Graphical abstract

Keywords: Mitral valve abscess, Mitral valve windsock aneurysm, Echocardiography, Infective endocarditis
Highlights
-
•
MV abscesses and aneurysms are uncommon complications of IE.
-
•
Serial echocardiography is important to identify perivalvular complications.
-
•
Three-dimensional transesophageal echocardiography has incremental value in diagnosis.
Introduction
Identifying complications of infective endocarditis (IE) is important to facilitate early surgical intervention. Mitral valve (MV) aneurysms and abscesses remain uncommon complications and reflect an often aggressive disease process with a poor prognosis if left untreated.1,2 We present an uncommon case of MV endocarditis, initially managed conservatively because of prohibitive comorbidities, with subsequent disease progression and complications with high-risk features, including windsock aneurysm, leaflet perforation, and abscess formation, eventually requiring operative management.
Case Presentation
A 73-year-old woman with a background history significant for coronary artery bypass grafting and mechanical aortic valve (AV) replacement 16 years previously presented with acute pulmonary edema and altered mental status. She had a protracted hospital admission 2 months prior for a non–ST-segment elevation myocardial infarction, which was complicated by a large right hemispheric cerebral intraparenchymal hematoma that was managed nonoperatively at an outside hospital. Her disease course was further complicated by methicillin-susceptible Staphylococcus aureus bacteremia due to suspected line infection, and she was commenced on 2 g cefazolin three times daily intravenously, with the infected venous catheter removed. Transthoracic echocardiography raised suspicion of a new MV vegetation, with evidence of new moderately severe mitral regurgitation (MR). Transesophageal echocardiography (TEE) revealed a large mobile echodensity (19 × 14 × 20 mm) involving the posterior leaflet with focal perforation (Figure 1; Videos 1 [two-dimensional (2D)] and 2 [three-dimensional (3D) left atrial view demonstrating a large, bulky vegetation on the posterior leaflet extending into the posterior annulus]). She was initially evaluated by cardiothoracic surgery and deemed at high risk for operative management, in the context of her recent intracranial event. Her blood cultures were negative, and she was subsequently discharged with a plan for 6 weeks of intravenous antimicrobial therapy.
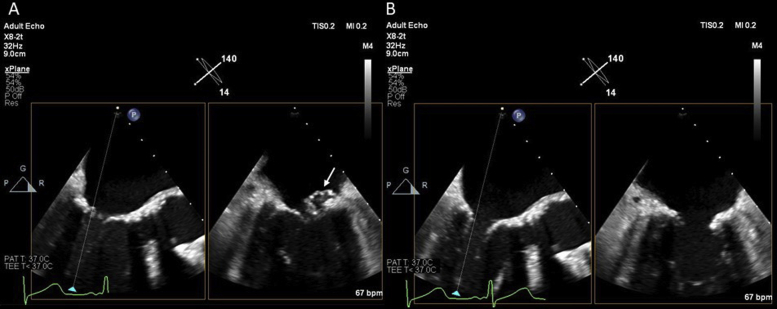
Figure 1.
(A) Initial transesophageal echocardiographic midesophageal biplane views at 0° and 90° demonstrated a large vegetation involving the posterior MV leaflet extending into the posterior mitral annulus (arrow). (B, C) Three-dimensional TEE (left atrial view) demonstrated a large vegetation on the posterior MV leaflet involving the posterior annulus (white arrow).
The patient presented again 1 week following discharge with altered mental status and acute pulmonary edema, requiring intubation and mechanical ventilation. Computed tomographic brain imaging demonstrated interval reduction of the cerebral hemorrhage, and she was transferred to our center for a second opinion regarding potential candidacy for cardiac surgery, having received clearance from neurology. Repeat TEE was pursued (18 days following the initial study) because of concern for perivalvular extension and ongoing pulmonary edema, which revealed a complex windsock-type infective aneurysm, arising from near the posterior mitral annular calcification (MAC) at the base of the posterior MV leaflet (Figure 2; Video 3, 2D biplane imaging showing the windsock aneurysm on the posterior leaflet with systolic expansion and diastolic collapse). There was also associated perforation of the posterior MV leaflet and resultant severe MR (Figures 3 and 4; Video 4, demonstrating severe MR through the perforated posterior leaflet). Three-dimensional TEE with multiplanar reconstruction analysis was able to further demonstrate the extent of the vegetation and associated aneurysm beyond the posterior leaflet involving the mitral annulus, highlighting the perivalvular spread of infection, which was relevant for operative planning (Figure 5; Video 5, 3D left atrial view of the MV showing aneurysm and perforation at P2 and extension into the posterior annulus). Her prosthetic AV leaflets appeared thickened (Figure 6), which was concerning for colonization in the setting of recent bacteremia, and this was also significant given her temporary cessation of anticoagulation following the intracranial event (transthoracic echocardiography demonstrated increased transvalvular gradients: peak gradient: 48 mm Hg, mean gradient: 27 mm Hg, dimensionless index: 0.34), although there was no obvious peri–aortic root abscess. Further cardiac imaging with cardiac computed tomography or 18F-fluorodeoxyglucose positron emission tomography to evaluate the prosthetic AV was not pursued given the patient's tenuous hemodynamic state and low likelihood to change management. Coronary angiography showed no obstructive coronary artery disease and a patent saphenous vein graft to the posterior descending coronary artery.
