Graphical abstract
Keywords: Echocardiography, Aortic regurgitation, Cardiac magnetic resonance imaging, Cardiac computed tomography, Takayasu arteritis
Highlights
-
•
Echocardiography is the first-line test to diagnose the presence and cause of AR.
-
•
Acute aortic syndromes should be excluded in patients with AR and a thickened aortic root.
-
•
Multimodal imaging with cardiac CT and MRI complement TTE and TEE to diagnose secondary causes of AR.
-
•
Aortitis is a potentially life-threatening disease, and is a rare cause of AR.
Introduction
Aortic regurgitation (AR) affects 8% to 13% of the population and results from failure of leaflet coaptation due to pathology affecting the ventricle, aortic valve (AV) annulus, AV cusps, or aorta.1 Most AR is chronic, from causes such as aortic dilatation, bicuspid AVs, rheumatic heart disease, or calcific valve disease.1 Chronic AR is tolerated because the left ventricle gradually dilates to adapt to the increasing regurgitant volume and so is able to preserve forward flow.1 Conversely, acute AR is poorly tolerated because the left ventricle is unable to accommodate the increased regurgitant volume, which may cause rapid clinical deterioration. These patients require immediate investigations to determine the etiology.1
We describe an unusual case of AR with increased aortic wall thickening. These findings should trigger urgent investigations to determine the etiology of the AR and to exclude life-threatening causes, specifically aortic dissection. Echocardiography is the primary imaging modality used to assess AR. It is complemented by magnetic resonance imaging (MRI) and computed tomography (CT), which offer better tissue characterization and assessment of extracardiac pathology. Through this case, we illustrate the value of each of these imaging modalities for the assessment and management of AR in the setting of increased aortic wall thickness.
Case Presentation
A 46-year-old woman presented with a 4-month history of abdominal fullness. She also reported intermittent flank pain. The patient denied experiencing shortness of breath, leg swelling, or exertional limitation. Her medical history included hyperthyroidism, which required no treatment, and constipation that was treated with laxatives.
On examination she was hypertensive with, a blood pressure of 166/77 mm Hg in the right arm and 159/81 mm Hg in the left arm. Her heart rate was 66 beats/min, and her respiratory rate was 16 breaths/min. She was afebrile, with a temperature of 36.6°C. Her oxygen saturation was normal at 98% on ambient air. Cardiac examination revealed normal S1 and S2 heart sounds. A grade 1/6 crescendo-decrescendo systolic murmur was audible at the left upper sternal border, and a grade 4/4 early diastolic murmur was heard at the left lower sternal border. Her peripheral pulses were bounding.
Laboratory testing at the time reported normal white blood cell count, hemoglobin, and platelets count of 6.0 × 109/L, 120 g/L, and 276 × 109/L, respectively. The patient's electrolytes and renal function were also normal (sodium 138 mmol/L, potassium 3.6 mmol/L, and creatinine 70 μmol/L). Electrocardiography showed normal sinus rhythm with a heart rate of 81 beats/min. Radiography of the chest and abdomen showed an enlarged cardiopericardial silhouette and mild fecal loading.
We were concerned for an aortic dissection because of the diastolic murmur and enlarged cardiac silhouette. Therefore, urgent transthoracic echocardiography was performed to assess the AV and pericardial space. This showed a tricuspid AV with moderate-to-severe AR (Figures 1A and 1B, Videos 1 and 2). The walls of the aortic root and proximal ascending aorta were thickened, measuring 9 mm (Figure 1, white arrow). There was suprapulmonic stenosis, with peak and mean systolic gradients of 53 and 32 mm Hg, due to extrinsic compression from periaortic tissue (Figures 1C-1E, Videos 3 and 4). There was mild tricuspid regurgitation and a right ventricular systolic pressure of 39 mm Hg. Left ventricular size was normal, but left ventricular systolic function was mildly reduced, with a left ventricular ejection fraction of 48%. Right ventricular size and systolic function were normal. A large, circumferential pericardial effusion was present without echocardiographic evidence of increased pericardial pressures.
Figure 1.
