Graphical abstract
Keywords: Rupture, Defect, VSD, Tamponade, Transcatheter
Highlights
-
•
Ventricular septal rupture and hemopericardium are rare postinfarction complications.
-
•
Contrast[HYPHEN]enhanced echocardiography can help identify pericardial effusion etiologies.
-
•
Transcaval percutaneous ventricular assist device implantation is a viable strategy.
Introduction
In the current era of coronary revascularization, postinfarction rupture is a rare complication of myocardial infarction (MI). We present a case of postinfarction ventricular septal rupture (VSR) identified by transthoracic echocardiography (TTE). The patient subsequently developed cardiac tamponade physiology and was diagnosed with a hemopericardium secondary to myocardial leak following contrast-enhanced TTE. A novel transcatheter strategy was used to place a percutaneous ventricular assist device, which was followed by surgery, and the patient survived to discharge.
Case Presentation
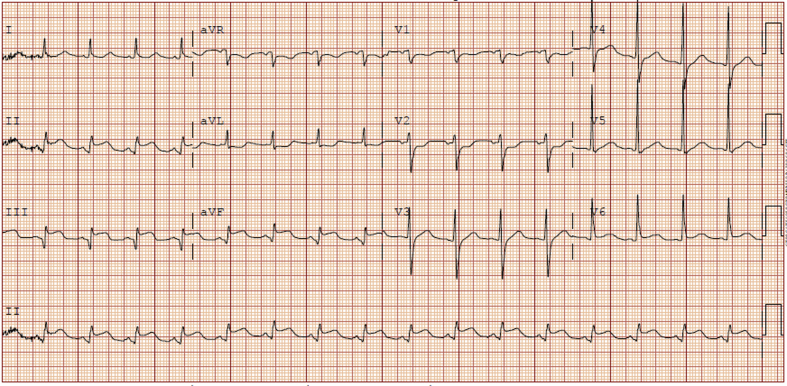
A 65-year-old man presented with 3 days of severe chest pain with minimal exertion. He described the pain as severe, burning, and substernal in nature, radiating to his back. He also noted feeling short of breath, nauseous, diaphoretic, and dizzy. His other medical conditions included hypertension and tobacco use disorder. His physical examination was notable for tachycardia and a cardiovascular examination without additional heart sounds. Electrocardiography was significant for ST-segment elevation in the inferior leads and left ventricular hypertrophy (Figure 1), and troponin was elevated at 5.45 ng/mL.
Figure 1.
The electrocardiogram from the patient's initial presentation. ST-segment elevation is notable in the inferior leads.
Emergent coronary angiography revealed 100% distal right coronary artery occlusion and 90% stenosis of the mid left anterior descending coronary artery. Four drug-eluting stents were placed in the right coronary artery, extending from the posterior descending artery to the ostium. However, flow was not restored to the posterior descending artery, as it was jailed by a right coronary artery stent.
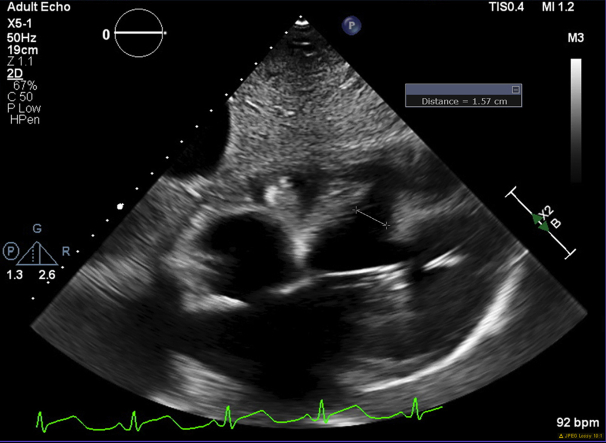
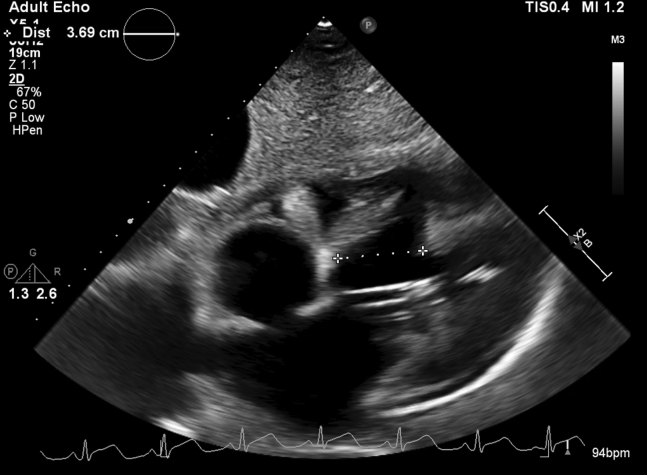
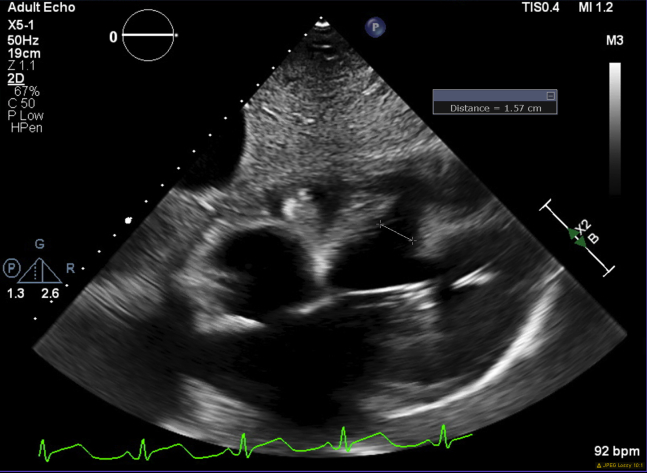
Two days later, the patient became hemodynamically compromised. TTE revealed a muscular postinfarction ventricular septal defect (PIVSD) that measured 1.57 cm at its narrowest dimension and 3.69 cm at its widest (Video 1, Figures 2 and 3). A trace pericardial effusion was also noted without tamponade physiology. Right heart catheterization showed a large left-to-right shunt (Qp/Qs 14.5) and a cardiac index of 1.71 L/min/m2.
