Abstract
The SARS-CoV-2 pandemic has affected communities, populations, and countries throughout the world. As the SARS-CoV-2 pandemic developed, the extent to which the disease interacted with already existing endemic, non-communicable and infectious diseases became evident, hence deeply influencing health outcomes. Additionally, a synergistic effect has been demonstrated also with socio-economic, cultural, and contextual determinants of health which seem to contribute to poorer health and accumulating social disadvantages.
In this essay, using as a starting point the syndemic theory that translates the cumulative and intertwined factors between different epidemics, we argue that the SARS-CoV-2 is a one health issue of a syndemic nature and that the failure to acknowledge this contributes to weakened policy-making processes and public health responses and ineffective health policies and programs.
Keywords: COVID-19, Health in all policies, One health, SARS-CoV-2, Syndemic
Abbreviations: NCD, Non Communicable Diseases; NTD, Neglected Tropical Diseases.; SDG, Sustainable Development Goals.
Highlights
-
•
COVID-19 is a one health issue.
-
•
COVID-19 has a syndemic nature.
-
•
Syndemic- informed approaches can lead to impactful multilevel prevention strategies.
-
•
Reducing limits between diseases, human and animal health encourage integrated, SDG-aligned approaches to planetary health.
1. Background
The SARS-CoV-2 infection was declared a public health emergency of international concern on 31st January and a pandemic on 11th March 2020. The disease was first reported in China in December 2019, although there is evidence from Italy and France that it was already circulating in Europe before that time [1]. In less than four months, the virus spread to all continents (excepting Antarctica), creating multiple asynchronous national epidemics of COVID-19 [2,3].
The pandemic infection caused by SARS-CoV-2 is unquestionably a one health issue due to the high possibility that the virus has crossed the species barrier to infect humans, then causing human-to-human transmission. Thus, there is a need to recognize the interconnection between people, animals, and their shared environment, and develop appropriate, collaborative and multidisciplinary approaches for its control and mitigation [4,5].
One perspective to the one health characteristics of the SARS-CoV-2 infection pandemics is its human-focused syndemic “side”.
In this essay we argue that the failure to consider the syndemic nature of this pandemic contributed to weakened policy-making processes and public health responses.
This essay is supported by a narrative review of the literature using a “synthesis of better evidence” approach [6] on syndemic theory and emerging evidence of the syndemic nature of the SARS-CoV-2 pandemic.
2. Syndemic theory
The 1990s saw the introduction of an innovative approach to comprehend health as part of a biocultural-synthesis that encompasses (i) the “determinant interconnections among pressing health problems”, (ii) “sufferer and community understandings of illness(es)/disease(s)” and (iii) the “relevant social, political, and economic forces in play”, as well as (iv) the environmental conditions that may lead to the development of health or disease. This dialectical thinking led to the term of “syndemic” as a new concept in epidemiological and public health thinking [[7], [8], [9], [10], [11], [12]].
The syndemic model shares the ecological premises of the fields with long tradition in health research and intervention (e.g. health promotion), which claim the need to overcome dichotomic understandings of individual behavior and social determinants, and between social and natural factors and further ties the syndemic model theory in with One Health. Consequently, the model discards conventional understandings of diseases as discrete entities distinct from each other and independent of the social contexts in which they are found. The model builds on a biosocial complex of co-occurrence and synergistic interaction, or simultaneous occurrence of diseases and their determinants that promote and enhance the overall negative effects on health and other conditions experienced by individuals and populations [13]. As such, it integrates and builds on concepts emerging concurrently, namely, biosocial models of health determinants [14], salutogenesis [15,16], late life compression of morbidity [17] and life course epidemiology [[18], [19], [20], [21]].
Further research on syndemics has underscored (i) a need to focus on social inequality as a root cause of syndemic interactions, and (ii) that population-level disease prevention can only occur through addressing the large-scale social forces that shape both individual and population health [[22], [23], [24]].
The term syndemic, at its simplest level, refers to two or more epidemics interacting synergistically and contributing, as a result of their interaction, to the clustering of excess burden of disease in a location or population, more than just the sum of both [25].
The syndemic concept also assumes biological synergisms that may vary from changes in blood biochemical markers or damage to organ systems caused by one pathogenic agent facilitating the spread of or impact on another agent, to gene mixing among pathogenic agents [25,26].
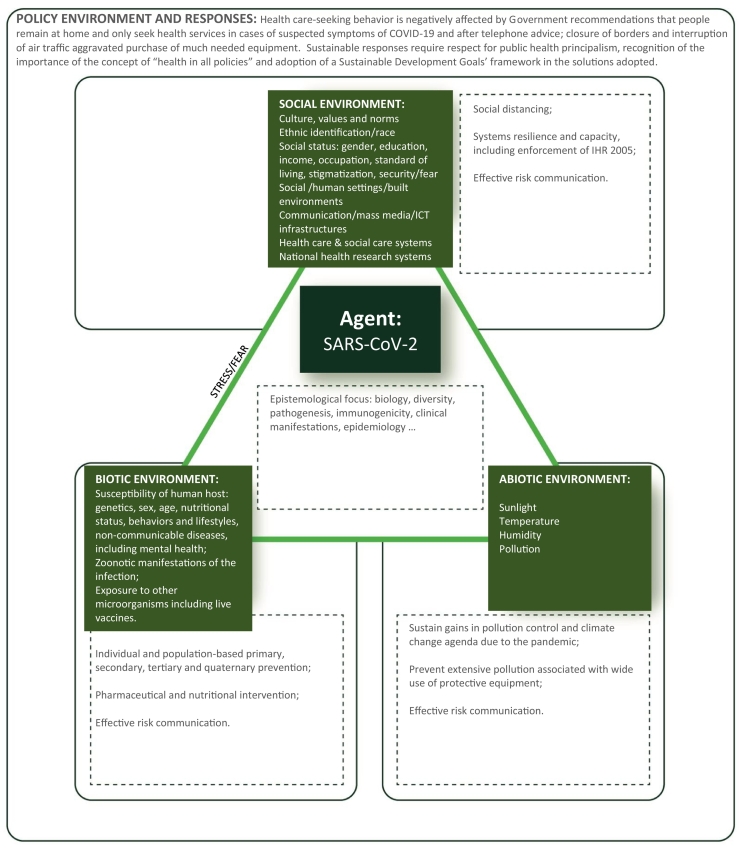
Lastly, the term syndemic also emphasizes the relevance of biological (hereditary factors, nutritional status, sex, age), social (wealth and poverty; culture and practices; networks and communities; economic, educational, dietary and leisure activities and behaviors; health care and social support systems; stigma and discrimination), environmental (natural and built environments) and political factors in the health of individuals and populations. Stress is a common pathway through which these social, environmental and political determinants affect the syndemics of health and disease (Fig. 1) [26].
