Graphical abstract
Keywords: Interventricular septal hematoma, Ventricular septal defect repair, Echocardiography
Highlights
-
•
IVSH is a rare complication after cardiac procedures.
-
•
It results from myocardial infarction, coronary intervention, and/or direct trauma.
-
•
It is associated with morbidity, mortality, and extracorporeal membrane oxygenation support.
-
•
Echocardiography aids in diagnosis and follow-up of IVSH.
-
•
Spontaneous fistula between the septal hematoma and RV led to decompression and recovery.
Introduction
Interventricular septal hematoma (IVSH) is a rare complication that has been described after several types of surgical procedures including patch closure of a ventricular septal defect (VSD),1 percutaneous coronary artery intervention,2 and internal mammary artery grafting to the left anterior descending coronary artery.3 Often IVSH causes hemodynamic compromise and is associated with significant morbidity and mortality.4 Commonly reported complications of IVSH include myocardial rupture, ventricular dysfunction, ventricular outflow tract obstruction, and abscess formation. The hematoma leading to coronary cameral fistula has been previously reported after stent placement in a coronary artery.2 We present a unique case of IVSH after closure of a VSD with development of a coronary fistulous communication associated with decompression of the hematoma into the right ventricle (RV) cavity and a favorable clinical outcome.
Case Presentation
A 5-month-old, 3.8 kg male was born at 34 weeks of gestation with a large perimembranous VSD diagnosed on an echocardiogram performed at 10 days of life for evaluation of heart murmur and tachypnea. The patient had symptoms of heart failure evidenced by failure to thrive requiring hospitalization and nasogastric tube feeding. His weight, height, and body mass index were less than the first percentile for age and gender, and he had only gained 6 g per day despite feeding on 30 Kilocalorie per ounce formula with medium chain triglycerides oil. On physical examination, there was mild tachypnea, a grade 2 holosystolic murmur heard with maximum intensity at the left lower sternal border, wide splitting of the second heart sound, and hepatomegaly. He was medically managed with oral furosemide twice daily. Preoperative echocardiogram (Figure 1) demonstrated a large perimembranous VSD with left to right shunt, dilated left atrium, and normal biventricular systolic function. The patient was referred for surgical closure.
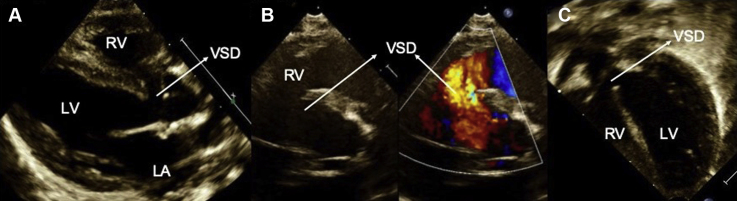
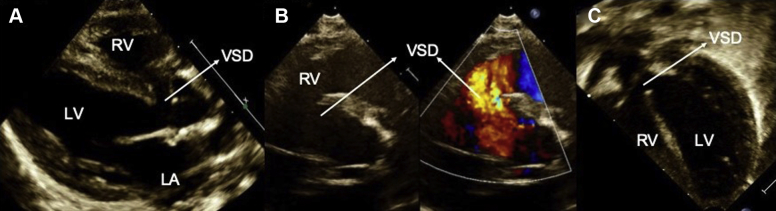
Figure 1.
Preoperative TTE demonstrating a large perimembranous VSD with left atrial dilation. (A) Parasternal long-axis view showing the perimembranous VSD; (B) parasternal short-axis view demonstrating the VSD in 2D and color with left to right shunt; (C) apical five-chamber view shows the perimembranous VSD. LA, Left atrium; LV, left ventricle.
