Abstract
Purpose
Patients with cancer are presumed to be more vulnerable to COVID-19. We evaluated a screening strategy combining chest computed tomography (CT) and reverse-transcription polymerase chain reaction (RT-PCR) for patients treated with radiation therapy at our cancer center located in a COVID-19 French hotspot during the first wave of the pandemic.
Methods and Materials
Chest CT images were proposed during radiation therapy CT simulation. Images were reviewed by an expert radiologist according to the COVID-19 Reporting and Data System classification. Nasal swabs with RT-PCR assay were initially proposed in cases of suspicious imaging or clinical context and were eventually integrated into the systematic screening. A dedicated radiation therapy workflow was proposed for COVID-19 patients to limit the risk of contamination.
Results
From March 18, 2020 to May 1, 2020, 480 patients were screened by chest CT, and 313 patients had both chest CT and RT-PCR (65%). The cumulative incidence of COVID-19 was 5.4% (95% confidence interval [CI], 3.6-7.8; 26 of 480 patients). Diagnosis of COVID-19 was made before radiation therapy for 22 patients (84.6%) and during RT for 4 patients (15.3%). Chest CT directly aided the diagnosis of 7 cases in which the initial RT-PCR was negative or not feasible, out of a total of 480 patients (1.5%) and 517 chest CT acquisitions. Four patients with COVID-19 at the time of the chest CT screening had a false negative CT. Sensitivity and specificity of chest CT screening in patients with both RT-PCR and chest CT testing were estimated at 0.82 (95% CI, 0.60-0.95) and 0.98 (95% CI, 0.96-0.99), respectively. Adaptation of the radiation therapy treatment was made for all patients, with 7 postponed treatments (median: 5 days; interquartile range, 1.5-14.8).
Conclusions
The benefit of systematic use of chest CT screening during CT simulation for patients undergoing radiation therapy during the COVID-19 pandemic seemed limited.
Introduction
The standard laboratory test to diagnose COVID-19 consists of reverse-transcription polymerase chain reaction (RT-PCR) to detect SARS-CoV-2 RNA extracted from nasopharyngeal samples. However, even though testing is very specific, false negative cases have been reported because the sensitivity of the test can be affected by the sample collection method and the timing of the sampling with respect to the course of the disease and the onset of symptoms.1 Because chest computed tomography (CT) has been shown to be valuable for COVID-19 diagnosis, with characteristic image findings,2 some authors initially suggested a pivotal role of chest CT for diagnosis and screening to alleviate the shortage of RT-PCR kits that characterized the beginning of the pandemic and the number of false-negative test results with nasopharyngeal and oropharyngeal swabs.3, 4, 5
The management of patients with cancer during this health crisis has been particularly challenging, with many uncertainties regarding the risk of severity of COVID-19 for patients undergoing anticancer treatment who are affected by COVID-19.6, 7, 8, 9, 10, 11 For patients undergoing radiation therapy, chest screening can be performed without modifying the patient workflow because the treatment planning requires a CT simulation for each patient. Therefore, screening based on chest CT scans and RT-PCR was implemented to control and mitigate the risk of COVID-19 spreading within our radiation oncology department, located in a coronavirus hotspot.
This study aimed to assess the feasibility of this COVID-19 screening procedure and to evaluate the performance of chest CT as a screening tool in our radiation therapy department in the context of limited availability of RT-PCR tests at the time of the first lockdown in France.
Methods and Materials
Study design and screening strategy
Between March 18, 2020 and May 10, 2020, a screening strategy for COVID-19 based on a chest CT acquisition was proposed by our radiation therapy department for all patients undergoing a CT simulation when feasible. We performed a unicenter, retrospective, observational study including all patients with cancer (solid tumors or hematologic malignancies) who underwent chest CT screening during CT simulation. The use of RT-PCR in this screening strategy evolved over time according to the availability of testing resources. Nasopharyngeal swabs for RT-PCR testing were first limited to symptomatic patients and asymptomatic patients with suspicious chest CT images. In early April, RT-PCR testing was extended to all patients undergoing chemoradiotherapy. As of April 20, 2020, systematic COVID-19 RT-PCR tests were performed before the first radiation therapy session because large-scale implementation of virus testing in local laboratories was available. Patients could also benefit from RT-PCR screening before the start of radiation therapy at our center (ie, before surgery or chemotherapy) or in the context of an ongoing observational study conducted at our center.
