Graphical abstract
Keywords: Echocardiography, Ultrasound enhancing agent, Cardiac mass, Hydatid cyst, Echinococcosis
Highlights
-
•
Cardiac masses are a rare but important finding requiring detailed investigation.
-
•
Echocardiography is a first-line imaging modality.
-
•
CT and MRI together allow precise structural and tissue characterization.
-
•
Surgical removal is a potentially curative treatment for cardiac hydatid cysts.
Introduction
A cardiac mass is a rare but important finding that requires a thorough clinical history and diagnostic evaluation. Masses may be due to tumors, thrombi, vegetations, calcific lesions, cysts, and other less common conditions. Clinical manifestations of cardiac masses relate to their size, anatomic location, rate of growth, invasion into adjacent tissues, and risk for embolization.1 Intracavitary masses may lead to symptoms such as chest pain, shortness of breath, palpitations, syncope, or stroke due to obstruction or embolization. Intramural growth may lead to conduction disturbances, ischemia, or congestive heart failure.1 However, a cardiac mass may be asymptomatic and incidentally found by radiography, computed tomography (CT), or echocardiography. When a cardiac mass is present, timely evaluation is imperative for diagnosis and treatment.2 Depending on the underlying etiology, management may include surveillance, medical therapy, percutaneous intervention, or surgical resection.1 Echocardiography plays an integral role in the initial screening and dynamic evaluation of patients with intracardiac masses.2,3
Case Presentation
Our patient was a 34-year-old woman who was born in Afghanistan and immigrated to Canada as a child in the 1990s. She had no medical history, was not taking any medications, and had no drug allergies. She was a lifelong nonsmoker and did not consume alcohol or any recreational drugs.
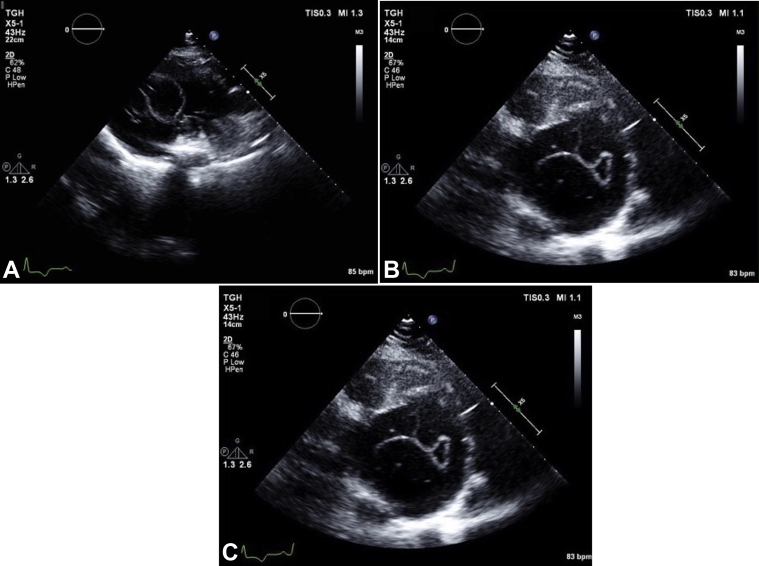
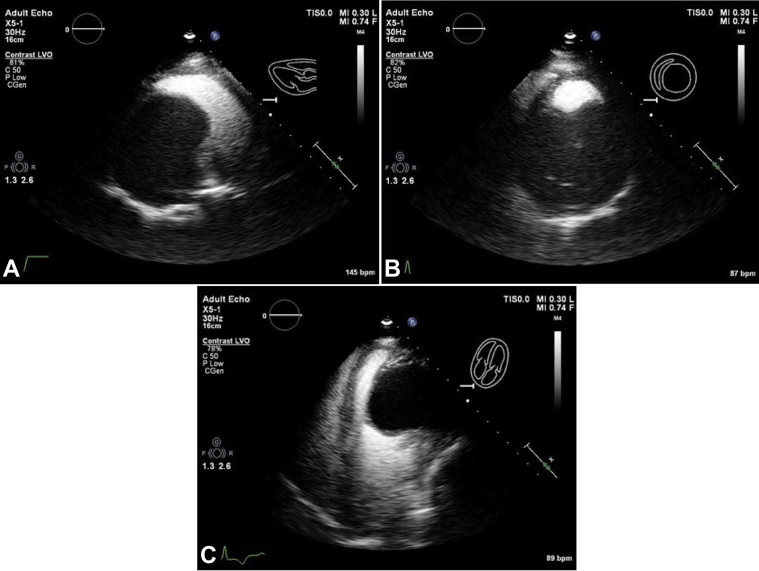
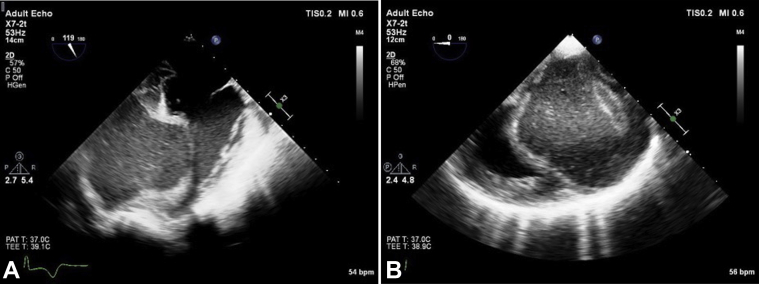
