movement enables a Rad6/Rad18 complex to encounter the P/T junction multiple times during each binding interaction, in accordance with the proposed model.
The time trajectories of the smFRET efficiencies can also be analyzed to estimate the one-dimensional (1D) diffusion coefficient of Rad6/Rad18 complexes. The changes in FRET efficiencies between two points separated by 3.45–5.4 s (n = 55–174) were utilized to calculate the mean-square displacements (MSDs) using a Förster radius of 5.4 nm for Cy3/Cy5.29 Shorter FRETon windows were removed from the analysis because the FRET dynamics within a short time window are dominated by noise. Longer FRETon windows were also removed from the analysis because the sample size with longer time windows becomes too small. We found the analysis window resulting in the highest R2 value of the fitting. MSDs were plotted as a function of diffusion time (t) and fit to a linear regression (MSD = 2Dt, where D represents the 1D diffusion coefficient) (Figure S4). This yields a lower limit for the 1D diffusion coefficient of 0.112 ± 0.00387 nm2 s−1 (R2 = 0.797; χ2 = 0.0167).
Collectively, the results presented here confirm that Rad6/Rad18 complexes are directly recruited to the vicinity of P/T junctions by the adjacent RPA filaments13,17,18 and reveal that Rad6/Rad18 complexes randomly diffuse along RPA filaments. These behaviors collectively enhance the catalytic activity of Rad6/Rad18 complexes by promoting the formation of productive complexes (kon) with PCNA encircling P/T junctions. These unforeseen results illuminate the undefined roles of Rad18·RPA interactions in regulating PCNA monoubiquitination, as discussed below.
DISCUSSION
Recent studies revealed that Rad6/Rad18 complexes directly interact (via Rad18) with RPA on ssDNA and these nonspecific interactions are required for PCNA monoubiquitination.13,17,18 A pressing issue that has remained unresolved is the functional role(s) of nonspecific Rad18·RPA interactions in PCNA monoubiquitination. In this study, we utilized transient-state kinetic assays and smFRET microscopy to directly monitor PCNA monoubiquitination and RPA·Rad18 interactions on ssDNA, respectively. Results from thorough experiments reveal that (1) Rad6/Rad18 complexes translocate along RPA filaments by random, thermally driven diffusion and (2) these translocations significantly enhance monoubiquitination of PCNA encircling distal P/T junctions. These results reveal a catalytic mechanism that is unique to the Rad6/Rad18 complex among PCNA-modifying enzymes and, to the best of our knowledge, the first example of ATP-independent translocation of a protein complex along a protein filament. Furthermore, this unique mechanism accounts for the many challenges that arise in vivo, namely, specificity and efficiency.
Monoubiquitination of PCNA elicits DNA synthesis by error-prone TLS pols and, hence, must be restricted to PCNA encircling P/T junctions stalled at DNA lesions, such as those generated by UVR exposure. UVR fluences similar to what an individual experiences from one hour of mid-day sun generate 1.6–2.2 million lesions in a human cell, with the vast majority (67–83%) undergoing very slow repair and persisting into S phase.30,31 However, DNA replication in a human cell emanates from 13 to 22 million P/T junctions, each encircled by PCNA. Thus, very few (≤10%) loaded PCNA clamps will ever encounter and subsequently idle at a UVR-induced lesion under physiologically relevant conditions.30,32,33 So, how is Rad6/Rad18 activity restricted to a such a small minority of loaded PCNA clamps? Compounding this specificity issue is the relative abundance of Rad6/Rad18 complexes. Approximately 50 proteins interact with loaded PCNA during S phase in human cells, and many are substantially enriched (as much as ~80-fold) at P/T junctions stalled at UVR-induced lesions.34 However, the abundance of Rad6/Rad18 complexes is maintained at a low level (approximately ≤795/cell) and does not change following UVR exposure.35,36 How can Rad6/Rad18 complexes effectively compete with the vast overabundance of competitive PCNA