Graphical abstract

Keywords: Drug-induced valvular heart disease, Selective serotonin reuptake inhibitor, Carcinoid-like heart disease, Cardiac ultrasound, Vigilance
Highlights
-
•
Drug-induced valvular heart disease (DI-VHD) is reminiscent of carcinoid heart disease.
-
•
DI-VHD is mediated through 5-hydroxytryptamine 2β receptor signaling pathways.
-
•
Serotonergic agents interacting with this 2β receptor may cause carcinoid-like VHD.
-
•
DI-VHD must be considered in patients with no reasons for showing VHD and using SSRI.
-
•
Echocardiographic surveillance is warranted in patients on serotonergic drugs, including SSRI.
Introduction
Serotonin, the “happiness” neurotransmitter, has complex cardiovascular effects. Serotonin-related mechanisms have been involved in valvular heart disease (VHD) in animals and humans in the setting of carcinoid tumors, fenfluramine-phentermine, and ergot alkaloids intake. Selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) are the most widely prescribed antidepressants. An association between heart valve lesions and use of serotonergic antidepressants is controversial. This case of a relatively young woman supports a potential causal relationship between SSRI use and VHD.
Case Presentation
A 41-year-old woman was referred for peripartum hypertension for which she had been treated with amlodipine for the last 2 weeks before delivery. The pregnancy and birth were further uncomplicated, and blood pressure was successfully managed with amlodipine and irbesartan. She had a history of essential arterial hypertension, major depression, anxiety, and obsessive-compulsive disorder for which she had been treated with psychotherapy and medication. Since 2010, she had been being treated with escitalopram 20 mg and later on switched to sertraline up to 100 mg, nortriptyline 50 mg, aripiprazole 5 mg, trazodone 100 mg, and clonazepam 0.5 mg.
Being a dance teacher, she had no complaints about and reported no cardiorespiratory symptoms or exercise intolerance. Physical examination showed a mentally and physically healthy woman, blood pressure of 126/80 mm Hg, a regular pulse of 72 bpm, normal head and neck examination, normal heart and lung auscultation, tender abdomen with no hepatosplenomegaly, and no peripheral cyanosis or edema. The electrocardiogram showed sinus rhythm with pronounced P waves, QRS morphology consistent with left anterior hemiblock, and normal repolarization.
Echocardiographic examination showed a nondilated, concentric remodeled left ventricle with preserved ejection fraction. Mild left-sided valve disease was observed, with thickened mitral and aortic valve leaflets, both showing mild regurgitation. The right ventricle (RV) was dilated (RV end-diastolic diameter/left ventricle end-diastolic diameter ratio of 1) and showed diastolic septal D shaping, suggestive of RV volume overload. Right ventricular function was preserved, and no regional RV dysfunction or wall abnormalities were observed. The right atrium was severely dilated (52 mL/m2). A severe, central tricuspid valve regurgitation (TR) with a dense, triangular continuous-wave Doppler signal (dagger-shaped Doppler profile) was present. Structurally, the subvalvular and valvular apparatus had a thickened and shortened appearance. The tricuspid leaflets showed restrictive motion, with the septal leaflet almost completely immobilized due to leaflet thickening and chordal retraction causing a large leaflet coaptation defect (Figure 1).
Figure 1.
Echocardiography (apical four-chamber view in systole) showed a moderately dilated RV and severely dilated right atrium. The subvalvular and valvular apparatus had a thickened and shortened appearance. The leaflets were restrictive due to chordal retraction causing noncoaptation (A). A severe TR was present (B).
An estimation of right ventricular pressure based on Bernoulli's principle was normal (20 mm Hg), and the central venous pressure estimate was also normal. Conditions that may cause RV volume overload (such as severe pulmonary regurgitation, high cardiac output, or cardiac or extracardiac left-to-right shunts) were excluded by echocardiography and right heart catheterization, which showed a normal cardiac output (4.9 L/minute), normal pulmonary pressures (mean pulmonary artery pressure 15 mm Hg), and normal pulmonary capillary wedge pressure of 1 mm Hg.
