Graphical abstract
Keywords: Primary cardiac sarcoma, Transthoracic echocardiography, Transesophageal echocardiography, Cardiac MRI, Cardiac CT
Highlights
-
•
Multimodality cardiac imaging can assess cardiac sarcomas and guide biopsy.
-
•
Cardiac MRI differentiates cardiac tumors from thrombus and identifies thrombus tumor.
-
•
Cardiac sarcoma treatment includes surgical and adjuvant options.
-
•
Survival rates of cardiac sarcoma are based on tumor location within the heart.
Introduction
Primary cardiac tumors are extremely rare, with the incidence ranging between 0.001% and 0.03%, of which 25% are malignant.1 Sarcomas are the most common malignant primary cardiac tumor, accounting for 10%-20% of all primary cardiac tumors.1 Presentation is often late, and disease burden is often high. Symptoms tend to relate to the structures involved. Multimodality cardiac imaging is integral in reaching a diagnosis in terms of both tissue characterization and guiding transcatheter biopsy. Cardiac sarcomas progress rapidly, and median survival is approximately 6-12 months.
Case Presentation
An 83-year-old woman with a history of coronary artery disease, hypertension, and hyperlipidemia initially presented to her primary care physician with a 3-week history of dyspnea on exertion. She denied orthopnea, paroxysmal nocturnal dyspnea, or peripheral edema at that time. On examination, she was noted to have a new murmur and was referred to a cardiologist for further evaluation.
A transthoracic echocardiogram (TTE) performed in the office that demonstrated a layering, noncalcified mass in the left atrium involving the anterior mitral valve leaflet obstructing mitral inflow (Figure 1, Videos 1 and 2). There was associated severe mitral stenosis (mean gradient = 22 mm Hg best seen on transesophageal echocardiography [TEE]; Figure 2), severe mitral regurgitation (Figure 2, Video 3), and severe pulmonary hypertension (pulmonary artery systolic pressure = 95 mm Hg). The interventricular septum was D shaped in systole consistent with elevated right heart pressures, and a small posterior pericardial effusion was noted (Figure 1B, Video 4). The patient was referred for cardiac magnetic resonance imaging (CMR) to better characterize the abnormality. Cardiac magnetic resonance imaging demonstrated a mass arising along the septum and inferior wall of the left atrium extending down along the anterior mitral valve leaflet (Figure 3, Video 5). The mass was not independently mobile and appeared to infiltrate these structures, resulting in significant mixed mitral valve disease. It measured 3.3 × 1.9 cm in the four-chamber view (Figure 3, Video 5). The mass was isointense to the myocardium on T1-weighted imaging with and without fat suppression and mildly hyperintense on T2-weighted imaging with fat suppression (Figure 4). The mass was found to perfuse poorly on first pass perfusion (Video 6), but there was evidence of hyperenhancement on early gadolinium enhancement imaging. Marked homogeneous hyperenhancement was noted on late gadolinium enhancement imaging along with evidence of a small amount of layered thrombus overlying the mass (Figure 4). There was no evidence of tumor infiltration in the pulmonary veins or right heart. Collectively the CMR findings were highly suggestive of a malignant cardiac tumor. The differential diagnosis at this point included primary cardiac sarcoma, lymphoma, or metastatic tumor. No definitive extracardiac malignancy was identified on computed tomography (CT) of the chest, abdomen, and pelvis, increasing our suspicion for a primary cardiac neoplasm.
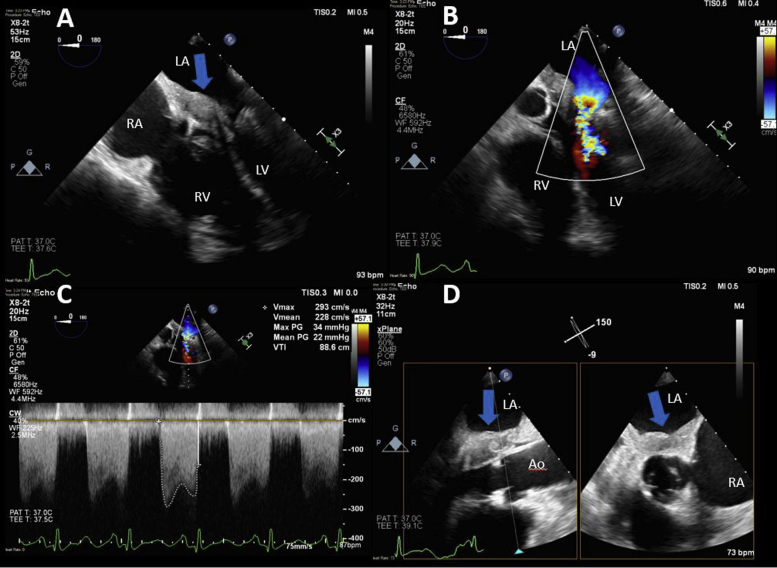
Figure 1.