binding proteins in human cells?37 This study along with previous work from our group and others suggests that the selectivity and efficiency of PCNA monoubiquitination are achieved through nonspecific Rad18·RPA interactions. On native DNA templates, RPA filaments adjacent to progressing P/T junctions are short and transient38 due to the minimal exposure of native ssDNA templates4 and their rapid conversion to double-stranded DNA duplexes by the replicative pols ε and δ (Figure 6, left).39 Here, Rad18·RPA interactions on ssDNA are prohibited and Rad6/Rad18 complexes must engage loaded PCNA directly from solution. These events are inhibited in vivo,40 likely by the continuous, rapid movement of PCNA engaged with replicating DNA polymerases13 and the vast overabundance of competitive PCNA binding proteins in human cells.34−37 In support of this, Rad18 is diffusely distributed throughout the nucleus during S phase in mock UVR-treated human cells40 and overexpression of Rad18 is required for PCNA monoubiquitination under these conditions.17 In stark contrast, P/T junctions stalled at UVR-induced lesions generate RPA filaments that range in length from 5 to 42 RPA molecules2,3,8−10 and persist for >8h.41 The unique properties of these RPA filaments promote Rad18·RPA interactions and localize Rad6/Rad18 complexes to rare target sites in a manner independent of PCNA binding (Figure 6, right). This avoids a biased competition with most cellular proteins that must localize to these same sites via direct binding to loaded PCNA.17 Given the relatively low abundance of Rad6/Rad18 complexes35,36 and the extended lengths of RPA filaments generated at UVR-induced lesions,2,3,8−10 a single Rad6/Rad18 complex is initially recruited to a random position along an RPA filament that is distal to the resident PCNA target. Once engaged, the Rad6/Rad18 complex translocates randomly along the RPA filament by thermally driven diffusion. In other words, the nonspecific binding interactions of a Rad6/Rad18 complex with RPA molecules along an RPA filament are correlated, allowing a Rad6/Rad18 complex to engage many RPA molecules during each encounter with an RPA filament. A correlated search of nonspecific sites for a target site is more efficient than a noncorrelated search.42 RPA undergoes constant microscopic dissociation from ssDNA, but these events manifest only as macroscopic dissociation into solution (i.e., direct/facilitated exchange) when proteins with comparable ssDNA affinities are present.43,44 The Rad6/Rad18 complex has immeasurably weak affinity for ssDNA at physiological ionic strength and, hence, must be recruited to DNA by RPA filaments.13,17,18 Altogether, this suggests that RPA is not displaced from ssDNA as a Rad6/Rad18 complex translocates along an RPA filament. As the resident PCNA is being stochastically sampled by the nucleoplasmic pool of PCNA binding proteins, diffusion of the Rad6/Rad18 complex along the adjacent RPA filament selectively increases the relative frequency of collisions between the resident PCNA target and the Rad6/Rad18 complex. Together, this promotes monoubiquitination of PCNA encircling stalled P/T junction despite the relatively low abundance of Rad6/Rad18 complexes.35,36
Figure 6.

RPA interacts with Rad18 to regulate PCNA monoubiquitination. (Left) Short RPA filaments adjacent to progressing P/T junctions are rapidly displaced by the replicative pols ε and δ. Here, interactions of Rad18 with RPA and PCNA on ssDNA are prohibited and Rad6/Rad18 complexes remain disengaged. (Right) Long, persistent RPA filaments generated at P/T junctions stalled at DNA lesions promote Rad18·RPA interactions and localize a Rad6/Rad18 complex to stalled P/T junctions in a manner independent of PCNA binding. Once engaged, the Rad6/Rad18 complex translocates randomly along the RPA filament by thermally driven diffusion. These movements selectively increase the relative frequency of collisions between the loaded PCNA target and the Rad6/Rad18 complex. Together, these Rad18 interactions promote monoubiquitination of PCNA encircling a stalled P/T junction.
