Abstract
Background:
The availability and affordability of antiepileptic drugs (AEDs) are critical to the success of public health initiatives enabling care for people with epilepsy in the community.
Objective:
To pilot survey the availability and affordability of AEDs in the community.
Methods:
Field workers used standard WHO–Health Action International approaches and collected data on the availability of, and maximum retail prices of originator brands and least price generics of AEDs in 46 randomly selected public (n = 29), private (n = 8), and charitable (n = 9) pharmacy outlets. Median price ratios were computed apropos international reference prices of corresponding medications and affordability gauged with reference to daily wage of lowest paid worker.
Results:
Only 10 outlets (7 – private, 3 – public, and none – charitable) stocked at least one essential AED. Median price ratios varied between 1.1 and 1.5 essentially reflecting the difference between the least price generics and originator brands. Of note, carbamazepine-retard, 200 mg put up the slightest difference in prices of originator and least price generic brands and also was the most affordable AED.
Conclusions:
The availability and affordability of most AEDs were poor and hence, this needs to be studied on a wider scale and thereafter efforts to improve both the availability and affordability are desirable in order to address the huge treatment gap for epilepsy in India.
Keywords: Affordability, antiepileptic drugs, availability, epilepsy
INTRODUCTION
Epilepsy is a chronic, disabling neurological disorder affecting nearly 50 million people worldwide of whom, a fifth live in India.[1,2,3,4] Disability-adjusted life years due to epilepsy have been estimated at 2 million in India, third among the list of neurological disorders after only Alzheimer’s disease and migraine.[5] The true burden of epilepsy includes years lost due to premature mortality,[6,7] years lived with disability due to seizures, side-effects of antiepileptic drugs (AEDs), a range of comorbid conditions[8,9,10] and the immeasurable consequences of the associated social stigma[11,12] and economic costs of treatment.[13] Despite the consequential burden, there remains a huge treatment gap, a term denoting the proportion of PWE who are inappropriately or “not at all” treated.[14,15,16] Bridging this treatment gap is a monumental task. It encompasses a range of approaches on the supply side – ensuring the availability of affordable medications and scaling up resources, manpower and expertise involved in treatment. Demand-side factors should be simultaneously addressed by improving adherence to treatment regimens, promoting self-management skills and disengaging stigma in PWE. A national-level epilepsy control program integrating these approaches in a comprehensive manner is desirable but regrettably, no policy-based initiatives exist at the moment.
The 67th World Health Assembly appealed to its member states to develop and implement pharmaceutical supply systems to ensure the delivery of safe, effective, affordable, and quality-assured essential medicines as a component of universal health coverage.[17] The availability of medicines at affordable prices is all the more important in resource poor countries as findings from several demonstration projects have supported the safety, efficacy, and cost-effectiveness of conventional AEDs, viz., phenobarbital, phenytoin, valproate, and carbamazepine.[18,19,20,21] Hence, “supply-side” factors are critical to the success of any epilepsy control program and merit investigation.[22,23,24]
We were recently sanctioned to pilot a community-based intervention that comprised dispensation of essential AEDs, provision of psycho-educational and self-management training and abrogation of stigma by primary-care community health workers.[25] Here, we report the results of a pilot survey to document AED prices, availability and affordability in the target area.
METHODS
The survey was undertaken at Ludhiana City, in Punjab state in northwest India. The city with a population of 2.06 million people has two district-level government hospitals, one run by the state government health corporation (Civil Hospital) and the other by the employees state insurance scheme.[25] Besides, there are 6 community health centers, 11 upgraded primary health centers, 10 revamped centers, 6 slum area dispensaries, and 4 urban kiosks as part of governmental health care infrastructure. The community health centers each cater to a population of 1,20000 and the primary health centers to 30,000 people. The urban kiosks are located in slum areas with high proportions of interstate migrants. The private sector comprises of two private referral tertiary-care hospitals, each attached to nationally reputed medical schools and a large but unspecified number of smaller private hospitals, nursing homes, and stand-alone clinics in the city. In addition, a number of homeopathic and ayurvedic physicians and quacks provide healthcare to the population. The city is serviced by over 1000 registered wholesale and retail pharmacies or drug-outlets, each staffed by a pharmacist.