Fig. 1.
Social, environmental and political determinants affect the syndemics of health and disease applied to the SARS-CoV-2 Syndemic.
The syndemic framework has been applied over the years to understand the dynamics of migrant health, health disparities, malnutrition, violence (including intimate partner violence), sexual health, substance abuse, mental health, oral health, HIV/AIDS, tuberculosis and other infectious diseases, and non-communicable diseases (NCD) (such as diabetes mellitus, cardiopulmonary conditions, obesity or kidney disease) [22,[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]].
The theory of syndemics is a conceptual tool in population health sciences that has the potential to help policymakers and program implementers in their endeavors to improve the health of populations. As originally theorized, three concepts underlie the theory: disease concentration, disease interaction, and the large-scale social forces that give rise to them. As theorized, syndemics are a complex, multilevel phenomenon. What makes the theory most noteworthy are its predictions about how interactions between different epidemics amplify the disease burden in a manner that differs if each disease would have been experienced separately. Additionally, the theory allows predictions about how public health planners can (or cannot) effectively intervene to mitigate this burden [24]. In short: syndemic translates the cumulative factors between different epidemics.
3. Evidence of the syndemic nature of the SARS-COV-2 pandemic
The SARS-CoV-2 epidemics overlaps with endemic diseases [NCD, malaria, schistosomiasis, tuberculosis, hepatitis C, HIV, dengue and other neglected tropical diseases (NTD)] and with seasonal diseases (such as influenza and other respiratory diseases), a host of cultural and social determinants (fear, stigmatization, racism, gender, economic inequalities, mis-information, risk behaviors, food and nutrition insecurity, occupation, climate, exposure to different types of pollution, supply of health and social care services, health care seeking behavior and violence, supply of drugs and addictive behaviors) and climate and environment [31,[42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57]] (Fig. 1).
A spatial overlap of high rates of NCD and COVID-19 suggests a broader syndemic health burden, in the sense that comorbidities intersect with nutrition, race and social determinants of health [56,[58], [59], [60]]. Substantial evidence points towards NCD being a major factor for poor outcomes resulting from COVID -19 [61,62] and vice-versa, COVID-19 leads to the neglect of NCD [61].
Biological interactions between SARS-CoV-2 infection and other coinfecting pathogens are also relevant. First, because of changes in host pathology related to indirect immune effects [63]. There are many examples of important interactions between malaria, NTD and other infectious diseases: malaria plays a role in Epstein–Barr virus infection, leading to Burkitt lymphoma [64]; several parasite – HIV coinfections are associated with increased HIV viral load, transmission and worsened immunosuppression [[65], [66], [67]]; deworming is associated with decreased HIV viral load and improved CD4+ counts among HIV-infected individuals [63].
Studies suggest that the balance between innate immunity and acquired immunity can be challenged by parasitic infections (e.g., parasite-induced disturbances to the helper cell type 1/type 2 balance and/or macrophage phenotype) and that effects of co-infections are immune-mediated. It has also been shown that these infections can affect disease susceptibility and pathogenesis and even interfere with accurate diagnose [68].
Hence it is possible that preexisting infection with tropical parasites and other NTDs may also lead to changes in susceptibility and/or severity of COVID-19, but it is unclear whether the resulting immune response will be beneficial or harmful when hosts are coinfected with SARS-CoV-2. It is worth mentioning the line of research that is looking at the nonspecific effects of BCG by studying the interplay between the prevalence of COVID-19 and the BCG vaccination and the hypothetical immunomodulatory effect of the BCG vaccine applied to the infection by the SARS-CoV-2 [69].
Actually, the low intensity and lethality of the national epidemics in most African countries suggest hypothetical protective interactions of the high burden of tuberculosis (and/or BCG coverage) and tropical parasitic diseases, along with the lack of health-care infrastructure capable of clinically detecting and confirming COVID-19 cases, the implementation of social distancing and hygiene, international air traffic flows, the climate, the relatively young and rural population, the genetic polymorphism of the angiotensin-converting enzyme 2 receptor, cross-immunity and the use of antimalarial drugs [[70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80]]. However, the detection of a new variant of the SARS-CoV-2 in South Africa (variant 501Y·V2) in middle December 2020 with preliminary studies suggesting that the variant is associated with a higher viral load, which may suggest potential for increased transmissibility, might challenge the low transmissibility, low lethality trend observed so far in most African Countries [81].
There are significant concerns that the emergence of COVID-19 is currently overlapping with other circulating viruses, particularly dengue, in various endo-epidemic regions across South America, South East Asia and Africa. The situation warrants observations and monitoring as both conditions may potentially lead to fatal outcomes, especially in patients with chronic comorbidities. Also, overlapping infections and co-occurrence may increase the number of patients requiring intensive care and mechanical ventilation, overwhelming the capacity of health services [82,83].
On the other hand, there is also evidence that non-pharmaceutical control measures (e.g., social distancing, school closures, travel restrictions, use of masks) being taken may prevent or postpone the occurrence of seasonal infections caused by virus such as influenza and respiratory syncytial virus. A buildup of susceptibility during these control periods may result in out of season peaks or in larger outbreaks in the coming years [84].
Non-pharmaceutical interventions also negatively affect many economic sectors, particularly in the informal economic sector (e.g., street vendors, informal traders), proximity economic activities, liberal workers and economic activities where the value chain is most dependent on the mobility of goods and people [85]. These include, for example, animal production and its related industries. Indeed, with the cessation of imports and exports between countries, it is not possible to provide feeds that are considered as basic raw materials in livestock raising. This situation impairs animal movements, decreases production inputs availability and negatively affects animal welfare. The sustainability of animal production is also affected by a shortage of workers due to the lockdown/curfew and the strong decrease in the purchasing power of the consumer. The result of the impact of non-pharmaceutical interventions, namely, lockdown, restrictions to movement of people and goods and physical distancing, among others, may result in economic collapse, increased unemployment, poverty deterioration and widespread famines, as food security becomes a global concern [[86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99]].