The left atrial mass was initially diagnosed by TTE. (A) Parasternal long axis-view shows a large layering left atrial mass extending to the anterior mitral leaflet (blue arrow). (B) Parasternal short-axis view shows septal flattening, which is present in systole and diastole, consistent with right ventricular pressure and volume overload (red arrow). A small posterior pericardial effusion is also noted (∗). (C, D) Apical four-chamber views again show a large layering left atrial mass that extends to the mitral valve and obstructs mitral inflow (blue arrow). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 2.
TEE two-dimensional imaging was performed to further evaluate the left atrial mass and guide the biopsy. (A) Large layering left atrial mass (blue arrow) extending to the mitral valve. The mass was associated with severe flow obstruction across the mitral valve (B, C). (D) Biplane imaging demonstrates the proximity of the mass (blue arrows) to the aortic valve. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 3.
Cardiac MRI. Panels A (three-chamber view) and B (four-chamber view) are steady state free precession still images showing the left atrial mass (blue arrows) arising from the septum and inferior wall of the left atrium and involving the mitral valve. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 4.
Cardiac MRI tissue characterization. The left atrial mass (blue arrows) is isointense on T1-weighted imaging with and without fat suppression (A, B) and mildly hyperintense on T2-weighted imaging with fat suppression (C). There is marked homogeneous hyperenhancement on late gadolinium enhancement imaging (D, four-chamber view; E three-chamber view). There is also some evidence of thrombus overlying the mass (green arrows in D, E, and F). (F) Late gadolinium enhancement long TI time image confirming thrombus (green arrow). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The patient proceeded to have a TEE-guided percutaneous transcatheter biopsy of the mass (Figure 5, Video 7). Following transseptal puncture, three-dimensional (3D) live multiplanar reconstruction was performed to guide the placement of the catheter and biotome over the left atrial mass. However, the biopsies revealed only thrombotic material. The patient became hemodynamically unstable when sedated for the procedure, and the interventionalist was reluctant to do a repeat biopsy. However, the imaging was reviewed again, and the highly suspicious CMR findings prompted a repeat attempt, which revealed a poorly differentiated intimal sarcoma. She was evaluated by cardiothoracic surgery and deemed inoperable due to extensive infiltration of cardiac tissue. Due to her advanced age, she was not considered for cardiac transplantation. She was referred to a sarcoma specialty center where she was treated with docetaxel/gemcitabine. Heart failure symptoms were refractory to chemotherapy and diuresis. The patient passed away 3 months later.
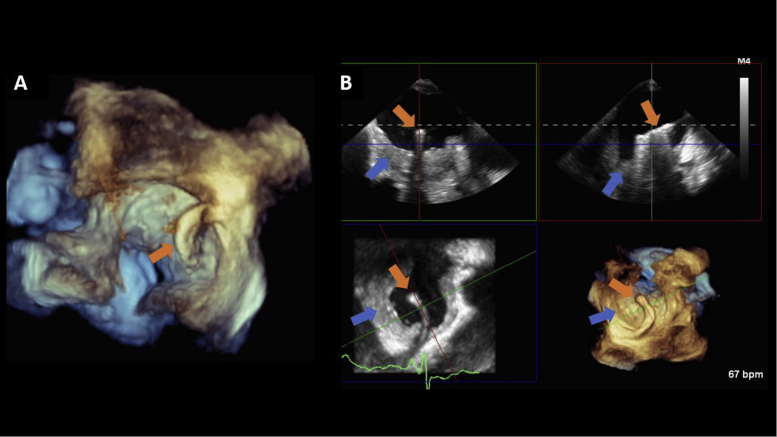
Figure 5.