Figure 2.
(A) TEE after second presentation: midesophageal biplane views at 140° and 50° (inverted right-left) subsequently demonstrated a windsock aneurysm arising from the posterior leaflet with systolic expansion (A; arrow) and diastolic collapse (B).
Figure 3.
(A) Transesophageal echocardiographic midesophageal long-axis view at 140° showing a prominent perforation in the posterior MV leaflet on 2D imaging (white arrow). (B) Transesophageal echocardiographic midesophageal long-axis view at 140° demonstrating severe MR resulting from the perforation on color Doppler imaging (black arrow).
Figure 4.
(A, B) Three-dimensional TEE (left atrial view) demonstrated a windsock aneurysm arising from the posterior MV leaflet with associated leaflet perforation (arrow). (C) Three-dimensional TEE (left atrial view) with color suppression showing a focal perforation (white arrow) at the central aspect of the posterior MV leaflet (P2). (D) Three-dimensional color TEE demonstrated severe MR due to perforation (black arrow).
Figure 5.
Three-dimensional TEE with multiplanar reconstruction (MPR) showing annular involvement on cross-sectional plane (white arrow). Three-dimensional MPR was able to clearly demonstrate extension into the posterior annulus indicative of perivalvular involvement. Ao, Aorta; LA, left atrium.
Figure 6.
(A) TEE of the mechanical AV: midesophageal biplane views at 140° and 50° demonstrating suspicious thickening of the prosthetic leaflets (arrow). (B) Three-dimensional TEE of the prosthetic AV (aortic view) also suggestive of leaflet thickening (arrow).
After further medical stabilization and discussion with family members, who stated that the patient was fully functioning and living independently before her illness, she underwent cardiac surgery. At the time of surgery, a large vegetation was found on the P2 and P3 scallops, with associated abscess formation eroding into the posterior mitral annulus (Figure 7). She had extremely friable tissue due to extensive abscess formation. A bioprosthesis (27-mm Biocor; St. Jude Medical, St. Paul, MN) was implanted in the mitral position, and because of intraoperative suspicion of colonization and to facilitate exposure of the MV, she underwent re-replacement of the AV with a 21-mm Carpentier-Edwards bioprosthesis (Edwards Lifesciences, Irvine, CA). Annular debridement was necessary because of significant pannus formation but no evident thrombus. The P2 scallop had to be resected, and the region affected by the abscess in the posterior annulus was reinforced by transferring the edge of the anterior mitral leaflet with chordal sparing. The explanted valves were sent for bacterial molecular testing (16S ribosomal-ribonucleic acid polymerase chain reaction), which was positive in the explanted MV but not in the explanted AV. In view of extreme deconditioning and recurrent hospitalization, the patient had a prolonged postoperative course but did not develop neurologic complications or end-organ dysfunction. She was discharged to a residential care facility to continue rehabilitation.
Figure 7.
Intraoperative findings showing a large P2/P3 leaflet vegetation (yellow arrow), perforation (white arrow), and abscess formation (dotted line area) eroding into the posterior mitral annulus. The patient's prior saphenous vein graft (SVG; asterisk) to the posterior descending artery (PDA) was preserved.