Clinical symptoms and temperature were collected from patients on arrival at the front desk of our department. Data regarding COVID-19 diagnosis, clinical presentation, treatment, and clinical outcomes, including impact on cancer management, were retrieved up to the date of the statistical analysis on June 17, 2020.
Written consent was obtained from all patients screened with a chest CT scan. This retrospective study was approved by the institutional review board and conducted in accordance with ethical standards and the 1964 Helsinki declaration and its later amendments.
CT examination and imaging evaluation
Breath-hold chest CT scans were obtained using a 16-row multidetector scanner (Siemens Sensation 16, Erlangen, Germany) with the following parameters: 120 kVp, 150 mA, 1.7 collimation, and 1.5:1 pitch. Images were reconstructed with a reconstruction matrix of 512 × 512, slice thickness of 1 mm, and BI57 (lung parenchyma) kernel (Siemens Healthineers). The width and level of the pulmonary window were set as 1500/–500. Free-breathing acquisition could be carried out for some patients with a deterioration of health conditions. A chest acquisition was not conducted when a CT simulation was obtained in the feet-first orientation (ie, lower limbs irradiation) to avoid additional repositioning.
All chest CT images were analyzed by a radiologist with 8 years of experience, without access to clinical or laboratory findings, within 48 hours of image acquisition. COVID-19–associated CT imaging features were described in accordance with American College of Radiology recommendations12 and included ground glass opacity, consolidation, interlobular septal thickening or crazy paving, subpleural line, lymph node enlargement, pleural effusion, and pericardial effusion.13 The COVID-19 Reporting and Data System (CO-RADS) classification was used to assess the pulmonary involvement and to stratify the level of suspicion of COVID-19 infection (Supplementary Text Appendix 1).2 CO-RADS provides a level of suspicion for pulmonary involvement of COVID-19 based on the features seen on a nonenhanced chest CT scan. The level of suspicion increases from very low (CO-RADS 1) to very high (CO-RADS 5). Two additional categories encode a technically insufficient examination (CO-RADS 0) and RT-PCR-proven SARS-CoV-2 infection at the time of examination (CO-RADS 6). In this study, CO-RADS scores of 4 and 5 were considered suspicious for COVID-19 infection.
RT-PCR screening was performed for all patients with concerning images before commencing radiation therapy and could be repeated in cases of negative COVID-19 results, depending on the presence of symptoms and level of suspicion after multidisciplinary evaluation with infectious disease specialists. Three radiation oncologists ensured prospectively that chest CT screening and RT-PCR results were analyzed within 48 hours.
COVID-19 RT-PCR testing
SARS-CoV-2 diagnostic testing by RT-PCR was mostly conducted at our center from March 23, 2020 (Supplementary Text Appendix 2), but could have been performed at an outside laboratory depending on patient preference.11
Definitions
Confirmed COVID-19 cases were defined as patients with positive RT-PCR results. Clinically positive COVID-19 cases were defined as patients with suspicious symptoms and chest CT images but negative RT-PCR test results.
A direct benefit of chest CT screening was defined by the diagnosis of confirmed or clinically positive COVID-19 in patients who were not known to be actually or recently infected or who were not specifically tested for COVID-19 (RT-PCR and/or diagnostic chest CT) due to acute symptoms and for whom a concomitant RT-PCR testing was not feasible or was considered a false-negative result.
Statistical analyses
Clinically positive COVID-19 cases were considered as the gold standard to assess the performance of RT-PCR testing and chest CT scans for the diagnosis of COVID-19. The accuracy of chest CT screening for the diagnosis of COVID-19 was estimated using data on patients who had both RT-PCR testing and chest CT. This limited bias linked to the risk of overestimation of patients considered negative by scan who had not been confirmed by PCR. The sensitivity and specificity of chest CT at the time of radiation therapy screening are presented. The analysis of RT-PCR accuracy for the diagnosis of COVID-19 included all serial RT-PCR results available in this cohort.