The patient presented with shortness of breath and was noted to have crackles in the left lower lobe. She was diagnosed with community-acquired pneumonia and initiated on antibiotic treatment. In the course of her evaluation, electrocardiography demonstrated T-wave inversions, raising suspicion for myocardial ischemia. This prompted further cardiac investigations. Transthoracic echocardiography (TTE) demonstrated preserved left ventricular (LV) systolic function with a calculated ejection fraction of 64% by the Simpson biplane method. A large echo-lucent structure was also noted bulging into the left ventricle from the myocardium of the inferior wall (Figures 1A-1C, Video 1, Video 2, Video 3). Multiple septations were noted within the mass, and deformation of the lesion was visualized, relating to LV contraction and relaxation. Administration of an ultrasound enhancing agent delineated the borders of the lesion and did not show vascularization or communication between the LV cavity and the inner cystic component (Figures 2A-2C, Video 4, Video 5, Video 6). Both the aortic and mitral valves were not influenced by the lesion. There wasa normal mitral valve inflow Doppler pattern.
Figure 1.
Transthoracic echocardiographic parasternal long-axis view (A), parasternal short-axis view (B), and apical four-chamber view (C) demonstrate a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Figure 2.
Transthoracic echocardiographic parasternal long-axis view (A), parasternal short-axis view (B), and apical four-chamber view (C) with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Over the course of the patient's hospitalization, blood cultures were negative, and further investigation for hepatitis B and C and human immunodeficiency virus were negative as well. Fungal serology for histoplasmosis, blastomycosis, and coccidioidomycosis were negative, as was a histoplasma urine antigen test. Serology for Echinococcus, Strongyloides, and toxoplasmosis was negative. Her eosinophil count was elevated (initially 0.36 × 109/L and increased to 0.71 × 109/L; normal range, 0.04 × 109/L to 0.40 × 109/L).