Stress and fear are likely to potentiate many of the interactions already cited. The epidemic of fear is well described by Delgado et al. and by Robalo et al. [100,101]. Fear in association with epidemics and pandemics has been acknowledged for centuries [102]. The peculiar aspect in relation to COVID-19 is that fear is as much caused by the novelty of the SARS-CoV-2 as it stems from the lack of preparedness of scientific communities and policymakers to help the populations to manage it [103,104].
Fear can lead to denial, including of scientific knowledge, and violence against neighbors and health care workers [[105], [106], [107]]. Fear is associated with social isolation, which is desirable to curtail the transmission of the infectious agent but impacts negatively the mental health of individuals [107] and facilitates intimate partner and child violence: social isolation contributes to unprotecting the victim and making escape, requesting help and reporting of violence difficult [27,30,32]. Fear is behind much of the stigmatization observed in association with infected persons and their contacts [108]. Fear influences health-seeking behavior in a negative way, resulting in avoidance of contact with health care facilities for preventive, promotive and treatment health care [61,109]. Fear leads also to the abandonment of the household [110,111] and to disruptive consumption patterns: one of the most immediate impacts of COVID-19 was a wave of panic buying by the public as lockdowns were announced [112].
The perception of a medical urgency is affected, leading to avoidance of emergency health care services [109]. This is further aggravated by the redeployment of scarce resources to the medical care of COVID-19 patients and to the reinforcement of public health teams involved with case finding, contact tracing and enforcement of non-pharmaceutical public health interventions [113,114].
Neglect of routine and emergency care is a reality that potentiates the negative effects of the SARS-CoV-2 pandemic. Government regulations, particularly at the beginning of the epidemics, usually recommended that people remained at home and only seek healthcare services, preferably through an initial telephone contact. With these restrictions, negative impacts on health were soon felt: endemic diseases such as malaria were neglected; chronic conditions worsened; women were left without access to family planning services, prenatal surveillance consultations, vaccinations or nutritional counseling, and institutional births were reduced considerably, associated in many developing countries with lack of public transport, leading to increases in mortality amenable to health care [24,29]. This situation, which accentuated the weaknesses of health systems and compromised the provision of services, is like a “triple burden of disease” [26] and contributed for an unwelcome “third wave” [29].
The relationship between COVID-19 and environmental factors is complex [78,115]. Recent studies have demonstrated a positive correlation between spread and decay of SARS-CoV-2 and temperature, absolute humidity and population density [116] and a positive correlation between the transmissibility of the SARS-CoV-2 and lower UV radiation, lower latitudes, lower elevation, and smaller populations with historical air pollution exposure [117]. No association was detected between deaths and country temperature or precipitation [118]. There are also preliminary findings attributing a significant fraction of worldwide COVID-19 mortality to anthropogenic air pollution [57]. On the other, a strong reduction of air pollution was identified in many locations during the lockdown measures [[119], [120], [121], [122]]. Studies measuring changes in air pollution and mortality have been related to a reduction in excess deaths due to air pollution (>4600 deaths in China, [119]. This can be viewed as a health co-benefit from the containment measures, which may reduce air pollution-induced COVID-19 mortality. Such benefits could also be achieved after the COVID-19 lockdown. Both perspectives of air pollution during the pandemic underscore the important role of fossil fuel-related and other anthropogenic emissions.
There is also evidence of SARS-CoV-2 viral RNA detectable in the inflow but not in the outflow wastewaters and its infectivity seems null [123].
An infodemic of fake news campaigns have significantly contributed for all the above, by disseminating misleading information and risk communication strategies, incoherent messages between politicians, public health authorities and scientists and an information overload that reduces trust and confidence, compromise economic activities and fail to mobilize the wider level of public support needed to enforce control measures effectively [30,31,124].
4. Conclusions
SARS-CoV-2 brought back to the forefront of the health policy debate significant issues already identified in past pandemics. Pledges were made then that we would learn from previous outbreaks and be better prepared when faced with another. That such has not happened and we are once again discussing predictable issues previously identified - such as health systems fragility and lack of resilience, inadequate surge capacities, and poor communication - is on the one end a moral failure [125] and, on the other, a reflection of the inadequate framework used to understand and respond to pandemics demonstrating the lack of preparedness for globally catastrophic risks [126]. It also identifies that the lack of capacity for governance is not a problem limited to low- and middle-income countries but widespread at all levels of development, highlighting the inability to correspond to the expectations created by the Sustainable Development Goals (SDG), of ministries exercising leadership beyond narrow sectoral boundaries, in pluralistic and multisectoral milieus, and also to predict and be prepared for emergent and future challenges while continuing to manage public services and institutions [127].
The interconnectivity of humans, animals and the social and abiotic environment (Fig. 1) is also highlighted by the current pandemic and it is most relevant in understanding and tackling any threats to food systems, agricultural production and other systems of economic livelihood. This is particularly important in economic systems where animals play an important role for society and food security – providing, income, transport, fuel and clothing as well as food, highlighting the relevance of a One-Health understanding of the pandemic [128].
The focus on finding a response to the COVID-19 pandemics, which should have been thought of previously (since for a long time, it was a given fact that a new epidemic would strike) led to a blurred approach to all other prevalent health issues and problems reflecting the “curse of piecemeal perspectives” and “siloed frameworks” [129,130].
Co-existent endemicendemic, and epidemic, NCD, NTD, and other (human and animal) infectious diseases as well as social systems' fragilities, long neglected environmental challenges, the level of health literacy and cultural issues were not considered and, now, the challenges loom larger than before as COVID-19 interacts syndemicaly with all these issues.
Understanding the impact of this syndemic requires first and foremost learning from documented experiences. Second, it requires the use of theoretical frameworks that can sufficiently conceptualize its multi-level, interacting and dynamic nature. It implies a need for assessment of how the public health system and communities can better respond to syndemics. Thus, we advocate for the adoption of a syndemic policy approach that enables a holistic and distinctive understanding of the intersection of prevalent (endemic) diseases and COVID-19-specific vulnerabilities and disparities experienced by individuals, populations, communities and societies. Syndemic-informed approaches can then, supported by insightful application of public health principalism [103], lead to impactful multi-level prevention strategies that simultaneously tackle both (endemic) diseases and their determinants, and COVID-19-specific factors and outcomes that lead to the clustering of health vulnerabilities and disparities over time (Fig. 1). This more holistic approach would integrate other conceptual frameworks such as “one health”, “health in all policies” and assume a SDG framework in the solutions adopted [113,[131], [132], [133], [134]].