In the current smFRET setup (Figure 5A), dissociation of a Rad6/Rad18 complex from an RPA filament cannot be defined. Thus, it is unknown how far a Rad6/Rad18 complex can diffuse along an RPA filament before dissociating into solution. Extensive diffusion would ensure monoubiquitination of PCNA encircling a stalled P/T junction regardless of where the Rad6/Rad18 complex initially engaged the adjacent RPA filament. However, this mechanism would likely be impacted by collisions with other proteins bound to the RPA filament, resulting in local trapping of the Rad6/Rad18 complex on small segments of the RPA filament.42,45−47 Thus, we envision that a Rad6/Rad18 complex diffuses along short segments of the RPA filament [≤6 RPA molecules (Figure 6)] before dissociating into solution. Given the wide distribution of UVR-induced lesions after exposure to physiologically relevant fluences (1 lesion every 3–4 kb) and the high, local concentration of RPA molecules on stalled P/T junctions, the disengaged Rad6/Rad18 complex likely reassociates with the same RPA filament at a random position. In this model, intermittent dissociation events between diffusive translocations allow a Rad6/Rad18 complex to escape local trapping and bypass other proteins bound to the RPA filament. However, short diffusion lengths require that a Rad6/Rad18 complex must ultimately engage the RPA filament at a position near the stalled P/T junction to monoubiquitinate the resident PCNA. This model is the focus of ongoing studies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank members of the Hedglin, Lee, and Benkovic laboratories for helpful insights, discussion, and critical reading of the manuscript.
Funding
This work was supported in part by National Institutes of Health Grants RO1 GM123164 to T.H.L. and RO1 GM013306 to S.J.B.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.0c00849.
DNA substrates utilized in this study, FRET-based active site titration of RPA, SDS–PAGE analysis of Cy5-labeled Rad6, and estimate of the 1D diffusion constant of the Rad6/Rad18 complex (PDF)
Accession Codes
Human replication protein A (RPA): RPA1 (P27694), RPA2 (P15927), and RPA3 (P35244). Human proliferating cell nuclear antigen (PCNA): PCNA (P12004). Human replication factor C (RFC): RFC1 (P35251), RFC2 (P35250), RFC3 (P40938), RFC4 (P35249), and RFC5 (P40937). Human Rad6/Rad18 complex: UBE2A/RAD6 (P49459) and RAD18 (Q9NS91). Human Uba1: UBA1 (P22314). Human ubiquitin: UBC (P0CG48).
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.0c00849
The authors declare no competing financial interest.
REFERENCES
- (1).Hedglin M, Kumar R, and Benkovic SJ (2013) Replication clamps and clamp loaders. Cold Spring Harbor Perspect. Biol 5, a010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Lehmann AR (1972) Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J. Mol. Biol 66, 319–337. [DOI] [PubMed] [Google Scholar]
- (3).Meneghini R, Cordeiro-Stone M, and Schumacher RI (1981) Size and frequency of gaps in newly synthesized DNA of xeroderma pigmentosum human cells irradiated with ultraviolet light. Biophys. J 33, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hedglin M, and Benkovic SJ (2017) Eukaryotic Translesion DNA Synthesis on the Leading and Lagging Strands: Unique Detours around the Same Obstacle. Chem. Rev 117, 7857–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sale JE, Lehmann AR, and Woodgate R (2012) Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol 13, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yoon JH, Prakash S, and Prakash L (2012) Requirement of Rad18 protein for replication through DNA lesions in mouse and human cells. Proc. Natl. Acad. Sci. U. S. A 109, 7799–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hedglin M, and Benkovic SJ (2015) Regulation of Rad6/Rad18 Activity During DNA Damage Tolerance. Annu. Rev. Biophys 44, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kim C, and Wold MS (1995) Recombinant human replication protein A binds to polynucleotides with low cooperativity. Biochemistry 34, 2058–2064. [DOI] [PubMed] [Google Scholar]
- (9).Kim C, Paulus BF, and Wold MS (1994) Interactions of human replication protein A with oligonucleotides. Biochemistry 33, 14197–14206. [DOI] [PubMed] [Google Scholar]