Analyses were performed using R software, version 3.6.0.14 Comparisons of demographic and clinical variables between patient groups were performed using the Fisher test for categorical variables and Wilcoxon signed-rank test or Kruskal-Wallis test for continuous variables (Table 1 and Table E5). Sensitivity, specificity, and negative and positive predictive value of chest CT screening and RT-PCR with exact binomial confidence interval were computed using the epiR R package, version 2.42. No imputation was made for missing values.
Table 1.
Patient characteristics
| Characteristics | Overall | CT and RT-PCR | CT only | P-value |
|---|---|---|---|---|
| N | 480 | 313 | 167 | |
| CT simulation, n (%) | ||||
| Yes | 480 (100.0) | 313 (100.0) | 167 (100.0) | 1.000 |
| No (already on treatment) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Age, median (IQR), y | 62.00 (50.00-70.00) | 62.00 (50.00-70.00) | 62.00 (50.00-70.50) | .942 |
| Age, y (%) | ||||
| <15 | 21 (4.4) | 11 (3.5) | 10 (6.0) | .218 |
| 15-44 | 72 (15.0) | 46 (14.7) | 26 (15.6) | |
| 45-64 | 203 (42.3) | 143 (45.7) | 60 (35.9) | |
| 65-74 | 129 (26.9) | 77 (24.6) | 52 (31.1) | |
| ≥75 | 55 (11.5) | 36 (11.5) | 19 (11.4) | |
| Sex, n (%) | ||||
| Female | 228 (47.5) | 157 (50.2) | 71 (42.5) | .133 |
| Male | 252 (52.5) | 156 (49.8) | 96 (57.5) | |
| Tumor type, n (%) | ||||
| Brain tumor | 20 (4.2) | 10 (3.2) | 10 (6.0) | .010 |
| Breast cancer | 68 (14.2) | 46 (14.7) | 22 (13.2) | |
| Endocrine cancer | 18 (3.8) | 8 (2.6) | 10 (6.0) | |
| Gastrointestinal cancer | 43 (9.0) | 34 (10.9) | 9 (5.4) | |
| Gynecologic cancer | 40 (8.3) | 33 (10.5) | 7 (4.2) | |
| Head and neck cancer | 67 (14.0) | 50 (16.0) | 17 (10.2) | |
| Hematologic cancer | 21 (4.4) | 13 (4.2) | 8 (4.8) | |
| Lung carcinoma | 78 (16.2) | 46 (14.7) | 32 (19.2) | |
| Pediatric cancer | 29 (6.0) | 14 (4.5) | 15 (9.0) | |
| Sarcoma | 29 (6.0) | 18 (5.8) | 11 (6.6) | |
| Skin cancer | 16 (3.3) | 12 (3.8) | 4 (2.4) | |
| Urologic cancer | 51 (10.6) | 29 (9.3) | 22 (13.2) | |
| Type of radiation therapy (%) | ||||
| Brachytherapy | 18 (3.8) | 17 (5.4) | 1 (0.6) | .016 |