These frameworks would contribute to (i) an approach which transcends geopolitical boundaries, based on good science, transparent communication and widespread global solidarity: (ii) tackle the transition out of market closures and lockdowns; (iii) more integrated and transparent surveillance infrastructures and monitoring of the occurrence of infectious diseases in humans, animals; (iii) environmental surveillance as an early warning system that monitors the levels of pathogens circulating in the population, identifies outbreaks, even before cases are notified to the healthcare system, and agents sharing similar genotypes across species as well as spatio-temporal spread of such infections; (iv) scrutiny of data, information, models and the processes by which the decisions are made and their rationale; (v) improve coordination and active collaboration among stakeholders representing diverse and apparently incompatible domains; (vi) highlight the need for an effective institutional landscape (national, regional and global), facilitating adequate regulation of hotspots for transmission of infectious agents among abiotic environment, animals and humans – regional Blocs such as the European Union, African Union, Association of Southeast Asian Nations, Caribbean Community and Mercado Común del Sur (MERCOSUR) should increase their role in health; (vii) emphasize the need for equitable solutions to infectious disease challenges, ensuring that policy response mechanisms and interventions are reflective of the disproportionate disease burdens borne by vulnerable and marginalized populations, or by persons providing health care and other essential services to those sick; (viii) further blur the border between infectious and NCD and between human and animal health encouraging an integrated approach to planetary health aligned with the SDG; and (ix) combine the short term approach in response to the crisis with a long term vision provided by the SDG.
Despite the widely recognition that the SARS-CoV-2 pandemic has become a learning opportunity, as we enter the “second epidemiological wave” much is still to be learned and one wonders if the way society deals with co-existing health problems is ever going to change. The SARS-CoV-2 has unraveled the complex dynamics between a novel, challenging health problem, co-existing, chronic and endemic ones and the capital of knowledge, competencies and practices of people all over the world and stressed its fundamental syndemic nature. As such, in this and incoming epidemics a syndemic understanding of health and disease is needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Paula Saraiva for the support with bibliography. Fundação para a Ciência e Tecnologia for funds to GHTM UID/04413/2020. CTDR is supported by CNPq, Brazil, through a Productivity Research Fellowship and is a “Cientista do Nosso Estado” by Faperj, Rio de Janeiro, Brazil.
References
- 1.Amendola A., Bianchi S., Gori M., Colzani D., Canuti M., Borghi E., Raviglione M.C., Zuccotti G.V., Tanzi E. Evidence of SARS-CoV-2 RNA in an Oropharyngeal Swab Specimen, Milan, Italy, Early December 2019. Emerg. Infect. Dis. 2020;27 doi: 10.3201/eid2702.204632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chams N., Chams S., Badran R., Shams A., Araji A., Raad M., Mukhopadhyay S., Stroberg E., Duval E.J., Barton L.M., Hajj Hussein I. COVID-19: a multidisciplinary review. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Listings of WHO's response to COVID-19. 2020. https://www.who.int/news/item/29-06-2020-covidtimeline
- 4.Walsh M.G., Sawleshwarkar S., Hossain S., Mor S.M. Whence the next pandemic? The intersecting global geography of the animal-human interface, poor health systems and air transit centrality reveals conduits for high-impact spillover. One Health. 2020;11:100177. doi: 10.1016/j.onehlt.2020.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.M. Rock, B.J. Buntain, J.M. Hatfield, B. Hallgrímsson, Animal-human connections, “one health,” and the syndemic approach to prevention, Soc. Sci. Med. 68 (2009) 991–995. doi: 10.1016/j.socscimed.2008.12.047. [DOI] [PubMed]
- 6.Green B.N., Johnson C.D., Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M. AIDS and the health crisis of the U.S. urban poor; the perspective of critical medical anthropology. Soc. Sci. Med. 1994;39:931–948. doi: 10.1016/0277-9536(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 8.Singer M. A dose of drugs, a touch of violence, a case of AIDS, part 2: further conceptualizing the SAVA syndemic. 2006;34:39–53. [Google Scholar]
- 9.Singer M. Toward a bio-cultural and political economic integration of alcohol, tobacco and drug studies in the coming century. Soc. Sci. Med. 2001;53:199–213. doi: 10.1016/s0277-9536(00)00331-2. [DOI] [PubMed] [Google Scholar]
- 10.Singer M. Stigma still: HIV stigmatization as social terrorism. Soc. Appl. Anthropol. Newslett. 2003;14:6–7. [Google Scholar]
- 11.Singer M. A dose of drugs, a touch of violence, a case of AIDS, part 2: further conceptualizing the SAVA syndemic. Free Inquiry Creat. Sociol. 2006;34:39–54. [Google Scholar]
- 12.Leatherman T., Goodman A. Building on the biocultural syntheses: 20 years and still expanding. Am. J. Hum. Biol. 2020;32 doi: 10.1002/ajhb.23360. [DOI] [PubMed] [Google Scholar]
- 13.Singer M., Bulled N., Ostrach B., Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 14.Dahlgren G., Whitehead M. Policies and Strategies to Promote Social Equity in Health. 1992. http://whqlibdoc.who.int/euro/-1993/EUR_ICP_RPD414(2).pdf WHO Regional Office for Europe.