Discussion
The incidence of IE has been increasing in the United States, with hospitalizations increasing from 15.9 per 100,000 adults in 2003 to 21.8 per 100,000 in 2016.3 Surgical intervention and timing are dependent on several factors, but the presence of heart failure, severe valvular dysfunction, prosthetic valve endocarditis, perivalvular complications, and large mobile vegetations (>10 mm) warrant early and aggressive treatment.2,4 Once a surgical indication exists, early intervention should be undertaken during hospitalization regardless of antimicrobial completion.4 However, surgical intervention must also be balanced by comorbidities and in particular the inherent risk for embolic cerebral complications, highlighting the critical importance of early and multidisciplinary involvement for IE management. In the setting of recent intracranial hemorrhage, operative delay is considered reasonable for ≥3 weeks.4,5 The unfortunate cerebral complication resulted in necessary operative delay in our patient, with subsequent evidence of disease relapse and perivalvular extension, demonstrating the increased risk for nonoperative management for progressive left-sided valve IE.
MV abscesses remain relatively uncommon findings in patients with IE, often reflecting a more aggressive disease process and a complication necessitating early surgical intervention.2 Perivalvular extension of IE occurs in the setting of uncontrolled infection and is associated with a poor prognosis.1,2 This process can be seen in aortic IE (10%–40% native valve endocarditis) but is more common in prosthetic valve endocarditis (56%–100%).2 MV abscesses are much less common, with a surgical series revealing an incidence of 14.3% (six of 42 annular abscesses from 106 consecutive surgical endocarditis cases) over a 16-year period.6 MV aneurysms are even rarer and are defined as focal saccular outpouchings of the mitral leaflet directed toward the left atrium with persistence throughout the cardiac cycle and associated systolic expansion and diastolic collapse.7 Most commonly, these are infective in nature, with other causes attributed to connective tissue disorders7 and are frequently located on the anterior leaflet in association with AV endocarditis.8 Aortic regurgitant jets directed at the anterior mitral leaflet and contiguous extension through the aorta-mitral intervalvular fibrosa are likely responsible for this finding.8 One series found an incidence of 0.02% over 17 years, with the majority (>80%) having an infective etiology.7 The most common complication of these aneurysms is perforation with resultant severe regurgitation,9 as occurred in our patient.
Our case demonstrated a particularly aggressive disease process, with serial TEE revealing a complicated, large vegetation associated with the MV annulus and posterior MAC, subsequently evolving into a windsock aneurysm with associated perforation. Intraoperatively, MV abscess formation was confirmed. This aggressive disease course likely reflects a highly virulent organism (methicillin-susceptible S aureus) in an elderly patient with previous cardiac surgery and subsequent ongoing bacteremia. We also postulate that the presence of MAC served as a nidus for endocarditis, ongoing infection, and phlegmon formation. There was limited postoperative imaging to accurately assess the degree of MAC after implantation of the new bioprosthetic MV. MAC is a common, chronic inflammatory process with a similar pathophysiologic basis to atherosclerosis, often associated with increasing age.10 It has previously been proposed that this inflammation and endothelial disruption may serve as a nidus for IE, with a prospective series over 5 years showing that 24% of patients with MV vegetations had annular calcification involvement and a more complicated disease course with significantly larger vegetations and a higher frequency of ring abscesses.11 Furthermore, in another study, S aureus was more commonly implicated in MAC-associated endocarditis: 57% of cases (16 of 28) compared with other bacterial species.10 Proposed mechanisms may relate to endocardial adherence and the underlying calcific substrate, with MAC being implicated in the transformation of fibroblasts to osteoblasts, which is a postulated pathophysiology in S aureus–associated osteomyelitis.10,11 We infer that our patient had line-associated methicillin-susceptible S aureus, and the presence of significant MAC made her particularly susceptible to seeding and extensive tissue destruction leading to the development of progressive MV aneurysm, MV leaflet perforation, and abscess formation. Indeed, it is also plausible that significant annular calcification promoted abscess formation because of poor antibiotic penetration.11