TEE 3D imaging. Two-dimensional, biplane, and 3D TEE imaging was performed to guide the transcatheter biopsy of the left atrial mass. Following transseptal puncture, 3D live multiplanar reconstruction (MPR) was performed to guide the placement of the Agilis catheter and biotome (orange arrows) over the left atrial mass (blue arrows). Samples were collected from different locations within the mass. (A) A volume-rendered image of the Agilis catheter across the interatrial septum; (B) 3D live MPR shows the catheter as it is being steered toward the left atrial mass. Three-dimensional imaging with live MPR allows for real-time manipulation of all imaging planes (coronal, sagittal, and transverse) to facilitate coaxial imaging of the catheter.
Discussion
Primary cardiac sarcomas are rare and rapidly progressing malignant tumors usually diagnosed in patients under the age of 65 years old. Retrospective series have found that most cases are diagnosed in the fourth and fifth decades of life.2 Cardiac sarcomas more frequently originate in the atria, followed by the pericardium.3 The ventricles are rarely the site of origin.3 They are often misdiagnosed due to nonspecific symptoms suggesting other cardiac or pulmonary conditions. Involvement of the mitral valve is exceedingly rare. Undifferentiated cardiac sarcomas affecting the left atrium tend to be asymptomatic until advanced local disease exists. A review of the literature revealed a wide array of nonspecific symptoms at presentation, including dyspnea, chest pain, heart failure, cough, arrhythmias, and symptoms relating to embolic events.
Early detection of cardiac sarcomas is challenging, and a combination of noninvasive imaging including echocardiography, CT, CMR, and occasionally positron emission tomography is frequently utilized to evaluate the hemodynamic impact of the mass and aid in establishing a diagnosis. Biopsy of the lesion remains the gold standard for diagnosis. Initial evaluation is usually performed with TTE. Echocardiographic features that support a diagnosis of a cardiac sarcoma include a broad-based, lobulated mass with heterogeneous echogenicity. If present, hypoechoic areas within the mass can indicate tumor necrosis or areas of hemorrhage. Comparatively, thrombus and myxomas tend to be homogenous on echocardiograph. The use of contrast echocardiography can enhance sarcoma features as they are vascular structures. This contrasts with other cardiac pathologies including myxomas, which tend to only partially enhance, and thrombi, which do not enhance. Other features that can aid in diagnosis can include pericardial involvement of the mass and pericardial effusion. Echocardiography is an excellent tool to assess the hemodynamic effects of the mass. While TEE provides a more comprehensive echocardiographic assessment, it is the modality of choice for the guidance of percutaneous transcatheter biopsy.4 Cardiac CT provides excellent high-resolution anatomic information and assessment for local invasion and further evaluates tissue characteristics of the mass as well as assessment of the pericardium and for metastatic disease.5 Cardiac MRI is a complementary imaging tool that provides further insight into tissue characteristics while also evaluating the hemodynamic effects, invasion, and impact on the surrounding structures. The CMR findings in this case encouraged the interventionalist to repeat the biopsy, which originally revealed only thrombotic material. Intracardiac tumors often have some degree of overlying thrombus, which increases the likelihood of a false-negative biopsy.