Sampling frame
We adapted the World Health Organization (WHO)/Health Action International (HAI) survey methodology and tool to determine the availability, pricing and affordability of various formulations and strengths (n = 40; Appendix 1) of 11 AEDs in 46 pharmacy outlets in the city.[26] The tool was jointly developed by WHO and HAI to reliably collect and analyze essential medicine prices, availability and affordability data across different healthcare sectors and regions within and across different countries. Both public (governmental) and private sectors were surveyed. The public-sector sample (n = 29) comprised of two district public hospitals, three community health centers, eight primary health centers, four slum area dispensaries, five revamped centers, and four urban kiosks, which were selected at random from the list of public health centers providing treatment and healthcare.[5] The private- sector sample (n = 8) included three private registered retail drug-outlets, selected at random in addition to the pharmacies of the two medical school-associated hospitals. A third (or “other”) sector surveyed comprised of nine charitable hospitals with outpatient facilities. In order to render the sample representative of the population, the sampling included both urban and peri-urban rural areas of the district.
Data collection
Study personnel were trained and they subsequently surveyed the facilities between 16.08 and 15.09.2017 and collected data in a structured format adapted from the WHO–-HAI workbook. The data referred to the availability and maximum retail prices (MRPs) of different formulations and strengths of the originator brands and least price generics (LPGs) of various AEDs listed in Appendix 1. When more than half of the listed items were unavailable at a particular site, the pricing data were collected from a backup site. Prices were accrued for only “in stock” items on the day of survey. Information collected was restricted to availability alone in those public-sector facilities, in which medicines were provided free of cost. A survey manager (KS) reviewed the field data accrued on a daily basis and also cross-validated a random sample of eight outlets.
Analysis
Data were double-entered in to Excel sheets for analysis. Median price ratios (MPRs) (with interquartile ranges) of the originator and LPGs were computed using international reference prices (IRPs) as denominators.[27] Group comparisons included availability and MPRs of the originator and LPGs and in the three different sectors (public, private, and others). Affordability was assessed by dividing the total daily cost of daily defined dose of the prescribed product by daily wage of the lowest-paid unskilled worker in Punjab state during the year of the survey.[26,28]
RESULTS
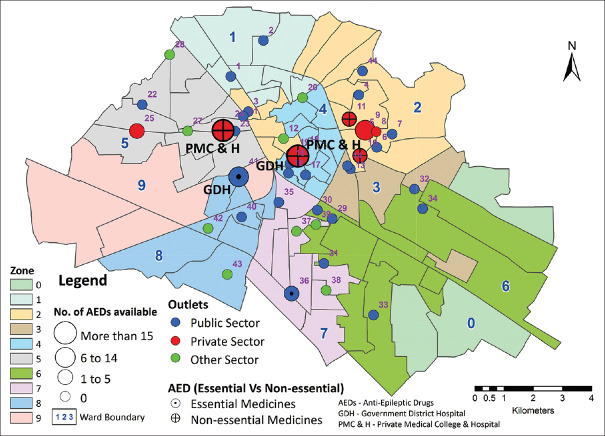
A total of 46 medicine outlets across the three sectors (29 across public sector, 8 across private sector, and 9 charitable hospitals) were surveyed and their locations were charted on the map of the city [Figure 1].
Figure 1.