Surgery was complicated by a moderate residual VSD after autologous pericardial patch closure; hence, a second bypass run was required. The entire VSD patch was taken down and redone using a Dacron patch through the septal leaflet of the tricuspid valve. The postoperative transesophageal echocardiogram (TEE) showed a hypokinetic interventricular septum, no residual VSD, moderate tricuspid valve regurgitation (TR), and normal biventricular systolic function (Video 1). After chest closure in the operating room, there was systemic hypotension with suspected cardiac tamponade. Transthoracic echocardiogram (TTE) demonstrated an underfilled/compressed RV with severe systolic dysfunction, moderate left ventricular systolic dysfunction, and a hypokinetic, echo-bright ventricular septum with a small hypoechoic space suggestive of IVSH measuring 0.75 × 0.15 cm (Figure 2A, Video 2). Surgical exploration revealed a mild-moderate thrombus in the pericardial space. Hemodynamics improved immediately after reopening the sternum. Despite the lack of significant hematoma in the pericardium, reopening of the sternum led to decompression and better filling of RV resulting in improved cardiac output. Systolic and presumed diastolic dysfunction after two runs of cardiopulmonary bypass and IVSH caused the clinical decompensation. Epicardial echocardiogram revealed thick interventricular septum and small hypoechoic space (Video 3). Four hours after leaving the operating room, the patient decompensated with a hypotensive arrest requiring resuscitation. On postoperative day (POD) 1, the IVSH increased in size (1.1 × 0.35 cm) and developed a fistulous connection into the RV, and biventricular systolic function was moderately depressed (Figure 2B, Videos 4 and 5). On POD 2, the fistulous connection was noted from the left anterior descending artery to the IVSH with diastolic flow, and it ultimately drained into the RV cavity during systole (Figure 2C and 3, Video 6, Video 7, Video 8). There was improvement in TR and biventricular systolic function (mildly decreased; Video 6, Video 7, Video 8). The patient had had 2:1 atrial flutter on POD 0 and accelerated junctional rhythm requiring AAI pacing. By POD 3, arrhythmias had resolved, the patient was weaned off low-dose epinephrine, the patient was hemodynamically stable with normal biventricular systolic function, and delayed sternal closure was performed (Video 9). The IVSH decreased in size (0.6 × 0.2 cm) by POD 18 (Video 10, Video 11, Video 12). The patient was discharged home on POD 20. Three months after the surgery, the echocardiogram demonstrated complete resolution of IVSH with normal biventricular systolic function and trivial TR (Videos 13 and 14).
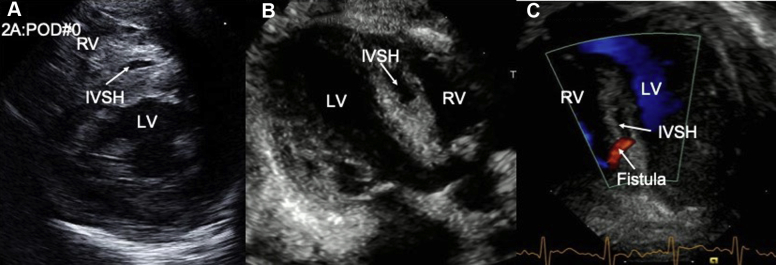
Figure 2.
Sequence of echocardiographic changes in IVSH. (A) IVSH detected on parasternal short axis view on POD 0; (B) increase in the size of the IVSH noted on parasternal long-axis view on POD 1; (C) development of fistulous connection between the IVSH and the RV shown in apical five-chamber view on POD 2. LV, Left ventricle.
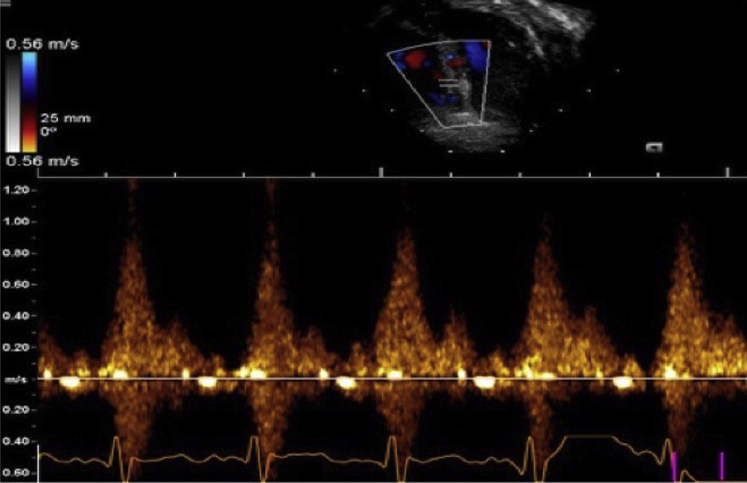
Figure 3.
Pulse-wave Doppler tracing of fistula from the IVSH demonstrating systolic flow into the RV.
