Abstract
Background and Purpose:
Epstein–Barr virus (EBV) meningoencephalitis can have variable and nonspecific brain magnetic resonance imaging (MRI) findings in children. This study was done with the purpose of describing brain MRI findings in children with EBV meningoencephalitis.
Materials and Methods:
The study included 45 pediatric patients that presented with variable neurological symptoms and were found to have EBV meningoencephalitis based on positive EBV deoxyribonucleic acid (DNA) in the cerebrospinal fluid. All these patients had undergone brain MRI. Clinical and radiological features were evaluated.
Results:
Fever was a presenting feature in all cases. Signs of meningitis and raised intracranial pressure (ICP) were seen in 24 (53.3%) cases, encephalopathy in 15 (33.3%), and seizures were present in 33 (73.3%). MRI was abnormal in 29 (64.4%) patients. The cortical/subcortical pattern was diagnosed in 9 (20%) cases, white matter involvement in 7 (15.5%), basal ganglia in 5 (11.1%), thalamic involvement in 4 (8.8%), brain stem involvement in 2 (6.2%), substantia nigra involvement in 2 (4.4%), and cerebellar involvement in 2 (4.4%). Diffusion restriction was present in 11 (24.4%) cases and susceptibility changes in 7 (15.5%). Meningeal enhancement was present in 10 (22.2%) cases. In addition, brain abscess and subdural effusion/empyema were present in 1 (2.2%) case each.
Conclusion:
Pediatric EBV meningoencephalitis has varied clinicoradiological spectrum and there is no specific MRI pattern to characterize the meningoencephalitis on imaging. Common MRI findings include cortical-subcortical involvement, white matter changes, basal ganglia, and thalamic involvement.
Keywords: Epstein–Barr virus, meningoencephalitis, magnetic resonance imaging
INTRODUCTION
Epstein–Barr virus (EBV) is commonly seen to involve pediatric patients and has a self-limiting course. Common manifestations of EBV infection seen in clinical practice include fever, lymph nodal involvement, pharyngitis, and otomastoiditis. Central nervous system (CNS) manifestations of EBV are uncommon; however, when present, they may be seen as encephalitis, meningitis, demyelination, meningoencephalitis, polyradiculomyelitis, nerve palsies, and cerebellar ataxia.[1,2] EBV encephalitis is seen in up to 5% of all causes of viral meningoencephalitis.[3] Common presenting features with EBV encephalitis are fever, neck rigidity, altered sensorium, irritability, or coma.[3] EBV virus has to be considered as a possible etiology in pediatric acute encephalitis as clinical and radiological findings are usually nonspecific. Diagnosis has to be made with the presence of EBV antibodies and polymerase chain reaction (PCR) in blood or cerebrospinal fluid (CSF). Magnetic resonance imaging (MRI) studies can be useful in these cases, particularly, to rule out other characteristic viral infections, such as herpes simplex, as well as to observe the extent of brain involvement in cases of a focal deficit. The purpose of this study was to evaluate the brain MRI findings in proven cases of EBV meningoencephalitis.
METHODS
This was a retrospective cohort study conducted in the department of pediatrics in a tertiary care research institute. The study was conducted from 2013 to 2015. Consecutive children of less than 12 years of age, admitted with acute febrile encephalopathy were enrolled in the study. These children were evaluated clinically, and underwent full general and neurological examination. The CSF was analyzed and was subjected to multiplex PCR for bacterial and viral agents. This study included 45 pediatric patients, who had various neurological symptoms and undergone brain MRI, with EBV meningoencephalitis (positive EBV deoxyribonucleic acid [DNA] in CSF). The MRI datasets were retrieved and reevaluated for imaging features. MRI was performed on 1.5T (Aera; Siemens, Erlangen, Germany) or 3T (Verio; Siemens, Erlangen, Germany) scanners. An experienced neuroradiologist evaluated all the MRI scans.
IMAGING EVALUATION
The MRI was performed within 2 weeks of the onset of acute febrile encephalopathy (AFE). All studies included standard MRI protocol—axial T1-weighted, T2-weighted, fluid-attenuated inversion-recovery (FLAIR), diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC) maps, and susceptibility-weighted images (SWI) images. Post-contrast three-dimensional magnetization prepared rapid acquisition gradient recalled echo (3D-MPRAGE) sequences were acquired in all the cases. The imaging features of EBV encephalitis were evaluated and assessed for location, presence of DWI restriction, presence of susceptibility changes on SWI images (to see for microhemorrhages and subarachnoid or intraparenchymal hemorrhage), and contrast enhancement.