Pharmacy outlets in Ludhiana showing the availability of AEDs (Source: Original)
Availability
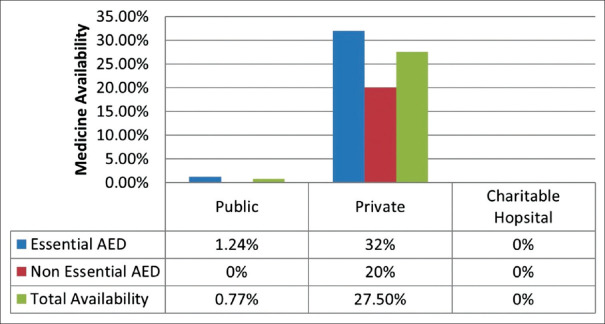
Of the formulations and strengths of different AEDs surveyed, the mean availability per outlet was 5.26 (±2.9)%. The difference in availability of AEDs between private sector and public and charitable sector outlets is presented in Figures 2 and 3 and demonstrates a nearly complete non-availability of AEDs in public and “other” sectors. At least one essential AED was available in 10 out of 46 (22%) outlets surveyed, in seven out of eight (87.5%) of the private sector outlets but in only three out of 29 (10.3%) of the public, and none of the “other” sector outlets. None of the outlets surveyed stocked the entire set of essential AEDs. Epilepsy medicines not included on the essential drug list were available in 4 out of 46 (8.7%) outlets, in none of the public and “other” sector outlets, and in 4 out of 8 (50%) private sector outlets. Pediatric formulations were available in 2 out of 29 (6.9%) public sector outlets (phenytoin syrup, 30 mg/5 ml and 125 mg/5 ml), four out of 8 (50%) private outlets and none of the “other” sector outlets.
Figure 2.
AED Availability across the three sectors surveyed (Source: Original)
Figure 3.
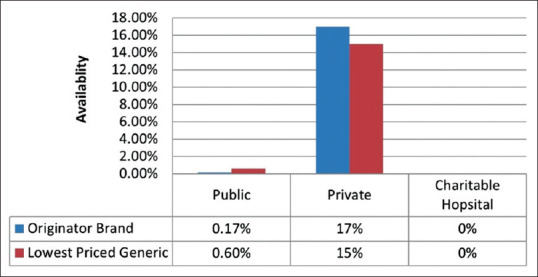
Availability of originator brand and lowest priced generic AEDs in the three sectors. (Source: Original)
Median price ratios (MPRs)
The MPRs were calculated for those drugs for which, MSH, 2015 IRPs were known and are shown in Table 1. Hence, MPRs could be calculated for 16 formulations of five AEDs [Table 1]. Overall, MPR of originator brand AEDs was 1.5 and that of LPGs was 1.1. Considerable difference in MPRs of originator brands and LPGs was observed for lamotrigine (particularly, 25 mg tablet strength) and phenytoin 100 mg (capsule/tablet) but not for carbamazepine-retard (200 mg and 400 mg) and sodium valproate (200 mg) tablets.
Table 1.
Median price ratios of AEDs with available 2015 Management Sciences of Health International Reference Prices[27] (Source: Original)
| AED formulation and strength | MPR (originator brand) | MPR (lowest price generic) |
|---|---|---|
| Carbamazepine R Tab 200 mg | 0.17 | 0.16 |
| Carbamazepine R Tab 400 mg | 0.35 | 0.31 |
| Carbamazepine syrup 100 mg/5 ml | 0.09 | |
| Carbamazepine Tab 400 mg | 2.85 | |
| Lamotrigine Tab 25 mg | 3.56 | 2.61 |
| Lamotrigine Tab 100 mg | 2.24 | 1.83 |
| Phenobarbital Tab 30 mg | 2.52 | |
| Phenobarbital Tab 60 mg | 3.59 | |
| Phenytoin Syrup 125 mg/5 ml | 0.13 | |
| Phenytoin Tab 50 mg | 0.35 | |
| Phenytoin Tab 100 mg | 2.46 | 2.06 |
| Sodium Valproate Syrup 200 mg/5 m | 0.33 | |
| Sodium Valproate Tab 200 mg | 0.68 | 0.67 |
| Sodium Valproate Tab CR 200 mg | 0.55 | |
| Topiramate Tab 25 mg | 0.72 | |
| Topiramate Tab 100 mg | 1.43 | |
| Average MPR | 1.50 | 1.13 |
Abbreviations:-AED: Antiepileptic drugs; MPR: Median price ratio; Tab: Tablet; CR: Controlled release; R: Retard
Affordability
The affordability analysis was restricted to private sector outlets as AEDs were mostly unavailable (or cost-free when available) in the other two sectors. Carbamazepine-retard, 200 mg was the most affordable AED [Table 2]. If lamotrigine, 25 mg is considered an outlier and hence excluded, the affordability ratios of all AEDs varied between 28 and 60.