| External radiation therapy | 462 (96.2) | 296 (94.6) | 166 (99.4) | |
| Hospitalization for brachytherapy, median (IQR), d | 3.00 (3.00-4.00) | 3.00 (3.00-4.00) | 7.00 (7.00-7.00) | .080 |
| No. of radiation therapy sessions, median (IQR) | 11.00 (5.00-24.00) | 10.00 (5.00-20.00) | 15.00 (5.00-25.00) | .374 |
| Symptoms at the time of inclusion, n (%) | ||||
| No | 426 (88.8) | 275 (87.9) | 151 (90.4) | .077 |
| Yes | 41 (8.5) | 32 (10.2) | 9 (5.4) | |
| NA | 13 (2.7) | 6 (1.9) | 7 (4.2) | |
| Chest CT scans received by patients, no. of patients (%) | ||||
| 1 | 447 (93.1) | 289 (92.3) | 158 (94.6) | .746 |
| 2 | 30 (6.2) | 21 (6.7) | 9 (5.4) | |
| 3 | 2 (0.4) | 2 (0.6) | 0 (0.0) | |
| 4 | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| CO-RADS score (%) | ||||
| 1 | 234 (48.8) | 139 (44.4) | 95 (56.9) | < .001 |
| 2 | 201 (41.9) | 132 (42.2) | 69 (41.3) | |
| 3 | 20 (4.2) | 18 (5.8) | 2 (1.2) | |
| 4 | 15 (3.1) | 14 (4.5) | 1 (0.6) | |
| 5 | 10 (2.1) | 10 (3.2) | 0 (0.0) | |
| RT-PCR tests received by patients, no. of patients (%) | ||||
| None | 167 (34.8) | 0 (0.0) | 167 (100.0) | < .001 |
| 1 | 207 (43.1) | 207 (66.1) | 0 (0.0) | |
| 2 | 68 (14.2) | 68 (21.7) | 0 (0.0) | |
| 3 | 27 (5.6) | 27 (8.6) | 0 (0.0) | |
| 4 | 10 (2.1) | 10 (3.2) | 0 (0.0) | |
| 6 | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| COVID-19 | ||||
| RT-PCR or clinically positive | 26 (5.4) | 25(8.0) | 1 (0.6) | .001 |
Abbreviations: CO-RADS = COVID-2019 Reporting and Data System classification; CT = computed tomography; IQR = interquartile range; RT-PCR = reverse-transcription polymerase chain reaction.
Results
Patients and feasibility of screening
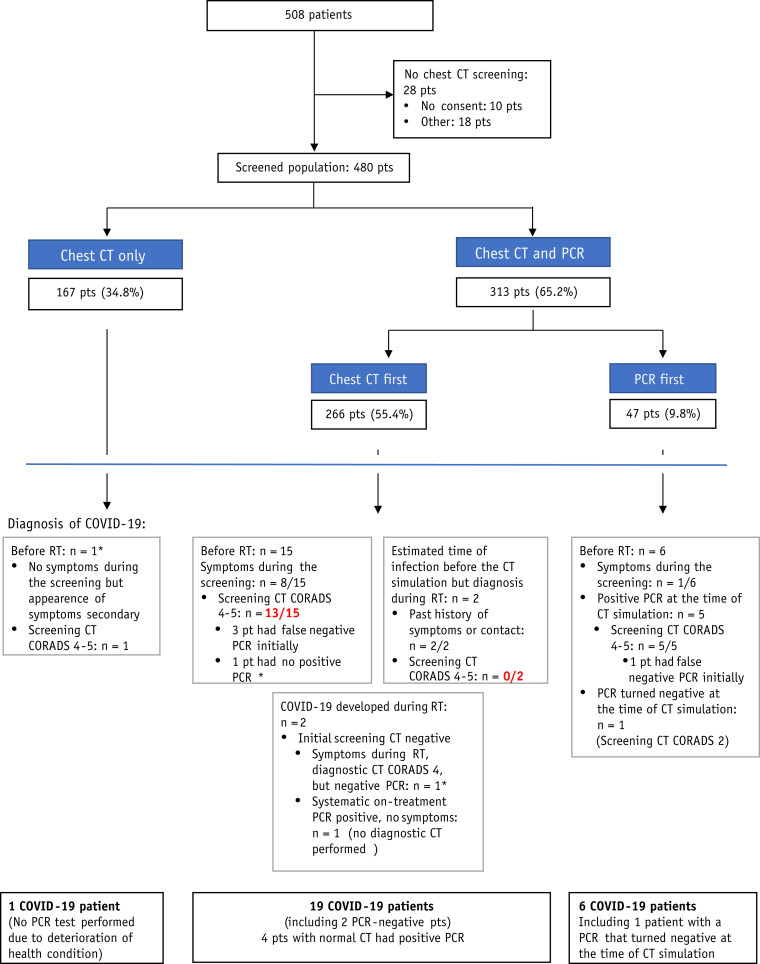
From March 18 to May 10, 508 patients with cancer underwent a CT simulation at our radiation therapy department. A chest acquisition was feasible for 480 patients (94.5%), which accounted for a total of 517 chest CT acquisitions. A breath-old acquisition was obtained for 455 patients (94.8%; Fig. 1 ). Median age was 62 years (interquartile range [IQR], 50-70; Table 1).
