Increased left ventricular (LV) myocardial extracellular volume fraction (ECVF) measured by cardiac magnetic resonance (CMR) is now a recognized consequence of anthracycline chemotherapy (1,2). It remains unknown, however, whether elevated ECVF may be attributable to a) an expansion of the LV myocardial extracellular space (due to interstitial fibrosis or edema)(1), b) a reduction of the total myocardial tissue volume (due to cardiomyocyte atrophy or loss), (2) or a combination thereof. Determining the etiology of increased LV ECVF after receipt of anthracyclines is important to guide therapy to reduce anthracycline associated cardiotoxicity. In this study, we performed CMR imaging (to measure ECVF) and collected histopathology from African Green Monkey (AGMs) that received chronic doses of doxorubicin (Dox, a widely used anthracycline) that closely recapitulated adjuvant anthracycline treatment for breast cancer.
This study conformed to the principles of the National Institutes of Health and all protocols were approved by Wake Forest University Animal Care and Use Committee. Five female AGMs aged 13±1 years (equivalent to a 39-year-old woman) underwent Dox treatment (five doses of 30 – 60 mg/m2/biweekly IV, cumulative dose: 240 mg/m2). CMR imaging was conducted before and 15 weeks after the last dose of Dox on a 3.0T Siemens Skyra scanner (Erlangen, Germany). LV volumes and myocardial mass were determined from cine white blood steady-state free precession images. ECVF was calculated from T1-maps acquired pre- and 15 minutes post-contrast administration (0.15 mmol/Kg of Prohance; Bracco Diagnostics, New Jersey) in a mid-cavity short-axis slice using a modified Look-Locker inversion recovery sequence (MOLLI). T2 maps were acquired pre-contrast (SSFP sequence) (3). Image analysis was completed on off-line work stations as previously published (3).
At 25 weeks after initiating Dox, euthanasia was induced in accordance with the American Veterinary Medical Association guidelines. A mid-section of the LV matching the LV mid-short axis plane used on CMR was fixed in 4% paraformaldehyde. Archival tissues of 5 age- and gender- matched healthy animals served as controls for histopathology. Five μm section were obtained and stained with picrosirius red (PSR) to quantify interstitial myocardial fibrosis and fluorescent-tagged wheat germ agglutinin (WGA) for quantification of cardiomyocytes. Paired and unpaired t-tests were used to compare continuous measures from baseline to final CMR and cardiac histological parameters between untreated controls and Dox-treated animals respectively; p values of ≤0.05 were considered significant.
After Dox, LV ejection fraction decreased by 25±5 percentage points (p<0.001) while LV myocardial mass indexed was significantly increased (Baseline: 19.3 ± 0.9 g/m2 vs. post-Dox: 27.2 ± 2.1 g/m2, p=0.006). ECVF was increased at final CMR (ECVF: 39.8 ± 1.3 %) compared to baseline (ECVF: 29.8% ± 1.7 %, p=0.04) and there was a trend for total cell volume to be increased (12.8 ± 0.7 vs. 15.5 ± 1.3, p=0.08). T2 values also increased after Dox (38.6 ± 1.5 ms vs. 49.4 ± 0.5 ms, p=0.001).
Histologically, Dox-treated animals exhibited an increase in interstitial LV collagen deposition (Controls: 3.1 ± 0.08% vs. Dox: 5.9 ± 0.26%, p=0.0001) along with an increase in cardiomyocytes cross- sectional areas (Controls: 242.7 ± 5.2 μm2 vs. Dox: 307.7 ± 8.2 μm2, p=0.0002) and a drop in the number of cardiomyocytes (Controls: 374 ± 8 vs. Dox: 295 ± 8, p<0.0001) (Figure 1).
Figure 1. Histopathological Mechanisms Leading to CMR-derived Extracellular Volume Fraction Expansion after Chronic Receipt of Doxorubicin.