Treatment of cardiac sarcoma is challenging given the high likelihood of advanced disease at the time of diagnosis, whether it be extensive local disease or distant metastasis.6 The treatment of choice is complete surgical resection; however, this presents technical and structural challenges given the location and invasive nature of these tumors. Surgery is feasible only if the benefits of excision outweigh the extent of myocardial tissue loss. These factors contribute to the overall poor prognosis for the disease. Complete surgical resection has been shown to offer an improved median survival of up to 24 months, which compares to all other patients who had a median survival of up to 10 months.7, 8, 9 Another surgical treatment option is cardiac autotransplantation, particularly for left-sided cardiac tumors with extensive local disease at presentation.10,11 In a study of 20 patients performed by Blackmon et al.,11 median overall survival of patients was 22 months with cardiac autotransplantation. Multiple sources advocate for an aggressive surgical approach to management, with some also advocating for additional therapies postoperatively, despite the lack of overall evidence for this. In a study performed by Bakaeen et al.,12 preoperative and postoperative chemotherapy resulted in an overall median survival of 23.5 months. Furthermore, patients with recurrent disease who underwent further treatment (including surgical resection or radiation) had improved median survival to 47 months. This is compared with those patients who had no further intervention and a median survival of 25 months.12 This contrasts with a study performed by Llombart-Cussac et al.13 who followed 15 cardiac sarcoma patients who received adjuvant chemotherapy with a doxorubicin-containing regimen within 6 weeks of surgery. The median survival in this study was 12 months.2
Studies investigating the treatment of primary cardiac sarcomas are limited by observational data and small sample size. More research is needed to clarify the most appropriate treatment strategies for this rare disease. Overall, the prognosis of primary cardiac sarcomas is poor due to disease burden at presentation. Historically, survival rates have ranged from 11 to 18 months, and 2-year survival rates have ranged from 14% to 26%.2 Treatment options are heavily influenced by location and stage of disease at diagnosis, with most sources advocating for aggressive initial management. Survival rate appears to be better in patients with left-sided cardiac sarcoma when compared with right.14,15 Right heart sarcomas are found to be bulky and infiltrative at the time of diagnosis and tend to metastasize early.15 Blackmon et al.11 noted that it was uncommon to develop right heart failure from obstruction until very late in the disease process. After treatment (an advised neoadjuvent chemotherapy followed by surgical resection) the median survival was 27 months. In comparison, left heart sarcomas were less infiltrative at time of diagnosis and tended to metastasize later than their right-sided counterparts. Left-sided tumors tended to present with heart failure symptoms. After proposed treatment (surgical resection with autotransplantation), cumulative survival was approximately 50% at 48 months. Despite the lack of evidence, adjuvant therapies, particularly chemotherapy, should be considered in all cases.
Conclusion
Primary cardiac sarcoma is a rare condition that tends to present late with advanced disease. Prognosis is generally poor. Multimodality cardiac imaging narrows down the differential diagnosis in these diagnostically challenging cases. Biopsy of the tumor is the gold standard for diagnosis and is effectively guided by TEE. Aggressive surgical management is the treatment of choice in selected cases, with most patients receiving adjuvant therapies thereafter.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.006.
Supplementary Data
TTE. Parasternal long-axis view demonstrating a large layering left atrial mass extending to the anterior mitral leaflet (blue arrow).
TTE. Apical four-chamber view demonstrating a large layering left atrial mass (blue arrow) that extends to the mitral valve and obstructs mitral inflow. The left ventricle is small and hyperdynamic. The right ventricle is enlarged with reduced right ventricular systolic function.
TEE two-dimensional imaging. TEE midesophageal views showing a large layering left atrial mass (blue arrow) extending to the mitral valve resulting in mitral stenosis (green arrow). Color-flow Doppler demonstrates significant mitral regurgitation.
TTE. Parasternal short-axis view demonstrating septal flattening in systole and diastole (red arrow), consistent with right ventricular pressure and volume overload. A small posterior pericardial effusion is also noted (∗).
Cardiac MRI. Three-chamber view and four-chamber steady state free precession cine images showing the left atrial mass (blue arrows) arising from the septum and inferior wall of the left atrium and involving the mitral valve.
Cardiac MRI perfusion. Cine images of first-pass perfusion with gadolinium revealing a poorly perfusing left atrial mass (blue arrows). A poorly perfusing intracardiac mass is suspicious for a neoplasm and is not consistent with thrombus.
Three-dimensional live multiplanar reconstruction (MPR) shows the catheter (orange arrows) as it is being steered toward the left atrial mass (blue arrows) following transseptal puncture. Three-dimensional imaging with live MPR allows for real-time manipulation of all imaging planes (coronal, sagittal, and transverse) to facilitate coaxial imaging of the catheter.