RESULTS
Of the total 45 patients diagnosed with EBV encephalitis through CSF examination, 28 (62.22%) were male and 17 (37.78%) were females. The mean age of all subjects was 3.6 years (range 3 months to 11 years). The most common presenting feature was fever in 45 (100%) cases. Signs of meningitis and raised ICP were seen in 24 (53.3%) cases, encephalopathy in 15 (33.3%), seizures in 33 (73.3%), focal deficits in 7 (15.5%), and rash in 4 (8.8%).
RADIOLOGICAL FINDINGS
A normal MRI scan was seen in 16 (35.5%) cases. The cortical/subcortical pattern was diagnosed in 9 (20%) cases [Figure 1], white matter involvement in 7 (15.5%), basal ganglia in 5 (11.1%) cases [Figure 2], thalamic involvement in 4 (8.8%) [Figure 3], brain stem involvement in 2 (6.2%) [Figure 3], substantia nigra involvement in 2 (4.4%), and cerebellar involvement [Figures 3 and 4] in 2 (4.4%). Diffusion restriction was present in 11 (24.4%) cases and susceptibility changes on SWI were seen in 7 (15.5%). Meningeal enhancement was present in 10 (22.2%) cases. In addition, brain abscess and subdural effusion/empyema were present in 1 (2.2%) case each. Multifocal involvement was seen in 6 (13.3%) cases.
Figure 1.

A 2-year male child with fever, seizures, and coma. Axial T2-weighted (a) MRI image showing diffuse cortical/subcortical involvement in bilateral cerebral hemispheres (Black arrows). Axial DWI (b) and corresponding ADC maps (c) showing patchy areas of diffusion restriction (White arrows). Postcontrast axial T1-weighted image (d) show diffuse leptomeningeal enhancement
Figure 2.
An 11-year male child with fever and encephalopathy. Axial T2 (a), FLAIR (b), and T1 (c) weighted images showing bilateral symmetrical changes involving striatum (caudate and putamen). Patchy diffusion restriction (d, DWI with b = 1000 sec/mm2) and susceptibility changes (e, SWI) are seen in the bilateral striatum. No parenchymal or meningeal enhancement seen on postcontrast T1-weighted image (f)
Figure 3.
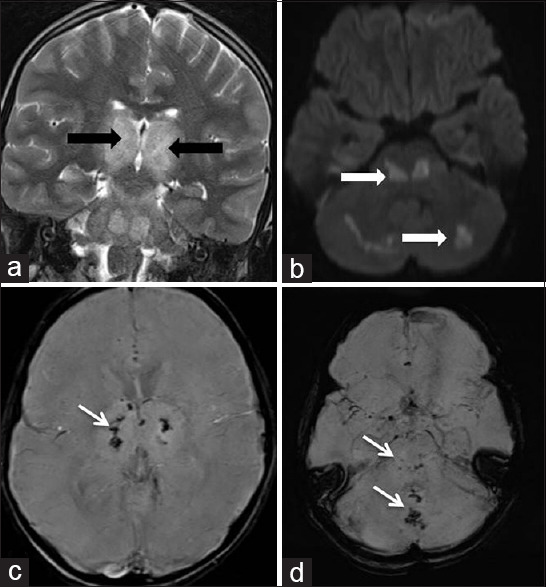
A 6-year male child with fever, seizures, and encephalopathy. Coronal T2-weighted (a) MRI showing hyperintensity involving bilateral thalami and brain stem (Black arrows). Axial DWI (b) images showing patchy areas of diffusion restriction in brain stem and cerebellum (White arrows). SWI images (c and d) show areas of hemorrhage in the bilateral thalami, cerebellar vermis, and brain stem (thin white arrows)
Figure 4.

A 5-year female child with fever, rash, and encephalopathy. Axial T2-weighted (a) and FLAIR (b) MRI showing hyperintensity involving the bilateral cerebral hemispheres (Black arrows) and dentate (White arrows). No diffusion restriction or susceptibility changes were seen
DISCUSSION
EBV encephalitis has been reported to be a cause of acute viral encephalitis in 5% of all cases. CNS involvement with EBV infection varies from 1 to 10%. The neurological complications of EBV range from acute encephalitis, transverse myelitis, nerve palsy, ataxia, seizures, movement disorders, and psychosis. The exact pathogenesis of EBV encephalitis is not clear; however, direct viral invasion, lymphocytes infiltration, and antibody-antigen complex deposition in neural tissue have been postulated as causative factors. EBV neurological syndromes can be seen in the absence of clinical infectious mononucleosis. Thus, it has been suggested that EBV should be considered as a possibility in all acute neurological presentations in pediatric patients, and specific antibody testing may be required to establish the diagnosis.[4]
In their study with proven EBV encephalitis cases, Hung et al.[5] found that MRI was abnormal in 5 out of 9 (56%) cases. One patient had cerebellar involvement, one mesial temporal involvement, and the other three had multifocal involvement in the brain stem, white matter, and basal ganglia. Shian et al.[6] reviewed the MRI findings retrospectively in their cohort of 29 patients. Out of these, eight (27.6%) patients had positive MRI findings. Out of which, four (50%) patients had brain atrophy, one (12.5%) had basal ganglia involvement, one (12.5%) had multifocal involvement, one (12.5%) had cerebral hemispheric involvement, and one (12.5%) had both cerebral and thalamic involvement. They found that these lesions disappeared on follow-up imaging done at approximately 2 weeks.