- (10).Kim C, Snyder RO, and Wold MS (1992) Binding properties of replication protein A from human and yeast cells. Mol. Cell. Biol 12, 3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hedglin M, Aitha M, and Benkovic SJ (2017) Monitoring the Retention of Human Proliferating Cell Nuclear Antigen at Primer/Template Junctions by Proteins That Bind Single-Stranded DNA. Biochemistry 56, 3415–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hedglin M, and Benkovic SJ (2017) Replication Protein A Prohibits Diffusion of the PCNA Sliding Clamp along Single-Stranded DNA. Biochemistry 56, 1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hedglin M, Aitha M, Pedley A, and Benkovic SJ (2019) Replication protein A dynamically regulates monoubiquitination of proliferating cell nuclear antigen. J. Biol. Chem 294, 5157–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pokhrel N, Caldwell CC, Corless EI, Tillison EA, Tibbs J, Jocic N, Tabei SMA, Wold MS, Spies M, and Antony E (2019) Dynamics and selective remodeling of the DNA-binding domains of RPA. Nat. Struct. Mol. Biol 26, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yates LA, Aramayo RJ, Pokhrel N, Caldwell CC, Kaplan JA, Perera RL, Spies M, Antony E, and Zhang X (2018) A structural and dynamic model for the assembly of Replication Protein A on single-stranded DNA. Nat. Commun 9, 5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Flynn RL, and Zou L (2010) Oligonucleotide/oligosaccharide-binding fold proteins: a growing family of genome guardians. Crit. Rev. Biochem. Mol. Biol 45, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Davies AA, Huttner D, Daigaku Y, Chen S, and Ulrich HD (2008) Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein a. Mol. Cell 29, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Huttner D, and Ulrich HD (2008) Cooperation of replication protein A with the ubiquitin ligase Rad18 in DNA damage bypass. Cell Cycle 7, 3629–3633. [DOI] [PubMed] [Google Scholar]
- (19).Masuda Y, Suzuki M, Kawai H, Suzuki F, and Kamiya K (2012) Asymmetric nature of two subunits of RAD18, a RING-type ubiquitin ligase E3, in the human RAD6A-RAD18 ternary complex. Nucleic Acids Res. 40, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Han J, Liu T, Huen MS, Hu L, Chen Z, and Huang J (2014) SIVA1 directs the E3 ubiquitin ligase RAD18 for PCNA monoubiquitination. J. Cell Biol 205, 811–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hibbert RG, Huang A, Boelens R, and Sixma TK (2011) E3 ligase Rad18 promotes monoubiquitination rather than ubiquitin chain formation by E2 enzyme Rad6. Proc. Natl. Acad. Sci. U. S. A 108, 5590–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Li MJ, Larsen L, and Hedglin M (2020) Rad6/Rad18 Competes with DNA Polymerases eta and delta for PCNA Encircling DNA. Biochemistry 59, 407–416. [DOI] [PubMed] [Google Scholar]
- (23).Johnson KA (1992) Transient-State Kinetic Analysis of Enzyme Reaction Pathways In The Enzymes (Sigman DS, Ed.) pp 1–61. [Google Scholar]
- (24).Lee J, Crickard JB, Reese JC, and Lee TH (2019) Single-molecule FRET method to investigate the dynamics of transcription elongation through the nucleosome by RNA polymerase II. Methods 159−160, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chen J, Le S, Basu A, Chazin WJ, and Yan J (2015) Mechanochemical regulations of RPA’s binding to ssDNA. Sci. Rep 5, 9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang QM, Yang YT, Wang YR, Gao B, Xi XG, and Hou XM (2019) Human replication protein A induces dynamic changes in single-stranded DNA and RNA structures. J. Biol. Chem 294, 13915–13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Murphy MC, Rasnik I, Cheng W, Lohman TM, and Ha T (2004) Probing single-stranded DNA conformational flexibility using fluorescence spectroscopy. Biophys. J 86, 2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Neupane K, Ritchie DB, Yu H, Foster DA, Wang F, and Woodside MT (2012) Transition path times for nucleic Acid folding determined from energy-landscape analysis of single-molecule trajectories. Phys. Rev. Lett 109, No. 068102. [DOI] [PubMed] [Google Scholar]