Table 2.
AED affordability of originator brand (OB) and lowest priced generic (LPG) (Source: Original)
| AED formulation and strength | Affordability (originator brand) | Affordability (lowest price generic) |
|---|---|---|
| Carbamazepine R Tab 200 mg | 5.99 | 5.57 |
| Carbamazepine R Tab 400 mg | 6.01 | 5.41 |
| Carbamazepine syrup 100 mg/5 ml | 32.93 | |
| Carbamazepine Tab 400 mg | 49.48 | |
| Lamotrigine Tab 25 mg | 296.18 | 217.02 |
| Lamotrigine Tab 100 mg | 46.66 | 38.06 |
| Phenobarbital Tab 30 mg | 58.29 | |
| Phenobarbital Tab 60 mg | 41.57 | |
| Phenytoin Syrup 125 mg/5 ml | 10.54 | |
| Phenytoin Tab 50 mg | 14.77 | |
| Phenytoin Tab 100 mg | 51.16 | 42.96 |
| Sodium Valproate Tab 200 mg | 35.38 | 34.75 |
| Sodium Valproate Tab CR 200 mg | 28.57 | |
| Topiramate Tab 25 mg | 60.16 | |
| Topiramate Tab 100 mg | 29.77 | |
| Average | 55.23 | 49.83 |
Abbreviations:-AED: Antiepileptic drugs; Tab: Tablet; CR: Controlled release;, R: Retard
CONCLUSION
Previous studies including meta-analyses have underscored the high treatment gap for epilepsy in LMICs.[15,16] Although reliable national-level estimates are not available, limited studies suggest particularly wide gaps in the treatment of epilepsy in India.[29,30,31] Gaps in several different aspects of epilepsy care add up to form the treatment gap. For instance, there is an enormous scarcity inside of the neurological work force available in LMICs.[32] The WHO recommends engaging primary care health personnel in order to close the gap till such time enough neurological experts are available to provide care for PWE in LMICs.[33] Two other gaps are relevant: gaps in supply of epilepsy medicines and affordability of treatment. This communication examines supply and affordability gaps in providing care to PWE in LMIC communities.
The timely and uninterrupted availability of epilepsy medicines at affordable rates is crucial to the success of epilepsy care at a community level. Conversely, the lack of access to epilepsy medicines translates to nonadherence, which in turn is associated poorer health and psycho-social outcomes.[34,35] Access to medications can be addressed by scaling up the supply side.
The limited geographic scope of this survey, notwithstanding, the data presented herein calls for attention the extreme lack of availability of epilepsy medicines even in nonremote urban locations. Leaving apart a few private outlets located close to leading tertiary care hospitals, the availability of AEDs was overall poor and fragmented. Overall, the mean availability fell well below the WHO-stipulated target of 80% availability for all essential medicines in LMICs.[36]
We encountered only minor differences in the MPRs of originator and LPGs of most AEDs. The small difference in MPRs of originator brands and LPGs indicates that appropriate policies are in place in order to regulate the prices of most epilepsy medicines. The only exception was lamotrigine at a dose of 25 mg. The wide disparity for this particular strength might be due to the absence of price regulation specifically for this strength.