Discussion
We present the echocardiographic features of a postoperative IVSH related to patch closure of a VSD that developed a fistulous connection to the RV cavity and subsequently resolved. There is currently a lack of consensus on the etiology and management of IVSH. The literature is limited to a few case reports and one case series of 12 patients. In the adult literature, IVSH has been described after myocardial infarction, percutaneous coronary artery intervention, coronary artery bypass graft procedures, or trauma. In the pediatric literature, IVSH has been reported following procedures such as isolated VSD closure, VSD closure along with repair of interrupted aortic arch, coarctation of aorta, transposition of great arteries, and repair of tetralogy of Fallot as well as after relief of pulmonary atresia/critical pulmonary stenosis with intact ventricular septum.5, 6, 7, 8, 9, 10 The etiology is speculated to be due to the insult to the septal perforator branches of the left anterior descending artery during the placement of the VSD patch, specifically the first septal perforator branch, which is in close proximity to the anterior rim of the VSD. This results in subendocardial bleeding, and further expansion of the hematoma occurs due to anticoagulation.4,11 However, this theory does not hold true in two reported cases where IVSH developed without VSD closure, one following relief of pulmonary atresia/critical stenosis with intact ventricular septum and the other following repair of total anomalous pulmonary venous return and atrial septal defect closure. The pathogenesis in the former case is thought to be due to hypertensive right ventricular pressures associated with endocardial fibroelastosis and coronary artery ischemia, and in the latter case due to possible high cardioplegia perfusion pressure leading to impaired myocardial perfusion.10,12 Jegatheeswaran et al.4 reported a series of 12 pediatric patients who developed IVSH after VSD closure. They identified that those under 2 months of age and median weight of 3.4 kg may be at higher risk of IVSH likely attributed to the difficulty in visualization of the defect.4 Among their 12 cases, 75% developed IVSH after a single cardiopulmonary bypass, while the remainder of the cases occurred following two bypass runs.
Complications of IVSH include decreased right and left ventricular systolic and diastolic function, myocardial rupture, thrombus formation, abscess formation, right and left ventricular outflow tract obstruction,2 conduction system abnormalities including heart block, junctional tachycardia, cardiac tamponade, requirement of extracorporeal membrane oxygenation support, and risk for multiorgan dysfunction and mortality.4 The mortality reported in the adult literature is higher, ranging up to 78%, especially in those managed convervatively.13 In comparison, the mortality reported in the pediatric literature is lower at 33% (in 12 pediatric patients after VSD closure); 16% (two cases) needed an intervention to drain the hematoma.4 Our patient's initial postoperative TEE did not demonstrate IVSH; however, the TTE in the operating room performed 2 hours later for acute decompensation showed the first sign of IVSH. This is consistent with the reported literature that only 55% (6 of 11 patients) of IVSH were discovered intraoperatively. The average time to diagnosis was 10.1 ± 7.9 hours.4 Echocardiography is the key investigation in the diagnosis and follow-up of IVSH. Characteristic features include echo bright, thickened interventricular septum with hypoechoic space. The IVSH can rapidly increase in size postoperatively, hence close follow-up is warranted. Development of fistulous connections to the coronary artery or the ventricular cavity and/or development of right or left ventricular outflow tract obstruction can be identified using two-dimensional (2D), color, and Doppler echocardiography. In addition, the literature has reported on the utility of contrast echocardiography, 2D speckle-tracking echocardiography, three-dimensional echocardiography, cardiac catheterization, and magnetic resonance imaging on diagnosing and evaluating IVSH. Speckle-tracking echocardiography demonstrates decreased myocardial strain in interventricular septal segments with normal strain in the other LV segments.14 Three-dimensional echocardiography demonstrates regional akinesia of the ventricular septum.15 In a rare case of spontaneous IVSH with endocardial fibroelastosis, cardiac magnetic resonance imaging in addition to echocardiography was useful in myocardial tissue characterization.16 Ventricular cine angiography demonstrated accumulation of contrast within the IVSH14 and identified injury to the first septal perforator artery.2
The management of the IVSH is often determined by its size and hemodynamic sequalae. Treatment options include conservative management, surgical drainage or needle aspiration of the IVSH. Support with extracorporeal membranous oxygenation has been recently described as a potential successful bridging therapy for hemodynamically unstable patients until the hematoma resolves.4 The mean time for resolution of the IVSH is reported to be variable at 20 ± 185 days. In one case series, 75% of pediatric patients had recovery of ventricular function. In our patient, the unique course of development of fistulous drainage between the IVSH and RV cavity resulted in decompression of the hematoma and likely contributed to spontaneous and early recovery. To our knowledge, this is the first report describing this sequence of echocardiographic changes in the evolution of postoperative IVSH with the development of a fistulous connection between the IVSH and the RV cavity and resolution of the IVSH. We highlight the utility of echocardiography in diagnosis and serial monitoring to evaluate the progress of IVSH.