- 15.Antonovsky A. Jossey-Bass Publishers; San Francisco: 1979. Health Stress and Coping. [Google Scholar]
- 16.Antonovsky A. Jossey-Bass Publishers, San Francisco; How People Manage Stress and Stay Well: 1987. Unraveling the Mystery of Health. [Google Scholar]
- 17.Fries J.F. The compression of morbidity. Milbank Q. 2005;83:801–823. doi: 10.1111/j.1468-0009.2005.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer J.T., Shanahan M.J., editors. Handbook of the Life Course. Springer US; 2003. [DOI] [Google Scholar]
- 19.Barker D.J.P. BMJ Publishing Group; Babies and Disease in Later Life: 1994. Mothers. [Google Scholar]
- 20.Barker D.J.P., Godfrey K.M., Gluckman P.D., Harding J.E., Owens J.A., Robinson J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 21.House J.S., Kessler R.C., Herzog A.R. Age, socioeconomic status, and health. Milbank Quart. 1990;68:383–411. doi: 10.2307/3350111. [DOI] [PubMed] [Google Scholar]
- 22.Mendenhall E. Syndemics: a new path for global health research. Lancet. 2017;389:889–891. doi: 10.1016/S0140-6736(17)30602-5. [DOI] [PubMed] [Google Scholar]
- 23.Tsai A.C., Venkataramani A.S. Syndemics and health disparities: a methodological note. AIDS Behav. 2016;20:423–430. doi: 10.1007/s10461-015-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai A.C., Mendenhall E., Trostle J.A., Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–982. doi: 10.1016/S0140-6736(17)30403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer M. Pathogen-pathogen interaction: a syndemic model of complex biosocial processes in disease. Virulence. 2010;1:10–18. doi: 10.4161/viru.1.1.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M., Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med. Anthropol. Q. 2003;17:423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 27.González-Guarda R.M., Florom-Smith A.L., Thomas T. A syndemic model of substance abuse, intimate partner violence, HIV infection, and mental health among Hispanics. Public Health Nurs. 2011;28:366–378. doi: 10.1111/j.1525-1446.2010.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kline N. There’s nowhere I can go to get help, and I have tooth pain right now: the oral health syndemic among migrant farmworkers in Florida. National Association for the Practice of Anthropology Bulletin. 2013;36:387–401. [Google Scholar]
- 29.O’Leary A., Jemmott J.B., Stevens R., Rutledge S.E., Icard L.D. Optimism and education buffer the effects of syndemic conditions on HIV status among African American men who have sex with men. AIDS Behav. 2014;18:2080–2088. doi: 10.1007/s10461-014-0708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons J.T., Grov C., Golub S.A. Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: further evidence of a syndemic. Am. J. Public Health. 2012;102:156–162. doi: 10.2105/AJPH.2011.300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline N. Syndemic statuses: intersectionality and mobilizing for LGBTQ+ Latinx health equity after the pulse shooting. Soc. Sci. Med. 2020;113260 doi: 10.1016/j.socscimed.2020.113260. [DOI] [PubMed] [Google Scholar]
- 32.Mendenhall E. 2012. Syndemic Suffering: Social Distress, Depression, and Diabetes among Mexican Immigrant Women. [Google Scholar]
- 33.Mendenhall E., Omondi G.B., Bosire E., Isaiah G., Musau A., Ndetei D., Mutiso V. Stress, diabetes, and infection: syndemic suffering at an urban Kenyan hospital. Soc. Sci. Med. 2015;146:11–20. doi: 10.1016/j.socscimed.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Mendenhall E., Kohrt B.A., Norris S.A., Ndetei D., Prabhakaran D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet. 2017;389:951–963. doi: 10.1016/S0140-6736(17)30402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer M. A dose of drugs, a touch of violence, a case of AIDS: conceptualizing the SAVA syndemic. Free Inquiry Creat. Sociol. 1996;24:99–110. [Google Scholar]
- 36.Singer M. Toward a critical biosocial model of Ecohealth in southern Africa: the Hiv/Aids and nutrition insecurity Syndemic. Ann. Anthropol. Pract. 2011;35:8–27. doi: 10.1111/j.2153-9588.2011.01064.x. [DOI] [Google Scholar]
- 37.Singer M., Bulled N., Ostrach B. Whither syndemics?: trends in syndemics research, a review 2015-2019. Glob Public Health. 2020;15:943–955. doi: 10.1080/17441692.2020.1724317. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan K.A., Messer L.C., Quinlivan E.B. Substance abuse, violence, and HIV/AIDS (SAVA) syndemic effects on viral suppression among HIV positive women of color. AIDS Patient Care STDs. 2015;29:S42–S48. doi: 10.1089/apc.2014.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willen S.S., Knipper M., Abadía-Barrero C.E., Davidovitch N. Syndemic vulnerability and the right to health. Lancet. 2017;389:964–977. doi: 10.1016/S0140-6736(17)30261-1. [DOI] [PubMed] [Google Scholar]
- 40.Bulled N., Singer M. In the shadow of HIV & TB: a commentary on the COVID epidemic in South Africa. Glob Public Health. 2020;15:1231–1243. doi: 10.1080/17441692.2020.1775275. [DOI] [PubMed] [Google Scholar]
- 41.Shiau S., Krause K.D., Valera P., Swaminathan S., Halkitis P.N. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020:1–6. doi: 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alpízar-Salazar M., Alpízar-Sánchez M.R., Aldecoa-Castillo J.M.D., Romero-Cervantes A., Alpízar M.F., Frydman T.D. Vol. 28. 2020. Chronic degenerative diseases before and after the COVID-19 pandemic in Mexico; p. 3. [Google Scholar]
- 43.Shrestha S., Bauer C.X.C., Hendricks B., Stopka T.J. Spatial epidemiology: an empirical framework for syndemics research. Soc. Sci. Med. 2020;113352 doi: 10.1016/j.socscimed.2020.113352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ong C.W.M., Migliori G.B., Raviglione M., MacGregor-Skinner G., Sotgiu G., Alffenaar J.-W., Tiberi S., Adlhoch C., Alonzi T., Archuleta S., Brusin S., Cambau E., Capobianchi M.R., Castilletti C., Centis R., Cirillo D.M., D’Ambrosio L., Delogu G., Esposito S.M.R., Figueroa J., Friedland J.S., Ho B.C.H., Ippolito G., Jankovic M., Kim H.Y., Klintz S. Rosales, Ködmön C., Lalle E., Leo Y.S., Leung C.-C., Märtson A.-G., Melazzini M.G., Fard S. Najafi, Penttinen P., Petrone L., Petruccioli E., Pontali E., Saderi L., Santin M., Spanevello A., van Crevel R., van der Werf M.J., Visca D., Viveiros M., Zellweger J.-P., Zumla A., Goletti D. Epidemic and pandemic viral infections: impact on tuberculosis and the lung. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01727-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gausman J., Langer A. Sex and gender disparities in the COVID-19 pandemic. J. Women’s Health (Larchmt) 2020;29:465–466. doi: 10.1089/jwh.2020.8472. [DOI] [PubMed] [Google Scholar]