Fig. 1.
Flowchart of patients according to the screening procedure. ∗False-negative reverse-transcription polymerase chain reaction test result. Red: False-negative chest computed tomography scan.
The median volume CT dose index related to chest CT scans was 8.08 mGy (IQR, 6.24-11.12), with a median dose-length product of 291.25 (IQR, 209.93-388.78; Table E1). Of note, a breath-hold chest acquisition was performed as part of the pretherapeutic CT simulation without any additional dose delivery required for 37 patients (7.3%) who were planned to be treated using the deep-inspiration breath-hold technique.
A total of 313 patients (65.2%) had both RT-PCR tests and chest CT screening at the time of CT simulation. A total of 470 RT-PCR tests for COVID-19 were available for these 313 patients, including 305 patients (97.4%) with RT-PCR testing performed <7 days before the CT simulation or after the CT simulation (n = 462 samples). Of the 470 samples, 311 (66.1%) were realized before the onset of radiation therapy (Fig. E1, Table E2). The percentage of positive tests in our cohort was 5.1%.
COVID-19 screening results
Clinical symptoms compatible with viral pneumonitis at the time of the simulation CT were reported in 41 of 467 patients for whom clinical data were available (8.5%) without a significant difference according to the screening procedure (P = .08; Table 1).
Half of the patients with chest CT imaging performed at the time of the simulation CT (246 patients of 480 [51.3%]) presented with lung abnormalities compatible with nonspecific lesions compatible with infection lesions (CO-RADS ≥2; Table 1). Twenty-four patients who underwent pretreatment screening (5.0%) presented with a COVID-19 infection (RT-PCR-confirmed or clinically positive for COVID-19) before the start of radiation therapy. However, 4 patients had false-negative chest CT screening, leading to a delayed diagnosis of COVID-19 after the start of radiation therapy for 2 patients.
Sensitivity and specificity of chest CT screening using a CO-RADS score of ≥4 for diagnosis of COVID-19 were 0.82 (95% confidence interval [CI], 0.60-0.95) and 0.98 (95% CI, 0.96-0.99), respectively, with positive and negative predictive values of 0.75 (95% CI, 0.53-0.90) and 0.99 (95% CI, 0.96-1.00), respectively (Table 2 and Table E3).
Table 2.
Contingency table of chest CT screening CO-RADS score according to COVID-19 status for patients with both chest CT and RT-PCR available at the time of the COVID-19 diagnosis
| CO-RADS score | COVID-19 positive, n (%) | COVID-19 negative, n (%) |
|---|---|---|
| 5 | 10 (100) | 0 (0) |
| 4 | 8 (57.1) | 6 (42.9) |
| 3 | 0 (0) | 18 (100) |
| 2 | 0 (0) | 132 (100) |
| 1 | 4 (2.9) | 135 (97.1) |
| CO-RADS score | COVID-19 positive, n | COVID-19 negative, n | Total, n |
|---|---|---|---|
| 4-5 | 18 | 6 | 24 |
| 1-3 | 4 | 285 | 289 |
| Total | 22 | 291 | 313 |
Abbreviations: CO-RADS, = COVID-2019 Reporting and Data System classification; CT = computed tomography.
The highest CO-RADS score for each patient was kept in case of multiple chest CT scans.
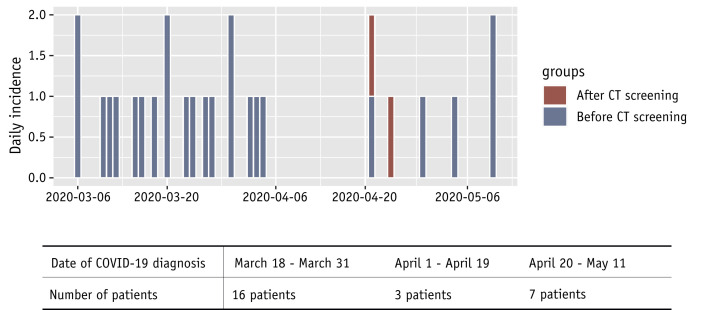
Of note, 2 other patients developed a COVID-19 infection after the start of radiation therapy, leading to a total of 26 COVID-19 patients during this period, with a cumulative incidence of 5.4% (95% CI, 3.6-7.8; Fig. 2 ). The details of RT-PCR tests carried out in COVID-19 patients are summarized in Table 3 and Table E3. Considering the false-negative RT-PCR results, the estimated sensitivity of nasopharyngeal samples in this cohort was 0.69 (95% CI, 0.51-0.83; Table 3).