Conclusion
Although rare, IVSH is a serious complication after surgical repair of VSD that is often overlooked in both adult and pediatric cases. Early recognition of this phenomenon is important as it is associated with high morbidity and mortality. Complications such as ventricular dysfunction, myocardial rupture, abscess formation, right or left ventricular outflow tract obstruction, and cardiac tamponade should be ruled out. In our patient, IVSH was associated with severe low cardiac output state, hypotensive arrest, and arrhythmia. During follow-up assessment, the IVSH fistulized to the right ventricular cavity leading to decompression and spontaneous recovery of biventricular systolic function, which led to a good clinical outcome. Recognition of this unusual phenomenon avoided an invasive procedure to drain the IVSH. Serial 2D echocardiography with color Doppler is useful in recognition of this sequence of changes.
Acknowledgments
We thank the cardiac sonographers at the Children's Health, University of Texas Southwestern Medical Center pediatric echocardiography laboratory, for their hard work and contribution.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.003.
Supplementary Data
TEE showing the VSD patch with an echo bright, hypokinetic interventricular septum.
TTE performed in the operating room shows the hypoechoic space in the interventricular septum consistent with IVSH.
Epicardial echocardiogram performed after sternal reopening demonstrates a small IVSH.
Echocardiogram performed on POD 1 shows increase in the size of the interventricular septal hematoma (1.1 × 0.35 cm).
Echocardiogram performed on POD 1 shows a small fistulous communication with the IVSH. The biventricular systolic function is moderately decreased.
Interventricular septal hematoma on POD 2 seen from 2D apical view (1.1 × 0.4 cm) with fistula from the left anterior descending artery (diastolic flow) into the IVSH and draining into the RV cavity (systolic).
Interventricular septal hematoma on POD 2 seen with color Doppler in the apical view (1.1 × 0.4 cm) with fistula from the left anterior descending artery (diastolic flow) into the IVSH and draining into the RV cavity (systolic).
Interventricular septal hematoma on POD 2 seen from the parasternal short-axis view. The biventricular systolic function is mildly decreased.
Apical four-chamber view demonstrates normalization of biventricular systolic function and hypokinetic interventricular septum on POD 3.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the apical five chamber view (2D) on the echocardiogram prior to discharge.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the apical five chamber view (color Doppler) on the echocardiogram prior to discharge.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the parasternal short-axis view on the echocardiogram prior to discharge.
Follow-up echocardiogram obtained 3 months after discharge shows complete resolution of interventricular septal hematoma with normal biventricular systolic function on apical four chamber view.
Follow-up echocardiogram obtained 3 months after discharge shows complete resolution of interventricular septal hematoma with normal biventricular systolic function on parastenral short axis view. There is improvement in the septal motion and normalization of the biventricular systolic function.
References
- 1.Drago M., Butera G., Giamberti A., Lucente M., Frigiola A. Interventricular septal hematoma in ventricular septal defect patch closure. Ann Thorac Surg. 2005;79:1764–1765. doi: 10.1016/j.athoracsur.2003.10.123. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Karim A.R., Vo M., Main M.L., Grantham J.A. Interventricular septal hematoma and coronary-ventricular fistula: a complication of retrograde chronic total occlusion intervention. Case Rep Cardiol. 2016;2016:8750603. doi: 10.1155/2016/8750603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettles D.F., Firth N., Nair R.U., Williams G.J. Fatal acute left ventricular outflow obstruction due to interventricular septal haematoma—diagnosis by transoesophageal echocardiography. Eur Heart J. 1989;10:479–481. doi: 10.1093/oxfordjournals.eurheartj.a059513. [DOI] [PubMed] [Google Scholar]
- 4.Jegatheeswaran A., Cohen M.S., Gaynor J.W., Mascio C.E., Spray T.L., Fuller S. Extracorporeal membrane oxygenation as a novel management strategy for interventricular septal hematoma following ventricular septal defect repair. J Thorac Cardiovasc Surg. 2020;159:1936–1940. doi: 10.1016/j.jtcvs.2019.09.150. [DOI] [PubMed] [Google Scholar]