Figure 2.
TTE showing the VSD with a measurement at the largest dimension of 3.69 cm. A trace pericardial effusion is also seen.
Figure 3.
TTE showing the VSD with a measurement at the smallest dimension of 1.57 cm. A trace pericardial effusion is also seen.
In an effort to stabilize the patient to allow tissue healing, an Impella 5.0 device (Abiomed, Danvers, MA) was emergently placed percutaneously to achieve rapid and maximal support (5 L/min; Video 2). The device was positioned within the apex, which allowed optimal hemodynamic support without interfering with the ventricular septal defect (VSD). A novel transcaval access strategy was used to minimize the risk for limb ischemia. In this approach, vascular access is gained through the femoral vein; the abdominal aorta is then entered from the neighboring abdominal inferior vena cava by the creation of an aortocaval connection (see Video 3).1
Six days later, the patient developed hypoxia and hypotension. TTE with contrast revealed a large pericardial effusion with cardiac tamponade physiology, and echocardiographic contrast bubbles were seen in the pericardial fluid, suggestive of oozing from a thinned and necrotic myocardium versus a small free wall rupture (FWR; Videos 4 and 5). A pericardial drain was placed with aspiration of sanguineous fluid, but this resulted in only temporary hemodynamic improvement. The patient underwent emergency coronary bypass and PIVSD patch repair. Intraoperative transesophageal echocardiograms display the VSD before closure and then after repair (Videos 6 and 7, respectively). Although intraoperative gross inspection showed no evidence of a true rupture, surgical pericardial drainage yielded frank blood. Accordingly, no FWR repair was performed. The patient was discharged 35 days later and remains alive. Postoperative follow-up transthoracic echocardiograms are shown in Videos 8 and 9, with notably no VSD or recurrence of effusion, suggesting that the damaged FWR myocardium had healed itself.
Discussion
VSR is a rare complication of MI. Previously occurring in 2% of MIs, in the era of coronary revascularization, VSR is now found in 0.2% of cases.2 This condition frequently results in cardiogenic shock and is associated with high mortality (>40%).2,3 PIVSDs have an average diameter of 1.2 cm, and larger defects are associated with worse outcomes.4,5 The PIVSD found in the presented case was much greater in size, measured to be 1.57 cm at its narrowest dimension and 3.69 cm at its widest. Considered in isolation from the subsequent hemopericardium and tamponade, it is remarkable that this patient survived with such a large PIVSD.