- 46.Kim E.J., Marrast L., Conigliaro J. COVID-19: magnifying the effect of health disparities. J. Gen. Intern. Med. 2020:1–2. doi: 10.1007/s11606-020-05881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mein S.A. COVID-19 and health disparities: the reality of “the great equalizer”. J. Gen. Intern. Med. 2020:1–2. doi: 10.1007/s11606-020-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.COVID-19 and Racial/Ethnic Disparities, Health Disparities, JAMA, JAMA Network 2020. https://jamanetwork.com/journals/jama/fullarticle/2766098 (accessed November 18, 2020)
- 49.Raifman M.A., Raifman J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. Am. J. Prev. Med. 2020;59:137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shokoohi M., Nasiri N., Sharifi H., Baral S., Stranges S. A syndemic of COVID-19 and methanol poisoning in Iran: time for Iran to consider alcohol use as a public health challenge? Alcohol. 2020;87:25–27. doi: 10.1016/j.alcohol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Escamilla R., Cunningham K., Moran V.H. COVID-19, food and nutrition insecurity and the wellbeing of children, pregnant and lactating women: A complex syndemic. Matern Child Nutr. 2020 doi: 10.1111/mcn.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sönmez S., Apostolopoulos Y., Lemke M.K., Y.-C. (Jerrie) Hsieh Understanding the effects of COVID-19 on the health and safety of immigrant hospitality workers in the United States. Tour. Manag. Perspect. 2020;35:100717. doi: 10.1016/j.tmp.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemke M.K., Apostolopoulos Y., Sönmez S. Syndemic frameworks to understand the effects of COVID-19 on commercial driver stress, health, and safety. J. Transp. Health. 2020;18:100877. doi: 10.1016/j.jth.2020.100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lembke A., Supply Unsafe. Why making controlled prescription drugs available for unsupervised use will not target the syndemic of HIV, Hepatitis C, overdose, and COVID-19-- a commentary on Bonn et al. J Stud Alcohol Drugs. 2020;81(2020):564–565. [PubMed] [Google Scholar]
- 55.Bonn M., Palayew A., Bartlett S., Brothers T.D., Touesnard N., Tyndall M. Addressing the Syndemic of HIV, hepatitis C, overdose, and COVID-19 among people who use drugs: the potential roles for decriminalization and safe supply. J Stud Alcohol Drugs. 2020;81:556–560. [PubMed] [Google Scholar]
- 56.Ramírez I.J., Lee J. COVID-19 emergence and social and health determinants in colorado: a rapid spatial analysis. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17113856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regional and global contributions of air pollution to risk of death from COVID-19 | Cardiovascular Research | Oxford Academic, (2020). https://academic.oup.com/cardiovascres/advance-article/doi/10.1093/cvr/cvaa288/5940460 (accessed November 22, 2020). [DOI] [PMC free article] [PubMed]
- 58.Freeman J. Something old, something new: the syndemic of racism and COVID-19 and its implications for medical education. Fam. Med. 2020;52:623–625. doi: 10.22454/FamMed.2020.140670. [DOI] [PubMed] [Google Scholar]
- 59.Gravlee C.C. Systemic racism, chronic health inequities, and COVID-19: a syndemic in the making? Am. J. Hum. Biol. 2020;32 doi: 10.1002/ajhb.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poteat T., Millett G.A., Nelson L.E., Beyrer C. Understanding COVID-19 risks and vulnerabilities among black communities in America: the lethal force of syndemics. Ann. Epidemiol. 2020;47:1–3. doi: 10.1016/j.annepidem.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer K., Monaco A., Kivipelto M., Onder G., Maggi S., Michel J.-P., Prieto R., Sykara G., Donde S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: consequences for healthy ageing. Aging Clin. Exp. Res. 2020:1–6. doi: 10.1007/s40520-020-01601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernández-Galdamez D.R., González-Block M.Á., Romo-Dueñas D.K., Lima-Morales R., Hernández-Vicente I.A., Lumbreras-Guzmán M., Méndez-Hernández P. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch. Med. Res. 2020;51:683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faure E. Malarial pathocoenosis: beneficial and deleterious interactions between malaria and other human diseases. Front. Physiol. 2014;5 doi: 10.3389/fphys.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulama D.H., Bailey J.A., Foley J., Chelimo K., Ouma C., Jura W.G.Z.O., Otieno J., Vulule J., Moormann A.M. Sickle cell trait is not associated with endemic Burkitt lymphoma: an ethnicity and malaria endemicity-matched case-control study suggests factors controlling EBV may serve as a predictive biomarker for this pediatric cancer. Int. J. Cancer. 2014;134:645–653. doi: 10.1002/ijc.28378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon G.G. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: scientific links. Int. J. Infect. Dis. 2016;42:54–57. doi: 10.1016/j.ijid.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Kwenti T.E. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123–136. doi: 10.2147/RRTM.S154501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wall K.M., Kilembe W., Vwalika B., Dinh C., Livingston P., Lee Y.-M., Lakhi S., Boeras D., Naw H.K., Brill I., Chomba E., Sharkey T., Parker R., Shutes E., Tichacek A., Secor W.E., Allen S. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mabbott N.A. The influence of parasite infections on host immunity to co-infection with other pathogens. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat. Rev. Immunol. 2020;20:335–337. doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martins H., Hansine R. Análise epidemiológica e demográfica da COVID-19 em África. Anais Do Instituto de Higiene e Medicina Tropical. 2020;19:7–42. doi: 10.25761/anaisihmt.353. [DOI] [Google Scholar]
- 71.Lalaoui R., Bakour S., Raoult D., Verger P., Sokhna C., Devaux C., Pradines B., Rolain J.-M. What could explain the late emergence of COVID-19 in Africa? New Microbes New Infect. 2020;38:100760. doi: 10.1016/j.nmni.2020.100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.COVID-19 in Africa: Dampening the storm? | Science. 2020. https://science.sciencemag.org/content/369/6504/624 (accessed November 18, 2020) [DOI] [PubMed]