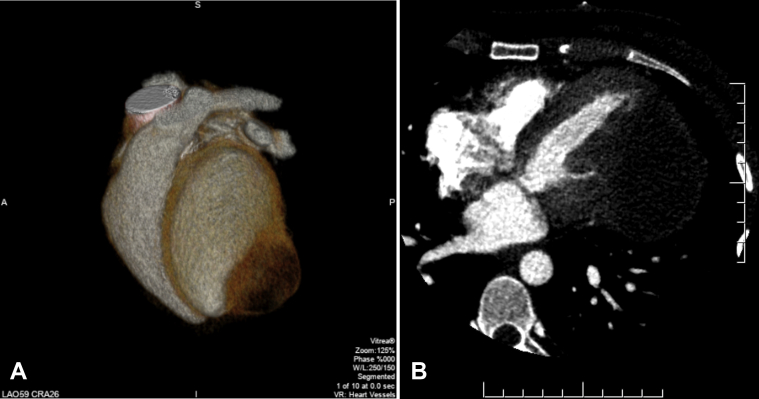
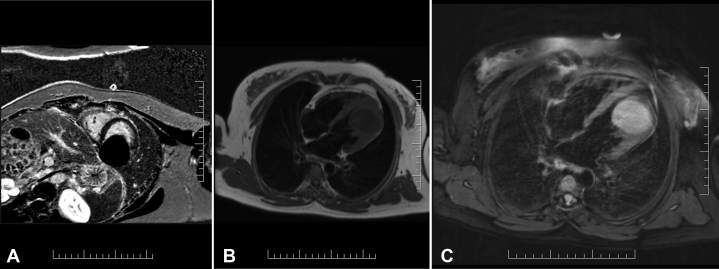
Cardiac CT with contrast was arranged to better assess the size and location of the mass and whether there was involvement of adjacent tissues. Findings were consistent with those on TTE, confirming the presence of a large 9-cm mass arising from the left ventricle. The mass appeared avascular and cystic and had a fibrotic capsule (Figures 3A and 3B).
Figure 3.
Cardiac computed tomographic three-dimensional volume-rendered image (A) demonstrating the mass along the LV apical lateral wall. Contrast-enhanced axial computed tomographic image (B) shows a large cystic appearing lesion likely embedded within the inferolateral LV wall.
Cardiac magnetic resonance imaging (MRI) was also arranged for better tissue characterization. This demonstrated a large, principal cystlike lesion arising within the myocardium of the distal lateral wall, encroaching upon the LV cavity (Figures 4A-4C, Video 7, Video 8, Video 9). Two smaller daughter cysts were also visualized within the principal lesion, and multiple thin curvilinear areas within the mass were suggestive of membranes. The cystlike lesion measured 5 × 5 × 4 cm and was located predominantly toward the apex of the heart. Biventricular systolic function was preserved (LV ejection fraction 69%), and both the aortic and mitral valves were not influenced by the lesion. The differential diagnosis was either a complex benign cystic lesion encapsulated in fibrotic capsule or hydatid cyst of the myocardium.
Figure 4.
Cardiac MRI short axis phase-sensitive inversion recovery delayed enhancement images (A). The cyst appears fully nulled, suggesting that it is fluid filled. Horizontal long-axis T1 weighted turbo-spin echo (B) showing a hypointense lesion. Horizontal long-axis, T2 spectral attenuated inversion recovery image (C) showing hyperintense signal from the mass, suggesting that it contains fluid. There is a hypointense peripheral ring, which likely represents a pericyst.
The infectious disease and cardiothoracic surgery services were consulted. A presumptive diagnosis of cardiac echinococcal infection was made on the basis of clinical and radiographic features. The patient was treated before surgery with oral albendazole (400 mg twice daily for 1 week) and for 6 months after her operation.
The patient was prepared for surgical resection of the mass. Additional MRI and ultrasound imaging of the abdomen ruled out cystic involvement of the abdomen. Transesophageal echocardiography was performed intraoperatively and confirmed the findings on TTE (Figures 5A and 5B, Videos 10 and 11).
Figure 5.
Intraoperative transesophageal echocardiographic four-chamber view (A) and short-axis view (B) demonstrate a large mass occupying the entire mid to distal LV cavity.
