Graphical abstract

Keywords: Left atrial appendage, Transesophageal echocardiography, Cardiac computed tomographic angiography
Highlights
-
•
LAA has a pathologic association with stroke and a role in AF.
-
•
The incidence of congenitally absent or rudimentary LAA is unknown.
-
•
Highlight multimodality imaging to identify congenitally absent and diminutive LAA.
Introduction
The left atrial appendage (LAA) is a complex anatomic structure that plays an important role in the pathophysiology of atrial fibrillation (AF) and associated stroke. The LAA accounts for 90% of thrombi seen in the setting of nonvalvular AF,1 and various studies have linked anatomic LAA subtypes to increased stroke risk.2 Multimodality imaging with transesophageal echocardiography (TEE), cardiac computed tomographic angiography (CCTA), and/or cardiac magnetic resonance imaging provides excellent sensitivity and specificity in diagnosing LAA morphology and pathology and also plays a key role in the implantation of LAA occlusion devices.
Here we describe two cases in which multimodality imaging played an important role in determining the etiology of nonvisualization of the LAA.
Case Presentations
Case 1: Congenital Absence of LAA
A 42-year-old man with hypertension, dyslipidemia, type 2 diabetes mellitus, paroxysmal AF, and morbid obesity underwent CCTA for assessment of chest pain and shortness of breath. No evidence of coronary artery disease was noted; however, the LAA was not visualized, raising the possibility of complete thrombosis versus congenital absence (Figures 1A and 1B). TEE was performed to further investigate the LAA pathology and confirmed a congenitally absent LAA (Figures 1C and 1D).
Figure 1.
Congenital LAA absence. (A, B) Multiplanar reconstruction axial image from CCTA demonstrates nonvisualization of the LAA adjacent to the left superior pulmonary vein (LSPV). (C) Three-dimensional TEE shows the surgeon's view and congenital absence of the LAA. (D) Transesophageal echocardiographic view at 60° confirms congenital absence of the LAA. AO, Aorta; AV, aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve.
Case 2: Diminutive LAA
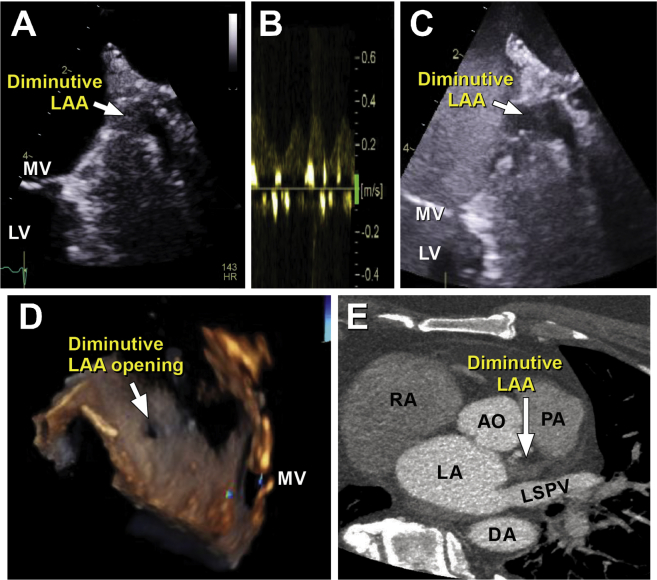
A 59-year-old man with morbid obesity presented with worsening shortness of breath and orthopnea over the course of 1 week. The workup revealed new-onset heart failure in the setting of AF with rapid ventricular response. Transthoracic echocardiography demonstrated mildly increased left ventricular size with global hypokinesis and an ejection fraction of 35%. Cardiac catheterization revealed normal coronary arteries. Tachycardia-induced cardiomyopathy was suspected, and TEE-guided cardioversion was planned. TEE showed only a small sliver of the LAA (Figure 2A), and minimal blood flow was noted on pulsed-wave Doppler evaluation (Figure 2B). Definity (Lantheus Medical Imaging, North Billerica, MA) contrast images showed minimal late filling of contrast in the sliver, raising concerns for LAA thrombosis (Figure 2C, Videos 1-3). Figure 2D shows the LAA orifice in three dimensions. Further interrogation with CCTA of the heart structure showed a diminutive appendage that did not opacify with contrast (Figure 2E). Because the possibility of thrombosis remained, the patient was continued on anticoagulation for 2 months, and repeat TEE was performed; the resulting images were identical to the initial images, confirming that the LAA was unusually small, with no evidence of thrombus.