As the TR seemed to be a primary or structural TR, several potential causes for degenerative TR were assessed. No leaflet vegetations were observed, and blood analysis showed no abnormalities (normal C-reactive protein, white blood cell counts, and eosinophil counts). Rheumatic valve disease, characterized by diffuse fibrous thickening of the leaflets, rather results in fusion of the commissures and in tricuspid valve (TV) stenosis, which was not present in our case. There were no features of Ebstein's anomaly or congenital TV dysplasia. Autoimmune disease and vasculitis, rarely causing valvulopathy, were excluded through laboratory testing (rheumatoid factor <11.30 U/mL, antinuclear antibodies negative, antineutrophil cytoplasmic antibody fluorescence negative). Importantly, the patient denied (former) use of known valvulopathic drugs such as ergotamine, methysergide, cabergoline, pergolide, (dex)fenfluramine, or benfluorex. The patient had no symptoms of carcinoid syndrome, and urinary 5-hydroxyindoleacetic acid levels (5.1 mg/24 h) and serum chromogranin A (123 μg/L) were within normal ranges. Finally, abdominal ultrasound revealed no abnormalities; in particular, no liver abnormalities were present.
Cardiac magnetic resonance imaging confirmed moderate RV dilatation with preserved RV ejection fraction. Flow mapping analysis yielded a TR volume of 54 mL with a regurgitant fraction of 44%. Anomalous pulmonary venous drainage was excluded, and no endomyocardial fibrosis was observed.
Because of intrinsic structural valve disease with severe TR and secondary right ventricular dilatation, the patient underwent minimal invasive surgery of the TV. The TV was tricuspid with the anterior and posterior leaflet having almost equal size. On gross examination, the free edges of the TV showed clear thickening (Figure 2). Papillary muscles were prominent with shortened chordae, and the septal leaflet was retracted. Because of the valve destruction, TV replacement with a bioprosthesis was performed.
Figure 2.

The free edges of the tricuspid valve were thickened and appeared as sclerotic plaque. Papillary muscles were big with very short chordae, and the septal leaflet was retracted.
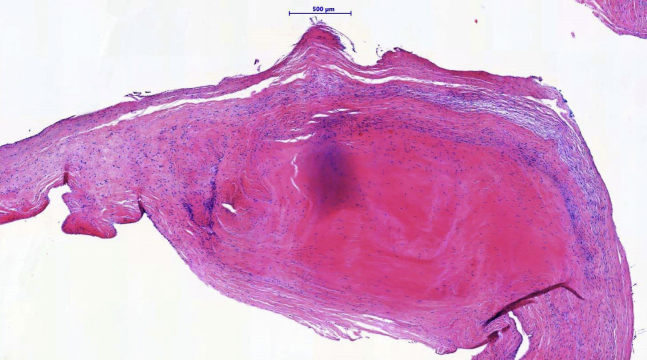
Microscopic analysis of the excised anterior tricuspid leaflet showed proliferation of myofibroblasts embedded in a collagenous and myxoid-enriched stroma. Inflammatory infiltrates, calcifications, and neovascularization were not observed in the specimen. Endocardial deposition of a “stuck-on” carcinoid-like plaque along the ventricular aspect was noted (Figure 3). Histopathological findings were compatible with features of carcinoid-like VHD.
Figure 3.
Histopathology of the anterior leaflet of the tricuspid valve: collagenous and myxoid stroma, with proliferation of myofibroblasts and the presence of a nodular, sclerotic plaque (hematoxylin and eosin staining).