Imaging remains critical in the timely and accurate diagnosis of patients presenting with complicated MV IE. The sensitivity of transthoracic echocardiography is <50%, compared with TEE with a significantly improved sensitivity of 90%,2 but other modalities, such as cardiac computed tomography and 18F-fluorodeoxyglucose positron emission tomography/computed tomography, may offer additional information, particularly in cases of perivalvular complications and prosthetic valve endocarditis.2,12 However, although TEE is the mainstay of diagnosis in most patients with aortic root abscesses, caution needs to be exercised when mitral annular abscesses are suspected. One retrospective cohort study demonstrated that preoperative detection of MV abscesses was considerably reduced compared with aortic root abscesses (30% [six of 20] vs 63% [15 of 24], P = .03).1 TEE was more likely to miss MV abscesses in the posterior annular position (61% [14 of 23]), often because of the presence of annular calcification (64% of missed cases).1 The lowered diagnostic sensitivity of TEE for MV abscesses (particularly when they are posterior in location) likely results from acoustic shadowing from significant calcification. Three-dimensional TEE may be incremental in these cases particularly through improved spatial resolution and delineation of anatomic relationships.13 However, this may not always be possible with significant acoustic shadowing similarly affecting 2D and 3D visualization, in which case off-axis and transgastric imaging is important to further interrogate the posterior aspects of the valve. Three-dimensional multiplanar reconstruction is additive and allows detailed anatomic assessment in multiple planes and identification of involved structures.14 Given the nature of abscesses to cross anatomic planes, 3D TEE can help better understand the extent and spatial relationships of such complications. Indeed, this was reflected in a small case series that demonstrated the utility of 3D TEE over 2D imaging in the diagnosis of endocarditis complications, including leaflet perforations and annular abscesses.15 In our case, 3D TEE was complementary to 2D imaging, allowing delineation of the complex posterior mitral leaflet vegetation involving primarily the P2 segment and the posterior mitral annulus with associated windsock aneurysm and perforation. Three-dimensional TEE is particularly beneficial in the preoperative setting, in which anticipating complications such as abscess or fistula formation, leaflet perforations, and commissural MR can facilitate operative planning and repair strategies.13
Although there are several reports in the literature of native MV endocarditis complicated by annular abscess16, 17, 18 and less commonly MV aneurysms,8,9 concurrent pathology represents a rarity. We were unable to find any previous reports of these uniquely uncommon endocarditis complications occurring in the same patient. Indeed, we postulate that the relatively long disease course (>3 weeks from diagnosis to operation) and initial conservative management were conducive to extensive tissue destruction and subsequent complications. Repeat TEE was also critical in highlighting the evolving pathology and emergence of new complications, demonstrating the importance of serial imaging in cases of clinical decompensation or unresponsive disease. This is even more critical in cases of suspected perivalvular extension with virulent organisms (such as S aureus), given the dynamic nature of abscess formation.2
Conclusion
MV aneurysms and MV abscesses remain relatively uncommon complications of IE, and concurrent existence is a rarity. We describe a case demonstrating evolution from a large vegetation involving the posterior MAC, with subsequent progression into a posterior leaflet windsock aneurysm, leaflet perforation with severe regurgitation, and abscess formation. This case highlights the importance of meticulous echocardiographic imaging, particularly with serial TEE in cases of altered clinical status or disease progression, to detect perivalvular complications and evolution. This can dramatically change surgical management and planning. Three-dimensional TEE is also an important modality that can be incremental in understanding extent of complications, characterizing anatomic relationships, and planning operative strategies.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.008.
Supplementary Data
Initial TEE demonstrating a large, bulky vegetation on the posterior leaflet extending into the posterior annulus.
Initial 3D TEE (left atrial view) showing the large, bulky vegetation on the posterior leaflet extending into the posterior annulus.
Follow-up transesophageal echocardiographic midesophageal biplane views revealing the windsock aneurysm on the posterior leaflet with systolic expansion and diastolic collapse.
Follow-up transesophageal echocardiographic midesophageal long-axis view showing a perforated posterior leaflet with severe MR.
Follow-up 3D TEE (left atrial view) showing the posterior leaflet aneurysm and perforation at P2, with extension into the posterior annulus.