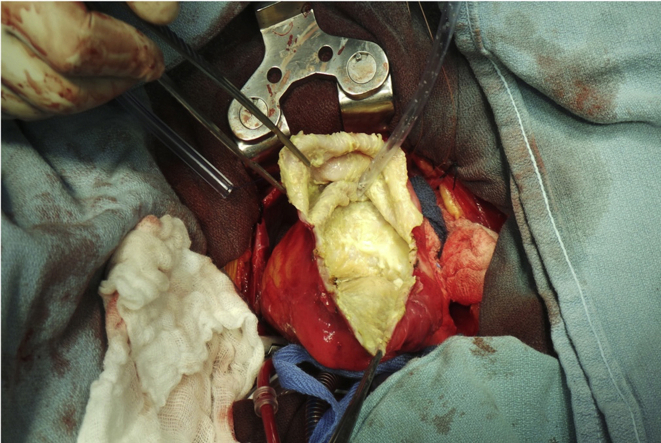
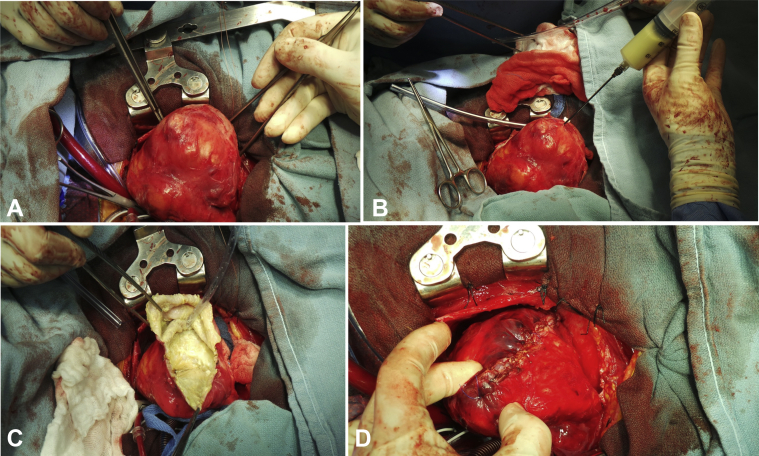
Surgical inspection revealed that the lesion adhered to the pericardium and that it contained a green- and yellow-colored liquid (Figures 6A-6D). Two daughter sacs were visualized connecting to the main sac. The appearance once again favored a hydatid cyst. However, the pathology report was not conclusive in confirming a hydatid cyst. It described thickened parts of the cyst wall showing large numbers of necrotic nodules, which were composed of a lining of inflammatory cells including macrophages, lymphocytes, and plasma cells. Debris in the cyst wall showed microcellular calcification, and none of this calcified debris had the appearance of hooklets.
Figure 6.
Gross intraoperative surgical inspection identified a large lesion adhered to the pericardium (A) containing a green- and yellow-colored liquid (B). The inner wall of the cyst (C) was dissected and resected before being sutured shut from the base of the heart to the apex of the heart (D).
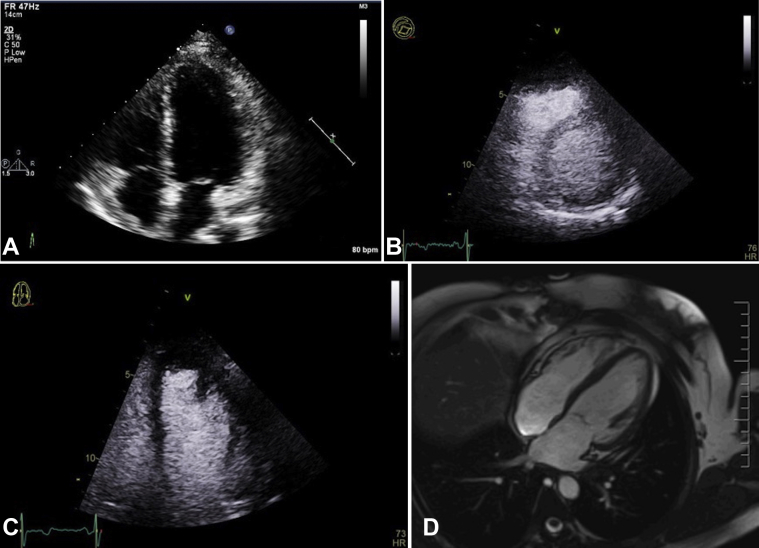
After resection, the patient's previous elevated eosinophil levels resolved. Both postoperative TTE with ultrasound enhancing agent administration and cardiac MRI showed an LV cavity devoid of masses and normal LV systolic function (LV ejection fraction 58% and 60%, respectively; Figures 7A-7D, Video 12, Video 13, Video 14, Video 15). The patient was last seen in the clinic in 2020, 3 years postoperatively. She has remained asymptomatic since her medical and surgical treatment. A series of follow-up transthoracic echocardiographic and cardiac MRI studies have shown normal LV function, with no evidence of residual or recurrent cysts. She will continue to undergo serial cardiac imaging and clinical follow-up on a yearly basis.