- (29).Lee S, Lee J, and Hohng S (2010) Single-molecule three-color FRET with both negligible spectral overlap and long observation time. PLoS One 5, No. e12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kemp MG, and Sancar A (2012) DNA excision repair: where do all the dimers go? Cell Cycle 11, 2997–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Niimi A, Brown S, Sabbioneda S, Kannouche PL, Scott A, Yasui A, Green CM, and Lehmann AR (2008) Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl. Acad. Sci. U. S. A 105, 16125–16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Macheret M, and Halazonetis TD (2018) Intragenic origins due to short G1 phases underlie oncogene-induced DNA replication stress. Nature 555, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Balakrishnan L, and Bambara RA (2013) Okazaki fragment metabolism. Cold Spring Harbor Perspect. Biol 5, a010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Srivastava M, Chen Z, Zhang H, Tang M, Wang C, Jung SY, and Chen J (2018) Replisome Dynamics and Their Functional Relevance upon DNA Damage through the PCNA Interactome. Cell Rep. 25, 3869–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Masuyama S, Tateishi S, Yomogida K, Nishimune Y, Suzuki K, Sakuraba Y, Inoue H, Ogawa M, and Yamaizumi M (2005) Regulated expression and dynamic changes in subnuclear localization of mammalian Rad18 under normal and genotoxic conditions. Genes Cells 10, 753–762. [DOI] [PubMed] [Google Scholar]
- (36).Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, and Aebersold R (2011) The quantitative proteome of a human cell line. Mol. Syst. Biol 7, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Li M, Larsen L, and Hedglin M (2020) Rad6/Rad18 Competes with DNA Polymerases eta and delta for PCNA Encircling DNA. Biochemistry 59, 407. [DOI] [PubMed] [Google Scholar]
- (38).Gourdin AM, van Cuijk L, Tresini M, Luijsterburg MS, Nigg AL, Giglia-Mari G, Houtsmuller AB, Vermeulen W, and Marteijn JA (2014) Differential binding kinetics of replication protein A during replication and the pre- and post-incision steps of nucleotide excision repair. DNA Repair 24, 46–56. [DOI] [PubMed] [Google Scholar]
- (39).Lewis JS, Spenkelink LM, Schauer GD, Yurieva O, Mueller SH, Natarajan V, Kaur G, Maher C, Kay C, O’Donnell ME, and van Oijen AM (2020) Tunability of DNA Polymerase Stability during Eukaryotic DNA Replication. Mol. Cell 77, 17–25.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Watson NB, Nelson E, Digman M, Thornburg JA, Alphenaar BW, and McGregor WG (2008) RAD18 and associated proteins are immobilized in nuclear foci in human cells entering S-phase with ultraviolet light-induced damage. Mutat. Res., Fundam. Mol. Mech. Mutagen 648, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Diamant N, Hendel A, Vered I, Carell T, Reissner T, de Wind N, Geacinov N, and Livneh Z (2012) DNA damage bypass operates in the S and G2 phases of the cell cycle and exhibits differential mutagenicity. Nucleic Acids Res. 40, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Suter DM (2020) Transcription Factors and DNA Play Hide and Seek. Trends Cell Biol. 30, 491–500. [DOI] [PubMed] [Google Scholar]
- (43).Ma CJ, Gibb B, Kwon Y, Sung P, and Greene EC (2017) Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament. Nucleic Acids Res. 45, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Gibb B, Ye LF, Gergoudis SC, Kwon Y, Niu H, Sung P, and Greene EC (2014) Concentration-dependent exchange of replication protein A on single-stranded DNA revealed by single-molecule imaging. PLoS One 9, No. e87922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Marechal A, and Zou L (2015) RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 25, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Marechal A, Li JM, Ji XY, Wu CS, Yazinski SA, Nguyen HD, Liu S, Jimenez AE, Jin J, and Zou L (2014) PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR activation via a ubiquitin-mediated circuitry. Mol. Cell 53, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yang XH, and Zou L (2009) Dual functions of DNA replication forks in checkpoint signaling and PCNA ubiquitination. Cell Cycle 8, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