References
- 1.Hill E.E., Herijgers P., Claus P., Vanderschueren S., Peetermans W.E., Herregods M.C. Abscess in infective endocarditis: the value of transesophageal echocardiography and outcome: a 5-year study. Am Heart J. 2007;154:923–928. doi: 10.1016/j.ahj.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouli M., Alqahtani F., Alhajji M., Berzingi C.O., Sohail M.R. Clinical and economic burden of hospitalizations for infective endocarditis in the United States. Mayo Clin Proc. 2020;95:858–866. doi: 10.1016/j.mayocp.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Pettersson G.B., Hussain S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8:630–644. doi: 10.21037/acs.2019.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang D.H. Timing of surgery in infective endocarditis. Heart. 2015;101:1786–1791. doi: 10.1136/heartjnl-2015-307878. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner F.J., Omari B.O., Robertson J.M., Nelson R.J., Pandya A., Pandya A. Annular abscesses in surgical endocarditis: anatomic, clinical, and operative features. Ann Thorac Surg. 2000;70:442–447. doi: 10.1016/s0003-4975(00)01363-1. [DOI] [PubMed] [Google Scholar]
- 7.Pena J.L.B., Bomfim T.O., Fortes P.R.L., Simão-Filho C., de Souza Andrade-Filho J. Mitral valve aneurysms: clinical characteristics, echocardiographic abnormalities, and possible mechanisms of formation. Echocardiography. 2017;34:986–991. doi: 10.1111/echo.13556. [DOI] [PubMed] [Google Scholar]
- 8.Guler A., Karabay C.Y., Gursoy O.M., Guler Y., Candan O., Akgun T. Clinical and echocardiographic evaluation of mitral valve aneurysms: a retrospective, single center study. Int J Cardiovasc Imaging. 2014;30:535–541. doi: 10.1007/s10554-014-0365-4. [DOI] [PubMed] [Google Scholar]
- 9.Moretti M., Buscaglia A., Senes J., Tini G., Brunelli C., Bezante G.P. Anterior mitral valve aneurysm is an uncommon complication of aortic valve infective endocarditis: a case report. Am J Case Rep. 2018;19:1146–1151. doi: 10.12659/AJCR.909922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pressman G.S., Rodriguez-Ziccardi M., Gartman C.H., Obasare E., Melendres E., Arguello V. Mitral annular calcification as a possible nidus for endocarditis: a descriptive series with bacteriological differences noted. J Am Soc Echocardiogr. 2017;30:572–578. doi: 10.1016/j.echo.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Eicher J.C., De Nadai L., Soto F.X., Falcon-Eicher S., Dobsák P., Zanetta G. Bacterial endocarditis complicating mitral annular calcification: a clinical and echocardiographic study. J Heart Valve Dis. 2004;13:217–227. [PubMed] [Google Scholar]
- 12.Wang T.K.M., Sánchez-Nadales A., Igbinomwanhia E., Cremer P., Griffin B., Xu B. Diagnosis of infective endocarditis by subtype using 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Circ Cardiovas Imaging. 2020;13:e010600. doi: 10.1161/CIRCIMAGING.120.010600. [DOI] [PubMed] [Google Scholar]
- 13.Yong M.S., Saxena P., Killu A.M., Coffey S., Burkhart H.M., Wan S.-H. The preoperative evaluation of infective endocarditis via 3-dimensional transesophageal echocardiography. Tex Heart Inst J. 2015;42:372–376. doi: 10.14503/THIJ-14-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sordelli C., Fele N., Mocerino R., Weisz S.H., Ascione L., Caso P. Infective endocarditis: echocardiographic imaging and new imaging modalities. J Cardiovasc Echogr. 2019;29:149–155. doi: 10.4103/jcecho.jcecho_53_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansalia S., Biswas M., Dutta R., Hage F.G., Hsiung M.C., Nanda N.C. The value of live/real time three-dimensional transesophageal echocardiography in the assessment of valvular vegetations. Echocardiography. 2009;26:1264–1273. doi: 10.1111/j.1540-8175.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 16.Dashti R., Al Jarallah M., Rajan R., Al Mulla K., Khalil M., Sayed W. Ruptured mitral valve abscess with mitral incompetence in culture negative infective endocarditis: case report. Eur Heart J Case Rep. 2018;2:yty003. doi: 10.1093/ehjcr/yty003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena P., Boyt A., Newman M.A. Mitral valve leaflet abscess complicating infective endocarditis. Heart Lung Circ. 2009;18:161–162. doi: 10.1016/j.hlc.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha B., Ishizuka N., Tanimoto K., Kawai A., Kurosawa H., Kasanuki H. Extremely rapid formation of mitral valve ring abscess in infective endocarditis. Echocardiography. 2004;21:531–536. doi: 10.1111/j.0742-2822.2004.03122.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Initial TEE demonstrating a large, bulky vegetation on the posterior leaflet extending into the posterior annulus.
Initial 3D TEE (left atrial view) showing the large, bulky vegetation on the posterior leaflet extending into the posterior annulus.
Follow-up transesophageal echocardiographic midesophageal biplane views revealing the windsock aneurysm on the posterior leaflet with systolic expansion and diastolic collapse.
Follow-up transesophageal echocardiographic midesophageal long-axis view showing a perforated posterior leaflet with severe MR.
Follow-up 3D TEE (left atrial view) showing the posterior leaflet aneurysm and perforation at P2, with extension into the posterior annulus.