Figure 7.
Postoperative transthoracic echocardiographic apical four-chamber view (A), parasternal short-axis view with ultrasound enhancing agent (B), apical four-chamber with ultrasound enhancing agent (C), and cardiac MRI horizontal long-axis cine (D) showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Discussion
This patient had a presumptive diagnosis of echinococcal infection with a large, isolated cardiac hydatic cyst on the basis of clinical and imaging investigations. The differential diagnosis included a complex benign cyst. The appearance and location were not consistent with tumor, vegetation, or thrombus.
Echinococcal infections result from infection of human hosts through the accidental ingestion of tapeworm eggs excreted in dog feces.4 The resulting cysts predominantly affect the liver (70%–80% of cases) and lung (15%–20% of cases).5 Cases with cardiac involvement are rare (0.02%–2%).6 They are most commonly noted in the left ventricle (50%–60%) but may also affect the interventricular septum (10%–20%), pericardium (10%–15%), right ventricle (5%–15%), and left or right atrium (5%–8%).6 Transmission of the larvae to the heart most likely occurs via direct hematogenous transmission.6 Such patients are typically exposed to the parasitic infection from animal contact during childhood. Echinococcus granulosis, the most common species of echinococcal disease, is endemic to South America, the Middle East and eastern Mediterranean, Central Asia, some sub-Saharan African countries, western China, and the former Soviet Union.7 Infected individuals may remain asymptomatic for many decades and occasionally for life.8 However, in those with cardiac involvement, symptoms typically occur in closer proximity to the infection if there is anatomic structural disruption leading to mass effect.8
Symptoms depend largely on the size and location of the cardiac hydatid cyst but are asymptomatic in approximately 30% of patients.9 Symptoms include chest pain, dyspnea, palpitations, and cough. Clinical presentations include cardiac tamponade, heart failure, syncope, arrhythmias, valvular stenosis or regurgitation, pulmonary hypertension, or peripheral embolism.9 Small cysts (<5 mm) rarely lead to any side effects. Larger cysts (>5 mm) may lead to mass effect resulting in obstruction of blood and lymphatic flow, invasion into tissue, and rupture leading to anaphylaxis.10
Physical examination findings depend on the location of the cyst. Involvement of the valvular apparatus may lead to obstruction or regurgitation across the valve. Involvement within the pericardial space may lead to a pericardial rub or signs of cardiac tamponade. Involvement within the septum may result in complete heart block.9 Similarly, findings on electrocardiography and chest radiography vary depending on the location of the cyst.
Two-dimensional echocardiography is the first-line test of choice to make the diagnosis of a cardiac hydatid cyst, with >90% sensitivity.2 It is a quick, highly sensitive, and noninvasive imaging modality that can demonstrate the presence of cardiac masses and characterize cysts using ultrasound. The use of an ultrasound enhancing agent may be helpful to clearly delineate the location and size of the mass.11 Furthermore, it can determine whether the cyst is enclosed or ruptured, whether it is communicating with the blood supply within the heart, and whether it is vascularized. Patients with exposure to or the presence of echinococcosis in any part of the body should undergo assessment using echocardiography of their heart and major vessels.9,12
Other noninvasive imaging modalities, such as CT and MRI, can provide details regarding size, composition, presence of daughter cysts, anatomic location, and involvement of adjacent organs.9 Mild eosinophilia is a common finding.9 Serologic testing is positive in approximately 80% of liver cysts and 65% of lung cysts, but the rates of seropositivity decline significantly if other organs are involved, and sensitivity may be <50% for cases involving the heart.13,14 Nevertheless, seronegativity does not exclude a diagnosis of hydatid cyst due to echinococcal infection. False-negative results may be observed when cysts are calcified (even if fertile) or inactive.14 Serologic tests do not replace clinical or imaging investigations.