(A) Transthoracic echocardiographic (TTE) image from the parasternal long-axis (PLAX) view showing increased aortic wall thickness (white arrow) and a large pericardial effusion. (B) The same TTE PLAX image showing increased aortic wall thickness (white arrow) with color demonstrating severe AR. (C) TTE imaging from the parasternal short-axis (PSAX) view at the level of the proximal ascending aorta demonstrating suprapulmonic compression of the main pulmonary artery by the thickened aortic root (white arrow). (D) The same TTE PSAX image with color demonstrating flow turbulence. (E) Continuous-wave Doppler tracing across the pulmonic valve illustrating increased peak and mean systolic gradients of 54 and 32 mm Hg. AT, Acceleration time; ET, ejection time; Grad, gradient; LA, Left atrium; LV, left ventricle; PA, pulmonary artery; PV, pulmonary valve; RVOT, right ventricular outflow tract; Vmax, maximal velocity; Vmean, mean velocity; VTI, velocity-time integral.
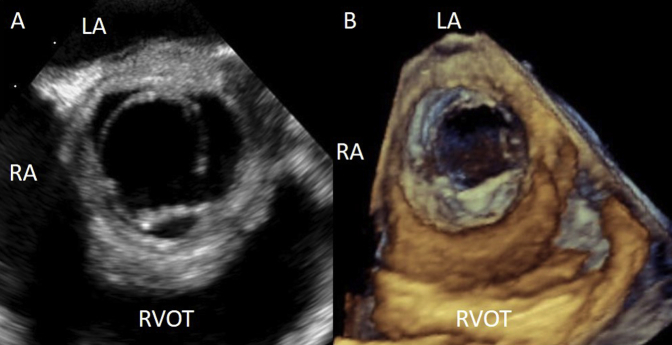
The presence of moderate to severe AR, a thickened aortic root, and a pericardial effusion increased suspicions for an acute aortic syndrome, such as aortic dissection or intramural hematoma. As per institutional practice, emergent transesophageal echocardiography was then performed and confirmed the findings on transthoracic echocardiography (Figures 2A and 2B, Video 5, Video 6, Video 7, Video 8). There was malcoaptation of the left and right coronary cusps of the AV resulting in a moderate to severe AR. The walls of the aortic root and proximal ascending aorta were thickened, and the sinotubular junction was effaced. There was no evidence of aortic dissection.
Figure 2.
(A) Transesophageal echocardiographic (TEE) image from the midesophageal window at 50° showing a tricuspid AV with a thickened aortic root. (B) TEE three-dimensional rendered image of the AV during systole as seen from the aorta. LA, Left atrium; RA, right atrium; RVOT, right ventricular outflow tract.
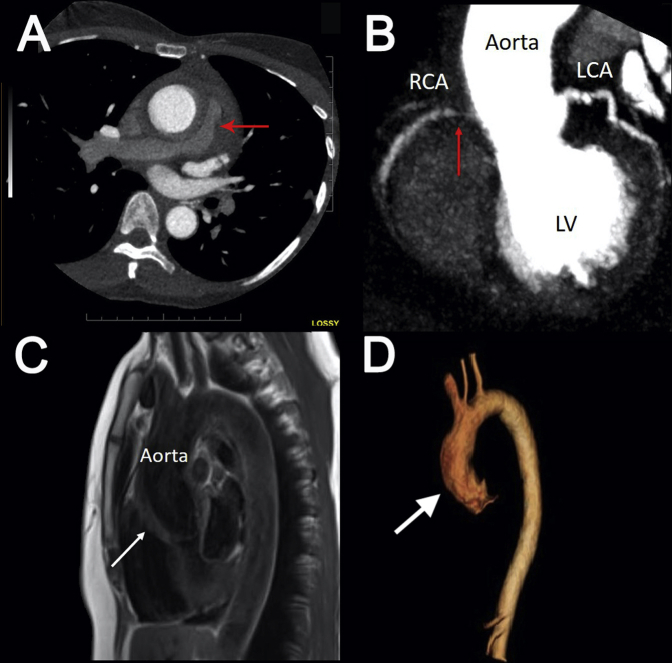
Contrast-enhanced cardiac CT was then arranged to definitively exclude an aortic dissection and intramural hematoma and to assess the coronary arteries. Cardiac CT demonstrated infiltrative soft tissue surrounding the aortic root and proximal ascending aorta, resulting in stenosis of the pulmonary trunk and right pulmonary artery (Figure 3A). There was infiltrative soft tissue surrounding the ostium of the right coronary artery, resulting in a long segment of 70% to 99% stenosis (Figure 3B, red arrow). There was infiltrative soft tissue around the left main coronary artery but no stenosis. There was no aortic dissection or intramural hematoma.