Discussion
Severe structural TV regurgitation was diagnosed in this patient. Based on extensive investigations to rule out other causes of valve disease, we favor the diagnosis of drug-induced VHD (DI-VHD), related to prolonged intake of SSRIs. Although the exact pathophysiological mechanism through which these pharmaceuticals may cause morphological and functional changes of heart valves is not fully understood, it has been observed that DI-VHD is reminiscent of carcinoid heart disease.1, 2, 3 Because of the similarities between DI-VHD and carcinoid heart disease, it was discovered that DI-VHD is mediated through 5-hydroxytryptamine 2β receptor signaling pathways.1,2 Serotonergic agents or their metabolites that specifically interact with this receptor may therefore cause carcinoid-like VHD. Yet the association between SSRI/SNRIs and VHD is controversial, and the pathophysiological mechanisms that may underpin a causal relationship remain enigmatic. Concerning SSRIs, limited and small case-control studies have been reported. Mast et al.4 reported a retrospective drug safety evaluation and failed to establish an association between SSRIs and heart valve regurgitation. A prospective French multicenter study did not support a valvulopathic role of SSRI in patients exposed to benfluorex.5 De Backer et al.6 retrospectively investigated the risk of VHD in relation to the use of SSRIs. They did observe an association between the use of serotonergic antidepressants and a dose-dependent increased rate of valvular regurgitation. In general, the difficulties in interpreting the controversial results in DI-VHD relate to the inherent risk of bias and confounding due to methodological issues in study design, type of populations studied, low numbers of patients included, retrospective nature of studies, short follow-up time, unblinded echocardiographic assessments, and variable definitions of echocardiography-assessed DI-VHD.7
Even if SSRIs or SNRIs truly cause DI-VHD, significant valve dysfunction may be a rare event notwithstanding the large number of patients treated with SSRIs or SNRIs. Rare events are (statistically) challenging with respect to establishing a correct cause-consequence relationship, as other concomitant causes of valve disease may be more prevalent in these patients. Therefore, exclusion of other potential causes for the current degenerative valve phenotype is mandatory. Based on (1) the exclusion of other potential causes for the TV phenotype, (2) the similar degenerative lesions observed on the mitral and aortic valve in our patient, and (3) the observation of stuck-on plaques on histopathological examination, we provide evidence for a SSRI/SNRI-induced valvulopathy. Admittedly, our case report triggered scrutiny on whether the histopathological identification of a “carcinoid-like plaque” is the pathognomonic lesion to diagnose serotonergic DI-VHD. Indeed, reporting bias in previous studies considering serotonergic-mediated valve disease may have neglected the occurrence of such degenerative lesions in other causes or pathways of alternative degenerative valve disease (without performing histologic examinations of resected valves in these cases). Therefore, further research on this issue may be required, and vigilance for potential DI-VHD in clinical practice remains necessary.
Conclusion
Serotonin-related mechanisms have been involved in VHD, as observed in the setting of carcinoid tumors, fenfluramine-phentermine, and ergot alkaloids intake. The association between SSRIs/SNRIs and VHD, however, remains controversial, and only limited case-control studies have been reported. Through extensive diagnostic workup, this case provides evidence for SSRI-induced valvulopathy. The final course of developing disease might be an interplay between the drug's pharmacological properties and a person's intrinsic genetic characteristics and environmental factors. This case report teaches us to maintain vigilance. When initiating drugs that are known to activate serotonin pathways, a risk-benefit analysis should be made on an individual basis, and clinical-echocardiographic surveillance during the treatment course is advised.
Footnotes
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
References
- 1.Fortier J.H., Pizzarotti B., Shaw R.E., Levy R.J., Ferrari G., Grau J. Drug-associated valvular heart diseases and serotonin-related pathways: a meta-analysis. Heart. 2019;105:1140–1148. doi: 10.1136/heartjnl-2018-314403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith S., Waggoner A., de las Fuentes L., Davila-Roman V. Role of serotoninergic pathways in drug-induced valvular heart disease and diagnostic features by echocardiography. J Am SocEchocardiogr. 2009;22:883–889. doi: 10.1016/j.echo.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oates J.A. The carcinoid syndrome. N Engl J Med. 1986;315:702–704. doi: 10.1056/NEJM198609113151109. [DOI] [PubMed] [Google Scholar]
- 4.Mast S., Gersing K., Anstrom K., Krishnan K., Califf R., Jollis J. Association between selective serotonin-reuptake inhibitor therapy and heart valve regurgitation. Am J Cardiol. 2001;87:989–993. doi: 10.1016/s0002-9149(01)01435-7. [DOI] [PubMed] [Google Scholar]
- 5.Maréchaux S., Jeu A., Jobic Y., Ederhy S., Donal E., Reant P. Impact of selective serotonin reuptake inhibitor therapy on heart valves in patients exposed to benfluorex: a multicentre study. Arch Cardiovasc Dis. 2013;106:349–356. doi: 10.1016/j.acvd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 6.De Backer T., Petrovic M., Audenaert K., Coeman M., De Bacquer D. A happy valve in a happy patient? Serotonergic antidepressants and the risk of valvular heart disease (SERVAL). A case-control study. Acta Clin Belg. 2016;71:57–62. doi: 10.1080/17843286.2015.1125563. [DOI] [PubMed] [Google Scholar]
- 7.De Backer T., Timmermans F. The sEROtonergic pathway: a hERO for the brain, a zERO for the valves? Heart. 2019;105:1134–1135. doi: 10.1136/heartjnl-2018-314551. [DOI] [PubMed] [Google Scholar]