- 5.Jensen R., Burg P., Anderson C., Garabedian C., Garabedian H., Siwek L. Postoperative ventricular septal hematoma: natural history of two pediatric cases. J Thorac Cardiovasc Surg. 2007;133:1651–1652. doi: 10.1016/j.jtcvs.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Mart C.R., Kaza A.K. Postoperative dissecting ventricular septal hematoma: recognition and treatment. ISRN Pediatr. 2011;2011:534940. doi: 10.5402/2011/534940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs S., Rega F., Vlasselaers D., Gewillig M., Meyns B. Dealing with a septal hematoma after switch operation with ventricular septal defect closure. Heart Surg Forum. 2010;13:E263–E264. doi: 10.1532/HSF98.20091171. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J., Liu H., Zhang J., Feng X., Wu S., Mei J. Interventricular septal hematoma after congenital cardiac surgery. Ann Thorac Surg. 2013;95:2171–2173. doi: 10.1016/j.athoracsur.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 9.Bailey F.J., Jivanji S.G., Kostolny M. Postoperative interventricular septal haematoma following tetralogy of Fallot repair and perimembranous ventricular septal defect repair. Interact Cardiovasc Thorac Surg. 2017;24:296–298. doi: 10.1093/icvts/ivw337. [DOI] [PubMed] [Google Scholar]
- 10.Suteu C.C., Muntean I., Benedek T., Toganel R. Giant dissecting ventricular septal haematoma associated with critical congenital heart disease. Interact Cardiovasc Thorac Surg. 2016;23:837–838. doi: 10.1093/icvts/ivw223. [DOI] [PubMed] [Google Scholar]
- 11.Yamazawa H., Takeda A., Nakajima H., Tachibana T., Aoki M. Interventricular septal hematoma following repair of a ventricular septal defect. J Card Surg. 2017;32:390–393. doi: 10.1111/jocs.13145. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi A., Cavalle-Garrido T., Redington A. Spontaneous intraoperative ventricular haematoma in a neonate. Heart. 2007;93:898. doi: 10.1136/hrt.2006.098475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas-Barron J., Roldan F.J., Romero-Cardenas A., Molina-Carrion M., Vazquez-Antona C.A., Zabalgoitia M. Dissecting intramyocardial hematoma: clinical presentation, pathophysiology, outcomes and delineation by echocardiography. Echocardiography. 2009;26:254–261. doi: 10.1111/j.1540-8175.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 14.Grohmann A., Elgeti T., Eddicks S., Knebel F., Rutsch W., Melzer C. Interventricular septum hematoma during cineventriculography. Cardiovasc Ultrasound. 2008;6:4. doi: 10.1186/1476-7120-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etman I., Abdelgawad H. P178 Dissecting ventricular septal hematoma; a rare complication of acute myocardial infarction. Eur Heart J Cardiovasc Imaging. 2020;21(Suppl 1):jez319.049. [Google Scholar]
- 16.Zhang N., Hu Q., Li Y., Liu J., Xu L., Sun Z. Spontaneous interventricular septum dissecting hematoma with endocardial fibroelastosis: imaging, diagnosis, surgical therapy and 6-year follow-up outcomes. Quant Imaging Med Surg. 2020;10:878–882. doi: 10.21037/qims.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TEE showing the VSD patch with an echo bright, hypokinetic interventricular septum.
TTE performed in the operating room shows the hypoechoic space in the interventricular septum consistent with IVSH.
Epicardial echocardiogram performed after sternal reopening demonstrates a small IVSH.
Echocardiogram performed on POD 1 shows increase in the size of the interventricular septal hematoma (1.1 × 0.35 cm).
Echocardiogram performed on POD 1 shows a small fistulous communication with the IVSH. The biventricular systolic function is moderately decreased.
Interventricular septal hematoma on POD 2 seen from 2D apical view (1.1 × 0.4 cm) with fistula from the left anterior descending artery (diastolic flow) into the IVSH and draining into the RV cavity (systolic).
Interventricular septal hematoma on POD 2 seen with color Doppler in the apical view (1.1 × 0.4 cm) with fistula from the left anterior descending artery (diastolic flow) into the IVSH and draining into the RV cavity (systolic).
Interventricular septal hematoma on POD 2 seen from the parasternal short-axis view. The biventricular systolic function is mildly decreased.
Apical four-chamber view demonstrates normalization of biventricular systolic function and hypokinetic interventricular septum on POD 3.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the apical five chamber view (2D) on the echocardiogram prior to discharge.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the apical five chamber view (color Doppler) on the echocardiogram prior to discharge.
Decrease in the size of the IVSH (0.6 × 0.2 cm) noted on the parasternal short-axis view on the echocardiogram prior to discharge.
Follow-up echocardiogram obtained 3 months after discharge shows complete resolution of interventricular septal hematoma with normal biventricular systolic function on apical four chamber view.
Follow-up echocardiogram obtained 3 months after discharge shows complete resolution of interventricular septal hematoma with normal biventricular systolic function on parastenral short axis view. There is improvement in the septal motion and normalization of the biventricular systolic function.