Analysis of affordability data suggested that a whole month’s stock of AEDs required nearly or over a month’s wages of the lowest paid, daily wages worker in India. The only exception to the lack of affordability was carbamazepine-retard (200 mg and 400 mg). These data emphasize that apart from carbamazepine-retard (200 mg and 400 mg), none of the epilepsy medicines was within the means of lowest paid daily wage workers in India. A medicine for a chronic disorder is considered affordable if the lowest paid, daily wage worker has to pay not more than a day’s wages to procure a month’s supply of the medicine. The poor affordability could be tackled by an equitable provision of essential AEDs. Indeed, a simulation analysis has demonstrated the feasibility of universal provision of all AEDs in India.[36]
Interpretation of the data from this survey has some limitations. We were unable to collect data on the quantity of epilepsy medicines available at the outlets surveyed because most storekeepers did not agree to divulge this information. Pricing analysis was restricted to only those AEDs for which IRPs were available. Moreover, the survey was geographically-restricted and hence data needs to be gathered from other diverse locations within the country. However, given the poor availability in urban regions, it may be assumed that the availability would be even poorer in remote rural locations.
The exceedingly low availability of epilepsy medicines underscores the need for a nation-wide survey of epilepsy medicines availability, pricing, and affordability. Scaling up the supply side is imperative from the governmental perspective in LMICs. Policy-level strategies that might address the supply gap might include but are not limited to allocation of a budget for epilepsy at national and sub-national levels and improving the efficiency of the supply chain. Clearly, governments need to invest more in procuring epilepsy medicines.
Financial support and sponsorship
The survey was an offshoot of a cluster-randomized trial of home-based care for epilepsy funded by Ad hoc grant no. No: 5/4-5/127/Neuro/2013-NCD-I of the Indian Council of Medical Research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The contribution of Manpreet and Sukhpreet, both field workers in executing the survey is acknowledged. We are grateful to Prof. Kurupath Radhakrishnan, Professor of Neurology at Amrita Institute of Medical Sciences, Cochin, India, and Dr. Meenakshi Sharma, Scientist G, Indian Council of Medical Research, New Delhi, India for helpful suggestions during the study appraisal meeting.
Appendix 1
Medicine Name and Strength
Carbamazepine R Tab 200 mg
Carbamazepine R Tab 300 mg
Carbamazepine R Tab 400 mg
Carbamazepine Syrup 100 mg/5 ml
Carbamazepine Tab 100 mg
Carbamazepine Tab 200 mg
Carbamazepine Tab 400 mg
Clobazam Tab 5 mg
Clobazam Tab 10 mg
Clobazam Tab 20 mg
Lacosamide Tab 100 mg
Lamotrigine Tab 25 mg
Lamotrigine Tab 100 mg
Levetracetam Syrup 500 mg/5 ml
Levetracetam Tab 250 mg
Levetracetam Tab 500 mg
Levetracetam Tab 750 mg
Levetracetam Tab 1000 mg
Oxcarbazepine Syrup 200 mg/ml
Oxcarbazepine Tab 150 mg
Oxcarbazepine Tab 300 mg
Oxcarbazepine Tab 450 mg
Oxcarbazepine Tab 600 mg
Phenobarbital Syrup 20 mg/5 ml
Phenobarbital Tab 30 mg
Phenobarbital Tab 60 mg
Phenytoin ER Tab 300 mg
Phenytoin Syrup 30 mg/5 ml
Phenytoin Syrup125 mg/5 ml
Phenytoin Tab 50 mg
Phenytoin Tab 100 mg
Sodium Valproate Syrup 200 mg/5 ml
Sodium Valproate Tab 200 mg
Sodium Valproate Tab 500 mg
Sodium Valproate Tab CR 200 mg
Sodium Valproate Tab CR 300 mg
Sodium Valproate Tab CR 500 mg
Topiramate Tab 25 mg
Topiramate Tab 100 mg
Zonisamide Tab 100 mg
REFERENCES
- 1.WHO. Atlas: Epilepsy care in the world. 2005. [[Last accessed on 2017 Oct 21]]. Available from: http://www.who.int/mental_health/neurology/Epilepsy_atlas_r1.pdf .