Figure 3.
Cardiac CT showing compression of the right pulmonary artery (A) proximal right coronary artery stenosis (red arrows) due to aortic root infiltration (B). (C) Cardiac MRI from the sagittal view demonstrating increased wall thickening and inflammation of the aortic root (white arrow), ascending aorta, and proximal aortic arch. (D) Cardiac MRI three-dimensional reconstruction showing thickening of the aortic root (white arrow), ascending aorta, and proximal aortic arch and sparing the other aortic segments. LCA, Left coronary artery; LV, left ventricle; RCA, right coronary artery.
In the absence of an acute aortic syndrome, cardiac MRI was ordered to characterize the infiltrative soft tissue surrounding the aorta. This showed diffuse wall thickening with late gadolinium enhancement (Figure 3C, white arrow) of the ascending aorta, which suggested diffuse inflammation. No such changes were seen in the descending thoracic aorta and abdominal aorta (Figure 3D, Video 9). On the basis of the cardiac MRI results, a diagnosis of aortitis was made.
We subsequently performed laboratory testing for infectious and noninfectious causes of aortitis. The results demonstrated that the patient's inflammatory markers were elevated, with an erythrocyte sedimentation rate of 51 mm/h and C-reactive protein of 37 mg/L. Results of tests for connective tissue diseases, such as antinuclear and antineutrophil cytoplasmic antibodies, rheumatoid factor, immunoglobulins, cryoglobulins, C3, and C4, were negative. There was no evidence of infection with negative blood cultures, syphilis serology, and tuberculin skin test. A diagnostic and therapeutic pericardiocentesis was performed and yielded 700 mL of exudative fluid that was predominantly lymphocytic and negative for bacterial cultures and acid-fast bacilli.
We diagnosed the patient with a large-vessel vasculitis, likely Takayasu arteritis. We initiated immunosuppression with oral prednisone 40 mg/d, followed by a slow taper in combination with oral azathioprine 100 mg/d. On this treatment, her inflammatory biomarkers improved (erythrocyte sedimentation rate 29 mm/h, C-reactive protein 13 mg/L). Cardiac surgery was considered to bypass the right coronary artery and replace the AV. However, it was deferred given the potential for improvement with immunosuppression and her elevated surgical risk due to inflamed and friable tissue. Two years later, she remains asymptomatic while receiving oral prednisone 5 mg/d and prefers to avoid surgery. Follow-up cardiac MRI performed 1 year after diagnosis demonstrated a slight reduction in aortic wall thickness (5 mm) and persistent moderate to severe AR (Figure 4).
Figure 4.
Cardiac magnetic resonance images obtained 1 year after diagnosis demonstrating a reduction in aortic wall thickness with steroid treatment.
Discussion
AR can result from aortic dilation or AV cusp perforation, prolapse, or restriction. Transthoracic echocardiography and TEE are first-line investigations used to identify the mechanism and quantify the severity of AR (Graphical Abstract).2 In this case, echocardiography identified increased aortic wall thickening as the probable mechanism of AR, which prompted urgent investigations for an acute aortic syndrome. Cardiac CT confirmed that there was no aortic hematoma or dissection and provided valuable information regarding coronary involvement. Cardiac MRI further demonstrated that the increased aortic wall thickness was due to active diffuse inflammation and so established the diagnosis of aortitis.