Abul-Kasim et al.[7] did a retrospective review involving 29 reports with 101 patients. The diagnosis in these cases was established through EBV antigen in the CSF or clinicoradiological CNS and serological signs of EBV infection. Radiological examinations were abnormal in 61 cases (61%). The cerebellum was the most common site of isolated involvement (18%) compared with isolated involvement of the cerebral hemispheres (16%), brain stem and mesencephalon (6.5%), basal ganglia (4.9%), thalamus (3.2%), and limbic system (3.2%). Meningeal involvement was reported in 4.9% of the cases. Multiple locations were involved in 31.1% of cases. They did not mention specific involvement of cortical/subcortical pattern. In addition, they correlated the anatomic localization of lesions with follow-up and found that those with isolated cerebral gray or white matter involvement had better recovery while those with thalamic involvement had residual sequelae. In addition, the mortality rate was higher in patients with isolated brain stem involvement.
We found abnormal imaging findings in 29 (64.5%) cases. Our study had included a more detailed description of the distribution of the lesions on MRI. The predominant pattern seen in our study was the involvement of the cortical/subcortical region. The rest of the previous studies and reports have mentioned it as cerebral hemispheric involvement; thus, the exact comparison cannot be made. We have also found the presence of diffusion restriction in 11 (24.4%) cases and susceptibility changes on SWI in 7 (15.5%) cases. Previous studies have not mentioned these findings in detail.
There are a few limitations to our study. A follow-up imaging was not performed; thus, the disappearance of lesions as demonstrated by Shian et al.[6] could not be commented upon. The purpose of the study was to demonstrate the imaging spectrum of EBV encephalitis and a follow up with prognostic implications depending on the site of involvement was not done as demonstrated in review by Abul-Kasim et al.[7]
CONCLUSIONS
We have shown the largest imaging cohort of patients with EBV meningoencephalitis to the best of our knowledge. Although there is an absence of any specific imaging pattern in EBV meningoencephalitis, it should be suspected in any acute pediatric encephalitis. The accurate knowledge of diverse clinical and brain MRI findings should be kept in mind while making a diagnosis.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Financial support and sponsorship
*Astrid Lindgren Children Hospital, Eugeniavägen 23, 171 64 Solna, Sweden.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tselis A, Duman R, Storch GA, Lisak RP. Epstein-Barr virus encephalomyelitis diagnosed by polymerase chain reaction: Detection of the genome in the CSF. Neurology. 1997;48:1351–5. doi: 10.1212/wnl.48.5.1351. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto H, Asaoka K, Imaizumi T, Ayabe M, Shoji H, Kaji M. Epstein-Barr virus infections of the central nervous system. Intern Med. 2003;42:33–40. doi: 10.2169/internalmedicine.42.33. [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Mitoma F, Vanast WJ, Tyrrell DL. Increased frequency of Epstein-Barr virus excretion in patients with new daily persistent headaches. Lancet. 1987;1:411–5. doi: 10.1016/s0140-6736(87)90119-x. [DOI] [PubMed] [Google Scholar]
- 4.Sumaya CV, Ench Y. Epstein-Barr virus infectious mononucleosis in children.I Clinical and general laboratory findings. Pediatrics. 1985;75:1003–10. [PubMed] [Google Scholar]
- 5.Hung KL, Liao HT, Tsai ML. Epstein-Barr virus encephalitis in children. Acta Paediatr Taiwan. 2000;41:140–6. [PubMed] [Google Scholar]
- 6.Shian WJ, Chi CS. Epstein-Barr virus encephalitis and encephalomyelitis: MR findings. Pediatr Radiol. 1996;26:690–3. doi: 10.1007/BF01356839. [DOI] [PubMed] [Google Scholar]
- 7.Abul-Kasim K, Palm L, Maly P, Sundgren PC. The neuroanatomic localization of Epstein-Barr virus encephalitis may be a predictive factor for its clinical outcome: A case report and review of 100 cases in 28 reports. J Child Neurol. 2009;24:720–6. doi: 10.1177/0883073808327842. [DOI] [PubMed] [Google Scholar]