- 2.Amudhan S, Gururaj G, Satishchandra P. Epilepsy in India II: Impact, burden, and need for a multisectoral public health response. Ann Indian Acad Neurol. 2015;18:369–81. doi: 10.4103/0972-2327.165483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amudhan S, Gururaj G, Satishchandra P. Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol. 2015;18:263–77. doi: 10.4103/0972-2327.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbareddy N, Santhosh SS, Satishchandra P. Epilepsy: Indian perspective. Ann Indian Acad Neurol. 2014;S1:S3–11. doi: 10.4103/0972-2327.128643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PHFI/ICMR/IHME. India state-level disease burden initiative: Preliminary estimates of mental and neurological disorders Unpublished. 2016 [Google Scholar]
- 6.Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, et al. Premature mortality of epilepsy in low- and middle-income countries: A systematic review from the mortality task force of the international league against epilepsy. Epilepsia. 2017;58:6–16. doi: 10.1111/epi.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diop AG, Hesdorffer DC, Logroscino G, Hauser WA. Epilepsy and mortality in Africa: A review of the literature. Epilepsia. 2005;46(Suppl 11):33–5. doi: 10.1111/j.1528-1167.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaitatzis A, Sisodiya SM, Sander JW. The somatic comorbidity of epilepsy: A weighty but often unrecognized burden. Epilepsia. 2012;53:1282–93. doi: 10.1111/j.1528-1167.2012.03528.x. [DOI] [PubMed] [Google Scholar]
- 9.Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110:207–20. doi: 10.1111/j.1600-0404.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- 10.Sander JW. Comorbidity and premature mortality in epilepsy. Lancet. 2013;382:1618–9. doi: 10.1016/S0140-6736(13)61136-8. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby A, Gorry J, Gamble C, Baker GA. Public knowledge, private grief: A study of public attitudes to epilepsy in the United Kingdom and implications for stigma. Epilepsia. 2004;45:1405–15. doi: 10.1111/j.0013-9580.2004.02904.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby A, Snape D, Baker GA. Epilepsy and social identity: The stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–8. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan A, Sahariah SU, Kapoor SK. Cost of epilepsy in patients attending a secondary-level hospital in India. Epilepsia. 2004;45:289–91. doi: 10.1111/j.0013-9580.2004.63102.x. [DOI] [PubMed] [Google Scholar]
- 14.Meyer AC, Dua T, Boscardin WJ, Escarce JJ, Saxena S, Birbeck GL. Critical determinants of the epilepsy treatment gap: A cross-national analysis in resource-limited settings. Epilepsia. 2012;53:2178–85. doi: 10.1111/epi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: A systematic review. Bull World Health Organ. 2010;88:260–6. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: A systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Assembly WRS-s. WHA67.22 - Access to Essential Medicines. 2014. [[Last accessed on 2017 Oct 21]]. Available from: http://apps.who.int/medicinedocs/documents/s21453en/s21453en.pdf .