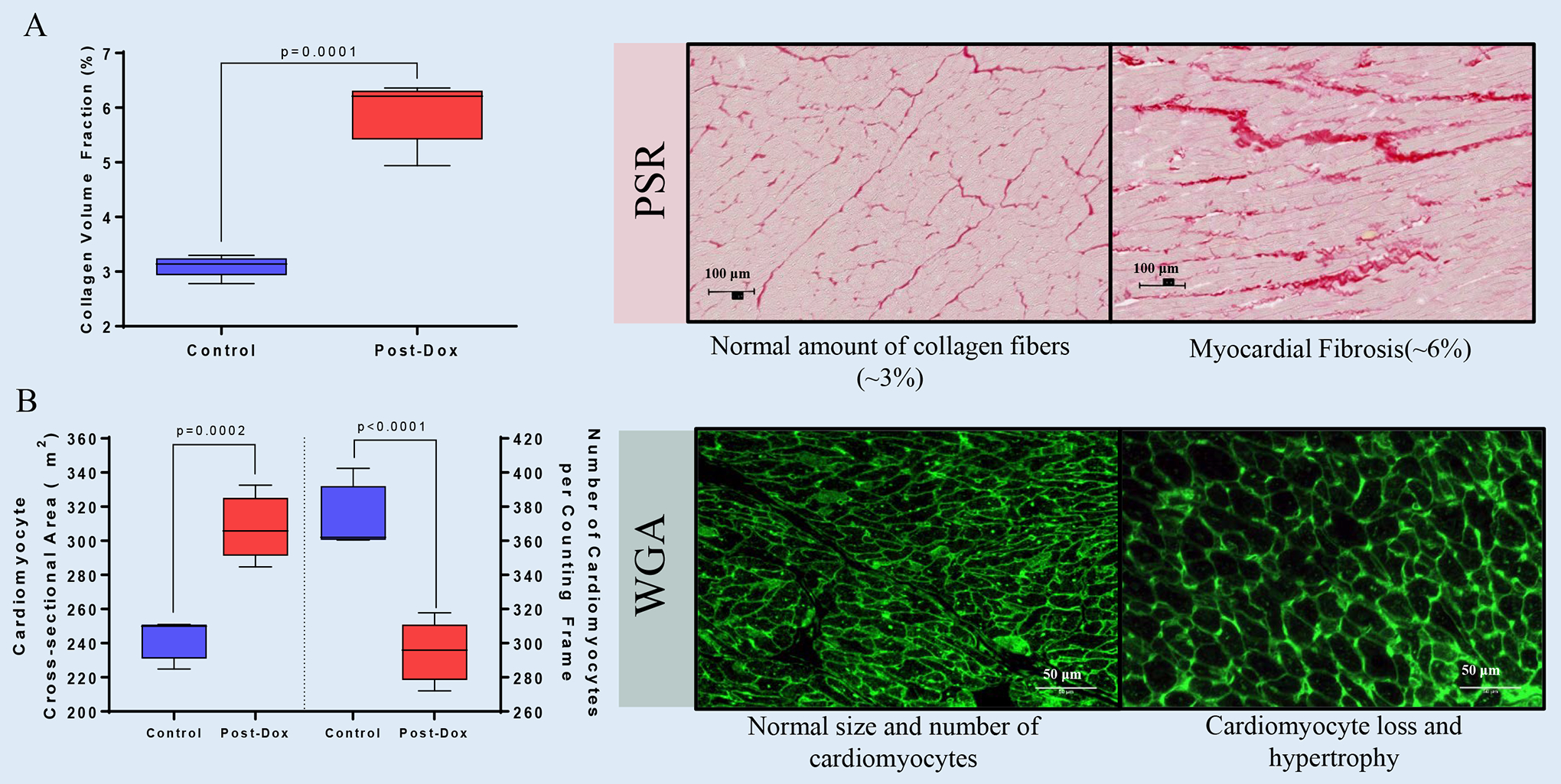
(A) Graphical representation of cardiac fibrosis and (B) cardiomyocyte size and numbers and representative microphotographs. All values are mean ± SEM at baseline.
Our results indicate that Dox-induced ECVF increases are primarily associated with accumulation of interstitial cardiac fibrosis. Cardiomyocyte loss resulted in compensatory hypertrophy of residual cardiomyocytes -reflected in the increased total cell volume and LV mass- deemphasizing the contribution of the LV myocardial compartment to ECVF increases. Despite the compensatory myocardial hypertrophy AGMs exhibited chemotherapy-induced cardiotoxicity. T2 elevations suggest that edema may partially contribute to the expansion of the interstitial space. Our observations underscore the complexity the LV and extracellular matrix remodeling induced by anthracyclines and the importance of critically assessing the mechanisms of CMR-derived expansion of ECVF to tailor therapeutic interventions prior or during to the establishment of irreversible processes such as myocardial fibrosis.
Further investigations are needed to comprehensively evaluate the continuum of myocardial and extracellular matrix remodeling utilizing CMR-derived metrics such as ECVF to assess the expansion of the interstitial space, T2 values to assess edema, and intracellular water lifetime to measure cardiomyocyte size as subclinical imaging markers. Such biomarkers may forecast the development of LV dysfunction after anthracycline chemotherapy and identify pathophysiologic processes that may be modified to thwart cardiotoxicity.
ACKOWLEDGEMENTS
The authors thanks Ms. Tonya Calhoun of Preclinical Translational Services, Wake Forest Innovations and Drs. Melaney Gee, Andre LeGrande for their technical and veterinary support in this study.
Financial Support: This research was supported in part by NIH grants 3R01HL118740-01S1 (GCM and WGH), and P40-OD010965 - Vervet Research Colony as a Biomedical Resource (JRK), Bethesda, MA; Wake Forest School of Medicine, Pathology Department “Spark” Grant (GCM), Winston-Salem, NC and Investigator Initiated Grant from Merck & Co., Kenilworth, NJ (JRK).
Relationship with Industry: Merck & Co. partially funded the present study.
ABBREVIATIONS
- AGM
African Green Monkey
- CMR
Cardiac Magnetic Resonance
- CVF
Collagen Volume Fraction
- Dox
Doxorubicin
- ECVF
Extracellular volume fraction
- LV
Left Ventricle
- LVEF
Left Ventricular Ejection Fraction
- LVEDV
Left Ventricular End-diastolic Volume
- LVESV
Left Ventricular End-systolic Volume
- MOLLI
Modified Look-Locker Inversion
- PSR
Picrosirius red
- WGA
Wheat germ agglutinin
References
- 1.Farhad H, Staziaki PV, Addison D et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated With Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circulation Cardiovascular imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira de Souza T, Quinaglia ACST, Osorio Costa F et al. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovascular imaging 2018;11:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]