Aortitis is a potentially life-threatening condition of inflammation of the aorta. The investigative approach to aortitis is guided by the differential diagnosis, which is classified by inflammatory and infectious causes. The most common noninfectious causes of aortitis are the large-vessel vasculitides, primarily giant-cell arteritis and Takayasu arteritis, which affect the aorta and its primary branches.3 The most common infectious causes are syphilis and bacterial and mycobacterial infections.3 The clinical presentation of aortitis varies from abdominal and back pain to severe aortic aneurysms and AR. Acute complications include aortic dissection, aortic rupture, aortic thrombosis, acute coronary syndrome, and AR.3 In our case, there was compression of the pulmonary artery by the increased aortic wall thickness. However, primary pulmonary artery involvement in Takayasu arteritis is common, occurring in 20% to 56% of patients, and can result in pulmonary hypertension.4 Although a pericardial effusion can be present during the initial presentation, such as in our case, overall, the occurrence is rare.5
Multimodal imaging is essential to the diagnosis of thoracic aortic disease such as aortitis.6 Digital subtraction angiography was the previous gold standard but has the disadvantage of being invasive and providing information related only to luminal changes, which are often a late feature. Echocardiography, cardiac CT, and cardiac MRI may show homogenous circumferential thickening of the aortic wall, but it is the uniform and smooth lumen that differentiates aortitis from atherosclerosis. Cardiac CT and MRI are important imaging tests in these patients, as they can definitively rule out acute aortic syndromes, which is part of the differential diagnosis.6 Moreover, MRI can demonstrate that the increase in aortic wall thickness is due to the presence of arterial wall edema.6 This MRI finding suggests aortic inflammation and is diagnostic for aortitis.6 Last, cardiac CT has the ability to noninvasively evaluate luminal and extraluminal coronary artery abnormalities, which are often present in aortitis.
Giant cell arteritis and Takayasu arteritis are the most common causes of aortitis and require immediate attention. These large-vessel vasculitides have distinct clinical features and epidemiology but may overlap in histopathologic findings.3 The diagnosis is based on clinical suspicion and guided by consensus criteria.7 Key diagnostic criteria for giant cell arteritis and Takayasu arteritis from the American College of Rheumatology are summarized in Table 1.8,9 Note that although these criteria are widely accepted for clinical use, they are not felt to be accurate. A multicenter international study, the Diagnostic and Classification Criteria in Vasculitis Study, was designed to revise and update the diagnostic and classification criteria in vasculitis. Although the study has been completed, results are pending.10,11 Briefly, Takayasu arteritis most commonly presents with symptoms resulting from occlusive disease of the aorta or its primary branches, including pulseless disease. As discussed earlier, multimodal imaging using echocardiography, cardiac CT, and cardiac MRI is central to the diagnostic approach.6
Table 1.
Diagnosis of Takayasu arteritis and giant cell arteritis using the American College of Rheumatology 1990 criteria
| Takayasu arteritis∗ | Giant cell arteritis† |
|---|---|
Age at disease onset ≤40 y
|
Age at disease onset ≥50 y
|
Claudication of extremities
|
New headache
|
Decreased brachial artery pulse
|
Temporal artery abnormality
|
Blood pressure difference >10 mm Hg
|
Elevated erythrocyte sedimentation rate
|
Bruit over subclavian arteries or aorta
|
Abnormal artery biopsy findings
|
Arteriographic abnormality
|
The presence of three or more criteria is consistent with a diagnosis of Takayasu arteritis with sensitivity of 90.5% and specificity of 97.8%.
The presence of three or more criteria is consistent with the diagnosis of giant cell arteritis with sensitivity of 93.5% and specificity of 91.2%.
Once a diagnosis of giant cell arteritis or Takayasu arteritis is made, immunosuppression with glucocorticoids should be initiated, typically with prednisone 1 mg/kg for Takayasu arteritis.3 Immunosuppression is slowly weaned over the course of months to years with careful surveillance for complications, as even acute aortic syndromes such as aortic dissection can be a late feature.3 Disease relapse is common (up to 50%) and may be surveilled with inflammatory biomarkers, including erythrocyte sedimentation rate and C-reactive protein, as well as advanced imaging with MRI or 18F-fluorodeoxygluocse positron emission tomography.3
Conclusion
This patient had a constellation of cardiac findings that were ultimately attributed to aortitis from large-vessel vasculitis. This is a rare cause of AR and a potentially life-threatening condition. This rare unifying diagnosis is one that could not be made using a single imaging modality. Multimodality imaging was also important to identify complications such as coronary artery stenosis and pulmonary artery compression. The active inflammation seen on cardiac MRI played a crucial role in directing management.
Footnotes
Conflicts of interest: Dr. Tsang is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. The other authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.009.