Fig. 2.
Epidemic curve of COVID-19 in the radiation therapy department.
Table 3.
Contingency tables of RT-PCR results according to COVID-19 status
| COVID-19 positive | COVID-19 negative | Total | |
|---|---|---|---|
| RT-PCR positive | 24 | 0 | 24 |
| RT-PCR negative | 11 | 435 | 446 |
| Total | 35 | 435 | 470 |
| Chest CT CO-RADS score 4-5 | Estimate | 95% confidence interval |
|---|---|---|
| Sensitivity | 0.82 | 0.60-0.95 |
| Specificity | 0.98 | 0.96-0.99 |
| Positive predictive value | 0.75 | 0.53-0.90 |
| Negative predictive value | 0.99 | 0.96-1.00 |
| RT-PCR | Estimate | 95% confidence interval |
|---|---|---|
| Sensitivity | 0.69 | 0.51-0.83 |
| Specificity | 1.00 | 0.99-1.00 |
| Positive predictive value | 1.00 | 0.86-1.00 |
| Negative predictive value | 0.98 | 0.96-0.99 |
Abbreviations: CO-RADS = COVID-2019 Reporting and Data System classification; CT = computed tomography; RT-PCR = reverse-transcription polymerase chain reaction.
Twenty COVID-19 patients (76.7%) experienced symptoms related to the infection (Table 4 ). Although 11 COVID-19 patients detected by chest CT screening, they were asymptomatic at the time of the screening; it was estimated that chest CT screening played a central role in the diagnosis of only 7 of 480 screened patients (1.5%; Table 4 and Table E4). Among these 7 patients, 5 patients with initially negative RT-PCR results presented with suspicious chest CT images (CO-RADS ≥4) on screening, prompting repeated nasopharyngeal swabs that finally confirmed SARS-CoV-2 infection. One patient refused the nasopharyngeal sample due to palliative status and health condition deterioration, and another patient had a negative RT-PCR test result; however, both patients had suspicious images and compatible symptoms (Table 4). They were considered clinically positive for COVID-19 and managed the same way as RT-PCR–positive patients.
Table 4.
Time and manner of COVID-19 diagnosis according to presence of symptoms at onset of disease
| Chest CT screening | No symptoms, n (%) | Actual or history of symptoms, n (%) | Total, n (%) | Chest CT screening direct benefit, n (%) | |
|---|---|---|---|---|---|
| Screening detected (RT-PCR or chest CT) | CO-RADS score 1-3 | 3 patients, including 2 with false-negative chest CT scans (no false-negative RT-PCR): - 1 patient first had a positive RT-PCR 1 wk before CT simulation, but at the time of the chest CT screening both RT-PCR and CT were negative |
2 patients, including 1 with false-negative chest CT: - 1 had initial false-negative RT-PCR; however, repeated tests motivated by a history of symptoms enabled patient diagnosis - 1 developed COVID-19 during RT (systematic RT-PCR performed before concomitant chemotherapy 1 mo after the start of RT. No diagnostic chest CT was performed at the time of the diagnosis). |
5 (19.2) | 0 (0) |
| CO-RADS score 4-5 | 3 (no false-negative RT-PCR) | 8 patients: - 4 had initial false-negative RT-PCR but repeated tests enabled diagnosis - 1 had no positive RT-PCR (1 test performed) - 1 refused the RT-PCR test |
11 (42.3) | 6 (23.1) | |
| Total | 6 (23.1) | 10 (38.5) | 16 (61.5) | ||
| COVID-19 infection known before RT management | CO-RADS score 1-3 | 0 | 1 false-negative chest CT | 1 (3.8) | 0 |
| CO-RADS score 4-5 | 0 | 7 patients: - 1 had a past infection of RT-PCR–confirmed COVID-19 1 mo before CT simulation. Two negative RT-PCR tests allowed planning of RT; however, suspicious images on chest CT screening led to a third RT-PCR test, which was positive) |
7 (26.9) | 1 (3.8) | |
| Total | 8 (30.8) | 8 (30.8) | |||
| Revealed by symptoms, n (%) | CO-RADS score 1-3 | 0 | 1 patient had negative chest CT screening: - symptoms of COVID-19 infection occurred during RT 1 mo after, with positive diagnostic chest CT. RT-PCR was negative, but patient was considered clinically positive. |
1 (3.8) | 0 (0)∗ |
| CO-RADS score 4-5 | 0 | 1 patient, no false-negative RT-PCR | 1 (3.8) | 0 | |
| Total | 0 (0) | 2 (7.7) | 2 (7.7) | ||
| TOTAL | 6 (23.1) | 20 (76.9) | 26 (100) | 7 (26.9) |
Abbreviations: CO-RADS = COVID-2019 Reporting and Data System classification; CT = computed tomography; RT = radiation therapy; RT-PCR = reverse-transcription polymerase chain reaction.