For patients with PIVSD with shock physiology, emergent intervention is necessary to promote hemodynamic stabilization. Although defect repair is considered the standard of care, friable tissue following MI yields suboptimal outcomes.6 Studies have found that survival rates nearly double in cases in which repair is delayed ≥7 days to allow adequate tissue healing.6,7 These studies do not include patients who underwent implantation of new percutaneous ventricular assistance devices. In a study by Arnaoutakis et al.,7 65% of patients were instead supported by intra-aortic balloon pump, and an ongoing debate remains regarding outcomes comparing percutaneous ventricular assistance devices with intra-aortic balloon pumps for patients undergoing percutaneous intervention for MI.8 Implantation of percutaneous ventricular assistance devices for tissue healing in patients with PIVSD has been previously documented only in case reports.9,10 The presented case further supports the use of this minimally invasive strategy in patients with PIVSD. To our knowledge, the presented case also describes the first use of the novel transcaval access strategy for this purpose.
Because of the patient's clinical instability and our institution's experience with percutaneous therapies, our multidisciplinary heart team initially planned to perform a transcatheter procedure for septal repair. The comparison of transcatheter and surgical VSD closure strategies has previously been studied only in pediatric patients with congenital defects, demonstrating significantly lower morbidity in patients who underwent transcatheter repair.11 To our knowledge, no data exist for the repair of defects that are caused by VSR after MI, a very different study cohort. However, this option became no longer viable with the subsequent discovery of a large hemopericardium resulting in cardiac tamponade physiology that was refractory to percutaneous drainage. The decision was then made to pursue surgical repair.
Uncertainty remains regarding the etiology of the hemopericardium in the presented case. Contrast-enhanced TTE demonstrated echocardiographic contrast bubbles in the pericardial fluid, thereby raising concern for FWR. In a case series of 96 bloody pericardial effusions, 11% resulted from MI, likely secondary to FWR.12 FWR has been reported in 1% to 2% of MIs, with exceptionally high rates of mortality.13 However, in the presented case, operative findings revealed no clear perforation. This suggests that blood may have oozed from a damaged myocardium containing microleaks. Myocardial leak or FWR progression to a large hemopericardium with postinfarction tamponade is uncommon, having been noted in only 0.85% of all MIs.14 Hemorrhagic postinfarction pericarditis, a rare consequence of myocardial ischemia and anticoagulation,15 can also be considered, although this would not account for contrast bubbles in the pericardial fluid. A leak from a thinned and necrotic myocardium remains the suspected etiology.
Conclusion
Cardiologists should be aware of VSR and FWR as uncommon complications of MI, particularly with late presentations. Imaging should be readily pursued in post-MI patients who become hemodynamically unstable, and echocardiography can quickly diagnose both conditions with minimal risk to the patient. In cases of pericardial effusions, contrast-enhanced TTE may also assists in determining the causative etiology. Best strategies in terms of timing of intervention and type of intervention (surgical vs percutaneous) for PIVSD repair remain uncertain.
Footnotes
Conflicts of Interest: Dr. Greenbaum is a proctor for Edwards Lifesciences and Medtronic and has an equity interest in Transmural Systems. Dr. Guyton has served as the national principal investigator on the Edwards Lifesciences transcatheter mitral valve replacement early feasibility trial. The other authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.10.010.
Supplementary Data
TTE showing the VSD with color Doppler. A trace pericardial effusion is also seen.
Aortogram with a sheath crossing the inferior vena cava into the abdominal aorta. An Impella device is also seen within the left ventricle.
Crossing the inferior vena cava into the abdominal aorta as part of the transcaval access procedure.
TTE showing the VSD and a large pericardial effusion with tamponade physiology.
Contrast-enhanced TTE showing a large pericardial effusion with contrast bubbles in the pericardial fluid suggesting blood oozing from a leaking myocardium. Surgical drainage yielded frank blood.
Intraoperative transesophageal echocardiography (midesophageal view) showing the VSD (before repair).
Intraoperative transesophageal echocardiography (midesophageal view) showing closure of the VSD.