Figure 2.
Diminutive LAA. (A) A small LAA sliver with minimal blood flow is seen on TEE in the 60° view. (B) Pulsed-wave Doppler evaluation shows low-velocity blood flow. (C) Definity contrast image shows minimal late filling of contrast in the LAA sliver. (D) The LAA orifice is seen on three-dimensional TEE. (E) CCTA shows a rudimentary appendage that did not opacify with contrast. AO, Aorta; AV, aortic valve; DA, descending aorta; LA, left atrium; LSPV, left superior pulmonary vein; LV, left ventricle; MV, mitral valve; PA, pulmonary artery; RA, right atrium.
Discussion
In these cases, we have illustrated the appearance of congenitally absent and diminutive LAAs on TEE and CCTA. Although the LAA serves as a decompression and reservoir chamber and has contractile functions, much of the attention paid to this structure in the past decade has been due to its pathologic association with stroke and its role in AF.
The LAA develops in the third to fourth weeks of embryonic life and is derived from the wall of the primary atrium, which is formed mainly by the absorption of primordial pulmonary veins and associated branches.3 It varies extensively in size, shape, and relationship with other cardiac structures. Most appendages have a well-defined orifice that leads to a neck region followed by the main body. The lobes arise from the main body, with two lobes being most common and three lobes being second most common.4 The number of lobes correlates with the risk for thrombosis.5
Congenital absence of the LAA is exceedingly rare.6,7 It is often diagnosed as an incidental finding during imaging for other purposes. Differential diagnosis for nonvisualization of the LAA includes congenital absence, flush thrombus, a diminutive or rudimentary LAA, or prior LAA isolation procedures. Multimodality imaging with either CCTA or cardiac magnetic resonance imaging in addition to TEE is needed to confirm the findings and provide the diagnosis. It is unknown whether a patient with AF can be considered at low risk for thrombus formation if he or she also has congenital absence of the LAA.
Conclusion
The role of the LAA as a common site of thrombus formation in nonvalvular AF as well as a trigger for AF has led to considerable interest in understanding the anatomic and physiologic aspects of this structure. A multimodality imaging approach is recommended to confirm congenital absence of or diminutive LAA in situations in which TEE or CCTA alone is unable to provide the diagnosis.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.09.006.
Supplementary Data
A small sliver with minimal blood flow is seen in the left atrial appendage.
A small opening of the LAA is highlighted.
Definity contrast imaging shows minimal late filling of contrast in the LAA sliver.
References
- 1.Odell J.A., Blackshear J.L., Davies E., Byrne W.J., Kollmorgen C.F., Edwards W.D. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg. 1996;61:565–569. doi: 10.1016/0003-4975(95)00885-3. [DOI] [PubMed] [Google Scholar]
- 2.Di Biase L., Santangeli P., Anselmino M., Mohanty P., Salvetti I., Gili S. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L., Burkhardt J.D., Mohanty P., Sanchez J., Mohanty S., Horton R. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 4.Veinot J.P., Harrity P.J., Gentile F., Khandheria B.K., Bailey K.R., Eickholt J.T. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M., Seo Y., Kawamatsu N., Sato K., Sugano A., Machino-Ohtsuka T. Complex left atrial appendage morphology and left atrial appendage thrombus formation in patients with atrial fibrillation. Circ Cardiovasc Imaging. 2014;7:337–343. doi: 10.1161/CIRCIMAGING.113.001317. [DOI] [PubMed] [Google Scholar]
- 6.Saleh M., Balakrishnan R., Castillo Kontak L., Benenstein R., Chinitz L.A., Donnino R. Congenital absence of the left atrial appendage visualized by 3D echocardiography in two adult patients. Echocardiography. 2015;32:1206–1210. doi: 10.1111/echo.12882. [DOI] [PubMed] [Google Scholar]
- 7.Collier P., Cavalcante J.L., Phelan D., Thavendiranathan P., Dahiya A., Grant A. Congenital absence of the left atrial appendage. Circ Cardiovasc Imaging. 2012;5:549–550. doi: 10.1161/CIRCIMAGING.112.975516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A small sliver with minimal blood flow is seen in the left atrial appendage.
A small opening of the LAA is highlighted.
Definity contrast imaging shows minimal late filling of contrast in the LAA sliver.