A diagnostic chest CT scan allowed for a diagnosis, despite a negative RT-PCR test result.
Diagnosis of COVID-19 allowed for treatment adaptation for all COVID-19 patients (Table E5). Radiation therapy was postponed for 7 patients (26%), with a median delay of 5 days (IQR, 1.50-14.75), and discontinued due to COVID-19 infection diagnosed during radiation therapy in 3 patients, but could be resumed within ≤2 days of discontinuation for all. Nineteen patients (73%) were treated on a dedicated accelerator.
Discussion
In this study, we report the results of active screening combining chest CT scans and RT-PCR tests of patients undergoing radiation therapy at a tertiary cancer center in Ile-de-France at the peak of the first wave of the COVID-19 pandemic.
Several authors have shown that chest CT scans may have a higher sensibility than RT-PCR for COVID-19 diagnosis,15 but its role for mass screening was highly debated due to radiation exposure and a possible low predictive value due to low specificity.16 In addition, guidelines did not recommend chest CT as a screening tool in the overall population.17 However, the downside of such examinations appeared to be low for patients with cancer undergoing radiation therapy and could have been interesting when access to RT-PCR tests was limited.18 CT simulation is needed in radiation therapy for treatment planning, and although free-breathing images are usually performed, breath-hold chest acquisition can be easily added without modifying the patient workflow. Moreover, radiation exposure might be considered negligible with regard to radiation therapy, even for extrathoracic treatments. Indeed, 0.05% to 0.7% of the prescribed dose is still delivered at 30 cm from the irradiated field. For example, a pelvic treatment of 45 Gy may deliver around 25 to 315 mGy to the chest, which is equivalent to 3 to 40 chest CT scans.19
Although we confirmed good sensitivity and reasonable specificity of chest CT for the diagnosis of COVID-19, the direct benefit of chest CT screening seemed limited in our study. Indeed, most cases detected by chest CT screening reported a history of symptoms or close contact with COVID-19–positive persons, and most could be detected by serial RT-PCR.
We acknowledge that this study has some limitations. First, the screening procedure, particularly the use of RT-PCR, has evolved over time depending on the availability of this test, bringing some heterogeneity in the management of patients. However, the screening results of all patients were discussed prospectively by the same team, which helped ensure homogeneous decisions regarding access to RT-PCR tests. Second, the number of COVID-19 patients detected in our cohort was relatively low, which might have brought uncertainties in the estimation of chest CT performance. Therefore, the results should be interpreted with caution.