- 73.Villalonga-Morales A. Why is Covid-19 epidemics no so intense in Africa? Rev. Esp. Anestesiol. Reanim. 2020 doi: 10.1016/j.redar.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bamgboye E.L., Omiye J.A., Afolaranmi O.J., Davids M.R., Tannor E.K., Wadee S., Niang A., Were A., Naicker S. COVID-19 pandemic: is africa different? J. Natl. Med. Assoc. 2020 doi: 10.1016/j.jnma.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonte L., Acosta A., Sarmiento M.E., Ginori M., García G., Norazmi M.N. COVID-19 lethality in Sub-Saharan Africa and helminth immune modulation. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.574910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguimkeu P., Tadadjeu S. Why is the number of COVID-19 cases lower than expected in sub-Saharan Africa? A cross-sectional analysis of the role of demographic and geographic factors. World Dev. 2021;138:105251. doi: 10.1016/j.worlddev.2020.105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lawal Y. Africa’s low COVID-19 mortality rate: a paradox? Int. J. Infect. Dis. 2021;102:118–122. doi: 10.1016/j.ijid.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meo S.A., Abukhalaf A.A., Alomar A.A., Aljudi T.W., Bajri H.M., Sami W., Akram J., Akram S.J., Hajjar W. Impact of weather conditions on incidence and mortality of COVID-19 pandemic in Africa. Eur. Rev. Med. Pharmacol. Sci. 2020;24:9753–9759. doi: 10.26355/eurrev_202009_23069. [DOI] [PubMed] [Google Scholar]
- 79.Anjorin A.A., Abioye A.I., Asowata O.E., Soipe A., Kazeem M.I., Adesanya I.O., Raji M.A., Adesanya M., Oke F.A., Lawal F.J., Kasali B.A., Omotayo M.O. Comorbidities and the COVID-19 pandemic dynamics in Africa. Tropical Med. Int. Health. 2020 doi: 10.1111/tmi.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musa H.H., Musa T.H., Musa I.H., Musa I.H., Ranciaro A., Campbell M.C. Addressing Africa’s pandemic puzzle: perspectives on COVID-19 infection and mortality in sub-Saharan Africa. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.09.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO WHO | SARS-CoV-2 Variants, WHO. 2020. http://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed February 9, 2021)
- 82.Cardona-Ospina J.A., Arteaga-Livias K., Villamil-Gómez W.E., Pérez-Díaz C.E., Bonilla-Aldana D.K., Mondragon-Cardona Á., Solarte-Portilla M., Martinez E., Millan-Oñate J., López-Medina E., López P., Navarro J.-C., Perez-Garcia L., Mogollon-Rodriguez E., Rodríguez-Morales A.J., Paniz-Mondolfi A. Dengue and COVID-19, overlapping epidemics? An analysis from Colombia. J. Med. Virol. 2020 doi: 10.1002/jmv.26194. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.da Silva S.J.R., de Magalhães J.J.F., Pena L. Simultaneous circulation of DENV, CHIKV, ZIKV and SARS-CoV-2 in Brazil: an inconvenient truth. One Health. 2021;12:100205. doi: 10.1016/j.onehlt.2020.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker R.E., Park S.W., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B.T. Proc Natl Acad Sci U S A. 2020. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Barros F.P.C., Hartz Z., Ferrinho P., editors. O enfrentamento da COVID-19 nos países da CPLP (Comunidade dos Países de Língua Portuguesa) Conselho Nacional de Secretários de Saúde; Brasília, Brasil: 2020. [Google Scholar]
- 86.Defo Deeh P.B., Kayri V., Orhan C., Sahin K. Status of novel coronavirus disease 2019 (COVID-19) and animal production. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.586919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashem N.M., González-Bulnes A., Rodriguez-Morales A.J. Animal welfare and livestock supply chain sustainability under the COVID-19 outbreak: an overview. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.582528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Khalaifah H., Al-Nasser A., Abdulmalek N., Al-Mansour H., Ahmed A., Ragheb G. Impact of SARS-Con-V2 on the poultry industry in Kuwait: a case study. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.577178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hafez H.M., Attia Y.A. Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schippers M.C. For the greater good? The devastating ripple effects of the Covid-19 crisis. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.577740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neff L.M. Hidden hunger: food insecurity in the age of coronavirus. Am. J. Clin. Nutr. 2020;112:1160–1161. doi: 10.1093/ajcn/nqaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asiedu E., Sadekla S.S., Bokpin G.A. Aid to Africa’s agriculture towards building physical capital: empirical evidence and implications for post-COVID-19 food insecurity. World Dev. Perspect. 2020;20:100269. doi: 10.1016/j.wdp.2020.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gundersen C., Hake M., Dewey A., Engelhard E. Food Insecurity during COVID-19. Appl Econ Perspect Policy. 2020 doi: 10.1002/aepp.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ayanlade A., Radeny M. COVID-19 and food security in sub-Saharan Africa: implications of lockdown during agricultural planting seasons. Npj Sci. Food. 2020;4:13. doi: 10.1038/s41538-020-00073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Erokhin V., Gao T. Impacts of COVID-19 on trade and economic aspects of food security: evidence from 45 developing countries. Int. J. Environ. Res. Public Health. 2020;17:5775. doi: 10.3390/ijerph17165775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pérez-Escamilla R., Cunningham K., Moran V.H. COVID-19 and maternal and child food and nutrition insecurity: a complex syndemic. Matern. Child Nutrit. 2020;16 doi: 10.1111/mcn.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valensisi G. COVID-19 and global poverty: are LDCs being left behind? Eur. J. Dev. Res. 2020 doi: 10.1057/s41287-020-00314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.M. Pereira, A.M. Oliveira, Poverty and food insecurity may increase as the threat of COVID-19 spreads, Public Health Nutrit. (undefined/ed) 1–5. doi: 10.1017/S1368980020003493. [DOI] [PMC free article] [PubMed]
- 99.Sliwa K., Yacoub M. Catalysing the response to NCDI poverty at a time of COVID-19. Lancet. 2020;396:941–943. doi: 10.1016/S0140-6736(20)31911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robalo M., Cardoso P., Bathy J., Cortez J., Schwartz R. O Enfrentamento Da COVID-19 Nos Países Da Comunidade Dos Países de Língua Portuguesa – CPLP. Conselho Nacional de Secretários de Saúde, Brasília; Brasil: 2020. A primeira onda COVID-19 nos estados membros da CPLP: o caso da GB. [Google Scholar]
- 101.Delgado A., Correia A., Lima Mendonça M.L., Freyre Monteiro F. O Enfrentamento Da COVID-19 Nos Países Da Comunidade Dos Países de Língua Portuguesa – CPLP, Conselho Nacional de Secretários de Saúde, Brasília: Brasil. 2020. A Primeira onda COVID-19 nos Estado Membros da CPLP: CV de Março a Agosto de 2020. [Google Scholar]