- 18.Mani KS, Rangan G, Srinivas HV, Srindharan VS, Subbakrishna DK. Epilepsy control with phenobarbital or phenytoin in rural south India: The Yelandur study. Lancet. 2001;357:1316–20. doi: 10.1016/s0140-6736(00)04516-5. [DOI] [PubMed] [Google Scholar]
- 19.Pal DK, Das T, Chaudhury G, Johnson AL, Neville BG. Randomised controlled trial to assess acceptability of phenobarbital for childhood epilepsy in rural India. Lancet. 1998;351:19–23. doi: 10.1016/S0140-6736(97)06250-8. [DOI] [PubMed] [Google Scholar]
- 20.Si Y, Liu L, Tian L, Mu J, Chen D, Chen T, et al. A preliminary observation of the adverse effects of phenobarbital among patients with convulsive epilepsy in rural West China. Epilepsy Behav. 2016;54:65–70. doi: 10.1016/j.yebeh.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Ding D, Hong Z, Chen GS, Dai XY, Wu JZ, Wang WZ, et al. Primary care treatment of epilepsy with phenobarbital in rural China: Cost-outcome analysis from the WHO/ILAE/IBE global campaign against epilepsy demonstration project. Epilepsia. 2008;49:535–9. doi: 10.1111/j.1528-1167.2007.01529_2.x. [DOI] [PubMed] [Google Scholar]
- 22.Asadi-Pooya AA. Availability of antiepileptic drugs: Politicians’ roles. Epilepsia. 2016;57:671. doi: 10.1111/epi.13323. [DOI] [PubMed] [Google Scholar]
- 23.Bergen DC. Restrictions on the availability of antiepileptic drugs. Arch Neurol. 2000;57:273–4. doi: 10.1001/archneur.57.2.273. [DOI] [PubMed] [Google Scholar]
- 24.Cameron A, Bansal A, Dua T, Hill SR, Moshe SL, Mantel-Teeuwisse AK, et al. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia. 2012;53:962–9. doi: 10.1111/j.1528-1167.2012.03446.x. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. Ludhiana city census data 2011. 2011. [[Last accessed on 2017 Oct 20]]. Available from: http://www.census2011.co.in/census/city/11-ludhiana.html .
- 26.World Health Organisation/Health Action International. Measuring medicine prices, availability, affordability and price components. 2008 [Google Scholar]
- 27.Management Sciences for Health International medical products price guide. 2015. [[Last accessed on 2017 Oct 20]]. Available from: http://mshpriceguide.org/en/home/
- 28.Anonymous. Punjab minimum wages, February 01, 2017 to July 31, 2017. 2017. Available from: https://paycheck.in/main/salary/minimumwages/punjab/punjab-minimum-wage-w-e-f-february-1-2017-to-july-31-2017 .
- 29.Nizamie SH, Akthar S, Banerjee and Goyal N. Health care delivery model in epilepsy to reduce treatment gap: World Health Organization study from a rural tribal population of India. Epilepsy Res. 2009;84:146–52. doi: 10.1016/j.eplepsyres.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Pandey S, Singhi P, Bhavneet B. Prevalence and treatment gap in childhood epilepsy in a North Indian City: A community-based study. J Trop Pediatr. 2014;60:118–23. doi: 10.1093/tropej/fmt091. [DOI] [PubMed] [Google Scholar]
- 31.Jagarlapudi M, Seshadri V. Determinants of the epilepsy treatment gap in a resource limited setting: A study in a rural community in South India. Neurology. 2016;86:P1082. [Google Scholar]
- 32.World Health Organization Atlas: Country Resources for neurological disorders. [[Last accessed on 2018 Feb 09]]. Available from: http://www.who.int/mental_health/neurology/en/
- 33.WHO. Global burden of epilepsy and the need for coordinated action at the country level to address its health, social and public knowledge implications. 2015. [[Last accessed on 2017 Oct 20]]. Available from: http://www.who.int/mental_health/neurology/epilepsy/resolution_68_20/en/
- 34.Lin CY, Chen H, Pakpour AH. Correlation between adherence to antiepileptic drugs and quality of life in patients with epilepsy: A longitudinal study. Epilepsy Behav. 2016;63:103–8. doi: 10.1016/j.yebeh.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad N, IzatyOthaman N, Islahudin F. Medication adherence and quality of life in epilepsy patients. Int J Pharm PharmSci. 2013;5:401–4. [Google Scholar]
- 36.Megiddo I, Colson A, Chisholm D, Dua T, Nandi A, Laxminarayan R. Health and economic benefits of public financing of epilepsy treatment in India: An agent-based simulation model. Epilepsia. 2016;57:464–74. doi: 10.1111/epi.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]