References
- 1.Burke A., Vermani R. Atlas of Tumor Pathology. 3rd series, fascicle 16. Armed Forces Institute of Pathology; Washington, D.C.: 1996. Tumors of the heart and great vessels. In: Burke A, Virmani R, editors. [Google Scholar]
- 2.Hamidi M., Moody J.S., Weigel T.L., Kozak K.R. Primary cardiac sarcoma. Ann Thorac Surg. 2010;9:176–181. doi: 10.1016/j.athoracsur.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siontis B.L., Zhao L., Leja M., McHugh J.B., Shango M.M., Baker L.H. Primary cardiac sarcoma: a rare, aggressive malignancy with a high propensity for brain metastases. Sarcoma. 2019:1960593. doi: 10.1155/2019/1960593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flachskampf F.A., Wouters P.F., Edvardsen T., Evangelista A., Habib G., Hoffman P. Recommendations for transoesophageal echocardiography: EACVI update 2014. Eur Heart J Cardiovasc Imaging. 2014;15:353–365. doi: 10.1093/ehjci/jeu015. [DOI] [PubMed] [Google Scholar]
- 5.Grebenc M.L., Rosado de Christenson M.L., Burke A.P., Green C.E., Galvin J.R. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–1112. doi: 10.1148/radiographics.20.4.g00jl081073. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro L.M. Cardiac tumours: diagnosis and management. Heart. 2001;85:218–222. doi: 10.1136/heart.85.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centofanti P., Di Rosa E., Deorsola L., Dato G.M., Patanè F., La Torre M. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236–1241. doi: 10.1016/s0003-4975(99)00700-6. [DOI] [PubMed] [Google Scholar]
- 8.Putnam J.B., Jr., Sweeney M.S., Colon R., Lanza L.A., Frazier O.H., Cooley D.A. Primary cardiac sarcomas. Ann Thorac Surg. 1991;51:906–910. doi: 10.1016/0003-4975(91)91003-e. [DOI] [PubMed] [Google Scholar]
- 9.Simpson L., Kumar S.K., Okuno S., Schaff H.V., Porrata L.F., Buckner J.C. Malignant primary cardiac tumors review of a single institution. Cancer. 2008;112:2440–2446. doi: 10.1002/cncr.23459. [DOI] [PubMed] [Google Scholar]
- 10.Reardon M.J., DeFelice C.A., Sheinbaum R., Baldwin J.C. Cardiac autotransplant for surgical treatment of a malignant neoplasm. Ann Thorac Surg. 1999;67:1793–1795. doi: 10.1016/s0003-4975(99)00343-4. [DOI] [PubMed] [Google Scholar]
- 11.Blackmon S.H., Patel A.R., Bruckner B.A., Beyer E.A., Rice D.C., Vaporciyan A.A. Cardiac autotransplantation for malignant or complex primary left-heart tumors. Tex Heart Inst J. 2008;35:296. [PMC free article] [PubMed] [Google Scholar]
- 12.Bakaeen F.G., Jaroszewski D.E., Rice D.C., Walsh G.L., Vaporciyan A.A., Swisher S.S. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg. 2009;137:1454–1460. doi: 10.1016/j.jtcvs.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Llombart-Cussac A., Pivot X., Contesso G., Rhor-Alvarado A., Delord J.P., Spielmann M. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78:1624–1628. doi: 10.1038/bjc.1998.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke A.P., Cowan D., Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Blackmon S.H., Reardon M.J. Surgical treatment of primary cardiac sarcomas. Tex Heart Inst J. 2009;36:451–452. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE. Parasternal long-axis view demonstrating a large layering left atrial mass extending to the anterior mitral leaflet (blue arrow).
TTE. Apical four-chamber view demonstrating a large layering left atrial mass (blue arrow) that extends to the mitral valve and obstructs mitral inflow. The left ventricle is small and hyperdynamic. The right ventricle is enlarged with reduced right ventricular systolic function.
TEE two-dimensional imaging. TEE midesophageal views showing a large layering left atrial mass (blue arrow) extending to the mitral valve resulting in mitral stenosis (green arrow). Color-flow Doppler demonstrates significant mitral regurgitation.
TTE. Parasternal short-axis view demonstrating septal flattening in systole and diastole (red arrow), consistent with right ventricular pressure and volume overload. A small posterior pericardial effusion is also noted (∗).
Cardiac MRI. Three-chamber view and four-chamber steady state free precession cine images showing the left atrial mass (blue arrows) arising from the septum and inferior wall of the left atrium and involving the mitral valve.
Cardiac MRI perfusion. Cine images of first-pass perfusion with gadolinium revealing a poorly perfusing left atrial mass (blue arrows). A poorly perfusing intracardiac mass is suspicious for a neoplasm and is not consistent with thrombus.
Three-dimensional live multiplanar reconstruction (MPR) shows the catheter (orange arrows) as it is being steered toward the left atrial mass (blue arrows) following transseptal puncture. Three-dimensional imaging with live MPR allows for real-time manipulation of all imaging planes (coronal, sagittal, and transverse) to facilitate coaxial imaging of the catheter.