- 102.Damme W.V., Lerberghe W.V. Editorial: epidemics and fear. Tropical Med. Int. Health. 2000;5:511–514. doi: 10.1046/j.1365-3156.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 103.Ferrinho P., Sidat M., Leiras G., de Barros F.P.C., Arruda H. Principalism in public health decision making in the context of the COVID-19 pandemic. Int. J. Health Plann. Manag. 2020;35:997–1000. doi: 10.1002/hpm.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Correia T. SARS-CoV-2 pandemics: the lack of critical reflection addressing short- and long-term challenges. Int. J. Health Plann. Manag. 2020;35:669–672. doi: 10.1002/hpm.2977. [DOI] [PubMed] [Google Scholar]
- 105.Sakthivel P., Rajeshwari M., Malhotra N., Ish P. Violence against doctors: an emerging epidemic amidst COVID-19 pandemic in India. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-138925. [DOI] [PubMed] [Google Scholar]
- 106.Goh K.K., Lu M.-L., Jou S. Impact of COVID-19 pandemic: social distancing and the vulnerability to domestic violence. Psychiatry Clin. Neurosci. 2020;74:612–613. doi: 10.1111/pcn.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Altheimer I., Duda-Banwar J., Schreck C.J. The impact of Covid-19 on community-based violence interventions. Am. J. Crim. Justice. 2020;45:810–819. doi: 10.1007/s12103-020-09547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bagcchi S. Stigma during the COVID-19 pandemic. Lancet Infect. Dis. 2020;20:782. doi: 10.1016/S1473-3099(20)30498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boserup B., McKenney M., Elkbuli A. The impact of the COVID-19 pandemic on emergency department visits and patient safety in the United States. Am. J. Emerg. Med. 2020;38:1732–1736. doi: 10.1016/j.ajem.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao T., Pan X., Pan C. The fate of house cats during the COVID-19 pandemic. Microbes Infect. 2020;22:157. doi: 10.1016/j.micinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singla R., Mishra A., Joshi R., Jha S., Sharma A.R., Upadhyay S., Sarma P., Prakash A., Medhi B. Human animal interface of SARS-CoV-2 (COVID-19) transmission: a critical appraisal of scientific evidence. Vet. Res. Commun. 2020:1–12. doi: 10.1007/s11259-020-09781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marchant-Forde J.N., Boyle L.A. COVID-19 effects on livestock production: a one welfare issue. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.585787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lancet T. COVID-19: a new lens for non-communicable diseases. Lancet. 2020;396:649. doi: 10.1016/S0140-6736(20)31856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rapid assessment of service delivery for NCDs during the COVID-19 pandemic. 2020. https://www.who.int/publications/m/item/rapid-assessment-of-service-delivery-for-ncds-during-the-covid-19-pandemic (accessed November 18, 2020)
- 115.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diao Y., Kodera S., Anzai D., Gomez-Tames J., Rashed E.A., Hirata A. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID-19: a comparative study of scenarios in China, England, Germany, and Japan. One Health. 2021;12:100203. doi: 10.1016/j.onehlt.2020.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Metelmann S., Pattni K., Brierley L., Cavalerie L., Caminade C., Blagrove M.S.C., Turner J., Sharkey K.J., Baylis M. Impact of climatic, demographic and disease control factors on the transmission dynamics of COVID-19 in large cities worldwide. One Health. 2021;100221 doi: 10.1016/j.onehlt.2021.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sobral M.F.F., Duarte G.B., da Penha Sobral A.I.G., Marinho M.L.M., de Souza Melo A. Association between climate variables and global transmission oF SARS-CoV-2. Sci. Total Environ. 2020;729:138997. doi: 10.1016/j.scitotenv.2020.138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen K., Wang M., Huang C., Kinney P.L., Anastas P.T. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. Lancet Planet Health. 2020;4:e210–e212. doi: 10.1016/S2542-5196(20)30107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. COVID-19 lockdowns cause global air pollution declines. PNAS. 2020;117:18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chekir N., Ben Salem Y. What is the relationship between the coronavirus crisis and air pollution in Tunisia? Euro-Mediterr J Environ Integr. 2020;6:3. doi: 10.1007/s41207-020-00189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khomsi K., Najmi H., Amghar H., Chelhaoui Y., Souhaili Z. COVID-19 national lockdown in Morocco: impacts on air quality and public health. One Health. 2020;11:100200. doi: 10.1016/j.onehlt.2020.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahmud R. COVID-19 and the future for Bangladesh’s poultry sector. One Health Poultry Hub. 2020 https://www.onehealthpoultry.org/blog-posts/covid-19-and-the-future-for-bangladeshs-poultry-sector/ (accessed November 23, 2020) [Google Scholar]
- 125.Smith M.J., Upshur R.E.G. Learning lessons from COVID-19 requires recognizing moral failures. Bioeth. Inq. 2020 doi: 10.1007/s11673-020-10019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu H.-Y., Lauta K., Maas M. 2020. Apocalypse Now? Initial Lessons from the Covid-19 Pandemic for the Governance of Existential and Global Catastrophic Risks. [Google Scholar]
- 127.Sheikh K., Sriram V., Rouffy B., Lane B., Soucat A., Bigdeli M. Governance roles and capacities of ministries of health: a multidimensional framework. Int. J. Health Policy Manag. 2020;0 doi: 10.34172/ijhpm.2020.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.FAO . FAO; Rome, Italy: 2020. Guidelines to Mitigate the Impact of the COVID-19 Pandemic on livestock Production and Animal Health. [DOI] [Google Scholar]
- 129.Sturmberg J.P., Tsasis P., Hoemeke L. COVID-19 – an opportunity to redesign health policy thinking. Int. J. Health Policy Manag. 2020;0 doi: 10.34172/ijhpm.2020.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walzer C. COVID-19 and the Curse of piecemeal perspectives. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.582983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lapão M.C. Conselho Nacional de Secretários de Saúde, Brasília; Brasil: 2020. A Agenda 2030 para o Desenvolvimento Sustentável no pós COVID 19. Exemplos para a CPLP., in: O Enfrentamento Da COVID-19 Nos Países Da Comunidade Dos Países de Língua Portuguesa – CPLP. [Google Scholar]
- 132.Mushi V. The holistic way of tackling the COVID-19 pandemic: the one health approach. Trop. Med. Health. 2020;48:69. doi: 10.1186/s41182-020-00257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruckert A., Zinszer K., Zarowsky C., Labonté R., Carabin H. What role for One Health in the COVID-19 pandemic? Can J Public Health. 2020:1–4. doi: 10.17269/s41997-020-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Forman R., Atun R., McKee M., Mossialos E. 12 lessons learned from the management of the coronavirus pandemic. Health Policy. 2020;124:577–580. doi: 10.1016/j.healthpol.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]