Biopsy of a cyst may be dangerous, as it may lead to seeding of the infection to other organs. Furthermore, release of the cystic content into the bloodstream may lead to an acute hypersensitivity reaction such as anaphylaxis, which may be fatal.15
Treatment of cardiac manifestations of echinococcosis includes observation, medical therapy with antiparasitic agents (albendazole), and surgical resection.3 Surgical removal remains the definitive treatment of choice, with low rates of recurrence.9,15 Special consideration must be given intraoperatively to avoid leakage of cystic fluid to avoid anaphylaxis or dissemination of infected scolices.15 Scolicidal solutions such hypertonic saline can reduce the spread. Resection under cardiopulmonary bypass is considered the safest method with the lowest risk for cystic content spillage intraoperatively.15 Thorough preoperative planning is necessary, as the postoperative mortality for those with cardiac involvement is 4% to 10%, compared with 0.5% to 4% for cysts involving other parts of the body.8
Conclusion
We present a unique case of presumed Echinococcus infection leading to a large hydatid cyst involving the left ventricle. TTE played a vital role in the early detection and characterization of the patient's cardiac mass. Multimodality noninvasive imaging including CT and MRI allowed more precise structural and tissue characterization. Surgical resection of this single hydatid cyst led to complete clinical and radiologic resolution of the patient's symptoms. Echocardiography has high sensitivity to evaluate for cardiac masses and is an excellent first-line modality to evaluate patients for a wide variety of clinical presentations.
Acknowledgments
We would like to acknowledge Dr. Jagdish Butany for reviewing and providing input on pathology specimens. We would like to acknowledge Dr. Christopher Kandel, a resident physician involved in the patient's care.
Footnotes
Conflicts of Interest: Dr. McClure receives speaking fees from Lantheus Medical Imaging for training physicians and sonographers in the use of the ultrasound enhancing agent Definity.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.002.
Supplementary Data
Transthoracic echocardiographic parasternal long-axis view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal short-axis view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal apical four-chamber view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal long-axis view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Transthoracic echocardiographic parasternal short-axis view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Transthoracic echocardiographic apical four-chamber view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Cardiac MRI horizontal long-axis cine demonstrating a large cystic-appearing lesion measuring 5 × 5 × 4 cm located predominantly within the apex of the heart and surrounded by a rim of myocardium.
On short axis steady-state free precession cine image, the lesion appears to be encroaching upon the LV cavity. Smaller walled “daughter cysts” can be visualized within the larger cyst. On horizontal long axis first pass perfusion imaging, there is no evidence of contrast uptake or perfusion within the lesion.
On horizontal long-axis first-pass perfusion imaging, there is no evidence of contrast uptake or perfusion within the lesion.
Intraoperative transesophageal echocardiographic four-chamber view demonstrating a large mass occupying the entire mid to distal LV cavity.
Intraoperative transesophageal echocardiographic short-axis view demonstrating a large mass occupying the entire mid to distal LV cavity.