This study was conducted in the context of the pandemic peak of COVID-19 in France, and because the impact of a screening procedure is dependent on disease prevalence, these results may only be representative of situations in which the prevalence of COVID-19 is similar. Moreover, this study was performed at the beginning of the pandemic, when the availability of RT-PCR testing was limited. Finally, although early detection of COVID-19 is important in this population, several weeks of daily sessions remains the rule for most curative treatments, also justifying the need for on-treatment screening. Currently, other approaches (eg, relying on weekly repeated use of RT-PCR or rapid antigenic testing) may allow for regular screening before and during treatment. Similarly, vaccination against COVID-19, which is currently being evaluated, could be a solution for controlling the spread of COVID-19.20
Conclusions
The COVID-19 pandemic is likely the greatest public health crisis in decades. Although still unclear how long the pandemic could last, with countries currently facing a third wave of infections, the need for high-level care in oncology remains a priority for patients with cancer. Although systematic strategies of screening are needed to limit disease transmission while allowing continuity of treatment, the benefit of systematic use of chest CT screening during CT simulation for patients undergoing radiation therapy during the COVID-19 pandemic seems limited.
Acknowledgments
The authors thank the patients, their families, and all investigators and caregivers involved in the COVID-19 pandemic management at Gustave Roussy. The authors thank especially Najma Douir, Gaëlle Mevel, Khadi Diop, Simon Corbin, Frederik Hubert, Gianfranco Brusadin, and all radiotherapists. The authors also thank Zaarour Tina Marie for the English editing.
Footnotes
Disclosures: R.S. reports research and travel grants from Fondation ARC and Université Paris-Saclay and support from Inserm and Fondation Bettencourt Schueller outside of the submitted work. L.A. reports consulting fees compensated to the institution for Pfizer, Novartis, Bristol-Myers Squibb, Ipsen, Roche, MSD, AstraZeneca, Merck, Amgen, Astellas, Exelixis, Corvus Pharmaceuticals, and Peloton Therapeutics outside of the submitted work. F.B. reports personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Eli Lilly Oncology, F.Hoffmann-La Roche Ltd, Novartis, Merck-Serono, MSD, PierreFabre, Pfizer, and Takeda outside of the submitted work. A.B. reports consulting fees and support from Bristol Myer Squibb, Ipsen, MSD, Astellas, and Janssen outside of the submitted work. C.C. reports personal fees from Takeda, MSD, GSK, and Elekta outside of the submitted work; nonfinancial support for research from TherAguiX; and being an investigator for clinical trials sponsored by Roche. E.D. has received consulting fees and support from Roche, BMS, Boehringer-Ingelheim, AstraZeneca, Lilly, Amgen, and Merck-Serono. All other authors have no conflicts of interest to declare.
Data Sharing Statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2021.02.022.
Supplementary Materials
References
- 1.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prokop M., van Everdingen W., van Rees Vellinga T., et al. CO-RADS - A categorical CT assessment scheme for patients with suspected COVID-19: Definition and evaluation. Radiology. 2020 doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Yang M., Yuan J., et al. Laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infection. Innovation. 2020;1:100061. doi: 10.1016/j.xinn.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita H., Mikami T., Chopra N., et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assaad S., Avrillon V., Fournier M.L., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: A systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150 doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albiges L., Foulon S., Bayle A., et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: Results from the Gustave Roussy cohort. Nature Cancer. 2020;1:965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 12.Simpson S., Kay F.U., Abbara S., et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothor Imaging. 2020;2 doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansell D.M., Bankier A.A., MacMahon H., et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team R: A language and environment for statistical computing. 2017. https://www.R-project.org/ Available at: Accessed March 13, 2021.
- 15.Herpe G., Lederlin M., Naudin M., et al. Efficacy of chest CT for COVID-19 pneumonia in France. Radiology. 2020 doi: 10.1148/radiol.2020202568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H., Hong H., Yoon S.H. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: A meta-analysis. Radiology. 2020 doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection#:∼:text=The%20Centers%20for%20Disease%20Control,19%20on%20CXR%20or%20CT Available at: Accessed March 13, 2021.
- 18.Sun R., Ammari S., Bockel S., et al. Optimization of patient management during the COVID-19 pandemic: Chest CT scan and PCR as gatekeepers of the radiation therapy workflow. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.556334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benadjaoud M.A., Bezin J., Veres A., et al. A multi-plane source model for out-of-field head scatter dose calculations in external beam photon therapy. Phys Med Biol. 2012;57:7725–7739. doi: 10.1088/0031-9155/57/22/7725. [DOI] [PubMed] [Google Scholar]
- 20.Ribas A., Sengupta R., Locke T., et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11:233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.