Supplementary Data
Transthoracic echocardiogram of a parasternal long axis view demonstrating increased aortic wall thickness and a large pericardial effusion.
Transthoracic echocardiogram of a parasternal long axis view with color demonstrating severe aortic regurgitation in the setting of increased aortic wall thickness and a large pericardial effusion.
Transthoracic echocardiogram of a parasternal short axis view at the level of great vessels demonstrating supra-pulmonic compression of the main pulmonary artery by the thickened aortic root.
Transthoracic echocardiogram of a parasternal short axis view at the level of great vessels with color Doppler demonstrating turbulent flow in the main pulmonary artery due to supra-pulmonic compression by the thickened aortic root.
Transesophageal echocardiogram of a midesophageal long axis view demonstrating the thickened walls of the aortic root and proximal ascending aorta.
Transesophageal echocardiogram of a midesophageal long axis view with color Doppler demonstrating severe AR.
Two-dimensional transesophageal echocardiogram of a midesophageal short axis view of the aortic valve demonstrating the thickened walls of the aortic root.
Three-dimensional transesophageal echocardiographic reconstruction of the aortic valve as viewed from the aorta demonstrating the thickened walls of the aortic root.
Cardiac magnetic resonance imaging 3D reconstruction showing thickened walls of the aortic root, ascending aorta, and proximal aortic arch, with sparing the other aortic segments.
References
- 1.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., Guyton R.A. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 2.Zoghbi W.A., Adams D., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Gornik H.L., Creager M.A. Aortitis. Circulation. 2008;117:3039–3051. doi: 10.1161/CIRCULATIONAHA.107.760686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Lv N., Dang A., Cheng N. Pulmonary artery involvement in patients with Takayasu arteritis. J Rheumatol. 2020;47:264–272. doi: 10.3899/jrheum.190045. [DOI] [PubMed] [Google Scholar]
- 5.Knockaert D.C. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–1804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein S.A., Evangelista A., Abbara S., Arai A., Asch F.M., Badano L.P. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–182. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F. 2012 Revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 8.Arend W.P., Michel B.A., Bloch D.A., Hunder G.G., Calabrese L.H., Edworthy S.M. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 9.Hunder G.G., Bloch D.A., Michel B.A., Stevens M.B., Arend W.P., Calabrese L.H. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 10.Craven A., Robson J., Ponte C., Grayson P.C., Suppiah R., Judge A. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS) Clin Exp Nephrol. 2013;17:619–621. doi: 10.1007/s10157-013-0854-0. [DOI] [PubMed] [Google Scholar]
- 11.Luqmani R.A., Suppiah R., Grayson P.C., Merkel P.A., Watts R. Nomenclature and classification of vasculitis—update on the ACR/EULAR Diagnosis and Classification of Vasculitis Study (DCVAS) Clin Exp Immunol. 2011;164(suppl 1):11–13. doi: 10.1111/j.1365-2249.2011.04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram of a parasternal long axis view demonstrating increased aortic wall thickness and a large pericardial effusion.
Transthoracic echocardiogram of a parasternal long axis view with color demonstrating severe aortic regurgitation in the setting of increased aortic wall thickness and a large pericardial effusion.
Transthoracic echocardiogram of a parasternal short axis view at the level of great vessels demonstrating supra-pulmonic compression of the main pulmonary artery by the thickened aortic root.
Transthoracic echocardiogram of a parasternal short axis view at the level of great vessels with color Doppler demonstrating turbulent flow in the main pulmonary artery due to supra-pulmonic compression by the thickened aortic root.
Transesophageal echocardiogram of a midesophageal long axis view demonstrating the thickened walls of the aortic root and proximal ascending aorta.
Transesophageal echocardiogram of a midesophageal long axis view with color Doppler demonstrating severe AR.
Two-dimensional transesophageal echocardiogram of a midesophageal short axis view of the aortic valve demonstrating the thickened walls of the aortic root.
Three-dimensional transesophageal echocardiographic reconstruction of the aortic valve as viewed from the aorta demonstrating the thickened walls of the aortic root.
Cardiac magnetic resonance imaging 3D reconstruction showing thickened walls of the aortic root, ascending aorta, and proximal aortic arch, with sparing the other aortic segments.