Postoperative transthoracic echocardiographic apical four-chamber view showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative parasternal short-axis view with ultrasound enhancing agent showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative apical four-chamber view with ultrasound enhancing agent showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative cardiac MRI horizontal long-axis cine showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
References
- 1.Basso C., Rizzo S., Valente M., Thiene G. Cardiac masses and tumours. BMJ Heart. 2016;102:1230–1245. doi: 10.1136/heartjnl-2014-306364. [DOI] [PubMed] [Google Scholar]
- 2.Peters P.J., Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocardiogr. 2006;19:230–240. doi: 10.1016/j.echo.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Hamda K., Maatouk F., Ben-Farhat M., Betbout F., Gamra H., Addad F. Eighteen-year experience with echinococcosus of the heart: clinical and echocardiographic features in 14 patients. Int J Cardiol. 2003;91:145–151. doi: 10.1016/s0167-5273(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 4.Moro P., Schantz P.M. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Brunetti E., Kern P., Vuitton D.A., Writing Panel for the WHO-IWGE Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Polat P., Kantarci M., Alper F., Suma S., Koruyucu M.B., Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–494. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 7.Wen H., Vuitton L., Tuxun T., Li J., Vuitton D.A., Zhang W. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 2019;32 doi: 10.1128/CMR.00075-18. e00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akar R., Eryilmaz S., Yazicioglu L., Eren N.T., Durdu S., Uysalel A. Surgery for cardiac hydatid disease: an Anatolian experience. Anadolu Kardiyol Derg. 2003;3:238–244. [PubMed] [Google Scholar]
- 9.Fennira S., Kamoun S., Besbes B., Ben Mrad I., Zairi I., Ben Moussa F. Cardiac hydatid cyst in the interventricular septum: a literature review. Int J Inf Dis. 2019;88:120–126. doi: 10.1016/j.ijid.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Junghanss T., da Silva A.M., Horton J., Chiodini P.L., Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301. [PubMed] [Google Scholar]
- 11.Porter T.R., Mulvagh S.L., Abdelmoneim S.S., Becher H., Belcik J.T., Bierig M. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31:241–274. doi: 10.1016/j.echo.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan M., Demirtas M., Cimen S., Ozler A. Cardiac hydatid cysts with intracavitary expansion. Ann Thorac Surg. 2001;71:1587–1590. doi: 10.1016/s0003-4975(01)02443-2. [DOI] [PubMed] [Google Scholar]
- 13.McManus D.P., Zhang W., Li J., Bartley P.B. Echinococcosis. Lancet. 2003;362:1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 14.Biava M.F., Dao A., Fortier B. Laboratory diagnosis of cystic hydatic disease. World J Surg. 2001;25:10–14. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 15.Hijazi E.M., Khamash M., Balas H., Heilat G.B. Intramural cardiac hydatid cyst—incidental finding. World J Cardiovasc Dis. 2014;4:5–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiographic parasternal long-axis view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal short-axis view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal apical four-chamber view demonstrating a large mass occupying almost the entire mid to distal LV cavity. An echo-bright layer appears to encompass a more echo-lucent center, with small linear densities within the structure.
Transthoracic echocardiographic parasternal long-axis view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Transthoracic echocardiographic parasternal short-axis view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Transthoracic echocardiographic apical four-chamber view with ultrasound enhancing agent. The large mass is seen along the mid-distal inferolateral, anterolateral, and apical walls without contrast enhancement, suggesting that the lesion is not vascular as per the American Society of Echocardiography 2018 guidelines on ultrasound enhancing agents in echocardiography.
Cardiac MRI horizontal long-axis cine demonstrating a large cystic-appearing lesion measuring 5 × 5 × 4 cm located predominantly within the apex of the heart and surrounded by a rim of myocardium.
On short axis steady-state free precession cine image, the lesion appears to be encroaching upon the LV cavity. Smaller walled “daughter cysts” can be visualized within the larger cyst. On horizontal long axis first pass perfusion imaging, there is no evidence of contrast uptake or perfusion within the lesion.
On horizontal long-axis first-pass perfusion imaging, there is no evidence of contrast uptake or perfusion within the lesion.
Intraoperative transesophageal echocardiographic four-chamber view demonstrating a large mass occupying the entire mid to distal LV cavity.
Intraoperative transesophageal echocardiographic short-axis view demonstrating a large mass occupying the entire mid to distal LV cavity.
Postoperative transthoracic echocardiographic apical four-chamber view showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative parasternal short-axis view with ultrasound enhancing agent showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative apical four-chamber view with ultrasound enhancing agent showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.
Postoperative cardiac MRI horizontal long-axis cine showing normal LV systolic function after mass resection. There is no evidence of residual or recurrent cyst.