One-month postoperative TTE showing VSD patch in place with no residual VSD by color flow. The lack of no recurrence of effusion should also be noted.
A side-by-side comparison of transthoracic echocardiograms demonstrating the change from preoperative to 1-month postoperative.
References
- 1.Greenbaum A.B., Babaliaros V.C., Chen M.Y., Stine A.M., Rogers T., O'Neill W.W. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol. 2017;69:511–521. doi: 10.1016/j.jacc.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murday A. Optimal management of acute ventricular septal rupture. Heart. 2003;89:1462–1466. doi: 10.1136/heart.89.12.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbadawi A., Elgendy I.Y., Mahmoud K., Barakat A.F., Mentias A., Mohamed A.H. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12:1825–1836. doi: 10.1016/j.jcin.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Maltais S., Ibrahim R., Basmadjian A.J., Carrier M., Bouchard D., Cartier R. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009;87:687–692. doi: 10.1016/j.athoracsur.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 5.O'Gara P.T., Kushner F.G., Ascheim D.D., Casey D.E., Jr., Chung M.K., de Lemos J.A. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 7.Arnaoutakis G.J., Zhao Y., George T.J., Sciortino C.M., McCarthy P.M., Conte J.V. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94:436–443. doi: 10.1016/j.athoracsur.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin A.P., Spertus J.A., Curtis J.P., Desai N., Masoudi F.A., Bach R.G. The evolving landscape of Impella Use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–284. doi: 10.1161/CIRCULATIONAHA.119.044007. [DOI] [PubMed] [Google Scholar]
- 9.La Torre M.W., Centofanti P., Attisani M., Patane F., Rinaldi M. Posterior ventricular septal defect in presence of cardiogenic shock: early implantation of the Impella Recover LP 5.0 as a bridge to surgery. Tex Heart Inst J. 2011;38:42–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Ibebuogu U.N., Bolorunduro O., Hwang I. Impella-assisted transcatheter closure of an acute postinfarction ventricular septal defect. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213887. bcr2015213887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J., Yang L., Yu S., Liu J., Zuo J., Chen W. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol. 2014;63:1159–1168. doi: 10.1016/j.jacc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Atar S., Chiu J., Forrester J.S., Siegel R.J. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999;116:1564–1569. doi: 10.1378/chest.116.6.1564. [DOI] [PubMed] [Google Scholar]
- 13.Garg P., Abdel-Rahman S.E., Greenwood J.P., Plein S. Free-wall rupture post-reperfused acute myocardial infarction: insights from multimodality cardiovascular imaging. Circulation. 2015;132:e245–e247. doi: 10.1161/CIRCULATIONAHA.115.018932. [DOI] [PubMed] [Google Scholar]
- 14.Patel M.R., Meine T.J., Lindblad L., Griffin J., Granger C.B., Becker R.C. Cardiac tamponade in the fibrinolytic era: analysis of >100,000 patients with ST-segment elevation myocardial infarction. Am Heart J. 2006;151:316–322. doi: 10.1016/j.ahj.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Cader F.A., Haq M.M., Nasrin S., Karim M.R. Pericardial tamponade due to haemorrhagic pericardial effusion as a complication of prasugrel: a case report. BMC Cardiovasc Disord. 2016;16:162. doi: 10.1186/s12872-016-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE showing the VSD with color Doppler. A trace pericardial effusion is also seen.
Aortogram with a sheath crossing the inferior vena cava into the abdominal aorta. An Impella device is also seen within the left ventricle.
Crossing the inferior vena cava into the abdominal aorta as part of the transcaval access procedure.
TTE showing the VSD and a large pericardial effusion with tamponade physiology.
Contrast-enhanced TTE showing a large pericardial effusion with contrast bubbles in the pericardial fluid suggesting blood oozing from a leaking myocardium. Surgical drainage yielded frank blood.
Intraoperative transesophageal echocardiography (midesophageal view) showing the VSD (before repair).
Intraoperative transesophageal echocardiography (midesophageal view) showing closure of the VSD.
One-month postoperative TTE showing VSD patch in place with no residual VSD by color flow. The lack of no recurrence of effusion should also be noted.
A side-by-side comparison of transthoracic echocardiograms demonstrating the change from preoperative to 1-month postoperative.